Abstract
The negative impact of smoking in multiple sclerosis is well established; however, there is much less evidence as to whether smoking cessation is beneficial to progression in multiple sclerosis.
Adults with multiple sclerosis registered on the United Kingdom Multiple Sclerosis Register (2011–20) formed this retrospective and prospective cohort study. Primary outcomes were changes in three patient-reported outcomes: normalized Multiple Sclerosis Physical Impact Scale (MSIS-29-Phys), normalized Multiple Sclerosis Walking Scale (MSWS-12) and the Hospital Anxiety and Depression Scale (HADS). Time to event outcomes were clinically significant increases in the patient-reported outcomes.
The study included 7983 participants; 4130 (51.7%) of these had ever smoked, of whom 1315 (16.5%) were current smokers and 2815/4130 (68.2%) were former smokers. For all patient-reported outcomes, current smokers at the time of completing their first questionnaire had higher patient-reported outcomes scores indicating higher disability compared to those who had never smoked (∼10 points difference in MSIS-29-Phys and MSWS-12; 1.5–1.8 points for HADS-Anxiety and HADS-Depression). There was no improvement in patient-reported outcomes scores with increasing time since quitting in former smokers.
Nine hundred and twenty-three participants formed the prospective parallel group, which demonstrated that MSIS-29-Phys [median (IQR) 5.03 (3.71, 6.34)], MSWS-12 [median (IQR) 5.28 (3.62, 6.94)] and HADS-Depression [median (IQR) 0.71 (0.47, 0.96)] scores worsened over a period of 4 years, whereas HADS-Anxiety remained stable. Smoking status was significant at Year 4; current smokers had higher MSIS-29-Phys and HADS-Anxiety scores [median (IQR) 3.05 (0.22, 5.88) and 1.14 (0.52, 1.76), respectively] while former smokers had a lower MSIS-29-Phys score of −2.91 (−5.03, −0.79).
A total of 4642 participants comprised the time to event analysis. Still smoking was associated with a shorter time to worsening event in all patient-reported outcomes (MSIS-29-Phys: n = 4436, P = 0.0013; MSWS-12: n = 3902, P = 0.0061; HADS-Anxiety: n = 4511, P = 0.0017; HADS-Depression: n = 4511, P < 0.0001). Worsening in motor disability (MSIS-29-Phys and MSWS-12) was independent of baseline HADS-Anxiety and HADS-Depression scores. There was no statistically significant difference in the rate of worsening between never and former smokers.
When smokers quit, there is a slowing in the rate of motor disability deterioration so that it matches the rate of motor decline in those who have never smoked. This suggests that smoking cessation is beneficial for people with multiple sclerosis.
Keywords: multiple sclerosis, public health, epidemiology
Rodgers et al. demonstrate the benefits of smoking cessation in a UK-wide, community-based registry population of almost 8000 people with multiple sclerosis. They show that, following smoking cessation, there is a deceleration in the rate of motor deterioration so that it matches the rate of motor decline in those who have never smoked.
Introduction
Retrospective studies have shown that people with multiple sclerosis who smoke have more significant motor symptoms,1 increased MRI activity and brain atrophy2,3 and more cognitive and psychological impairment.4,5 In relapsing multiple sclerosis smoking can lead to earlier death,6 reaching disease milestones earlier 7 and earlier onset of secondary progressive multiple sclerosis.8,9 While the negative impact of smoking in multiple sclerosis is well established, there is less evidence whether smoking cessation is beneficial. Retrospective analyses have shown that smoking cessation may reduce the risk of reaching disability milestones,9 and quitting earlier is associated with stronger reductions in risk.5 These milestones, however, are confounded by other factors such as mood.10
Studying the effects of exposures known to be harmful, such as smoking, poses specific challenges. One approach is to use registry data where the self-directed choice of each study subject determines their exposure. Registry studies are subject to several biases, in particular those associated with geographical and temporal variations in data collection.11 Some cross-site and longitudinal stability can be introduced by the use of patient-reported outcomes (PROs) in which participants answer questions about certain aspects of their own health. PROs are not widely used as standardized clinical outcome measures in multiple sclerosis and preference has been for tools such as the Expanded Disability Status Scale (EDSS). However, PROs have been shown to be the better predictor of outcome when integrated into a patient-specific, personalized approach.12 Two validated PROs have established use in assessing the motor impact of multiple sclerosis: the Multiple Sclerosis Walking Scale 12 (MSWS-12)13 and the motor component of the Multiple Sclerosis Impact Scale 29 (MSIS-29-Phys).14 Clinically relevant changes, corresponding to established EDSS outcomes, have been validated both for the MSWS-1215 and the MSIS-29.16,17 The MSIS-29 has also been correlated with mortality.18
The United Kingdom Multiple Sclerosis Register (UKMSR) is a primarily patient-driven registry of PRO-based data that has been active since 2011. We have used UKMSR data from almost 8000 participants to conduct a PRO-based study investigating the effects of smoking, and of smoking cessation, in people with multiple sclerosis. To our knowledge, this represents the largest investigation into the effects of smoking cessation in multiple sclerosis and the largest study of smoking in any neurological disease to use PROs. We have used MSWS-12, MSIS-29-Phys and Hospital Anxiety and Depression Scales (HADS).19 First, we utilized historical data on smoking and smoking cessation to assess current impact on functional status. We then used prospectively collected data in the same cohort yearly over 4 years to measure average change in the PROs. Finally, we modelled the PRO results for use in time to event analyses using confirmed clinically relevant worsening events, to dissect out the impact of smoking and smoking cessation on disease progression in multiple sclerosis.
Materials and methods
Ethics approval
The UK Multiple Sclerosis Register has research ethics approval from South West Central Bristol Research Ethics Committee 16/SW/0194.
Population demographics
The UKMSR is an online, UK-wide register supported by National Health Service (NHS) clinical centres (Resarch Ethics Committee: South West Central Bristol NRES 16/SW/0194). The register includes independent verification of treatments and EDSS outcomes from NHS centres in a separate but overlapping population. Participants enter data regularly (3 monthly from 2011 to 2018 and 6 monthly subsequently) and are sent reminders by email. Since September 2018 participants have a 28-day window in which to complete the PROs, although often they are all completed in one day. For this study demographics and disease-specific data were assessed at the first questionnaire (baseline) or within 12 months prior or 6 months after completion of the first questionnaire. Demographic data collected included age, gender, ethnicity (Black Asian or minority ethnic group/not). Disease-specific data were also obtained, including disease length (in years, from initial symptoms), disease type at diagnosis (secondary and primary progressive/not progressive) and whether the participant was on a disease-modifying treatment (DMT) or not for their multiple sclerosis (highly active/normally active/none). Highly active treatments were defined as: alemtuzumab, cladribine, daclizumab, fingolimod, mitoxantrone, natalizumab, crelizumab, ofatumumab and rituximab. A univariate statistical analysis was completed on these data; chi-squared test for categorical, one-way ANOVA tests for comparison of parametric means and Kruskal–Wallis tests for non-parametric data.
