Abstract
Neuromyelitis optica is an autoimmune inflammatory disorder targeting aquaporin-4 water channels in CNS astrocytes. Histopathological descriptions of astrocytic lesions reported in neuromyelitis optica so far have emphasized a characteristic loss of aquaporin-4, with deposition of IgG and complement and lysis of astrocytes, but sublytic reactions have been underappreciated.
We performed a multi-modality study of 23 neuromyelitis optica autopsy cases (clinically and/or pathologically confirmed; 337 tissue blocks). By evaluating astrocytic morphology, immunohistochemistry and AQP4 RNA transcripts, and their associations with demyelinating activity, we documented a spectrum of astrocytopathy in addition to complement deposition, microglial reaction, granulocyte infiltration and regenerating activity.
Within advanced demyelinating lesions, and in periplaque areas, there was remarkable hypertrophic astrogliosis, more subtle than astrocytic lysis. A degenerative component was suggested by ‘dystrophic’ morphology, cytoplasmic vacuolation, Rosenthal fibres and associated stress protein markers. The abundance of AQP4 mRNA transcripts in sublytic reactive astrocytes devoid of aquaporin-4 protein supported in vivo restoration following IgG-induced aquaporin-4 endocytosis/degradation. Astrocytic alterations extending beyond demyelinating lesions speak to astrocytopathy being an early and primary event in the evolving neuromyelitis optica lesion. Focal astrocytopathy observed without aquaporin-4 loss or lytic complement component deposition verifies that astrocytic reactions in neuromyelitis optica are not solely dependent on IgG-mediated aquaporin-4 loss or lysis by complement or by IgG-dependent leucocyte mediators.
We conclude that neuromyelitis optica reflects a global astrocytopathy, initiated by binding of IgG to aquaporin-4 and not simply definable by demyelination and astrocytic lysis. The spectrum of astrocytic morphological changes in neuromyelitis optica attests to the complexity of factors influencing the range of astrocytic physiological responses to a targeted attack by aquaporin-4-specific IgG. Sublytic astrocytic reactions are no doubt an important determinant of the lesion’s evolution and potential for repair. Pharmacological manipulation of the astrocytic stress response may offer new avenues for therapeutic intervention.
Keywords: astrocytes, autoimmune, gliosis, Rosenthal fibres, emperipolesis
Guo et al. describe global sublytic astrocytic changes in neuromyelitis optica, beyond demyelinated lesions. Compensatory AQP4 synthesis and leucocyte emperipolesis indicate endogenous repair and restriction of infiltrating immune cells. Stress responses independent of AQP4 loss, or complement/leucocyte lysis, favour therapeutic intervention.
Introduction
Neuromyelitis optica (NMO) is a disabling CNS inflammatory autoimmune disease, characterized by recurrent severe attacks of optic neuritis and transverse myelitis.1 A serum autoantibody specific for aquaporin-4 water channel (AQP4) aids the distinction of NMO from other CNS demyelinating diseases.2 AQP4 is the principal CNS water channel, and is expressed predominantly in astrocytes. Astrocytopathic features recognized in NMO include cytolysis and clasmatodendrosis, as well as AQP4 loss.1,3 AQP4-specific IgG is acknowledged to be the primary driver of NMO neuropathology.4,5 The range of astrocyte reactions occurring in NMO is incompletely characterized, beyond loss of AQP4 and cytolysis. Knowledge of sublytic reactions, the frequency of astrocytic morphological differences and their relationship to demyelinating lesions represent a particular void.
The classic astrocytic reaction, termed ‘reactive astrogliosis’, ‘reactive gliosis’ or ‘gliosis’, manifests as a proliferation of hypertrophic astrocytes with upregulated glial fibrillary acidic protein (GFAP) immunoreactivity.6-9 Does ‘hypertrophic’ morphological change always happen during astrocytic reactions? Astrogliosis is considered a nonspecific, chronic reaction to a wide variety of insults. Most observations are based on CNS diseases that do not selectively target astrocytes. Little is known about the spectrum of astrocytic reactions occurring in primary astrocytopathies, which reflect both the initial astrocytic response and secondary cellular interactions of astrocytes undergoing lethal stress.
NMO provides a unique opportunity to observe the astrocytic response to a cell-specific attack. The best-studied of the few identified primary astrocytic disorders is Alexander disease, a genetic disorder that presents clinically as a leukoencephalopathy.8 Most cases are due to GFAP gene mutations. Similarities and differences in the astrocyte reactions of Alexander disease and NMO remain to be addressed. Until now, models of autoimmune astrocytopathy induced in experimental animals by injecting AQP4-IgG only partly recapitulate histopathological features of NMO.1,3,10–13 Human astrocytes are more complex and diverse than rodents’.14 The direct study of human CNS tissues is invaluable for defining subtle pathological features of NMO. To record the spectrum and frequency of astrocyte pathology in NMO, we present here findings gained from a systematic histopathological, immunohistochemical and molecular biological study of astrocytes in brain, spinal cord and optic nerve tissues obtained from autopsied NMO patients.
Material and methods
Study design and series
This study was approved by the Institutional Review Board of Mayo Clinic, Rochester, MN (IRB 2067–99). Inclusion criteria were: (i) clinical and pathological diagnosis of NMO or NMOSD and no evidence of an alternative diagnosis; and (ii) available archival tissue for pathological analysis. Twenty-three autopsy cases met the inclusion criteria and 337 tissue blocks were included. The mean number of blocks per case was 15.9 (range 4–36). Table 1 provides demographics for the patient cohort. For simplicity, we use the terminology NMO throughout this communication.
Table 1.
Clinical demographics
| Characteristic | Summary |
|---|---|
| Number of subjects | 23 |
| Sex, male:female | 2:21 |
| Age at symptom onset, years | 49 (12–73) |
| Age at death, years | 52 (16–80) |
| Diagnosis, n (%) | |
| NMO | 18 (78%) |
| NMO spectrum disorder | 5 (22%) |
| AQP4-IgG serostatus, positive: negativea | 9:0 |
| Number of clinical attacks | 3 (2–7) |
| Disease duration, months | 36 (8–240) |
Values shown are median (range); age of symptom onset and disease duration missing for one patient; number of clinical attacks missing for two patients; AQP4-IgG serology missing for 14 patients.
Sera available for testing in nine patients. Other subjects either lacked sera or preceded the availability of serological testing.
Histochemistry
Formalin-fixed paraffin-embedded 5-µm thick sections were stained with haematoxylin and eosin (H&E), Luxol fast blue and periodic acid Schiff (LFB/PAS) for myelin, and modified Bielschowsky silver for axons. Immunohistochemistry was performed with the avidin–biotin-complex method,13 using primary antibodies specific for GFAP (1:100, DAKO), neurofilament protein (1:800, steam antigen retrieval with citric acid buffer pH 6.0, DAKO), AQP4 (1:250, Sigma-Aldrich), myelin proteolipid protein (PLP) (1:500, Serotec), myelin-associated glycoprotein (MAG) (1:1000, Abcam), myelin-oligodendrocyte glycoprotein (MOG) (1:1000, Abcam), CD68 KP1 (1:100, DAKO), C9neo (monoclonal B7 and polyclonal, 1:200, from Professor Paul Morgan, Cardiff, UK) and Alpha-B-crystallin (1:1000, Millipore).
