A substantial proportion of persons who develop COVID-19 experience postacute sequelae of SARS-CoV-2 infection (PASC). This article reports baseline findings from an ongoing longitudinal cohort study that seeks to characterize the risk factors, clinical findings, laboratory features, and natural history of PASC.
Visual Abstract. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings.

A substantial proportion of persons who develop COVID-19 experience postacute sequelae of SARS-CoV-2 infection (PASC). This article reports baseline findings from an ongoing longitudinal cohort study that seeks to characterize the risk factors, clinical findings, laboratory features, and natural history of PASC.
Abstract
Background:
A substantial proportion of persons who develop COVID-19 report persistent symptoms after acute illness. Various pathophysiologic mechanisms have been implicated in the pathogenesis of postacute sequelae of SARS-CoV-2 infection (PASC).
Objective:
To characterize medical sequelae and persistent symptoms after recovery from COVID-19 in a cohort of disease survivors and controls.
Design:
Cohort study. (ClinicalTrials.gov: NCT04411147)
Setting:
National Institutes of Health Clinical Center, Bethesda, Maryland.
Participants:
Self-referred adults with laboratory-documented SARS-CoV-2 infection who were at least 6 weeks from symptom onset were enrolled regardless of presence of PASC. A control group comprised persons with no history of COVID-19 or serologic evidence of SARS-CoV-2 infection, recruited regardless of their current health status. Both groups were enrolled over the same period and from the same geographic area.
Measurements:
All participants had the same evaluations regardless of presence of symptoms, including physical examination, laboratory tests and questionnaires, cognitive function testing, and cardiopulmonary evaluation. A subset also underwent exploratory immunologic and virologic evaluations.
Results:
189 persons with laboratory-documented COVID-19 (12% of whom were hospitalized during acute illness) and 120 antibody-negative control participants were enrolled. At enrollment, symptoms consistent with PASC were reported by 55% of the COVID-19 cohort and 13% of control participants. Increased risk for PASC was noted in women and those with a history of anxiety disorder. Participants with findings meeting the definition of PASC reported lower quality of life on standardized testing. Abnormal findings on physical examination and diagnostic testing were uncommon. Neutralizing antibody levels to spike protein were negative in 27% of the unvaccinated COVID-19 cohort and none of the vaccinated COVID-19 cohort. Exploratory studies found no evidence of persistent viral infection, autoimmunity, or abnormal immune activation in participants with PASC.
Limitations:
Most participants with COVID-19 had mild to moderate acute illness that did not require hospitalization. The prevalence of reported PASC was likely overestimated in this cohort because persons with PASC may have been more motivated to enroll. The study did not capture PASC that resolved before enrollment.
Conclusion:
A high burden of persistent symptoms was observed in persons after COVID-19. Extensive diagnostic evaluation revealed no specific cause of reported symptoms in most cases. Antibody levels were highly variable after COVID-19.
Primary Funding Source:
Division of Intramural Research, National Institute of Allergy and Infectious Diseases.
SARS-CoV-2 is a novel coronavirus not previously known to infect humans. COVID-19, the clinical syndrome caused by infection with SARS-CoV-2, was first recognized in December 2019. As of April 2022, there have been more than 496 million COVID-19 cases worldwide (1). A significant number of persons who contract COVID-19 report symptoms that persist after the acute illness (2). Current studies of postacute sequelae of SARS-CoV-2 infection (PASC) have largely been based on questionnaire data and analysis of electronic medical records (3–8). To more objectively determine the long-term medical and mental health consequences of COVID-19, a longitudinal cohort study of participants recovering from COVID-19 and control participants without a history of SARS-CoV-2 infection was initiated in June 2020. The primary objectives of the study are to characterize the risk factors, clinical findings, laboratory features, and natural history of PASC. This article describes baseline findings from this ongoing study.
Methods
Study Design
The protocol (Supplement 1) was approved by the National Institutes of Health (NIH) Institutional Review Board. Written informed consent was obtained from all participants. The authors were responsible for the study design, the collection and analysis of the data, and the preparation of the manuscript; they vouch for the accuracy and completeness of the data and the fidelity of the study to the approved protocol.
Enrollment in this study is ongoing. This article contains findings on the 309 participants who enrolled during the first year of the study. Given the lack of publications with detailed clinical information and diagnostic data about PASC, we believe that the data generated by our study to date provide new insights into the nature and severity of this syndrome and offer important information for physicians evaluating and treating these patients.
Participants
This article is part of a longitudinal study being conducted at the NIH Clinical Center in Bethesda, Maryland. Adults with laboratory-confirmed SARS-CoV-2 infection were eligible if they were at least 6 weeks past onset of COVID-19 symptoms, had no fever within 7 days before enrollment, and did not have worsening respiratory symptoms. Persons with asymptomatic disease were eligible 4 weeks after the first positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) test result. Both study groups were recruited from within a 100-mile radius of Bethesda, Maryland, an area that includes Maryland, northern-central Virginia, the District of Columbia, and parts of southern Pennsylvania. For the COVID-19 group, there was no active study recruitment process other than posting details of the study on ClinicalTrials.gov and the NIH Clinical Center websites. Participants in the COVID-19 group were enrolled regardless of the presence of PASC. Adults with no history of COVID-19 were recruited as a control group via the aforementioned websites and the NIH Office of Patient Recruitment Listserv, an e-mail list maintained for persons interested in receiving information about participation in studies being conducted at the NIH Clinical Center. Control participants were recruited without regard for their current health status or active medical conditions and were not intentionally matched with the COVID-19 group for age, gender, race, or other variables. Both groups were required to have a negative result from a nasopharyngeal SARS-CoV-2 RT-PCR test performed at the protocol screening visit. For the COVID-19 group, PASC was defined as any symptom or medical condition that began or worsened after the onset of the COVID-19 illness (or the first positive RT-PCR result for those with asymptomatic infection) and was still present at the study enrollment visit. The mean time from onset of COVID-19 symptoms (or positive RT-PCR result in asymptomatic cases) to study enrollment was 162 days. To maintain balance in the amount of time during which symptoms were counted, for control participants, the only symptoms or conditions that were included for comparison were those that began or worsened no more than 162 days before their enrollment date and were still present at the study enrollment visit.