Patient-reported outcome questionnaires
Three PROs were used: MSIS-29-Phys, MSWS-12 and the HADS scale. The MSIS-29-Phys subscore version 1 (MSIS-29v114) was used prior to April 2012 and version 2 (MSIS-29v220) was used subsequently. Answers to the 20 questions that form the MSIS-29 Phys subscore are each scored between 1 and 5 in version 1 and between 1 and 4 in version 2. These scores give a total ranging from 20 to 100 for MSIS-29v1 and of 20 to 80 for MSIS-29v2. In order to account for the changes in scales, totals were rescaled to a value in the range of 0 to 100 using a unity-based normalization procedure.21 A 10-point increase in the normalized score corresponds to an 8-point increase in the MSIS-29v1 physical score. This change reflects clinically relevant worsening.16,17 MSWS-12 version 213 was used to assess walking function. Participants were only excluded from the MSWS-12 assessment if they indicated that they could not walk. The score was normalized as above. A 10-point change in the normalized score corresponds to a 5.4-point change in the raw score, which reflects a clinically relevant change.15 The HADS provides scores for anxiety and depression.19 A 2-point change in either of these subscores corresponds to a clinically relevant change.22
Retrospective and prospective cohort study design
The retrospective analysis was conducted using the population in whom valid data were available for date of birth, gender and smoking status. Participants needed to have answered at least one of the three PROs and the first questionnaire answered for each PRO was used.
Two approaches were taken for the prospective cohort analysis. In the first, for the 4-year prospective parallel group analysis, participants were identified who had completed each of the 3 PROs at baseline and every year (±60 days) over a 4-year period. Second, for the time to event analyses ‘streaks’ of longitudinal data were obtained from participants who fulfilled specific criteria. To be included in a streak, participants had to have completed at least three sets of PRO questionnaires. The minimum time interval between each questionnaire was 15 days and the maximum interval could not be longer than 240 days. The longest sequence of questionnaires completed by each participant defined by these criteria was selected for each participant. In the event that a participant had more than one longest streak (of equal length), then the most recent was chosen. A separate set of streaks was built for each questionnaire. Clinically significant step changes were used as ‘events’ for time to event analysis. Information about the time to event was provided from timestamps which were automatically appended by the database tables. Censoring occurred at the date when the PRO score increased by a clinically significant step or, alternatively, when the last questionnaire of the streak was completed. The maximum number of PROs answered was used as a confounding variable in the time to event analysis.
Smoking status
Questions about smoking status were available to answer at any time and were reviewable at the time of every email reminder when the regular battery of PROs were completed. Participants were asked if they had ever smoked and, if they answered yes, whether they continued to smoke. This provided three distinct categories: never, former and current smoker. If the participant answered that they had stopped smoking, then the cessation date was requested. The number of cigarettes smoked per day in current and former smokers was also requested. This was classified as light (≤7), moderate (7–12) and heavy (≥13) based on the distribution of the data. Pack years smoked is defined by the rate of daily smoking in percentage of standard 20 cigarette packs multiplied by the total time smoked in days.
Statistical analysis
All statistical analysis was performed using the R statistical programming language, version 3.5.1, in the RStudio environment, version 1.1.463. Boxplots were used to illustrate the retrospective analysis (minimum score, lower quartile, median, upper quartile, maximum score). Generalized linear modelling was used for the retrospective analysis and Cox regression modelling was used to analyse the effects of being a never-, current- and former smoker on the rate of clinically significant events using the ‘survival’ package (v2.43–3) in R (v3.5.3). Baseline variables considered were age at first assessment, disease length, multiple sclerosis type at diagnosis (progressive as reference versus not progressive), gender (female as reference), ethnicity group (Black Asian or minority ethnic group versus white) and pack years smoked. DMT, either highly active, normally active or none, was modelled as a time-varying covariate. Linear mixed models for repeated measures were used to analyse the prospective parallel groups using the ‘lme4’ package (v1.1–7) optimized using restricted maximum likelihood estimates. Dependent variables were the normalized MSIS-29 Phys, MSWS-12, HADS anxiety and depression scores. Fixed effects for time, smoking status, age, gender, time since onset, treatment type, pack years and ethnicity were added, as well as the interaction terms between time and smoking status. The study participants were included as a random effect. Estimates of the fixed effects and their 95% CIs are reported.
Data availability
Access to the data for this study is available in a secure environment subject to governance approval.
Results
Demographics
Seven thousand, nine hundred and eighty-three people with multiple sclerosis who had a valid smoking status and had completed at least one PRO were identified from the UKMSR database (n = 16 187; valid date of birth and gender). Details of the excluded population are provided in Supplementary Table 1. In turn, 4642 people with multiple sclerosis in turn had the required information to produce a streak of prospective time to event data. For the 4-year prospective parallel cohort analysis, 923 people with multiple sclerosis were available given the selection criteria (Table 1). Using simultaneously collected data (n = 4591) we confirmed that the MSIS-29-Phys was highly correlated with the MSWS-12 [r = 0.87, 95% CI (0.86, 0.87)]. MSIS-29 was less so with the HADS-depression [r = 0.61, 95% CI (0.59, 0.63)] and HADS-anxiety [r = 0.37, 95% CI (0.35, 0.40)]. The correlation of the MSWS-12 to the HADS-depression was higher [r = 0.50, 95% CI (0.48, 0.53)] than the MSWS-12 correlation with the HADS-anxiety [r = 0.21, 95% CI (0.18, 0.23)]. HADS-depression and HADS-anxiety were also correlated with each other [r = 0.60, 95% CI (0.59, 0.62)].
Table 1.