Histopathological evaluation
To evaluate histopathological features of NMO lesions and their associations, we reviewed stained tissue sections using an Olympus BX51 microscope with special attention to: tissue destruction, demyelinating activity, complement product deposition, AQP4 immunoreactivity, microglial reaction, granulocyte infiltration and astrocyte morphology. Specific astrocyte morphological changes evaluated or graded were: (i) cell body profile: (a) normal, unremarkable change in cellular size; (b) hypertrophic, increased size of cell bodies and (c) dystrophic, decreased or variable cellular size with irregular configuration and decreased finely branching processes; (ii) bipolar or unipolar morphology; (iii) nucleus, single or multiple nuclei; (iv) lysis, loss of astrocytes, astrocytic fragments present and/or GFAP-positive debris within or outside macrophages; and (v) Rosenthal fibres, mild [one to two/high-power microscopic field (HPF)], moderate (three to nine/HPF) and marked (≥10/HPF).
The following four stages of demyelinating activity were defined based on myelin debris within macrophages15: (i) early active demyelination, myelin debris within macrophages immunoreactive for both minor (MOG, MAG) and major myelin proteins (PLP); (ii) late active demyelination, macrophages immunoreactive for major myelin proteins only; (iii) inactive demyelination, absence of myelin-laden macrophages; and (iv) periplaque white matter, which is non-demyelinated white matter around a demyelinating plaque.
To specify the topographic association and frequency of these pathological features of NMO, we classified the aforementioned for each block. We coded each feature (e.g. demyelination, AQP4 loss, astrocyte abnormality) and defined the region of interest with a combination of codes. We recorded regions of interest with the same combination of features only once per tissue block, to avoid repeatedly recording small lesions with the same pathological features. Subsequently, the frequencies of pathological features were calculated to reflect the percentage of blocks and patients for whom a given abnormality was found. In total, we analysed 337 blocks from 23 CNS autopsies and classified 1048 regions of interest. The number of regions of interest for each specific pathological feature is <1048 because: (i) the specific pathological feature was sometimes lacking; (ii) regions of interest were sometimes lacking due to exhaustion of tissue blocks; and (iii) limited availability of unstained sections. The actual number of regions of interest analysed for any specific pathological feature is listed in the ‘Results’ section.
In situ hybridization
PCR was performed targeting human AQP4 mRNA sequences with upstream primer 5ʹ-gcatgagtgacagacccaca-3ʹ and downstream primer 5ʹ-catggccagaaattccgctg-3ʹ. The 137-basepair PCR product was subcloned into the pGEM-T vector (Promega) and confirmed by DNA sequencing and BLAST in the NCBI database. The plasmid was then amplified by transformation into the JM109 High-Efficiency Competent Cells (Promega). The extracted and purified plasmid was then linearized with NcoI or SalI and subsequently used for in vitro RNA transcription with Sp6 or T7 RNA polymerase to generate antisense or sense probes, respectively. The RNA probe was labelled with digoxigenin with the DIG RNA labelling kit (Roche). The sequence of the sense probe is 5ʹ-gcatgagtgacagacccacagca aggcggtggggtaagtgtggacct ttgtgtaccagagagaacatcatggt ggctttcaaaggggtctggactcaagcttt ctggaaagcagtcacagcggaatttctggccatg-3ʹ. In situ hybridization followed a previously described method.16 Specificity for the in situ hybridization study was controlled by comparing hybridization with antisense and sense probes in both NMO tissue and normal CNS control tissue. The colour reaction developed with NBT/BCIP system (Promega) appeared purple-blue when tissue sections yielded positive RNA signals. To confirm that the detected AQP4 mRNA resided in astrocytes, we subsequently stained for GFAP immunoreactivity using DAB substrate (Sigma); GFAP signals appeared brown.
Data availability
More detailed data are provided in the Supplementary material. The original pathology slides and clinical information that support findings in this study are available from the corresponding author on reasonable request.
Results
Classic astrocyte pathology in neuromyelitis optica
Consistent with previous reports of NMO pathology, including our own,3,13,17 the present group of NMO autopsies exhibited classical features of astrocytopathy, including astrocytic lysis (Supplementary Fig. 1), AQP4 loss, hypertrophic and ‘dystrophic’ astrocytes (Supplementary Fig. 2), cytoplasmic vacuolation (Supplementary Fig. 3), variable nuclear number and morphology, and bipolar/unipolar astrocytes (Supplementary Fig. 4). Astrocytopathy was more severe in demyelinating lesions. However, evidence of astrocytopathy extended beyond demyelinating lesions (Supplementary Fig. 5). The Supplementary material summarizes detailed semi-qualitative analysis of these classic astrocytopathies, including relationships to lesional stage.
Rosenthal fibres: evidence of astrocyte stress in neuromyelitis optica
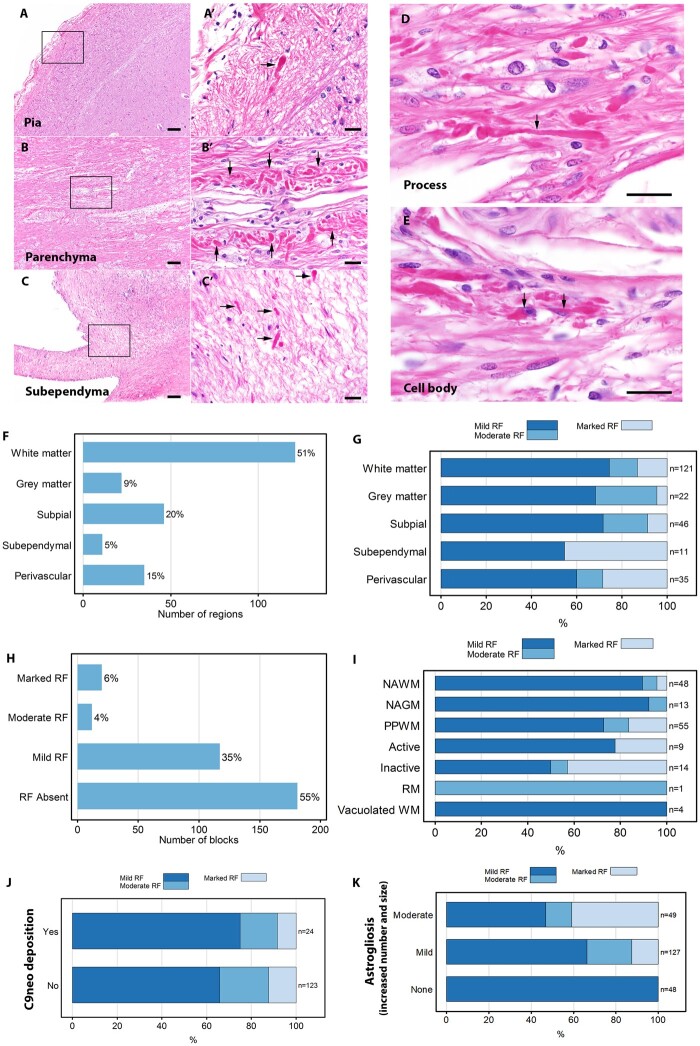
Rosenthal fibres, seen by H&E staining as eosinophilic rod-shaped protein-aggregates in astrocytic processes, have been reported in Alexander disease, a variety of reactive and neoplastic disorders (e.g. astrocytoma) and occasionally in multiple sclerosis.18,19 They contain GFAP, ubiquitin, α-B-crystallin and heat shock proteins. We found Rosenthal fibres in 96% of NMO cases (22/23), in both lesional and non-lesional areas. In 52% of cases (12/23) Rosenthal fibres were located in foci of moderate or marked high-density (Fig. 1). Semi-quantitative analyses revealed most Rosenthal fibres were typically scattered throughout in a low-density pattern (78%), but 22% occurred in moderate and marked high-density patterns (Fig. 1). Immunohistochemically, they were α-B-crystallin-positive and variably positive for GFAP (Fig. 2). Rosenthal fibres were located mainly in processes or peripheral regions of astrocytes, and occasionally in perinuclear cytoplasm. Most regions with evidence of Rosenthal fibre deposition also showed gliosis, and the fibres tended to occur in highest density in regions with more severe astrogliosis (Fig. 1N). Regions with Rosenthal fibres showed both well-preserved astrocytes (74%, 114/154) and astrocytic lysis (26%, 40/154). Rosenthal fibres were distributed in subpial, perivascular and subependymal regions, as well as in white and grey matter (Fig. 1), in lesions of all stages, and in both the centre and periphery of lesions. They also extended beyond areas of demyelination (Fig. 2), but were more abundant in demyelinated lesions than in non-demyelinated areas. Rosenthal fibres were observed more frequently in inactive demyelinating lesions (32%, 35/109) than in active lesions (16%, 31/198) (Supplementary Fig. 5).
