Abstract
Dermatitis herpetiformis (DH) is an autoimmune skin disease that causes itchy, blistering rash, typically on the elbows, knees and buttocks. DH and coeliac disease share the same genetic background, glutendependent enteropathy and antibody response against tissue transglutaminase. DH is currently considered a cutaneous manifestation of coeliac disease, and the prevailing hypothesis is that DH develops as a late manifestation of subclinical coeliac disease. The incidence of DH is decreasing contemporarily with the increasing incidence of coeliac disease. The IgA immune response in DH skin is directed against epidermal transglutaminase, while the autoantigen in the gut is tissue transglutaminase. Granular IgA deposition in the papillary dermis is pathognomonic for DH, and is a finding used to confirm the diagnosis. The treatment of choice for DH is a life-long gluten-free diet, which resolves the rash and enteropathy, increases quality of life, and offers a good long-term prognosis.
Key words: dermatitis herpetiformis, coeliac disease, glutenfree diet, transglutaminase, immunoglobulin A, villous atrophy
Dermatitis herpetiformis (DH) is an intensively itching skin disease, which causes papulovesicular eruption, predominantly on the elbows, knees and buttocks. DH is considered an autoimmune-based disease, since pathognomonic granular immunoglobulin A (IgA) response in the dermis, directed against epidermal transglutaminase (TG3), and circulating autoantibodies against tissue transglutaminase (TG2) and TG3 exist in DH (1, 2). Moreover, the predisposing genetic background, more specifically HLA DQ2 or DQ8 haplotypes, is a necessity for development of the disease (3). DH is considered a specific variant of coeliac disease, manifesting primarily in the skin, but coeliac-type enteropathy also exists in DH, albeit more subtle than in coeliac disease (4). Currently approximately 13% of patients with coeliac disease have DH (5, 6) and the highest reported prevalence of DH to date has been 75 per 100,000 from Finland (5). The prevalence is lower in some areas of the globe and in specific populations, for example in Asia and in African-Americans (7, 8) and, overall, the geographical differences in the prevalence of DH and, likewise, coeliac disease, have been explained mainly by HLA genetics and wheat consumption habits (9). Also the incidence figures of DH have ranged from 0.4 to 3.5/100 000/year, even in different studies performed in Europe or North America (5, 10). DH is typically diagnosed during adulthood, and the incidence of DH is highest in females and males aged 50–69 years (5, 6). Interestingly, the diagnostic age of DH has increased (5) and, although the reasons for this increase remain largely obscure, a possible explanation could be changes in dietary habits. Nonetheless, even though childhood diagnosis is rare in northern Europe (5, 6, 11) it seems to be more common in Italy and Hungary (12, 13).
The focus of this review is to describe the current, clinically relevant, concepts of DH diagnostics, treatment and prognosis. In addition, the close link between DH and coeliac disease is elaborated, and unique features of DH, the cutaneous manifestation of coeliac disease, are presented.
SIGNIFICANCE
Dermatitis herpetiformis is an itchy, blistering rash, which occurs on the elbows, knees and buttocks. Dermatitis herpetiformis is considered a cutaneous manifestation of coeliac disease. Even though obvious gastrointestinal symptoms are rare in dermatitis herpetiformis, intestinal coeliac-type villous atrophy or inflammation is present at diagnosis. The diagnosis is confirmed by skin biopsy revealing typical IgA deposits, and the majority of patients also have coeliac autoantibodies in the serum. The treatment of choice for dermatitis herpetiformis is a life-long gluten-free diet, which resolves the rash and enteropathy, increases quality of life, and offers a good long-term prognosis.
SKIN MANIFESTATION OF COELIAC DISEASE
The clinical manifestations of DH were first described as early as 1884 by Louis Duhring (14) and, 4 years later, the classical abdominal and malabsorptive symptoms of coeliac disease were described by Samuel Gee (15). The link between DH and coeliac disease was found when Marks et al. (16) detected that coeliac-type enteropathy was also a common finding in DH, and importantly, when gluten-free diet (GFD), the treatment of choice in coeliac disease, was shown to heal small bowel mucosal changes in DH, and to alleviate DH rash (17, 18). Subsequent family and genetic studies have coupled DH and coeliac disease even more convincingly together: DH and coeliac disease have been shown to occur often in the same families and even in monozygotic twins, and furthermore, predominantly HLA DQ2 and, more rarely, DQ8 haplotypes have been shown to be the predisposing haplotypes in both (19–21). Moreover, it has been shown that the phenotype of coeliac disease is not invariably constant, since it can convert from classical disease into DH, especially when dietary compliance is poor (22).