Demographics and PRO outcomes of the total population with a given smoking status (n = 7983), the time-to-event (n = 4642) and parallel group (n = 923) population
| N, smoking status P value | Never | Former | Current | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Time to event | Parallel | Total | Time to event | Parallel | Total | Time to event | Parallel | Total | Time to event | Parallel | |
| n below | n below | n below | (n = 3853) | (n = 2378) | (n = 487) | (n = 2815) | (n = 1589) | (n = 309) | (n = 1315) | (n = 675) | (n = 127) | |
| Age at baseline, mean (SD) | 7983 | 4642 | 923 | 48 (11.5) | 49.6 (11) | 51.5 (10) | 50.7 (11.2) | 52.6 (10.7) | 54.7 (9.8) | 45.3 (11.1) | 47.3 (10.9) | 49.1 (10.6) |
| Time since multiple sclerosis onset, mean (SD) | 7826 | 4596 | 919 | 13.3 (10.6) | 14.3 (10.8) | 16.1 (10.7) | 15.6 (11.8) | 16.5 (12) | 17.5 (11.4) | 11.9 (9.8) | 13 (10.2) | 14.2 (10.5) |
| Gender: female, n (%) | 7983 | 4642 | 923 | 3016 (78.3) | 1856 (78) | 375 (77) | 1934 (68.7) | 1070 (67.3) | 185 (59.9) | 940 (71.5) | 457 (67.7) | 84 (66.1) |
| Multiple sclerosis at diagnosis: known-progressive, n (%) | 7983 | 4642 | 923 | 682 (17.7) | 459 (19.3) | 116 (23.8) | 602 (21.4) | 380 (23.9) | 98 (31.7) | 225 (17.1) | 134 (19.9) | 32 (25.2) |
| On DMT: known-DMT, n (%) | 7983 | 4642 | 923 | 1167 (30.3) | 810 (34.1) | 171 (35.1) | 656 (23.3) | 407 (25.6) | 73 (23.6) | 359 (27.3) | 209 (31) | 41 (32.3) |
| On highly active DMT n (%) | 7983 | 4642 | 923 | 59 (1.5) | 38 (1.6) | 6 (1.2) | 38 (1.3) | 21 (1.3) | 2 (0.6) | 25 (1.9) | 13 (1.9) | 4 (3.1) |
| Black, Asian and minority ethnic, n (%) | 7534 | 4408 | 868 | 271 (7) | 137 (5.8) | 17 (3.5) | 177 (6.3) | 75 (4.7) | 7 (2.3) | 118 (9) | 57 (8.4) | 9 (7.1) |
| MSIS-29-Phys, median [IQR] | 7840 | 4436 | 922 | 36.7 [16.7–60] | 36.7 [18.3–58.6] | 40.6 [21.7–61.7] | 43.3 [23.3–64.4] | 45 [25–65] | 48.3 [28.3–66.7] | 48.3 [26.7–69.1] | 50 [30–70] | 48.8 [30–65.6] |
| MSWS-12, median [IQR] | 7318 | 3902 | 885 | 47.6 [14.3–78.6] | 50 [16.7–78.6] | 61.9 [26.2–85.7] | 59.5 [23.8–85.7] | 59.5 [26.2–85.7] | 71.4 [40.5–90.5] | 59.5 [28.6–85.7] | 64.3 [31–85.7] | 66.7 [39.3–86.9] |
| HADS-anxiety, median [IQR] | 7923 | 4511 | 923 | 7 [4–10] | 7 [4–10] | 7 [4–10] | 8 [5–11] | 7 [5–11] | 7 [4–9] | 9 [6–12] | 9 [6–12] | 8 [5–11] |
| HADS-depression, median [IQR] | 7923 | 4511 | 923 | 6 [3–9] | 6 [3–9] | 5 [3–8.5] | 6 [4–9] | 6 [4–10] | 6 [4–9] | 8 [5–11] | 7 [5–10] | 7 [4–10] |
Smoking prevalence in the UKMSR population
Smoking status was independently verified in our data provided by NHS clinical centres. There were 858 people with multiple sclerosis who had smoking data on both the portal submitted by people with multiple sclerosis and in records collected by their clinical team. Of these, 265 records were independently collected within 2 months of each other since 2015. In the clinical data 11.0% were current smokers versus 14.7% for the online portal (P = 0.0491); 10 people with multiple sclerosis had told their healthcare team they do not smoke but revealed they did smoke on the portal.
Of the total multiple sclerosis population, 4130/7983 (51.7%) were ever smokers; 1315/7983 (16.5%) were current smokers, which is similar to the 10-year average prevalence across the entire UK from 2011 (16.68%, P = 0.619)23; 2815/4130 (68.2%) of the smokers had stopped at the time of data collection; this proportion is higher than for the total UK population between 2011 and 2019, (57.2%, P < 0.001). In the time to event population, 675/4642 (14.5%) were current smokers and 130/923 (13.7%) of the 4-year prospective population were current smokers. As the populations studied became more selective and required longer follow-up (total > time to event > 4-year parallel group) age and disease length increased, as did the proportion of people with multiple sclerosis with progressive diagnoses but also the proportion of males (Table 1).
Retrospective analysis of smoking impact
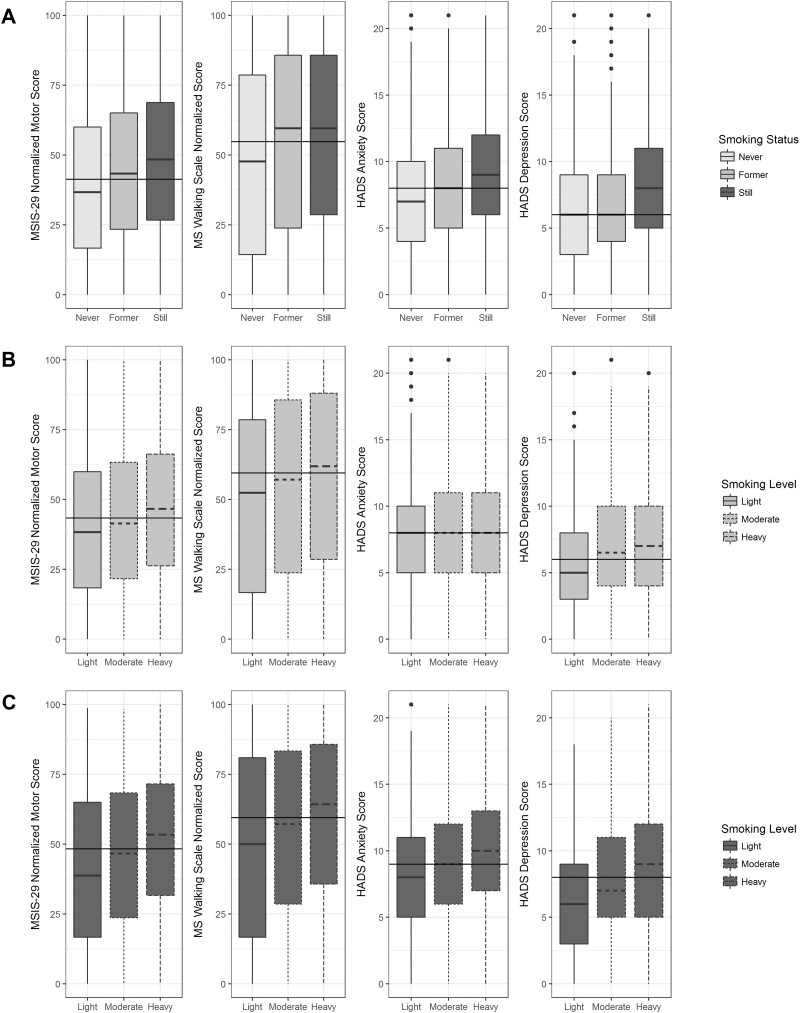
The total population was used for the retrospective analysis (n = 7983; Table 1). For all PROs, those who were current smokers at the time of completing their first questionnaire had higher disability, depression and anxiety compared to those who had never smoked (Fig. 1A). Smoking cessation was associated with a range of PRO scores depending on the PRO. HADS-depression scores were similar in former smokers compared to never smokers (Fig. 1A, panel 4). MSIS-29-Phys and HADS-anxiety scores were lower in former smokers than those of current smokers but higher than those of never smokers (Fig. 1A, panels 1 and 3). There was no change in the MSWS-12 scores compared to current smokers (Fig. 1A, panel 2). In those who were still smoking, heavier smoking burden (light, moderate, heavy) was associated with a higher PRO score in all cases (Fig. 1B). In those who were former smokers, the effects of increased smoking burden were still evident except for in the HADS-anxiety score (Fig. 1C). PRO score was not correlated with time since quitting in former smokers in all PROs; MSIS-29-Phys [r = 0.04, 95% CI (0.003, 0.078), n = 2754], MSWS-12 [r = 0.11, 95% CI (0.07, 0.15), n = 2581], HADS-anxiety [r = −0.19, 95% CI (−0.22, −0.15), n = 2779] and HADS-depression [−0.08, 95% CI (−0.11, −0.04), n = 2779].