Figure 1.
Distribution of Rosenthal fibres in NMO. (A–C) Rosenthal fibres (RFs) in subpial (A, non-lesion area), perivascular parenchyma (B, within an active demyelinating lesion) and subependymal regions (C, non-lesion area) appear as amorphous pink structures by haematoxylin and eosin staining (H&E). (A′–C′) Magnified images of the framed regions (A–C) show highly eosinophilic homogeneous intracellular inclusions characteristic of RFs (arrows). RFs are most abundant in astrocytic processes (D, arrow), but some are seen in cell bodies (E, arrow). (F) The column schematic figures illustrate that most RFs are in white matter. (G) RF density relative to anatomical distribution. (H) Forty-five per cent of analysed CNS tissue blocks show RFs, predominantly in low density. (I) RF density relative to demyelinating activity. (J) Most RFs are in regions lacking complement membrane attack complex (C9neo) deposits. (K) RFs tended to occur in the highest density in regions with more severe astrogliosis. Scale bars = 100 µm (A–C) and 20 µm (A′–C′, D and E). Active = active demyelination; Inactive = inactive demyelination; NAGM = normal-appearing grey matter; NAWM = normal-appearing white matter; PPWM = periplaque white matter; RM = remyelination; WM = white matter.
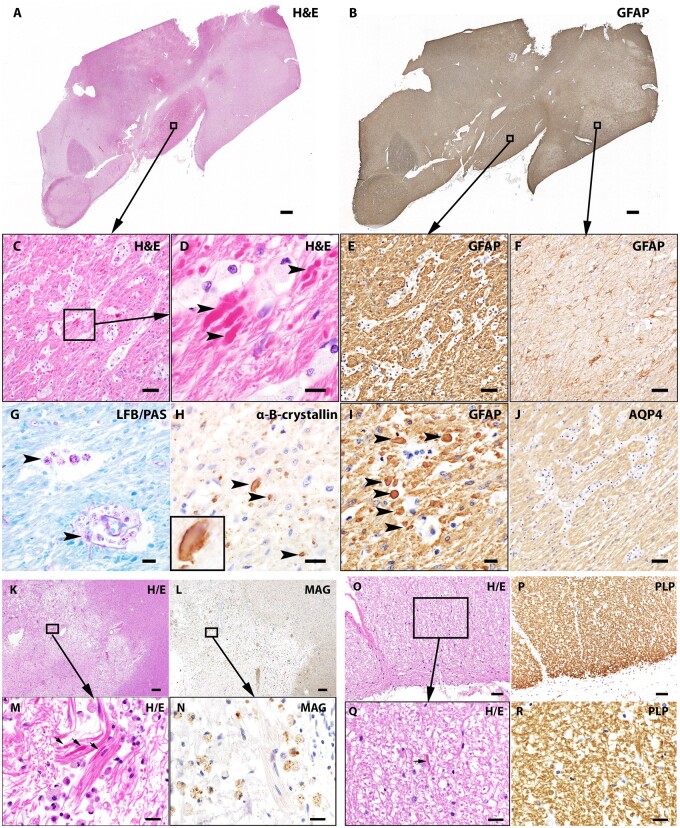
Figure 2.
Characteristics of Rosenthal fibres in NMO. The interpeduncular region of this patient’s brain shows (A) highly eosinophilic RFs (framed, H&E staining) and (B) intense GFAP immunoreactivity. High-power views (C and D) of (A) framed region reveal prominent gliosis and RFs (arrowheads). (E and I) GFAP stained optic tract in consecutive sections indicate RFs have variable GFAP-positivity (arrowheads, I). (F) Adjacent globus pallidus in the same tissue block (framed in B) shows relatively unremarkable astrocytes. (G) LFB/PAS shows preservation of myelin (blue) in a region containing RFs; the purple-pink granules in PAS-positive macrophages (arrowheads) suggest myelin degradation from a previous pathological event. (H) Immunostaining reveals the α-B-crystallin-positivity characteristic of RFs. Note the greater immunoreactivity at the periphery of the Rosenthal fibre structure in magnified image (inset). (J) AQP4 immunoreactivity is increased in this region. Rosenthal fibres are present in both demyelinating lesions and normal-appearing white matter: (K) White matter is rarefied in early active demyelinating lesion (H&E staining); (L) myelin loss is indicated by paucity of myelin MAG immunoreactivity in this area. (M) High magnification of the framed region in (A) shows RF accumulation (arrows) in this macrophage-enriched lesion. (N) High-power view of the MAG-stained lesion reveals myelin debris within macrophages, consistent with early active demyelinating activity.15 (O) and higher power image (Q) show RF deposition (arrow) in normal-appearing white matter. (P and R) Preserved PLP immunoreactivity in myelinated structures in the same region. Scale bars = 200 µm (A, B, K and L), 20 µm (D, G, H, I, M, N, Q and R) and 50 µm (C, E, J, O and P).
Emperipolesis: astrocytes containing plasma cells and neutrophils
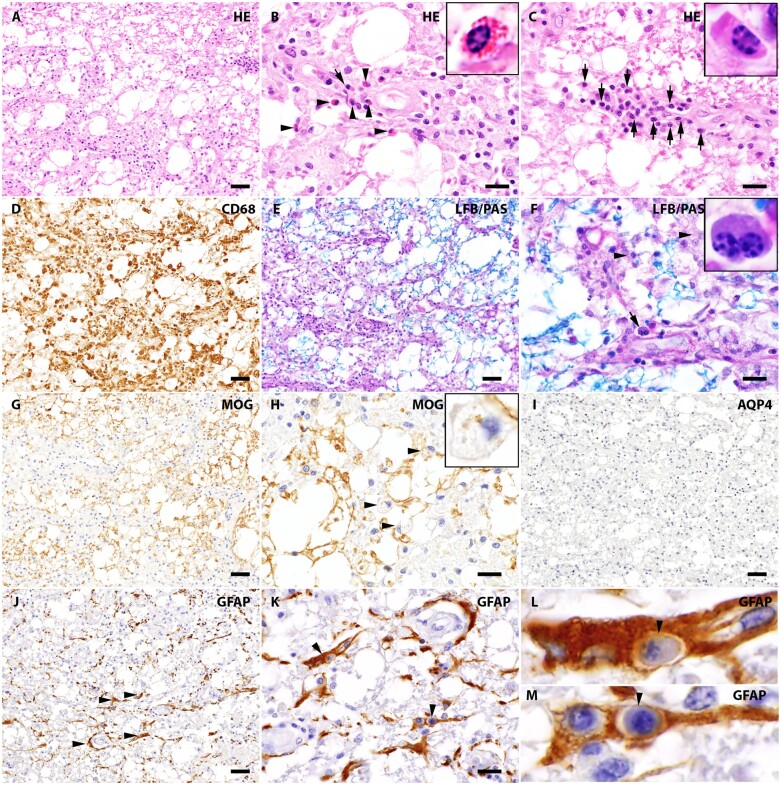
Astrocytes occasionally showed unusual ‘engulfment’ of peripheral immune cells. Figures 3 and 4 illustrate examples of plasma cells or neutrophils enclosed within astrocytic cytoplasm. Immunohistochemistry revealed single astrocytes incorporating multiple plasma cells (characterized by cartwheel nuclei and an apparently intact plasma membrane surrounded by GFAP-immunoreactive cytoplasm). Engulfed neutrophils also were observed. Figure 4 illustrates GFAP-positive astrocytes in a spinal cord lesion containing intact intracytoplasmic neutrophils, consistent with emperipolesis.