Baseline Evaluation
All participants underwent evaluations at the enrollment visit, including past medical history, a full review of systems, and physical examination. For past medical history (including details of acute symptoms of COVID-19 and preexisting medical conditions), information was collected from participants during their initial enrollment visit and from review of existing medical records. Thus, examiners were not blinded to study group. In addition, participants in both groups were asked about a set of 17 specific symptoms (see Section 1.4 of Supplement 2) reported to occur after acute COVID-19 (9–11). Blood was collected at the enrollment visit for routine chemical analysis; hematologic assessment; measurement of D-dimer, high sensitivity C-reactive protein, rheumatoid factor, antinuclear antibody, anticardiolipin antibodies, troponin I, pro–B-type natriuretic peptide, serum immunoglobulins, and SARS-CoV-2 antibodies; and research evaluations of immunologic parameters (see Section 1.2 of Supplement 2).
Procedures done at the enrollment visit consisted of neurocognitive assessment, pulmonary function testing, 6-minute walk test, and echocardiography (Section 1.3 of Supplement 2). Cognition was measured with NIH Toolbox tasks that evaluate processing speed, episodic memory, and executive functioning (12). The NIH Toolbox is a validated multidimensional standard set of measures assessing cognitive, emotional, motor, and sensory function (www.healthmeasures.net/explore-measurement-systems/nih-toolbox). Neurocognitive performance on each task was assessed using standardized T scores (mean, 50 [SD, 10]) corrected for demographic variables, including age, gender, race, ethnicity, and education.
Participants were asked to complete online questionnaires about their mental and physical health. This article includes survey data on health-related quality of life, anxiety, and depression. Quality of life was assessed using the Short Form-36 Health Survey (SF-36), version 2, tabulated using QualityMetric software to yield physical and mental health component scores. The SF-36 is an extensively validated survey that applies norm-based scoring based on a mean score of 50 (SD, 10) in the U.S. general population, with higher scores indicating better quality of life (13, 14). Depression and anxiety were assessed using the ultra-brief Patient Health Questionnaire-2 (PHQ-2) and the Generalized Anxiety Disorder-2 (GAD-2). Both questionnaires are validated and standardized assessments of anxiety and depression (15, 16).
Statistical Analysis
Only PASC that were reported by at least 1% of participants in the COVID-19 group were included and compared with the number of participants in the control group reporting the same symptoms. For continuous variables, unadjusted comparisons were made using the t test or the Wilcoxon rank-sum test, and adjusted comparisons were made using multivariate linear regression. For binary variables, unadjusted comparisons were made using the Fisher exact test, and adjusted comparisons were made using multivariate logistic regression. For the COVID-19 group, associations between predictors and the presence of PASC were quantified using the Fisher exact test for binary predictors and univariate logistic regression for continuous predictors. The association between time and binding inhibition percentage was quantified using the Spearman correlation. The Benjamini–Hochberg procedure for controlling the expected false discovery rate at 10% was used to determine which results were significant. All P values are 2-sided. Adjustment variables were specified before analysis based on a subjective synthesis of literature review and clinical experience. Missing data were minimal and were assumed to be missing completely at random. All analyses were performed using R, version 4.1.1 (R Foundation for Statistical Computing). We used Fisher.exact for the Fisher exact test, wilcox.test for the Wilcoxon rank-sum test, t.test for the t test, cor.test and the bootstrap for the Spearman correlation, glm for multivariate regressions, and p.adjust with method=“BH” to calculate false discovery rate–adjusted P values. The original protocol anticipated an approximately equal number of survivors and control participants. Based on this assumption, for a binary outcome with 5% incidence in the control group, using a univariate logistic regression with 200 survivors and 200 control participants would allow for detection of a relative risk of 2.6 with 80% power at a 2-sided significance level of 0.05. Section 1.1 of Supplement 2 provides additional details on the statistical methods, and Section 1.2 of Supplement 2 gives additional details on the analytic methods for the high-dimensional flow cytometry data.
Role of the Funding Source
This study was funded by the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases and, in part, with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. 75N91019D00024. All analyses of biological material were done in a blinded manner at laboratories affiliated with the funding source.