Figure 1 Box plots demonstrating the effect of smoking cessation (A) and smoking amount (light, moderate, heavy) in current (B) and former smokers (C) for the MSIS-29-phy (panel 1), MSWS-12 (panel 2), HADS-anxiety (panel 3) and HADS-depression (panel 4).
Carrying out a multivariable linear regression adjusting for age at baseline, time since onset, multiple sclerosis type at diagnosis, ethnicity and whether the subject was receiving a DMT (Table 2) confirmed the expected impact of age and disease length and having progressive disease on PRO scores, but also demonstrated benefits of being on a DMT and being non-white. The analysis confirmed that smokers had higher PROs scores than never smokers with a mean increase in 4.7 and 3.7 points for the MSIS-29-Phys and MSWS-12, respectively, and 0.79 and 0.74 for the HADS-anxiety and -depression, respectively. There was no significant difference between former smokers and never smokers in any of the PROs. However, for each additional pack year of smoking there was a significant increase in all PROs: 0.19 point for the MSIS-29-Phys, 0.21 point for the MSWS-12 and 0.03 points for both HADS scores, indicating a cumulative effect of smoking on disability.
Table 2.
Multivariable linear regression of retrospective data (MSIS-29-Phys n = 7840, MSWS-12 n = 7318, HADS n = 7923)
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | ||
|---|---|---|---|---|---|
| MSIS-29-Phys | MSWS-12 | ||||
| (Intercept) | 31.45 (28.45, 34.44) | <0.0001 | 26.25 (22.36, 30.13) | <0.0001 | |
| Smoking status (ref: Never) | Former | −0.21 (−2.12, 1.7) | 0.83 | −0.63 (−3.11, 1.84) | 0.62 |
| Current | 4.65 (1.87, 7.42) | 0.001 | 3.68 (0.09, 7.28) | 0.044 | |
| Age | 0 (−0.07, 0.07) | 0.97 | 0.24 (0.15, 0.32) | <0.0001 | |
| Gender (ref: Female) | Male | −0.86 (−2.12, 0.39) | 0.18 | 1.04 (−0.6, 2.68) | 0.21 |
| Time since onset | 0.54 (0.48, 0.59) | <0.0001 | 0.7 (0.62, 0.78) | <0.0001 | |
| Progressive (ref: No) | Yes | 12.67 (11.14, 14.2) | <0.0001 | 20.9 (18.87, 22.93) | <0.0001 |
| Treatment (Ref: No treatment) | Normally active | −5.41 (−6.71, −4.12) | <0.0001 | −6.37 (−8.02, −4.72) | <0.0001 |
| Highly active | −6.63 (−11.06, −2.2) | 0.0034 | −6.37 (−11.9, −0.85) | 0.024 | |
| Black, Asian, minority ethnic (ref: No) | Yes | −3.71 (−5.83, −1.59) | 0.00059 | −5.54 (−8.29, −2.79) | <0.0001 |
| Pack Years | 0.19 (0.11, 0.26) | <0.0001 | 0.21 (0.11, 0.31) | <0.0001 | |
| HADS-Anxiety | HADS-Depression | ||||
| (Intercept) | 11.79 (11.27, 12.3) | <0.0001 | 6.7 (6.21, 7.2) | <0.0001 | |
| Smoking status (ref: Never) | Former | 0.15 (−0.18, 0.47) | 0.38 | −0.13 (−0.45, 0.18) | 0.41 |
| Current | 0.79 (0.31, 1.26) | 0.0012 | 0.74 (0.28, 1.2) | 0.0015 | |
| Age | −0.09 (−0.1, −0.07) | <0.0001 | −0.02 (−0.03, −0.01) | 0.00016 | |
| Gender (ref: Female) | Male | −0.84 (−1.05, −0.62) | <0.0001 | 0.06 (−0.14, 0.27) | 0.56 |
| Time since onset | 0.02 (0.01, 0.03) | 0.0031 | 0.03 (0.02, 0.04) | <0.0001 | |
| Progressive (ref: No) | Yes | 0.14 (−0.12, 0.4) | 0.29 | 0.81 (0.56, 1.06) | <0.0001 |
| Treatment (Ref: No treatment) | Normally active | −0.3 (−0.52, −0.08) | 0.0081 | −0.52 (−0.74, −0.31) | <0.0001 |
| Highly active | −1.52 (−2.27, −0.76) | <0.0001 | −1.25 (−1.98, −0.52) | 0.00075 | |
| Black, Asian, minority ethnic (ref: No) | Yes | −0.18 (−0.54, 0.18) | 0.32 | −0.24 (−0.59, 0.11) | 0.18 |
| Pack Years | 0.03 (0.01, 0.04) | <0.0001 | 0.03 (0.02, 0.05) | <0.0001 | |
Prospective parallel group analysis of smoking impact over 4 years
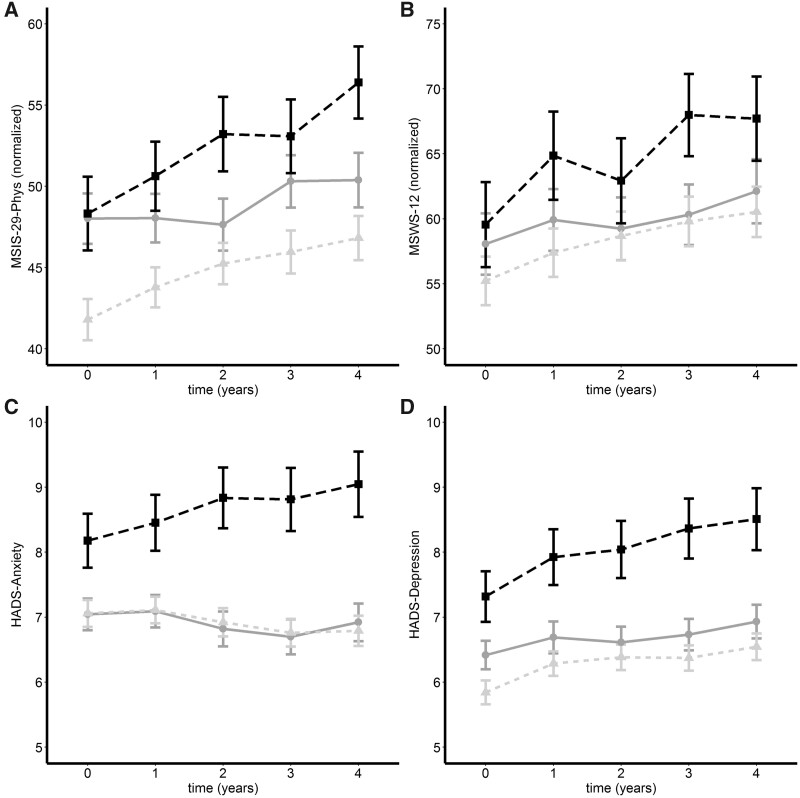
To determine the impact of smoking on PROs over the longer term we utilized a subgroup of people with multiple sclerosis (n = 923; Table 1) who had completed the PROs every year over 4 years. Average scores were plotted for each category of smoking status (Fig. 2). The MSIS-29-phy, MSWS-12 and HADS-depression score worsened over time, whereas the HADS anxiety remained stable. Smoking status was significant controlling for time across all PROs. Linear mixed modelling demonstrated that at Year 4 MSIS-29-Phys [OR (95% CI) 5.03 (3.71, 6.34)], MSWS-12 [5.28 (3.62, 6.94)], and HADS-depression [0.71 (0.47, 0.96)] scores increased, whereas the HADS-anxiety did not change. Being a current smoker was associated with a higher score than never smokers for MSIS-29-Phys [3.05 (0.22, 5.88)] and HADS-anxiety [1.14 (0.52,1.76)], whereas former smokers had a lower score at Year 4 by −2.91 (−5.03, −0.79) MSIS-29-Phys points. Average scores for MSIS-29, MSWS-12 and HADS-depression increased steadily for each year after baseline when accounting for both fixed and random effects (see Supplementary Table 2 for full results).