A major breakthrough occurred in coeliac disease research in the 1990s when TG2 enzyme was identified as the autoantigen of the disease (23). Subsequently an enzyme-linked immunosorbent assay (ELISA)-based method for detecting TG2 antibodies was developed and found to be accurate in coeliac disease (24) and, furthermore, a similar TG2 antibody reaction was shown to occur in the serum of patients with DH (2). Moreover, TG2-targeted autoimmune response has been detected in the small bowel mucosa of untreated coeliac disease and DH patients (25, 26).
DH, however, has some distinct features compared with coeliac disease in general. DH is more rarely diagnosed during childhood compared with coeliac disease (11, 27). Furthermore, DH is slightly more common among males than females (5), which contradicts the female predominance known to exist in coeliac disease (6, 28). Moreover, the incidence of DH has decreased, but in coeliac disease a marked increase in the incidence figures has been detected (5, 6, 28). One prevailing hypothesis is that DH develops as a late manifestation of coeliac disease, affecting individuals with subclinical or neglected coeliac disease. It has, moreover, been suggested that the TG3 immune response typical for DH develops as an epitope spreading phenomenon from an autoimmune response initially targeting TG2 (29). Coeliac-type dental enamel defects detected in adults diagnosed with DH indicate that these individuals were already sensitive to gluten in early childhood (30). Moreover, the rarity of childhood DH and the changing phenotype of coeliac disease during poor dietary adherence support this hypothesis, and furthermore, the divergent trend of incidences of DH and coeliac disease also fits well with this hypothesis: better diagnostics of coeliac disease due to increased awareness, availability of accurate serum autoantibody tests, and screening of risk groups has resulted in a smaller pool of patients with undiagnosed coeliac disease and, consequently, fewer individuals with potential for development of DH.
DIAGNOSING DERMATITIS HERPETIFORMIS
The suspicion of DH typically arises from the characteristic skin symptoms, which are an intensely pruritic rash with small blisters and papules affecting most commonly the extensor surfaces of the elbows, knees and buttocks (Fig. 1a, b and Table I). Occasionally other sites, such as the scalp, face, upper back and neck, are also affected. There is individual variation in the severity of the rash and pruritus, but commonly due to the intense itch and scratching, the blisters are broken and only erosions, crusts and post-inflammatory hyperpigmentation are consequently present. Acral purpura is one, albeit quite rare, finding in DH and can be found either as a sole presentation or concomitantly with the typical DH rash (31–33). Despite the gluten-sensitive enteropathy, obvious gastrointestinal symptoms and signs of malabsorption are rare in DH, but some kind of abdominal symptoms have, however, been reported in up to one-third of patients (34, 35). Interestingly, although the clinical picture of coeliac disease has been shown to become milder and more heterogenic with increasingly common non-classical symptoms (36–38), it seems that the clinical picture and the severity of DH rash have remained quite unchanged during recent decades (39).
Fig. 1.
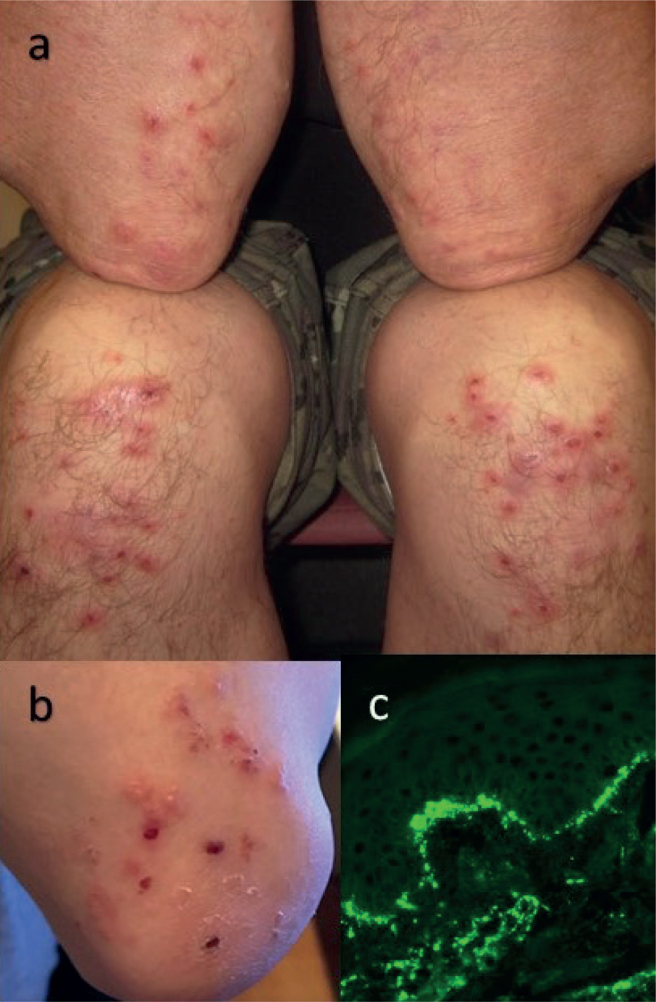
Clinical characteristics of dermatitits herpetiformis (DH). (a) A typical clinical picture of dermatitis herpetiformis (DH) with excoriated blisters and papules on the elbows and knees. (b) Intact and excoriated blisters, papules and crusts on the elbow. (c) Direct immunofluorescence (×40) finding in DH; granular IgA deposits in the basal membrane zone and in the dermis.