Results
Participants
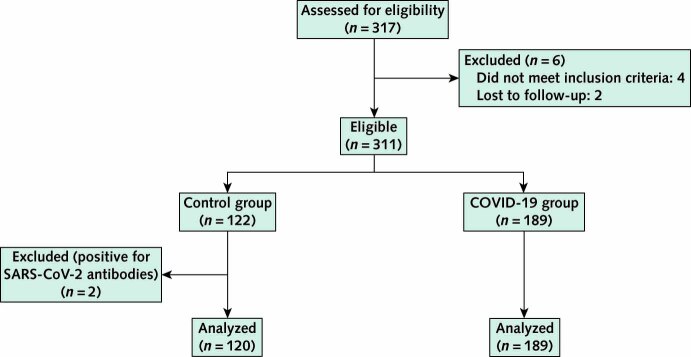
From 30 June 2020 to 1 July 2021, the study enrolled 189 persons with prior laboratory-documented SARS-CoV-2 infection and 122 control participants with no history of COVID-19–like illness. Two of the control participants had antibodies to SARS-CoV-2 nucleocapsid protein and were not included in the analysis (Appendix Figure). The control group was smaller than the COVID-19 group because of slower accrual of the former. Baseline participant characteristics are shown in Table 1, and the period of enrollment in each cohort is shown in Figure 1 of Supplement 2.
Appendix Figure. Study flow diagram.
Table 1.
Baseline Characteristics of Participants
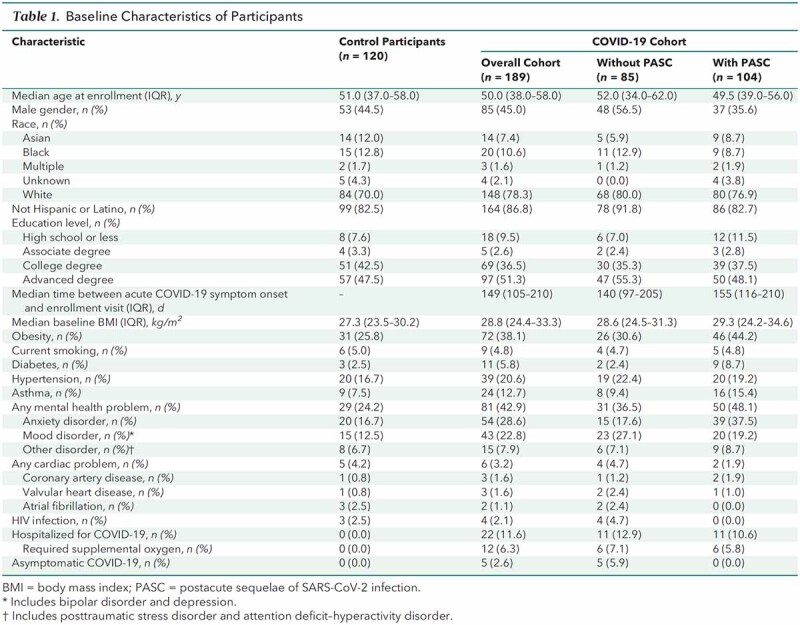
In the COVID-19 group, 167 participants (88%) had an illness that did not require hospitalization. The median time from onset of COVID-19 symptoms (or positive RT-PCR result in asymptomatic cases) to study enrollment was 149 days (IQR, 105 to 210 days). At the time of enrollment, 104 (55%) participants in the COVID-19 group reported 1 or more PASC (Table 1).
Because SARS-CoV-2 vaccines were introduced during the study period, serologic testing for antibody to the SARS-CoV-2 nucleocapsid protein was used to document prior infection. Antibody was detected in 159 participants with COVID-19 at the time of enrollment. Of the 30 participants with COVID-19 who were antibody-negative at enrollment, 27 had documented detection of SARS-CoV-2 RNA by RT-PCR testing of a nasopharyngeal sample. The remaining 3 participants had documented SARS-CoV-2 antinucleocapsid protein antibody detected within 90 days of an acute respiratory illness consistent with COVID-19 but tested negative for antibodies at the enrollment visit. Fourteen of the 30 nucleocapsid antibody–negative participants had 1 or more PASC.
Clinical and Laboratory Findings
Participants in the COVID-19 group reported more symptoms than those in the control group. Table 2 lists the prevalence of specific symptoms reported in both groups. For participants with PASC, the median number of symptoms was 2 (IQR, 1 to 4). The most frequent PASC were fatigue, dyspnea, parosmia, concentration impairment, headache, memory impairment, insomnia, chest discomfort, and anxiety.
Table 2.
Selected Symptoms, Physical Findings, Questionnaires, and Cognitive Testing Results
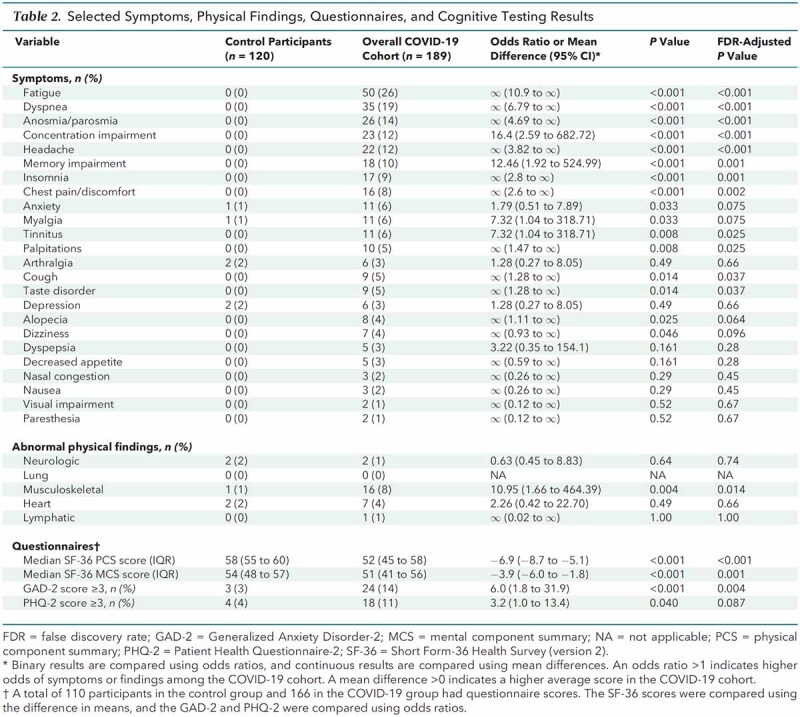
Abnormal findings on physical examination were less common than reported symptoms and did not correlate with the presence of specific symptoms in either group (Table 2; Section 2.1 of Supplement 2). The only significant difference in physical findings between groups was the proportion of participants with abnormal musculoskeletal findings (8% in the COVID-19 group vs. 1% in the control group; odds ratio [OR], 10.95 [95% CI, 1.66 to 464.39]; P = 0.004; adjusted P = 0.014). The most common abnormal musculoskeletal findings in the COVID-19 and control groups, respectively, were localized bursa, muscle, or tendon tenderness (3% vs. 0.8%); unilateral bony swelling consistent with osteoarthritis (1.5% vs. 0%); and postoperative changes (1.0% vs. 0%). No participant in either group had physical findings of inflammatory synovitis or myositis.