Figure 3.
Interaction between reactive astrocytes and plasma cells in the NMO lesion. (A) White matter lesion with prominent tissue vacuolization in a patient’s spinal cord (H&E stain). (B) Higher magnification view of A reveals infiltrating eosinophils (arrowheads, enlarged in the inset) and a neutrophil (arrow). (C) A high-power view of a different region of A shows prominent infiltration by plasma cells (arrows, enlarged in the inset), characterized by an eccentric nucleus with typical cartwheel arrangement of heterochromatin. (D) CD68 highlights prominent microglial reaction and macrophage infiltration. (E) LFB/PAS staining reveals partial loss of myelin (blue). (F) High-power view shows macrophages with intracellular LFB positive debris, consistent with active demyelination (arrowheads). A binucleated plasma cell in this NMO lesion (arrow) illustrates the variable plasma cell component. (G) Foci of reduced MOG immunoreactivity indicate myelin loss. (H) High-power view of MOG immunoreactivity reveals myelin debris within macrophages (arrowhead, inset), indicating early active demyelinating activity. (I) This area is completely lacking AQP4 immunoreactivity. (J) GFAP staining reveals the few remaining immunoreactive astrocytes in this early active demyelinating lesion are ‘dystrophic’ (arrowheads). (K–M) Of note, high-power view of the GFAP stained lesion shows plasma cells (arrowheads) within astrocytes (emperipolesis). The clear profiles of plasma cells and astrocytes, as well as the space between the plasma cell and astrocytic cytoplasm, are consistent with intact plasma membranes of both cell types. Scale bars = 50 µm (A, D, E, G, H, I and J) and 20 µm (B, C, F, H and K).
Figure 4.
Interaction between reactive astrocytes and neutrophils in the early active NMO lesion. (A) Tissue vacuolation, perivascular exudation and hypercellularity are evident in this patient’s spinal cord white matter (H&E stain). (B) High-power view reveals infiltrating perivascular and parenchymal (arrowheads) neutrophils. (C) GFAP-immunoreactive astrocytes are rare in the consecutive section, and some have dystrophic morphology (arrowheads). (D and E) High-magnification images show emperipolesis of neutrophils (characteristic multilobulated nuclei) within GFAP-positive astrocytes. (F) AQP4 immunoreactivity is lost in this lesion. (G) MOG immunostain reveals beading and broken myelin fragments. (H) High-power view reveals MOG-laden macrophages (arrowheads), compatible with early active demyelination. (I) Complement C9neo immunoreactivity (pink) reveals complement membrane attack complex deposited in a vasculocentric rosette pattern, a characteristic feature of NMO pathology. Scale bars = 100 µm (A, C, F, G and I) and 20 µm (B, D, E and H).
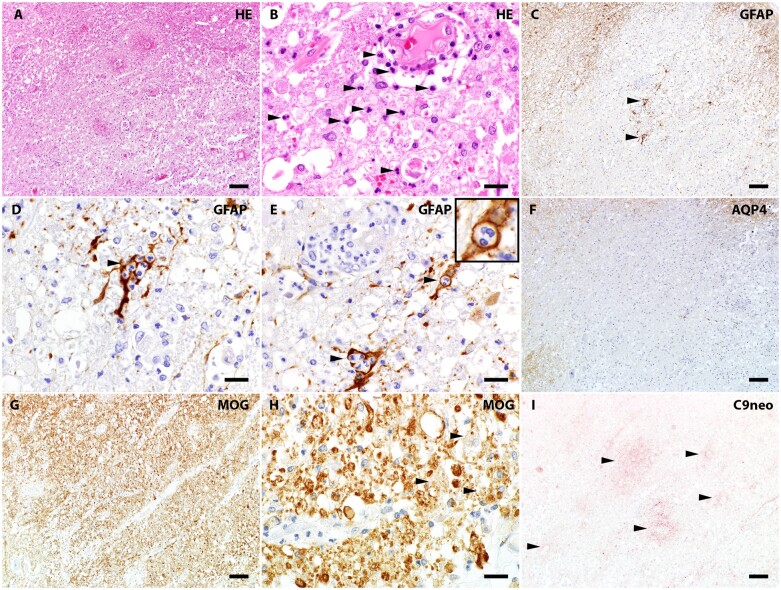
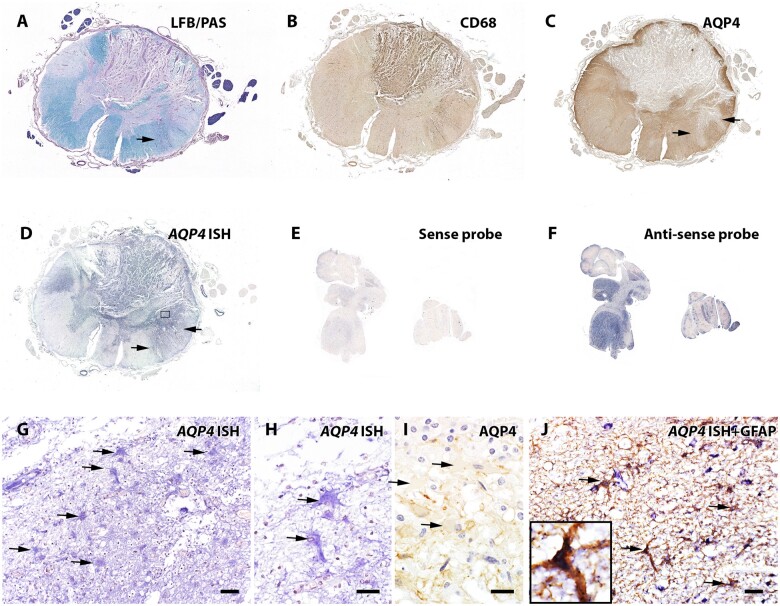
Dissociation of AQP4 mRNA expression and AQP4 protein loss in sublytic reactive astrocytes
In situ hybridization revealed AQP4 mRNA transcripts in both normal control and NMO patient CNS tissues (Fig. 5). Simultaneous staining for GFAP confirmed that the AQP4 mRNA was expressed by GFAP-immunoreactive astrocytes. Astrocytes within demyelinating lesions were devoid of AQP4 immunoreactivity but showed intense AQP4 mRNA signals (Fig. 5). This indicated preservation of AQP4 mRNA transcription, and its apparent upregulation, in reactive astrocytes. Furthermore, within myelin-preserved lesions, AQP4 mRNA signal intensity in reactive astrocytes lacking AQP4 protein was greater than in astrocytes residing in normal-appearing white matter surrounding the lesion. The intensity of AQP4 mRNA signals in astrocytes lacking AQP4 protein indicated that AQP4 loss in NMO lesions is not due to gene downregulation, but is consistent with NMO-IgG-induced internalization and degradation of AQP4 antigen and compensatory AQP4 synthesis, as has been demonstrated in vitro.20,21
Figure 5.