Figure 2.
Parallel group analysis of mean change (±standard error) for former (grey line), current (black dashes) and never (light grey dots) smokers. Plots are over 4 years for MSIS-29-Phys (A: n = 731: 382 never-smokers, 105 current-smokers and 244 former smokers), MSWS-12 (B: n = 573: 317 never-smokers, 81 current-smokers and 175 former smokers), HADS-anxiety (C) and HADS-depression (D: n = 766: 407 never-smokers, 107 current-smokers and 252 former smokers).
Time to event analyses
The prospective time to event analysis was performed using streaks of data created using the criteria described above. Streak length ranged from 180 days to 8 years. Median streak length (in years) for MSIS-29-Phys was 3.22 (IQR 3.65), MSWS 2.82 (IQR 3.65) and HADS 3.19 (IQR 3.63). Median time (in days) between each questionnaire in the streak was 115 (IQR 77) for the MSIS-29-Phys, 111 (IQR 65) for the MSWS and 114 (IQR 74) for the HADS.
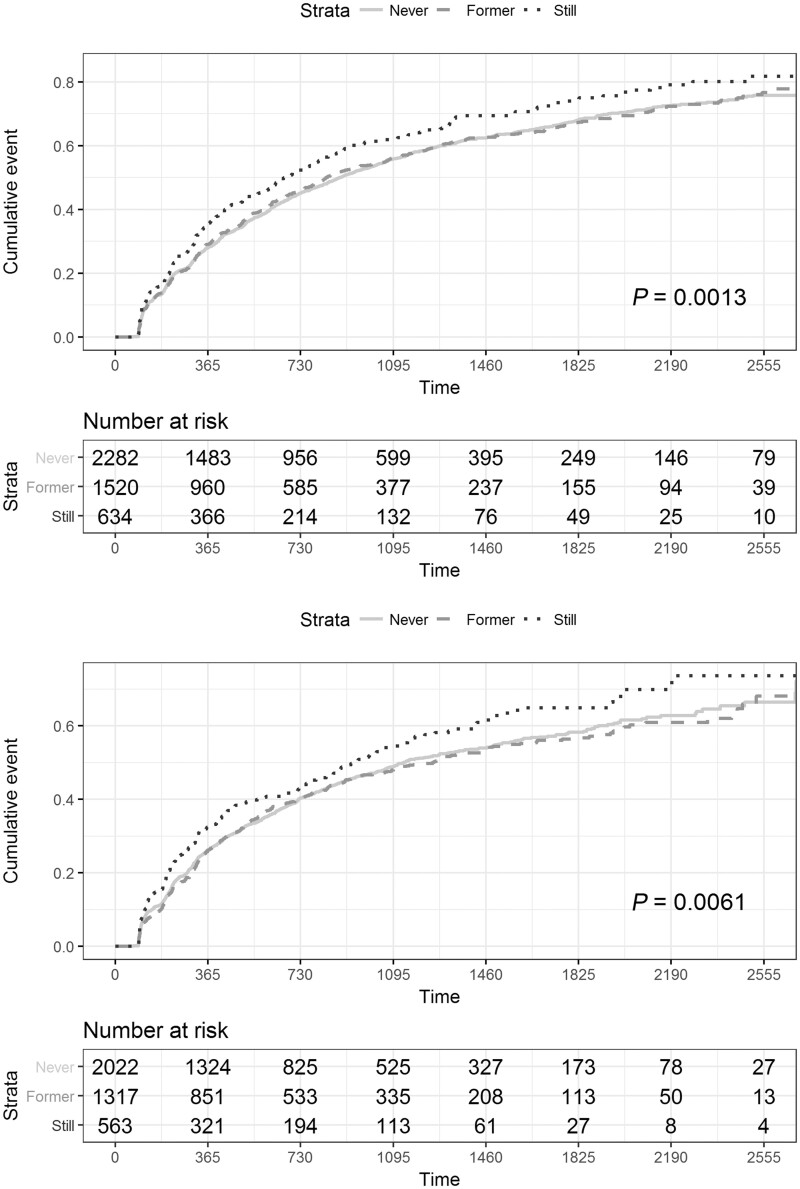
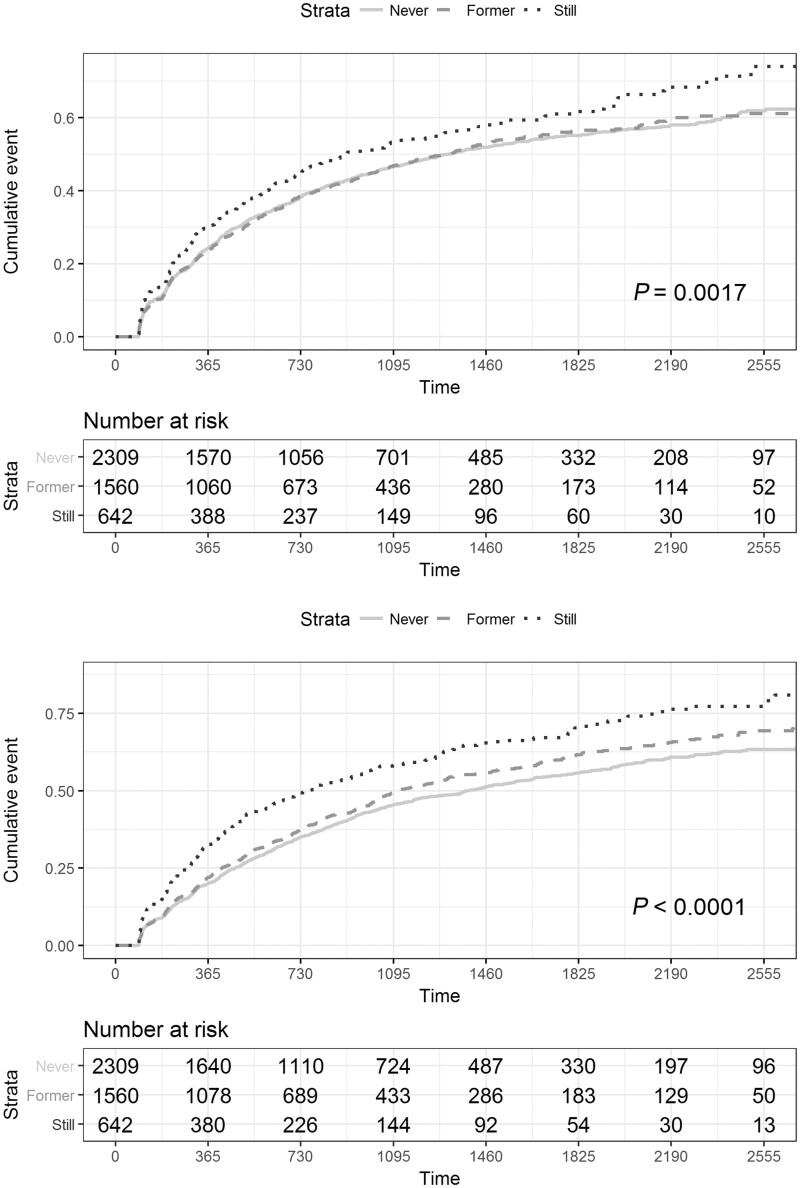
Cumulative event probabilities for time to worsening event were calculated for each PRO (Figs 3 and 4). Cox regression models were created for time to worsening, controlling for age at baseline, gender, baseline score, multiple sclerosis type at diagnosis with DMT treatment as a time-varying covariate (Table 3). Together, these demonstrated that current smoking was associated with a shorter time to worsening of MSIS-29-Phys (Table 3 and Fig. 3A), MSWS-12 (Table 3 and Fig. 3B), HADS-anxiety events (Table 3 and Fig. 4A) and HADS-depression (Table 3 and Fig. 4B). There was no significant difference in the rate of events between never and former smokers.