Plasma levels of C-reactive protein, D-dimer, and biomarkers of cardiac injury or dysfunction (troponin I, pro–B-type natriuretic peptide) and of brain injury (neurofilament light chain) also did not differ significantly between groups (Table 3). Results of laboratory measurements assessing renal, hepatic, and hematopoietic function did not reveal clinically relevant differences between groups (Table 1 of Supplement 2).
Table 3.
Selected Laboratory and Diagnostic Testing Results*
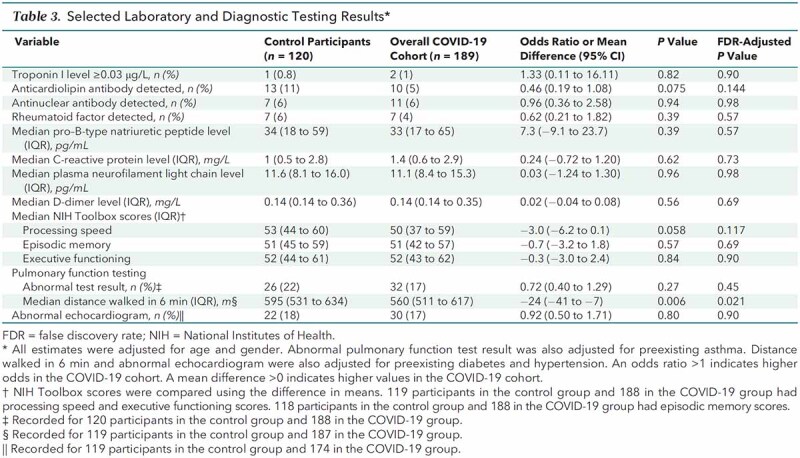
The prevalence of antinuclear antibodies, rheumatoid factor, and anticardiolipin antibodies did not differ significantly between groups (Table 3). No participant in either group who tested positive for an autoantibody had any clinical or laboratory findings compatible with systemic lupus erythematosus, polymyositis, rheumatoid arthritis, or thrombotic events.
The proportion of participants with abnormal findings on pulmonary function testing (spirometry, lung volumes, and diffusion capacity) was similar between groups (Table 3). The most common abnormality reported in both groups was a mild defect in diffusion of carbon monoxide (8% in the COVID-19 group and 13% in the control group) (Table 2 of Supplement 2).
Abnormal findings on transthoracic echocardiography were found in 17% of participants in the COVID-19 group versus 18% in the control group (Table 3). The most common abnormality reported in both groups was chamber enlargement (12% in both groups) (Table 2 of Supplement 2).
The median distance walked during standardized 6-minute walk testing was shorter in the COVID-19 group than the control group (560 vs. 595 m; mean difference, −24 m [CI, −41 to −7 m]; P = 0.006; adjusted P = 0.021) (Table 3). Two participants in the COVID-19 group showed a decrease in oxygen saturation (5% for both) during the walk test, as did 2 in the control group (7% and 4%).
In addition to protocol-defined procedures, other diagnostic testing was performed if clinically indicated to evaluate specific symptoms. The results of that testing are described in Section 2.2 of Supplement 2.