AQP4 mRNA transcripts revealed by in situ hybridization in astrocytes devoid of AQP4 protein in a non-demyelinated lesion of NMO patient’s spinal cord. (A) LFB/PAS stain shows demyelinating lesions in both the dorsal and lateral spinal cord. (B) CD68 immunohistochemistry reveals extensive macrophage infiltration/microglial activation. (C) Loss of AQP4 immunoreactivity in demyelinating lesions. In a focal area of the ventral spinal cord (arrows in A and C), myelin is preserved despite loss of AQP4. (D) In situ hybridization targeting AQP4 mRNA indicates AQP4 gene transcripts (purple-blue colour) in both demyelinating and non-demyelinated lesions exhibiting AQP4 loss, by comparison with regions retaining AQP4 protein. (E) Specificity of the hybridization signal was demonstrated by applying the sense probe to normal human cerebellar tissue (very weak signal), while the antisense probe (F) shows a strong signal in the grey matter. (G and H) Magnified view of the framed region in (D) reveals AQP4 mRNA within hypertrophic reactive astrocytes (arrows). (I) Magnified view of AQP4 immunoreactivity in the same region of (H) shows AQP4 protein lost from the hypertrophic reactive astrocytes (arrows). (J) Dual-labelling of normal control spinal cord tissue identifies the AQP4 mRNA-positive cells (purple-blue hybridization signal) as GFAP-positive astrocytes (brown immunostained product, arrows). Scale bars = 20 µm (H and I) and 50 µm (G and J).
Evidence for widespread extra-lesional astrocytic reactivity
Normal-appearing grey and white matter without AQP4 loss
To determine whether the astrocytic changes that we observe in NMO require antibody-mediated AQP4 loss, we analysed the correlation of astrocytopathic changes to deposition of complement products and more advanced pathological features. We found that astrocytopathic changes extended beyond lesioned tissues. Evidence of reactive astrogliosis or Rosenthal fibres was detected in normal-appearing parenchyma that lacked demyelination, AQP4 loss, or complement deposition (Fig. 2O–R).
Discussion
This focused study of NMO pathology concentrates on the distribution and type of individual astrocytic morphologies, their Rosenthal fibre content, AQP4 mRNA transcripts, interacting immune cells and associations with demyelinating activity. The observations we made in autopsied human CNS tissues demonstrate that (i) NMO is a global astrocytopathy with a wide spectrum of manifestations, ranging from sublytic to lytic; (ii) ‘dystrophic’ morphology, prominent cytoplasmic vacuolation, lysis and Rosenthal fibre deposition are degenerative accompaniments; (iii) emperipolesis of invading leukocytes (plasma cells and neutrophils) is a newly recognized astrocytic reaction to immune attack; and (iv) astrocyte reactivity in NMO does not necessarily require direct binding of IgG at the individual cell level; it is also observed in non-lesional areas that retain AQP4 protein.
Pathological studies that have focused on cytolytic mechanisms in NMO have underappreciated sublytic astrocytic reactions. Such reactions plausibly reflect the complex pathophysiological events that underlie observed morphological and functional changes, such as metabolic perturbation and cytokine and chemokine release.22–27 Studies of astrogliosis have emphasized hypertrophy and gain of physiological functions, but have largely neglected astrocytic degeneration. The basis of the dystrophic morphology and extreme cytoplasmic vacuolation of astrocytes that we have documented in NMO remains to be explained. Previous reports have correlated astrocytic process fragmentation and cytoplasmic vacuolation with degeneration.28–30 The frequency with which we observed astrocytic degeneration in NMO pathology is greater than we have observed in other CNS demyelinating diseases, including multiple sclerosis. However, astrocytic degenerative changes, in some cases supported by upregulation of cell death markers,31 are not confined to NMO, and have been correlated with disease process severity.28 For example, astrocytic process fragmentation is described in chronic traumatic encephalopathy and, in the osmotic demyelination syndrome, astrocytic degeneration in regions prone to demyelination is manifest as clasmatodendrosis, intracellular aggregates, organelle swelling, aquaporin dysregulation and loss of gap junctions.29,31,32
Takai and colleagues33 recently reported the stages of astrocytopathy observed in a Japanese NMO population with respect to AQP4 loss, inflammatory cell infiltration, complement deposition and demyelination activity. The authors proposed the concept of ‘astrocyte dystrophy’ in NMO, and recommended staging of NMO pathology on the basis of astrocytic changes.33
Our study complements that of Takai et al.33 in addressing the topic of astrocytopathy histopathologically in NMO targeting AQP4. Both studies reveal extensive astrocytic pathologies encompassing a wide morphological spectrum in NMO lesions at different stages. The study of Takai et al.33 provided quantitative data concerning inflammatory infiltration, complement activation and axonal injury, while our study extended the characterization of sublytic astrocytopathy to reveal Rosenthal fibre accumulation, emperipolesis of leukocytes, astrocytic reaction extending beyond regions that exhibit AQP4 loss and deposited complement membrane attack products, and upregulation of AQP4 mRNA in regions of AQP4 protein loss. The results from both studies indicate that astrocytes exhibiting diverse morphologies are a central feature of NMO pathology. However, our interpretation of the results differs somewhat. Takai and colleagues33 suggested a chronological progression between the stages of astrocytopathy and other CNS pathological changes. Our interpretation is less conventional on this point (Fig. 6). We agree that the staging of astrocytopathy may facilitate interpretation of astrocyte fate at the single-cell level in terms of evaluating the disease evolution driven by pathogenic AQP4 IgG. However, at the tissue level, the contributions of reactive astrocytes, other resident CNS cells and infiltrating immune cells act independently and in concert, both through complement signalling, cell–cell contact and through indirect interaction via cytokines, chemokines and gap junction communication through the astrocytic syncytium. Secreted pro-inflammatory and anti-inflammatory/reparative mediators, both humoral and extracellular-vesicle-borne cargos,36 all determine the histopathological and functional outcome. These interactions may alter or obscure the chronological order of astrocytopathic progression and reverse pathological transitions. Caution is therefore warranted in judging lesional age per se solely based on astrocyte morphology. Nevertheless, despite differences in interpretation, our observations and those of Takai and colleagues33 offer complementary evidence of diversity in the spectrum of astrocytic responses and phenotypes within and around NMO lesions. Beyond the binary phenotype implied by astrocyte lysis, the emerging spectrum of sublytic changes reflects ongoing physiological and pathophysiological responses of astrocytes within normal-appearing CNS parenchyma and within acute and chronic lesions.
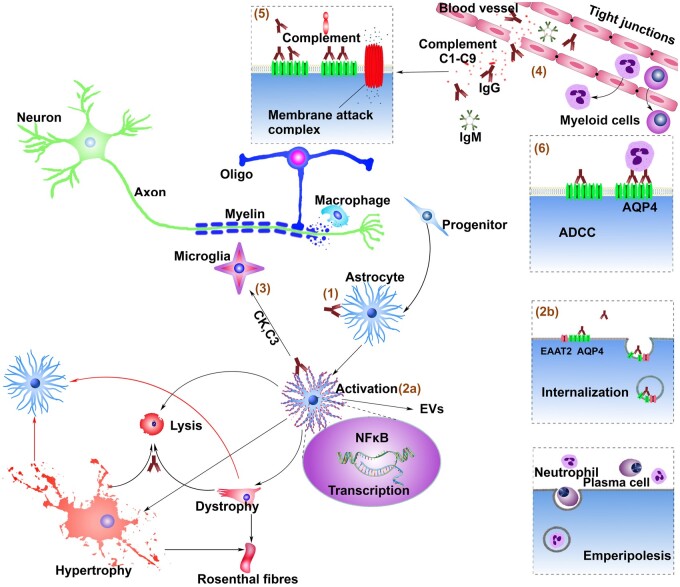
Figure 6.