Figure 3 .
Cumulative event (1 − Kaplan–Meier) curves for MSIS-29-Phy (A: n = 4436) and MSWS-12 (B: n = 3902). Being a current smoker (dots) was associated with a higher rate of worsening events in both MSIS-29-Phys [Wald test chi-square, (df = 2) =13.32, P = 0.0013; median time (95% CI): 673 days (600, 787)] and MSWS-12 [Wald test chi-square, (df = 2) =10.16, P = 0.0061; median time 936 days (803, 1135)] compared to never [line; MSIS-phys median time 883 days (819, 960); MSWS-12 median time 1131 (1035, 1317)] and former smokers [dashes; MSIS-29-Phys median time 829 days (772, 930); MSWS-12 median time 1250 (1029, 14 567)].
Figure 4 .
Cumulative event (1 − Kaplan–Meier) curves for HADS-anxiety (A: n = 4511) and HADS-depression (B: n = 4511). Being a current smoker (dots) was associated with a higher rate of both HADS-anxiety [Wald test chi-square, (df = 2) =12.68, P = 0.0017; median time 907 days (742, 1239)] and HADS-depression [Wald test chi-square, (df = 2) =54.25, P < 0.0001; median time 760 days (629, 934)]. PRO worsening events compared to never [line; HADS-anxiety median time 1318 days (1168, 1519); HADS-depression median time 1392 (1207, 1563)] and former smokers [dashes; HADS-anxiety median time 1318 days (1118, 1483); HADS-depression median time 1110 (1034, 1270)].
Table 3.
Cox regression models for the time to worsening of the PROs
| MSIS-29-Phys | MSWS-12 | HADS-Anxiety | HADS-Depression | ||
|---|---|---|---|---|---|
| Smoking status (ref: Never) | Former | 1.02 (0.88, 1.17) | 0.97 (0.84, 1.13) | 1.02 (0.88, 1.17) | 1.01 (0.88, 1.17) |
| Still | 1.3 (1.04, 1.62) | 1.16 (0.92, 1.47) | 1.25 (1, 1.57) | 1.25 (1, 1.56) | |
| PRO baseline score | 0.99 (0.99, 1) | 1 (1, 1.01) | 1 (0.99, 1.01) | 1 (0.99, 1.01) | |
| Max number of PROs | 1 (0.99, 1.01) | 1 (0.99, 1.01) | 1 (0.99, 1) | 1 (0.99, 1.01) | |
| Age at baseline (years) | 1 (1, 1.01) | 1 (0.99, 1) | 1 (1, 1.01) | 1 (1, 1.01) | |
| Gender (ref: Female) | Male | 0.97 (0.88, 1.06) | 0.96 (0.87, 1.06) | 0.98 (0.89, 1.07) | 0.98 (0.89, 1.07) |
| Time Since Onset (years) | 1 (1, 1.01) | 1 (0.99, 1) | 1 (0.99, 1) | 1 (0.99, 1) | |
| Progressive (ref: No) | Yes | 1.22 (1.09, 1.36) | 1.05 (0.94, 1.18) | 1.11 (1, 1.24) | 1.11 (1, 1.24) |
| Treatment (Ref: No treatment) | Normally active | 1 (0.91, 1.1) | 1.06 (0.96, 1.17) | 1.03 (0.94, 1.13) | 1.03 (0.94, 1.14) |
| Highly active | 1.11 (0.77, 1.62) | 1.1 (0.76, 1.6) | 1.13 (0.77, 1.64) | 1.13 (0.78, 1.65) | |
| Black, Asian, minority ethnic (ref: No) | Yes | 0.86 (0.72, 1.04) | 0.85 (0.7, 1.04) | 0.88 (0.73, 1.06) | 0.88 (0.74, 1.06) |
| Pack years | 1 (1, 1.01) | 1 (0.99, 1.01) | 1 (0.99, 1.01) | 1 (0.99, 1.01) |
Values are presented as hazard ratio (95% CI).
The relationship between anxiety, depression and motor events
The HADS-anxiety and HADS-depression PROs were separately modelled against each motor PRO (Table 4). One thousand, eight hundred and sixty participants shared time to event data starting at the same time for all PROs. Using Cox modelling controlling for baseline MSIS-29-Phys (Table 4, column 1) and MSWS-12 (Table 4, column 2) score, age, gender, multiple sclerosis type at diagnosis, ethnicity and DMT as a time-varying covariate, current smoking was associated with an increased risk of having a higher MSIS-29-Phys and MSWS-12 score independently of the baseline HADS-anxiety and HADS-depression scores. An increasing baseline HADS-depression score was independently associated with a worsening of both the MSIS-29-Phys and MSWS-12. Next, we used Cox models to investigate the impact of the MSIS-29-motor on the HADS-anxiety (Table 4, column 3) and HADS-depression scores (Table 4, column 4). In both cases, being a current smoker was associated with a higher MSIS-29-Phys score and HADS-anxiety score controlling for age, gender, multiple sclerosis type at diagnosis, ethnicity and DMT as a time-varying covariate.
Table 4.