Risk Factors and Associations With PASC
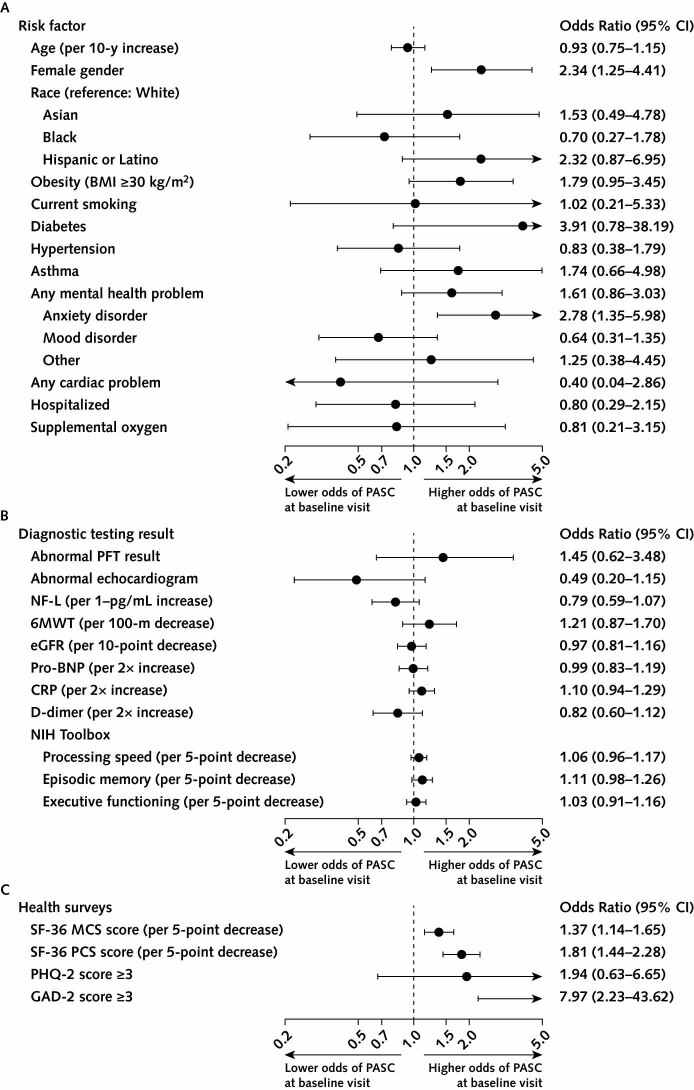
Of the potential pre–COVID-19 risk factors shown in panel A of Figure 1, only female gender (OR, 2.34 [CI, 1.25 to 4.41]; P = 0.005; adjusted P = 0.033) and self-reported history of anxiety disorder (OR, 2.78 [CI, 1.35 to 5.98]; P = 0.003; adjusted P = 0.027) were significantly associated with increased risk for PASC.
Figure 1. Risk factors and associations with PASC.
6MWT = 6-minute walk test distance (in meters); BMI = body mass index; CRP = C-reactive protein; eGFR = estimated glomerular filtration rate; GAD-2 = Generalized Anxiety Disorder-2; MCS = mental component summary; NF-L = neurofilament light chain; NIH = National Institutes of Health; PASC = postacute sequelae of SARS-CoV-2 infection; PCS = physical component summary; PFT = pulmonary function test; PHQ-2 = Patient Health Questionnaire-2; pro-BNP = pro–B-type natriuretic peptide; SF-36 = Short Form-36 Health Survey (version 2). A. Odds ratios with 95% CIs quantifying univariate associations between pre–COVID-19 characteristics and presence of any PASC at the baseline visit. Mood disorders include bipolar disorder and depression. B. Odds ratios with 95% CIs quantifying univariate associations between results of diagnostic testing done at the baseline visit and presence of any PASC. C. Odds ratios with 95% CIs quantifying univariate associations between scores on health surveys completed by participants at the baseline visit and presence of PASC.
We found minimal to no associations between results of diagnostic testing and presence of PASC (Figure 1, B). The distance walked in 6 minutes, an abnormal pulmonary function test result, or an abnormal echocardiogram were not significantly associated with the presence of PASC (Figure 1, B) or the presence of persistent cardiopulmonary symptoms (dyspnea, chest pain, cough, palpitations), neurologic symptoms (concentration impairment, memory impairment, headache, parosmia, paresthesia), or fatigue (Figure 2 of Supplement 2). Laboratory biomarkers of inflammation (C-reactive protein, D-dimer) and organ-specific tissue damage (pro–B-type natriuretic peptide, troponin I, neurofilament light chain, estimated glomerular filtration rate) were also not significantly associated with PASC or with persistent fatigue, cardiopulmonary symptoms, or neurologic symptoms (Figure 1, B; Figure 2 of Supplement 2).
Neurocognitive Testing and Health Surveys
No significant differences were found between groups in NIH Toolbox scores for processing speed, episodic memory, and executive functioning (Table 3). Performance scores for the 3 NIH Toolbox domains were not significantly associated with PASC or with persistent fatigue, neurologic symptoms, or cardiopulmonary symptoms (Figure 1, B; Figure 2 of Supplement 2).
Quality of life was assessed using the SF-36 physical and mental health component scores. Scores were lower in participants in the COVID-19 group than the control group (Table 2). This difference was driven by participants with PASC, who had significantly lower values for both the physical and mental health component scores compared with those without PASC (Figure 3 of Supplement 2). Lower SF-36 scores were associated with presence of PASC (Figure 1, C).
The GAD-2 and PHQ-2 surveys were used to screen for current anxiety and depression symptoms, respectively. A total score of 3 or greater is a recommended cutoff on each measure for identifying persons who warrant further evaluation for generalized anxiety or depressive disorder (16). The proportion of participants with GAD-2 anxiety scores above the cutoff was significantly higher in the COVID-19 group than in the control group (14% vs. 3%; OR, 6.0 [CI, 1.8 to 31.9]; P < 0.001; adjusted P = 0.004) (Table 2). A GAD-2 score of 3 or above was significantly associated with the presence of any PASC (OR, 7.97 [CI, 2.23 to 43.62]; P < 0.001; adjusted P = 0.004) (Figure 1, C). The proportion of participants in the COVID-19 group with a PHQ-2 depression score above the cutoff was also significantly higher (11% vs. 4%; OR, 3.2 [CI, 1.0 to 13.4]; P = 0.040; adjusted P = 0.087) (Table 2); however, a higher PHQ-2 score was not significantly associated with the presence of PASC (OR, 1.94 [CI, 0.63 to 6.65]) (Figure 1, C).