Proposed pathways to astrocytopathy in NMO from observations of human immunohistopathology, and in vitro and in vivo effects of AQP4-IgG on live glial cells. (1) Binding of pathogenic IgG to AQP4 on the astrocytic membrane initiates (2a) Astrocytic activation and (2b) internalization of AQP4 and coupled glutamate transporters.34 Astrocytic activation leads to production/release of complement C3 and cytokines (CK) and (3) secondary activation of microglia11 leading to C1q production/release. (4) Ensuing myeloid cell chemotaxis and enhanced endothelial tight junction permeability amplify IgG influx and enable influx of C1–C9 and rheumatoid factor-like IgM, thus initiating/amplifying (5) complement-mediated astrocytic lysis and (6) antibody-dependent cell-mediated cytotoxicity. Sequelae of NFκB activation include astrocytic hypertrophy (GFAP upregulation)35 and surface AQP4 replenishment. The latter would enable repeated IgG binding and lytic and non-lytic sequelae, including astrocytic dystrophy and signalling to progenitors and remote astrocytes via gap junctions, cytokines and extracellular vesicles (EVs). Demyelination in NMO is plausibly attributable to a cascade of toxic bystander effects mediated by extracellular glutamate accumulation, the cytokine milieu and oxidative stress with secondary oligodendrocyte damage. Accumulation of GFAP protein and other stress proteins during the astrocytic reactions would favour Rosenthal fibre formation. The emperipolesis of infiltrating polymorphonuclear and plasma cells by activated astrocytes may serve to limit the immune attack. Proliferation and differentiation of neural progenitor cells would compensate for astrocytes lost in cytolytic lesions.
Reduction of AQP4 immunoreactivity in morphologically preserved astrocytes is consistent with antigenic modulation, a process that involves IgG-induced cross-linking and clearance of cell surface AQP4 protein (and its coupled glutamate transporter, EAAT2) via endocytosis and degradation.23 This in vitro-demonstrated mechanism is evidenced in the CNS pathology of NMO by the immunohistopathological observation of AQP4 ‘internalized’ in astrocytes (increased cytoplasmic AQP4 immunoreactivity, with decreased plasma membrane AQP41).21 The present study’s demonstration that reactive astrocytes in NMO exhibit robust AQP4 mRNA transcripts indicate that (i) astrocytes lacking surface AQP4 protein are ‘alive’; (ii) AQP4 protein loss is not due to AQP4 gene downregulation; and (iii) astrocytes attempt to compensate for surface protein loss and restore physiological function by continuing to synthesize AQP4. Endocytosis of AQP4 induced by IgG cross-linking may contribute to some of the astrocytic morphological changes observed in NMO. Cytoplasmic vacuolation, for example, might reflect vesicular exocytosis/endocytosis, phagocytosis or a step in the cell’s degeneration. Integration of newly synthesized AQP4 into the astrocytic plasma membrane requires spatiotemporal regulation of vesicle trafficking.37–40 The trafficking of AQP4-bearing vesicles increases under conditions of activation or oedema.38 Astrocytes also can engage in phagocytic activity.41 Experimentally induced lack of MLC1, a normally abundant astrocytic protein that associates with several astrocytic membrane proteins, causes cytoplasmic vacuolation and membrane ruffling.42,43 MLC1 co-localizes with AQP4 in processes at blood–brain and cerebrospinal fluid-brain barriers.44,45 Thus IgG-induced internalization of AQP4 protein might affect MLC1 homeostasis and cytoplasmic vesicle composition.
Our histopathological findings indicate a robust astrocytic stress response involving regions of normal-appearing white matter tissue, i.e. lacking evidence of myelin loss or complement-mediated destruction. Early development of a pronounced astrocytic morphological response before evident ‘lesion’ formation may be a hallmark of primary astrocytopathy. Despite having distinctly different aetiologies, both NMO and Alexander disease (caused by GFAP gene mutation) exhibit early formation of Rosenthal fibres with demyelination as secondary outcome. In both diseases, the density of cytoplasmic Rosenthal fibres correlates with the severity of astrogliosis.46 Early mortality commonly occurs in Alexander disease, and is indicative of a severe outcome from astrocyte dysfunction.47 Some features of the Rosenthal fibres we observed appear to be distinctive for NMO pathology, as they are present in all lesion stages as well as in normal-appearing white matter, i.e. extending beyond the classic lesions characterized by AQP4 loss, demyelination or complement deposition.13,17,48 The large numbers of Rosenthal fibres in demyelinating lesions contrasted with their sparse scattering in non-demyelinated areas. Rosenthal fibre formation is thought to result from aberrant intermediate filament assembly or protein degradation. GFAP overexpression in transgenic mice causes fatal encephalopathy.49,50 The basis of Rosenthal fibre accumulation in NMO remains to be determined. Perhaps the binding of IgG to AQP4 at the astrocytic foot process initiates an astrocytic stress response (Fig. 6), by upregulating GFAP synthesis beyond the astrocyte’s catabolic capacity.
Rosenthal fibres and hypertrophic gliosis occurring in non-lesional areas retaining AQP4 immunoreactivity suggest that the astrocytic stress response in NMO does not require binding of IgG to the individual astrocyte. Cell–cell cross-talk between IgG-bound and non-IgG-bound astrocytes may be responsible, either through the astroglial syncytium, or via cytokines secreted from IgG-activated astrocytes, or secondarily activated microglia or infiltrating peripheral immune cells.24,27 We previously showed that mixed cultures of astrocytes and microglia produce and release multiple cytokines via an NFκB-dependent pathway induced by NMO patients’ serum or purified IgG.24,27 Since NFκB is a primary driver and regulator of both inflammatory and stress responses, the pathological hallmarks of stress that we describe in NMO tissue may derive from a therapeutically tractable upstream signalling event. The abundance of AQP4 mRNA detected in hypertrophic GFAP-positive astrocytes in demyelinated as well as non-demyelinated regions may also be driven by NFκB, which regulates both AQP4 and GFAP expression.35 Our earlier finding that inflammatory cytokine production induced by applying NMO patients’ IgG to mixed glial cultures involves immunoproteasome activation is consistent with a model in which the binding of IgG skews astrocytes toward an inflammatory state that results in proteostatic stress, heat shock protein activation and Rosenthal fibre formation.51
Emperipolesis is a special cell-to-cell interaction in which a host cell engulfs intact host cells. It is a well-documented physiological phenomenon in haematopoietic cells,52 and is also observed in pathological conditions, such as Rosai–Dorfman disease, autoimmune haemolytic anaemia, multiple myeloma, neuroblastoma and Creutzfeldt–Jakob disease.53 Astrocyte engulfment of oligodendrocytes and lymphocytes has been described in multiple sclerosis, Creutzfeldt–Jakob disease and some astrocytic neoplasms.52,54,55 The mechanism and biological significance are unknown. Astrocytic emperipolesis of plasma cells and neutrophils has not previously been reported. It may be a protective mechanism restricting the activity and migration of CNS-infiltrating immune cells.56
The immunopathological findings we report here have significant implications for patients with NMO. Expert opinion in the past decade has emphasized the importance of attack prevention in patient management. However, the concept that all NMO disability is attack-related may be incorrect. The newly recognized spectrum of astrocytopathy supports the concept of attack-independent structural changes in evolving NMO pathology.57,58 The disability measures used in recently reported phase 3 trials have focused on ambulation (i.e. EDSS, Hauser ambulation index), vision (e.g. visual-analogue scale), pain (visual-analogue scale score for pain), fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue) and quality of life (SF-36 and EQ-5D-3L).59–63 Although these trials demonstrated stability or improvements in such measures over the randomized controlled periods, with persistence of benefit during the open label extension periods, the studies were not designed to monitor subtle or even large changes in cognition or psychological disorders such as depression and anxiety.