Cox regression models for the time to worsening of the PROs incorporating anxiety and depression
| MSIS-29-Phys | MSWS-12 | HADS-Anxiety | HADS-Depression | ||
|---|---|---|---|---|---|
| Smoking status (ref: Never) | Former | 1.02 (0.88, 1.18) | 0.98 (0.84, 1.14) | 1 (0.86, 1.16) | 0.99 (0.85, 1.15) |
| Still | 1.28 (1.02, 1.6) | 1.16 (0.92, 1.47) | 1.27 (1.01, 1.6) | 1.22 (0.97, 1.55) | |
| MSIS-29-Phys | 0.99 (0.99, 0.99) | 0.97 (0.96, 0.97) | 0.96 (0.96, 0.97) | ||
| MSWS-12 | 1 (1, 1.01) | 1.03 (1.02, 1.03) | 1.03 (1.02, 1.03) | ||
| HADS-anxiety | 0.99 (0.98, 1.01) | 0.99 (0.98, 1.01) | 1.04 (1.02, 1.05) | ||
| HADS-depression | 1.05 (1.03, 1.06) | 0.99 (0.98, 1.01) | 1.05 (1.04, 1.07) | ||
| Max MSIS-29 | 1.02 (0.97, 1.07) | 1.02 (0.97, 1.07) | 1.01 (0.96, 1.07) | ||
| Max MSWS-12 | 1.03 (1, 1.05) | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.03) | ||
| Max HADS | 0.98 (0.94, 1.03) | 0.97 (0.96, 0.99) | 0.97 (0.93, 1.03) | 0.98 (0.93, 1.03) | |
| Age at baseline | 1 (1, 1.01) | 1 (0.99, 1) | 1 (0.99, 1) | 1 (0.99, 1) | |
| Gender (ref: Female) | Male | 0.94 (0.86, 1.04) | 0.96 (0.87, 1.05) | 0.94 (0.86, 1.04) | 0.9 (0.82, 0.99) |
| Time since onset | 1 (1, 1.01) | 1 (0.99, 1) | 1 (0.99, 1) | 1 (0.99, 1) | |
| Progressive (ref: No) | Yes | 1.24 (1.12, 1.39) | 1.06 (0.94, 1.19) | 1.01 (0.89, 1.13) | 1.02 (0.91, 1.15) |
| Treatment (Ref: No Treatment) | Normally active | 0.99 (0.9, 1.09) | 1.05 (0.95, 1.16) | 1.03 (0.93, 1.14) | 1.02 (0.93, 1.13) |
| Highly active | 1.11 (0.76, 1.62) | 1.07 (0.73, 1.57) | 1.06 (0.74, 1.53) | 1.07 (0.75, 1.54) | |
| Black, Asian, minority ethnic (ref: No) | Yes | 0.86 (0.72, 1.03) | 0.85 (0.7, 1.04) | 0.87 (0.71, 1.05) | 0.87 (0.72, 1.06) |
| Pack years | 1 (1, 1.01) | 1 (1, 1.01) | 1 (1, 1.01) | 1 (1, 1.01) |
Values are presented as hazard ratio (95% CI).
Discussion
The UKMSR has enabled the identification of a UK-wide, community-based registry population of almost 8000 people to demonstrate the benefits of smoking cessation in multiple sclerosis. Smoking is associated with a dose-related worsening of motor function and smokers experience an accelerated rate of worsening compared to non-smokers. Once accrued, the damage does not resolve when smoking is stopped. Importantly, however, we have shown that, following smoking cessation, there is a deceleration in the rate of motor deterioration so that it matches the rate of motor decline in those who have never smoked.
The use of registry data has allowed us to overcome some of the challenges associated with studying harmful interventions. The use of PROs has, in turn, negated some of the limitations that are associated with registry data. One drawback of registry data is the potential variability associated with data drawn from multiple sites and multiple operators. This variability is particularly true of the EDSS, a quantifiable neurological examination and the most commonly used outcome in multiple sclerosis.24 The use of validated PROs has allowed a more uniform UK-wide approach to data collection. It is especially reassuring that the retrospective analysis highlights the known benefits of DMTs in multiple sclerosis as this has previously been shown with other registries using the EDSS.25 Interestingly, we found that non-white ethnicity is associated with lower PRO scores. Generally, non-white populations have similar disability to white populations. However, socioeconomic factors including participation, health literacy and health behaviours differ in non-white populations. As our population is a volunteer population, this non-white population could therefore be biased towards those with a better outcome.26 Furthermore, we have reinforced the appropriateness of PROs in this setting by using a prospective parallel group analysis to show that PROs related to motor disability worsen over 4 years irrespective of smoking status, as has been previously documented with the EDSS.
We have extended the use of PROs by adapting them for use in time to event analyses. Such analyses are common in multiple sclerosis trials.27 Regular data capture has allowed us to identify a population in whom smoking status can be confirmed at each data time point and in whom clinically significant PRO step changes can be identified and timestamped over a period of up to 8 years. PROs tied to clinically relevant outcomes in this way offer the opportunity to determine the interaction with key potential confounders such as depression and anxiety. Here, we have shown that, uniquely among the tested PROs, anxiety does not worsen over time, even though anxiety is higher in current smokers, improves with smoking cessation and is independently associated with the motor score. This implies that anxiety it is not directly linked to MS. Depression, on the other hand, does appear to be linked with the disease itself, worsening over time and, notably, deteriorating more rapidly in former smokers compared to never smokers. There is a potential that depression could drive both continued smoking and lack of exercise.
There are several limitations of our study. The UKMSR is predominantly a self-declared register, but here we have confirmed smoking status against independent healthcare team verification. Interestingly, we find that the rate of smoking declaration is higher in the self-reported data than in the clinical documentation. This discrepancy raises questions about how clinical teams can target smoking cessation advice if they are not aware of a patient’s true smoking status. A further major issue with registries is that selection bias can be augmented when participants are effectively allocating themselves into study groups. Therefore, we cannot exclude the indirect effects of other beneficial health-related activity that may go hand in hand with smoking cessation. The UKMSR is representative of the UK multiple sclerosis population,11 but here, by using a subset of the total study population, we find that completing more PROs and with greater regularity is associated with lower rates of smoking. Participants who have completed more PROs also tend to be older, male and to have more progressive multiple sclerosis. Despite these apparent biases, we are still able to demonstrate the impact of smoking cessation in all populations.
Despite longstanding knowledge that smoking is associated with a poor outcome in multiple sclerosis, we show that the rate of smoking in people with multiple sclerosis is on par with the national rates. The number of former smokers is higher than the national average, indicating the rates of smoking may have previously been higher still in people with multiple sclerosis in common with prior populations studied.7,9 This suggests that people with multiple sclerosis may not be receiving sufficient encouragement and support to stop smoking. This failure is in common with a number of other conditions in which smoking is known to have a negative impact. Recognition of such a failure has led to calls for advice about smoking cessation to be included in standard clinical guidelines for relevant diseases28 and adds to the arguments for generating evidence for the effectiveness of smoking cessation interventions.29 Here we have provided further impetus for people with multiple sclerosis to stop smoking by showing that the rate of motor deterioration is not only accelerated in smokers, but that it returns to the rate of deterioration in non-smokers following smoking cessation.
Supplementary Material
Acknowledgements
Ed Holloway and Emma Gray, UK Multiple Sclerosis Society.
Abbreviations
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- HADS
Hospital Anxiety and Depression Scale
- MSIS-29
Multiple Sclerosis Impact Scale
- MSWS-12
Multiple Sclerosis Walking Scale
- PRO
patient-reported outcome
- UKMSR
United Kingdom Multiple Sclerosis Register
Funding
The UK Multiple Sclerosis Society (Award Reference 112), The Berkeley Foundation, The Multiple Sclerosis Trials Collaboration.