Immunologic and Virologic Studies
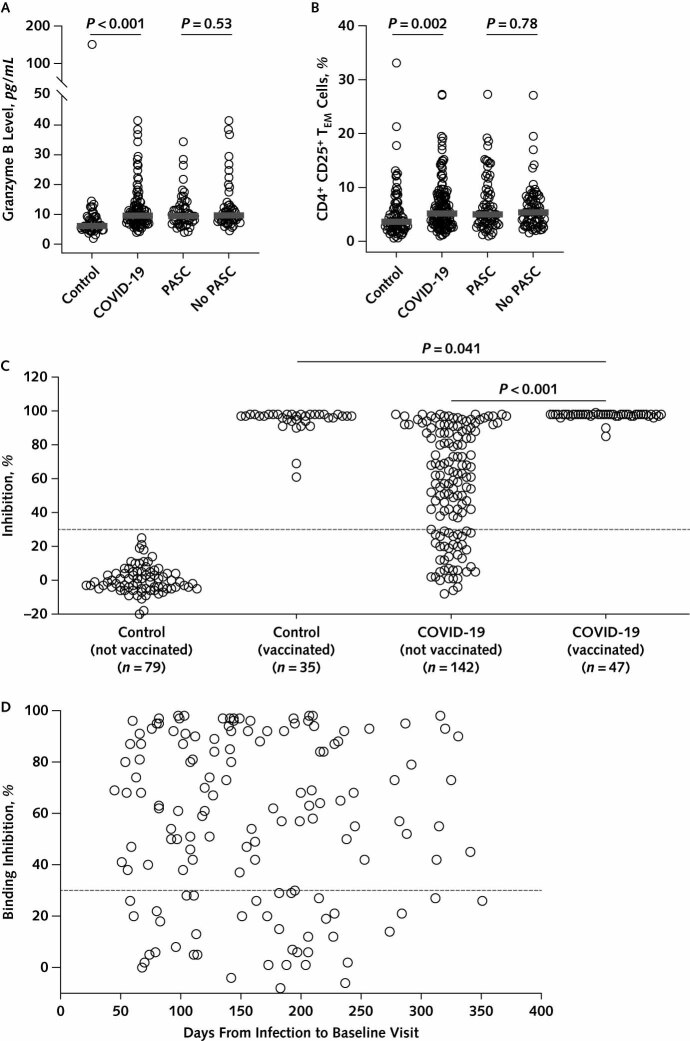
To address the possibility that persistent activation of the immune system might play a role in the pathogenesis of PASC, plasma samples from a subgroup of participants were selected for inflammatory biomarker analysis. Because recent vaccination could affect plasma levels of inflammatory biomarkers and confound the interpretation of results, we selected samples from a subgroup of 48 participants with PASC, 52 without PASC, and 50 control participants who had not received a SARS-CoV-2 vaccine before blood sample collection. No significant differences were detected between groups in plasma levels of macrophage inflammatory protein-1β, interferon-γ, tumor necrosis factor-α, programmed cell death ligand-1, interferon γ–induced protein 10, interleukin-2 receptor α, interleukin-1β, interleukin-6, interleukin-8, RANTES (regulated on activation, normal T cell expressed and secreted), and CD40 (Figure 4 of Supplement 2). We did find that plasma levels of granzyme B were higher in the COVID-19 group (P < 0.001; adjusted P < 0.001) (Figure 2, A); however, no significant difference in granzyme B levels was found between participants with and without PASC (P = 0.53) (Figure 2, A).
Figure 2. Characterization of immune parameters in study participants.
PASC = postacute sequelae of SARS-CoV-2 infection; TEM = effector memory T cells. A. Plasma levels of granzyme B among study participants. P values were calculated using the Wilcoxon rank-sum test. B. Comparison of the frequency of CD4+ CD25+ TEM cells (cluster 6) in the peripheral blood of study participants. P values were calculated using the Wilcoxon rank-sum test. C. Percentage inhibition of angiotensin-converting enzyme 2 (ACE2) receptor binding to the SARS-CoV-2 receptor-binding domain (RBD) in sera from study participants, using a surrogate neutralizing antibody binding assay (17). The dashed line represents the assay cutoff (30% inhibition). “Not vaccinated” refers to participants who had not received a SARS-CoV-2 vaccine dose before study enrollment, and “vaccinated” refers to participants who had received ≥1 SARS-CoV-2 vaccine dose after infection but before study enrollment. P values were calculated using the t test. D. Percentage inhibition of ACE2 receptor binding to the SARS-CoV-2 RBD as a function of time from COVID-19 symptom onset to study enrollment. The dashed line represents the assay cutoff (30% inhibition).
High-dimensional flow cytometry was performed on peripheral blood T cells from a subset of 139 unvaccinated participants in the COVID-19 group and 78 unvaccinated control participants to evaluate phenotypic markers of T-lymphocyte activation. Frequencies of CD4+ and CD8+ T-lymphocyte subsets did not differ significantly between groups (Figure 5 of Supplement 2). Optimized t-distributed stochastic neighbor embedding and FlowSOM analyses identified 15 distinct T-cell clusters (Figure 6 of Supplement 2). Among these, only the frequency of CD4+ T-cell cluster 6, an effector memory phenotype with elevated expression of CD25, met the criteria for significance, being higher in the COVID-19 group than in the control group (P = 0.002; adjusted P = 0.029) (Figure 2, B; Figure 6 of Supplement 2). However, there was no significant difference for this T-cell subpopulation in the COVID-19 group between those with and those without PASC (P = 0.78) (Figure 2, B).