Depression is significantly greater in NMO patients than in healthy subjects and MS controls64–67; anxiety also is reported to influence the quality of life in NMO.68,69 The multifactorial aetiology of these behavioural manifestations complicates assigning cause. Chronic disease itself associates with psychological disorders. On the other hand, despite the fact that NMO compared to MS is generally regarded as a disease with less brain involvement, cognitive impairment is common in NMO, which can itself cause psychological disorders. This study provides a pathological explanation for a more diffuse CNS injury beyond the optic nerve and spinal cord that could drive a recently proposed ‘smouldering’ (subclinical) disease process independent of clinical attacks, to account for neuropsychological symptoms, including cognitive impairment. Inter-attack deterioration has been attributed to antibody-mediated perturbation of AQP4 that plays an important role in regulating long-term synaptic plasticity.70 AQP4-null mice appear normal neurologically but, unlike wild-type mice, their motor performance does not improve with Rotarod training.11 Richard et al.71 reported altered expression and function of astrocytic connexins after application of NMO-IgG. As components of tripartite CNS synapses, and the anatomical basis of the connexin-sustained astroglial syncytium that regulates the flow of water and small molecules throughout the CNS, astrocytes are crucial for synaptic plasticity and cognitive function. Richard et al. proposed astrocyte dysfunction as a contributor to cognitive impairments in NMO patients with cortical lesions characterized by neuronal loss without demyelination, in consideration of their decreased AQP4 expression without astrocyte loss.72 The diffuse astrocytopathy we report here in NMO CNS tissues may similarly explain the frequent neuropsychological symptoms and signs encountered in NMO patients.
Limitations of our study include lack of serological data for some archival pathological samples. NMO diagnosis in those cases was based on clinical history, imaging and NMO-compatible pathological findings. The patients’ unknowable serum AQP4-IgG status may affect the findings by introducing heterogeneity. In addition, our observations are limited to a specific NMO cohort; the specificity of the changes we observed will require further testing in a large group of neurological disease controls. Finally, our histopathological studies may overlook the very earliest stages of lesion formation. Confirmation of our interpretations awaits future experimental studies using in vivo imaging approaches.
Conclusions
Detailed neuropathological study of patients’ tissues demonstrates that NMO targeting the AQP4 water channel is a global astrocytopathy not simply definable by demyelination and astrocytic lysis. The observed astrocytic reactions are not solely dependent on IgG-mediated AQP4 loss or lysis by complement or by IgG-dependent leukocyte mediators. The diverse severity and complexity of changes suggest that autoimmune astrocytopathy is an evolving degenerative process. ‘Dystrophic’ astrocytic morphology, Rosenthal fibres and prominent cytoplasmic vacuolation indicate degeneration. The sublytic astrocytic reaction is an important determinant of the lesion’s evolution and potential for repair. Pharmacologically manipulating the astrocytic stress response may serve as a new avenue for therapeutic intervention in NMO.
Supplementary Material
Acknowledgements
The authors acknowledge Patricia Ziemer, Department of Neurology, Mayo Clinic for expert technical assistance. The authors acknowledge Stephen D Weigand, Department of Health Sciences Research, Mayo Clinic for help with preparing statistical figures. The authors acknowledge assistance of the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology.
Funding
This study was supported in part by the research grant to V.L. from the US National Institutes of Health (R01 NS110949).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- NMO
neuromyelitis optica
References
- 1. Lucchinetti CF, Guo Y, Popescu BF, Fujihara K, Itoyama Y, Misu T.. The pathology of an autoimmune astrocytopathy: Lessons learned from neuromyelitis optica. Brain Pathol. 2014;24(1):83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG.. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. [DOI] [PubMed] [Google Scholar]
- 3. Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–1205. [DOI] [PubMed] [Google Scholar]
- 4. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR.. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. [DOI] [PubMed] [Google Scholar]
- 6. Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24(3):312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khakh BS, Deneen B.. The emerging nature of astrocyte diversity. Annu Rev Neurosci. 2019;42:187–207. [DOI] [PubMed] [Google Scholar]
- 8. Olabarria M, Goldman JE.. Disorders of astrocytes: Alexander disease as a model. Annu Rev Pathol. 2017;12:131–152. [DOI] [PubMed] [Google Scholar]
- 9. Sofroniew MV, Vinters HV.. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradl M, Misu T, Takahashi T, et al. Neuromyelitis optica: Pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66(5):630–643. [DOI] [PubMed] [Google Scholar]
- 11. Chen T, Lennon VA, Liu YU, et al. Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J Clin Invest. 2020;130(8):4025–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geis C, Ritter C, Ruschil C, et al. The intrinsic pathogenic role of autoantibodies to aquaporin 4 mediating spinal cord disease in a rat passive-transfer model. Exp Neurol. 2015;265:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125(Pt 7):1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oberheim NA, Wang X, Goldman S, Nedergaard M.. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29(10):547–553. [DOI] [PubMed] [Google Scholar]
- 15. Bruck W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38(5):788–796. [DOI] [PubMed] [Google Scholar]
- 16. Breitschopf H, Suchanek G, Gould RM, Colman DR, Lassmann H.. In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol. 1992;84(6):581–587. [DOI] [PubMed] [Google Scholar]
- 17. Misu T, Hoftberger R, Fujihara K, et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013;125(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishie M, Mori F, Ogawa M, et al. Multinucleated astrocytes in old demyelinated plaques in a patient with multiple sclerosis. Neuropathology. 2004;24(3):248–253. [DOI] [PubMed] [Google Scholar]
- 19. Wippold FJ 2nd, Perry A, Lennerz J.. Neuropathology for the neuroradiologist: Rosenthal fibers. AJNR Am J Neuroradiol. 2006;27(5):958–961. [PMC free article] [PubMed] [Google Scholar]
- 20. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–2231. [DOI] [PubMed] [Google Scholar]
- 21. Hinson SR, Romero MF, Popescu BF, et al. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci USA. 2012;109(4):1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belanger M, Allaman I, Magistretti PJ.. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. [DOI] [PubMed] [Google Scholar]
- 23. Hinson SR, Roemer SF, Lucchinetti CF, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008;205(11):2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howe CL, Kaptzan T, Magana SM, Ayers-Ringler JR, LaFrance-Corey RG, Lucchinetti CF.. Neuromyelitis optica IgG stimulates an immunological response in rat astrocyte cultures. Glia. 2014;62(5):692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossi DJ, Brady JD, Mohr C.. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10(11):1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner DA, Adamson DC.. Neuronal-astrocyte metabolic interactions: understanding the transition into abnormal astrocytoma metabolism. J Neuropathol Exp Neurol. 2011;70(3):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker-Caulfield ME, Guo Y, Johnson RK, et al. NFkappaB signaling drives pro-granulocytic astroglial responses to neuromyelitis optica patient IgG. J Neuroinflammation. 2015;12:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broe M, Kril J, Halliday GM.. Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain. 2004;127(10):2214–2220. [DOI] [PubMed] [Google Scholar]
- 29. Hsu ET, Gangolli M, Su S, et al. Astrocytic degeneration in chronic traumatic encephalopathy. Acta Neuropathol. 2018;136(6):955–972. [DOI] [PubMed] [Google Scholar]