Competing interests
R.N., Support from advisory boards and travel from Novartis, Roche and Biogen. He has grant support from the UK Multiple Sclerosis Society and is a member of a NICE HTA committee. T.F., personal fees from Bayer, BiosenseWebster, Boehringer Ingelheim, CSL Behring, Daiichi-Sankyo, Fresenius Kabi, Galapagos, Immunic, Janssen, LivaNova, Novartis, Roche, Vifor; all outside the submitted work. J.C., In the last 3 years, J.C. has received support from the Efficacy and Evaluation (EME) Programme, a Medical Research Council (MRC) and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment (HTA) Programme (NIHR), the UK Multiple Sclerosis Society, the US National Multiple Sclerosis Society and the Rosetrees Trust. He is supported in part by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in multiple sclerosis funded by the Canadian Multiple Sclerosis society. A local principal investigator for commercial trials funded by: Actelion, Biogen, Novartis and Roche; has received an investigator grant from Novartis; and has taken part in advisory boards/consultancy for Azadyne, Biogen, Celgene, Janssen, MedDay, Merck, Novartis and Roche. T.H., received support to attend meetings, provide advice for adboards, delivering lectures, etc. from: Merck Serrono, Biogen, UCB, Eisai, Brittania, Ipsen, Allergan, Merz, Revance, GSK, GWP Pharma, Solvay Health Care. All other authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Emre M, de Decker C.. Effects of cigarette smoking on motor functions in patients with multiple sclerosis. Arch Neurol. 1992;49(12):1243–1247. [DOI] [PubMed] [Google Scholar]
- 2. Healy BC, Ali EN, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zivadinov R, Weinstock-Guttman B, Hashmi K, et al. Smoking is associated with increased lesion volumes and brain atrophy in multiple sclerosis. Neurology. 2009;73(7):504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozcan ME, Asil T, Ince B, et al. Association between smoking and cognitive impairment in multiple sclerosis. Neuropsychiatr Dis Treat. 2014;1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanasescu R, Constantinescu CS, Tench CR, Manouchehrinia A.. Smoking cessation and the reduction of disability progression in multiple sclerosis: A cohort study. Nicotine Tob Res. 2018;20(5):589–595. [DOI] [PubMed] [Google Scholar]
- 6. Manouchehrinia A, Weston M, Tench CR, Britton J, Constantinescu CS.. Tobacco smoking and excess mortality in multiple sclerosis: A cohort study. J Neurol Neurosurg Psychiatry. 2014;85(10):1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manouchehrinia A, Tench CR, Maxted J, Bibani RH, Britton J, Constantinescu CS.. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain. 2013;136(Pt 7):2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernan MA. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128(6):1461–1465. [DOI] [PubMed] [Google Scholar]
- 9. Ramanujam R, Hedström A-K, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117–1123. [DOI] [PubMed] [Google Scholar]
- 10. Binzer S, McKay KA, Brenner P, Hillert J, Manouchehrinia A.. Disability worsening among persons with multiple sclerosis and depression: A Swedish cohort study. Neurology. 2019;93(24):e2216–e2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Middleton R, Rodgers W, Chataway J, et al. Validating the portal population of the United Kingdom multiple sclerosis register. Mult Scler Relat Disord. 2018;24:3–10. [DOI] [PubMed] [Google Scholar]
- 12. Pellegrini F, Copetti M, Bovis F, et al. A proof-of-concept application of a novel scoring approach for personalized medicine in multiple sclerosis. Mult Scler J. 2020;26(9):1064–1073. [DOI] [PubMed] [Google Scholar]
- 13. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.. Measuring the impact of MS on walking ability: The 12-item MS Walking Scale (MSWS-12). Neurology. 2003;60(1):31–36. [DOI] [PubMed] [Google Scholar]
- 14. Hobart J. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient-based outcome measure. Brain. 2001;124(5):962–973. [DOI] [PubMed] [Google Scholar]
- 15. Mehta L, McNeill M, Hobart J, et al. Identifying an important change estimate for the Multiple Sclerosis Walking Scale-12 (MSWS-12v1) for interpreting clinical trial results. Mult Scler J - Exp Transl Clin. 2015;1:2055217315596993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costelloe L, O'Rourke K, Kearney H, et al. The patient knows best: Significant change in the physical component of the Multiple Sclerosis Impact Scale (MSIS-29 physical). J Neurol Neurosurg Amp Psychiatry. 2007;78(8):841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phillips GA, Wyrwich KW, Guo S, et al. Responder definition of the Multiple Sclerosis Impact Scale physical impact subscale for patients with physical worsening. Mult Scler J. 2014;20(13):1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raffel J, Wallace A, Gveric D, Reynolds R, Friede T, Nicholas R.. Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale-29. PLoS Med. 2017;14(7):e1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zigmond AS, Snaith RP.. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 20. Hobart J, Cano S.. Improving the evaluation of therapeutic interventions in multiple sclerosis: The role of new psychometric methods. Health Technol Assess. 2009;13(12):63–88. [DOI] [PubMed] [Google Scholar]
- 21. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): A randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383(9936):2213–2221. [DOI] [PubMed] [Google Scholar]
- 22. Lemay KR, Tulloch HE, Pipe AL, Reed JL.. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J Cardiopulm Rehabil Prev. 2019;39(6):E6–E11. [DOI] [PubMed] [Google Scholar]
- 23. Adult smoking habits in the UK: 2019 - Office for National Statistics . https://www.ons.gov.uk/releases/adultsmokinghabitsintheuk2019. Accessed 17 February 2022.
- 24. Bovis F, Signori A, Carmisciano L, et al. Expanded disability status scale progression assessment heterogeneity in multiple sclerosis according to geographical areas: EDSS Progression Heterogeneity. Ann Neurol. 2018;84(4):621–625. [DOI] [PubMed] [Google Scholar]
- 25. Kalincik T, Diouf I, Sharmin S, et al. Effect of disease modifying therapy on disability in relapsing–remitting multiple sclerosis over 15 years. Neurology. 2020;95(5):e783–e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D.. Does multiple sclerosis-associated disability differ between races? Neurology. 2006;66(8):1235–1240. [DOI] [PubMed] [Google Scholar]
- 27. EMA . Clinical investigation of medicinal products for the treatment of multiple sclerosis. European Medicines Agency. Published 31 March 2015. Accessed 17 February 2022. https://www.ema.europa.eu/en/clinical-investigation-medicinal-products-treatment-multiple-sclerosis
- 28. Ekezie W, Murray RL, Agrawal S, Bogdanovica I, Britton J, Leonardi-Bee J.. Quality of smoking cessation advice in guidelines of tobacco-related diseases: An updated systematic review. Clin Med. 2020;20(6):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marck CH, das Nair R, Grech LB, Borland R, Constantinescu CS.. Modifiable risk factors for poor health outcomes in multiple sclerosis: The urgent need for research to maximise smoking cessation success. Mult Scler J. 2020;26(3):266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to the data for this study is available in a secure environment subject to governance approval.