To assess for evidence of persistent SARS-CoV-2 infection in the COVID-19 cohort, plasma samples from 125 participants (63 with PASC and 62 without) were tested for SARS-CoV-2 nucleocapsid protein. One participant without PASC had a low-level positive result (10 pg/mL [assay cutoff, 3 pg/mL]), and a single participant with PASC had a detectable signal that was below 3 pg/mL. No signal was detected in the remaining 121 samples.
Serologic Testing
All participants were tested for antibodies to the SARS-CoV-2 spike protein using a surrogate neutralizing antibody binding assay (17). For the 142 participants in the COVID-19 group who had not received a SARS-CoV-2 vaccine before the enrollment visit, the median binding inhibition level was 61.5% (IQR, 28.0% to 88.8%), with 39 (27%) participants showing a level below the assay cutoff of 30% (Figure 2, C). The percentage binding inhibition in the unvaccinated COVID-19 group did not correlate with time from COVID-19 symptom onset to study enrollment (Spearman correlation, −0.03 [CI, −0.19 to 0.13]; P = 0.71) (Figure 2, D).
Forty-seven (25%) of the participants in the COVID-19 group received a SARS-CoV-2 vaccine after infection but before study enrollment, and 35 (29%) control participants were vaccinated before enrollment. The median time from vaccination to the baseline visit was 31 days (IQR, 15.5 to 57 days) in the COVID-19 group and 45 days (IQR, 27 to 94 days) in the control group. For the COVID-19 group, the percentage inhibition value in those who were vaccinated before enrollment was higher than in those who had not been vaccinated (mean difference, 40.3% [CI, 34.8% to 45.6%]; P < 0.001) (Figure 2, C). The percentage inhibition was similar between vaccinated participants in the COVID-19 group and vaccinated control participants (mean difference, 2.9% [CI, 0.1% to 5.6%]; P = 0.041) (Figure 2, C).
Discussion
In this COVID-19 cohort, 55% of participants reported 1 or more persistent postacute symptoms, such as fatigue, dyspnea, chest discomfort, parosmia, headache, insomnia, memory impairment, anxiety, and concentration impairment. These symptoms are similar to what has been reported in questionnaire-based studies of PASC (3–7). In our cohort, the risk factors for developing PASC were female gender and pre–COVID-19 history of anxiety.
In contrast to other reports describing persistent post–COVID-19 symptoms (3–8, 18), our study used protocol-prespecified diagnostic evaluations that were conducted in all participants. We enrolled persons with a spectrum of initial COVID-19 disease severity and without regard for the presence of PASC. In addition, we concurrently enrolled a control group similar to the COVID-19 group in age and demographic characteristics with no clinical history or serologic evidence of prior SARS-CoV-2 infection. Thus, we were able to compare findings in persons with PASC versus both a control group without evidence of SARS-CoV-2 infection and a COVID-19 group without PASC.
Abnormal findings on physical examination and routine laboratory evaluation were uncommon and occurred with similar frequency in the COVID-19 and control groups. Levels of plasma inflammatory markers, levels of biomarkers for cardiac and central nervous system injury, and presence of select autoantibodies were similar between groups and were not associated with the presence of PASC. Results of pulmonary function testing, 6-minute walk testing, and echocardiograms were normal in the majority of participants in both groups. Mild to moderate abnormalities on pulmonary function testing and echocardiography were detected at a similar frequency in both groups and were not associated with the presence of PASC. No significant differences were found in neurocognitive testing scores between groups, and scores were not associated with PASC or self-reported post–COVID-19 neurocognitive symptoms.
Despite the largely normal findings on objective testing, the presence of PASC had a significant effect on self-reported physical and mental health. Participants with PASC reported lower quality of life than either participants with COVID-19 without PASC or control participants, as measured by the mental and physical health components of the SF-36 Health Survey. Self-reported current anxiety, as measured by the GAD-2 questionnaire, was significantly associated with PASC. This finding suggests that reported anxiety after COVID-19 may reflect the uncertainty and worry felt by those experiencing persistent unexplained symptoms.
Aberrant immune activation, possibly secondary to persistent SARS-CoV-2 infection, has been suggested as a cause of PASC (19, 20). We did not find evidence of persistent viral infection when we tested for SARS-CoV-2 RNA in nasopharyngeal specimens and the presence of viral nucleocapsid protein in plasma. These negative results cannot rule out possible occult viral infection in deep tissues that cannot be easily accessed. Using measurements of soluble markers of inflammation and high-dimensional T-cell phenotyping, we were unable to find evidence of ongoing systemic inflammation or immune activation in participants in the COVID-19 group who had PASC. Only plasma levels of granzyme B and a CD4+ CD25+ T-lymphocyte subset with an effector memory phenotype were significantly increased in the COVID-19 group compared with control participants. However, neither of these parameters differed significantly in participants with versus without PASC, suggesting they reflect recent SARS-CoV-2 infection rather than being a factor in the pathogenesis of PASC. These negative findings combined with the lack of objective evidence of tissue damage or organ dysfunction on diagnostic evaluations suggest that persistent, abnormal immune activation, if present, is not causing ongoing organ damage in persons in our COVID-19 cohort.
Evaluation of neutralizing antibodies using a surrogate binding inhibition assay showed that 39 of the 142 unvaccinated participants in our COVID-19 cohort had binding inhibition levels below the cutoff of 30% and thus would be considered antibody-negative by this emergency use–authorized test. The 47 participants in the COVID-19 cohort who were vaccinated after infection but before study enrollment showed significantly higher antibody levels than the unvaccinated group, with all having binding inhibition levels greater than 85%. These findings indicate that there may be a subpopulation of recovered patients with antibody concentrations below a protective level and suggest that vaccination after natural infection provides a significant boost in neutralizing antibodies.