- 30. Isobe I, Maeno Y, Nagao M, et al. Cytoplasmic vacuolation in cultured rat astrocytes induced by an organophosphorus agent requires extracellular signal-regulated kinase activation. Toxicol Appl Pharmacol. 2003;193(3):383–392. [DOI] [PubMed] [Google Scholar]
- 31. Nicaise C, Marneffe C, Bouchat J, Gilloteaux J.. Osmotic demyelination: from an oligodendrocyte to an astrocyte perspective. Int J Mol Sci. 2019;20(5):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bouchat J, Couturier B, Marneffe C, et al. Regional oligodendrocytopathy and astrocytopathy precede myelin loss and blood-brain barrier disruption in a murine model of osmotic demyelination syndrome. Glia. 2018;66(3):606–622. [DOI] [PubMed] [Google Scholar]
- 33. Takai Y, Misu T, Suzuki H, et al. Staging of astrocytopathy and complement activation in neuromyelitis optica spectrum disorders. Brain. 2021;144(8):2401–2415. [DOI] [PubMed] [Google Scholar]
- 34. Hinson SR, Clift IC, Luo N, Kryzer TJ, Lennon VA.. Autoantibody-induced internalization of CNS AQP4 water channel and EAAT2 glutamate transporter requires astrocytic Fc receptor. P Natl Acad Sci USA. 2017;114(21):5491–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee Y, Messing A, Su M, Brenner M.. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56(5):481–493. [DOI] [PubMed] [Google Scholar]
- 36. Wang K, Ye L, Lu H, et al. TNF-alpha promotes extracellular vesicle release in mouse astrocytes through glutaminase. J Neuroinflammation. 2017;14(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ciappelloni S, Bouchet D, Dubourdieu N, et al. Aquaporin-4 surface trafficking regulates astrocytic process motility and synaptic activity in health and autoimmune disease. Cell Rep. 2019;27(13):3860–3872.e4. [DOI] [PubMed] [Google Scholar]
- 38. Potokar M, Stenovec M, Jorgacevski J, et al. Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia. 2013;61(6):917–928. [DOI] [PubMed] [Google Scholar]
- 39. Potokar M, Vardjan N, Stenovec M, et al. Astrocytic vesicle mobility in health and disease. Int J Mol Sci. 2013;14(6):11238–11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zorec R, Verkhratsky A, Rodriguez JJ, Parpura V.. Astrocytic vesicles and gliotransmitters: slowness of vesicular release and synaptobrevin2-laden vesicle nanoarchitecture. Neuroscience. 2016;323:67–75. [DOI] [PubMed] [Google Scholar]
- 41. Wakida NM, Cruz GMS, Ro CC, et al. Phagocytic response of astrocytes to damaged neighboring cells. PLoS ONE. 2018;13(4):e0196153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duarri A, Lopez de Heredia M, Capdevila-Nortes X, et al. Knockdown of MLC1 in primary astrocytes causes cell vacuolation: MLC disease cell model. Neurobiol Dis. 2011;43(1):228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hwang J, Vu HM, Kim MS, Lim HH.. Plasma membrane localization of MLC1 regulates cellular morphology and motility. Mol Brain. 2019;12(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo Y, Weigand SD, Popescu BF, et al. Pathogenic implications of cerebrospinal fluid barrier pathology in neuromyelitis optica. Acta Neuropathol. 2017;133(4):597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teijido O, Casaroli-Marano R, Kharkovets T, et al. Expression patterns of MLC1 protein in the central and peripheral nervous systems. Neurobiol Dis. 2007;26(3):532–545. [DOI] [PubMed] [Google Scholar]
- 46. Heaven MR, Flint D, Randall SM, et al. Composition of Rosenthal fibers, the protein aggregate Hallmark of Alexander disease. J Proteome Res. 2016;15(7):2265–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li R, Messing A, Goldman JE, Brenner M.. GFAP mutations in Alexander disease. Int J Dev Neurosci. 2002;20(3-5):259–268. [DOI] [PubMed] [Google Scholar]
- 48. Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: Distinction from multiple sclerosis. Brain. 2007;130(Pt 5):1224–1234. [DOI] [PubMed] [Google Scholar]
- 49. Hagemann TL, Gaeta SA, Smith MA, Johnson DA, Johnson JA, Messing A.. Gene expression analysis in mice with elevated glial fibrillary acidic protein and Rosenthal fibers reveals a stress response followed by glial activation and neuronal dysfunction. Hum Mol Genet. 2005;14(16):2443–2458. [DOI] [PubMed] [Google Scholar]
- 50. Messing A, Head MW, Galles K, Galbreath EJ, Goldman JE, Brenner M.. Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol. 1998;152(2):391–398. [PMC free article] [PubMed] [Google Scholar]
- 51. Tang G, Xu Z, Goldman JE.. Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem. 2006;281(50):38634–38643. [DOI] [PubMed] [Google Scholar]
- 52. Shintaku M, Yutani C.. Oligodendrocytes within astrocytes (‘emperipolesis’) in the white matter in Creutzfeldt-Jakob disease. Acta Neuropathol. 2004;108(3):201–206. [DOI] [PubMed] [Google Scholar]
- 53. Gupta N, Jadhav K, Shah V.. Emperipolesis, entosis and cell cannibalism: demystifying the cloud. J Oral Maxillofac Pathol. 2017;21(1):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Furer M, Hartloper V, Wilkins J, Nath A.. Lymphocyte emperipolesis in human glial cells. Cell Adhes Commun. 1993;1(3):223–237. [DOI] [PubMed] [Google Scholar]
- 55. Muller W, Dahmen HG.. Lymphocytes within glial cells (‘emperipolesis’) in a case of a granular cell tumor. Acta Neuropathol. 1978;44(2):163–165. [DOI] [PubMed] [Google Scholar]
- 56. Barcia C, Sanderson NS, Barrett RJ, et al. T cells' immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS ONE. 2008;3(8):e2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Felix CM, Levin MH, Verkman AS.. Complement-independent retinal pathology produced by intravitreal injection of neuromyelitis optica immunoglobulin G. J Neuroinflammation. 2016;13(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jeong IH, Kim HJ, Kim NH, Jeong KS, Park CY.. Subclinical primary retinal pathology in neuromyelitis optica spectrum disorder. J Neurol. 2016;263(7):1343–1348. [DOI] [PubMed] [Google Scholar]
- 59. Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352–1363. [DOI] [PubMed] [Google Scholar]
- 60. Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614–625. [DOI] [PubMed] [Google Scholar]
- 61. Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114–2124. [DOI] [PubMed] [Google Scholar]
- 62. Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: A randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tahara M, Oeda T, Okada K, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(4):298–306. [DOI] [PubMed] [Google Scholar]
- 64. Chavarro VS, Mealy MA, Simpson A, et al. Insufficient treatment of severe depression in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016;3(6):e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y, Tang X.. Depressive syndromes in autoimmune disorders of the nervous system: Prevalence, etiology, and influence. Front Psychiatry. 2018;9:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pan J, Zhao P, Cai H, et al. Hypoxemia, sleep disturbances, and depression correlated with fatigue in neuromyelitis optica spectrum disorder. CNS Neurosci Ther. 2015;21(7):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. He D, Chen X, Zhao D, Zhou H.. Cognitive function, depression, fatigue, and activities of daily living in patients with neuromyelitis optica after acute relapse. Int J Neurosci. 2011;121(12):677–683. [DOI] [PubMed] [Google Scholar]
- 68. Shi ZY, Chen HX, Lian ZY, Liu J, Feng HR, Zhou HY.. Factors that impact health-related quality of life in neuromyelitis optica spectrum disorder: anxiety, disability, fatigue and depression. J Neuroimmunol. 2016;293:54–58. [DOI] [PubMed] [Google Scholar]
- 69. Ebadi Z, Saeedi R, Hashemi SN, Gheini MR, Sahraian MA, Naser Moghadasi A.. Evaluation of types of psychological disorders in patients with neuromyelitis optica spectrum disorder (NMOSD). Mult Scler Relat Disord. 2020;42:102128. [DOI] [PubMed] [Google Scholar]
- 70. Scharfman HE, Binder DK.. Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochem Int. 2013;63(7):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Richard C, Ruiz A, Cavagna S, et al. Connexins in neuromyelitis optica: A link between astrocytopathy and demyelination. Brain. 2020;143(9):2721–2732. [DOI] [PubMed] [Google Scholar]
- 72. Saji E, Arakawa M, Yanagawa K, et al. Cognitive impairment and cortical degeneration in neuromyelitis optica. Ann Neurol. 2013;73(1):65–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
More detailed data are provided in the Supplementary material. The original pathology slides and clinical information that support findings in this study are available from the corresponding author on reasonable request.