Our results have several limitations. First, most participants with COVID-19 in our study had mild to moderate initial illness that did not require hospitalization. Thus, our findings may not represent the full spectrum and severity of PASC experienced by persons with severe disease requiring hospitalization. Second, although we enrolled participants regardless of the presence of PASC, our study probably overestimated the prevalence of persistent post–COVID-19 symptoms because persons with PASC were likely more motivated to enroll. Third, control participants were not intentionally age- or gender-matched to participants with COVID-19. Fourth, PASC that resolved before study enrollment were not captured in our cohort. Finally, this report contains detailed data on 189 COVID-19 survivors, a sample size that may not fully capture all PASC.
In summary, these initial observations in a cohort of persons with predominantly mild to moderate COVID-19 and control participants without evidence of prior SARS-CoV-2 infection provide insights into the nature and severity of PASC. For participants with PASC, an extensive diagnostic evaluation failed to reveal a cause of reported symptoms in most cases. Exploratory studies did not show evidence of abnormal systemic immune activation or persistent viral infection in participants with PASC. The constellation of subjective symptoms in the absence of objective abnormalities on diagnostic evaluation resembles what has been described with other illnesses, including chronic fatigue syndrome/myalgic encephalomyelitis (21), postinfection syndromes described after resolution of certain viral and bacterial infections (22–25), and mental health disorders such as depression and anxiety (26). The pathogenesis of PASC remains unclear and requires further study.
Supplementary Material
Footnotes
This article was published at Annals.org on 24 May 2022.
References
- 1. World Health Organization. COVID-19 Weekly Epidemiological Update. Edition 87. 12 April 2022.
- 2. Nalbandian A,Sehgal K,Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-15. [PMID: ] doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blomberg B,Mohn KG,Brokstad KA, et al; Bergen COVID-19 Research Group. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607-13. [PMID: ] doi: 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Logue JK,Franko NM,McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. [PMID: ] doi: 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menges D,Ballouz T,Anagnostopoulos A, et al. Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One. 2021;16:e0254523. [PMID: ] doi: 10.1371/journal.pone.0254523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taquet M,Dercon Q,Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:e1003773. [PMID: ] doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Townsend L,Dyer AH,Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15:e0240784. [PMID: ] doi: 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Aly Z,Xie Y,Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-64. [PMID: ] doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 9. Carfì A,Bernabei R,Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603-5. [PMID: ] doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopkins C,Surda P,Whitehead E, et al. Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49:26. [PMID: ] doi: 10.1186/s40463-020-00423-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tenforde MW,Kim SS,Lindsell CJ, et al; IVY Network Investigators. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993-8. [PMID: ] doi: 10.15585/mmwr.mm6930e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gershon RC,Cella D,Fox NA, et al. Assessment of neurological and behavioural function: the NIH Toolbox [Letter]. Lancet Neurol. 2010;9:138-9. [PMID: ] doi: 10.1016/S1474-4422(09)70335-7 [DOI] [PubMed] [Google Scholar]
- 13. Scoggins JF,Patrick DL. The use of patient-reported outcomes instruments in registered clinical trials: evidence from ClinicalTrials.gov. Contemp Clin Trials. 2009;30:289-92. [PMID: ] doi: 10.1016/j.cct.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ware JE Jr. Using generic measures of functional health and well-being to increase understanding of disease burden [Editorial]. Spine (Phila Pa 1976). 2000;25:1467. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 15. Kroenke K,Spitzer RL,Williams JB, et al. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50:613-21. [PMID: ] doi: 10.1176/appi.psy.50.6.613 [DOI] [PubMed] [Google Scholar]
- 16. Löwe B,Wahl I,Rose M, et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122:86-95. [PMID: ] doi: 10.1016/j.jad.2009.06.019 [DOI] [PubMed] [Google Scholar]
- 17. Tan CW,Chia WN,Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073-8. [PMID: ] doi: 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 18. Huang L,Yao Q,Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747-58. [PMID: ] doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peluso MJ,Lu S,Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224:1839-48. [PMID: ] doi: 10.1093/infdis/jiab490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips S,Williams MA. Confronting our next national health disaster—long-haul Covid. N Engl J Med. 2021;385:577-9. [PMID: ] doi: 10.1056/NEJMp2109285 [DOI] [PubMed] [Google Scholar]
- 21. Prins JB,van der Meer JW,Bleijenberg G. Chronic fatigue syndrome. Lancet. 2006;367:346-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 22. Carson PJ,Konewko P,Wold KS, et al. Long-term clinical and neuropsychological outcomes of West Nile virus infection. Clin Infect Dis. 2006;43:723-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 23. Lettinga KD,Verbon A,Nieuwkerk PT, et al. Health-related quality of life and posttraumatic stress disorder among survivors of an outbreak of Legionnaires disease. Clin Infect Dis. 2002;35:11-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24. White PD,Thomas JM,Kangro HO, et al. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet. 2001;358:1946-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25. Wills AB,Spaulding AB,Adjemian J, et al. Long-term follow-up of patients with Lyme disease: longitudinal analysis of clinical and quality-of-life measures. Clin Infect Dis. 2016;62:1546-51. [PMID: ] doi: 10.1093/cid/ciw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon GE,VonKorff M,Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341:1329-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.