Table I.
Diagnostic procedures in dermatitis herpetiformis (DH) and recommendations regarding when they should be applied
| Procedure | Recommendation |
|---|---|
| Patient history and physical examination | |
| Duration, severity and type of skin symptoms | Always |
| Presence of gastrointestinal and malabsorptive symptoms and signs | Always |
| Family history of coeliac disease and DH | Always |
| Presence of associated autoimmune diseases | Always |
| Diagnostic procedures | |
| Direct immunofluorescence examination of perilesional skin biopsy | Always |
| Histopathological analysis of lesional skin biopsy | In obscure cases |
| Serum tissue transglutaminase or endomysial antibodies | Always |
| Small bowel biopsy examination | Only if gastrointestinal symptoms not compatible with coeliac disease exist |
| HLA DQ2 and DQ8 typing | Only in obscure cases |
The differential diagnosis of DH includes other subepidermal blistering diseases, especially linear IgA disease and bullous pemphigoid. In addition, other itchy skin diseases, such as atopic and nummular dermatitis, lichen planus, urticaria and scabies may sometimes be difficult to differentiate from scratched DH rash, although the typical predilection sites of these diseases differ from those of DH (40).
The gold-standard method to verify DH diagnosis is direct immunofluorescence (IF) examination, which shows the pathognomonic granular IgA deposits in the papillary dermis and/or at the dermoepidermal junction (Fig. 1c). IgA deposits are widespread, but not totally uniformly distributed in the skin of patients with DH, and therefore the ideal site for the diagnostic skin biopsy is uninvolved perilesional skin, where the deposits are found in greater amounts (41). The immune response in DH skin is directed against TG3, an enzyme closely related, but not identical, to TG2 (1). It has recently been demonstrated that TG3 disappears from the dermis of patients with DH on a GFD, in parallel with IgA, but the disappearance is prolonged, often taking years even on a strict diet (42). There are a few rather interesting studies reporting that granular IgA also exists in the skin of coeliac disease patients with healthy skin or with inflammatory skin diseases other than DH (43, 44). However, the number of the patients in these studies has been small, and further research evidence is needed before conclusions can be drawn about the existence of granular IgA in non-DH skin. For the time being, at least, this finding can be considered DH-specific.
In addition to the characteristic granular deposition of IgA, mostly sporadic cases of fibrillary IgA deposits in DH have been presented (45–47). The fibrillar pattern of IgA appears to be more common in Japan, where it has been reported to occur in approximately one-third of patients with DH. However, Japanese patients with DH also show other distinct features that differ from Caucasian patients; the Japanese patients with DH do not carry the predisposing HLA-DQ2 and HLA-DQ8 haplotypes, the occurrence of gluten-sensitive enteropathy is rare, and coeliac-disease-specific autoantibodies are seen only in low proportion of patients. These findings suggest that the pathogenesis of Japanese DH differs from that of Caucasian DH, and may not be dependent on gluten (48, 49).
Histopathological examination of lesional skin biopsy is not required for diagnosis of DH, but, in obscure cases, compatible findings with DH support the diagnosis (40). Ideal areas for histopathological biopsy specimen are an intact vesicle or erythematous skin, and the typical findings include non-specific subepidermal blister and papillary microabscesses, together with neutrophil and a few eosinophil infiltrates (50). However, the abovementioned findings alone do not allow the differentiation of DH from other autoimmune bullous disorders.
A recent study from Finland demonstrates that diagnosis of DH is not always easy. The study investigating the diagnostic delay of DH during the last 45 years detected that the duration of skin symptoms before the diagnosis was 2 years or more in one-third of patients with DH. Female sex, villous atrophy at diagnosis, and a DH diagnosis prior to the year 2000 were significantly associated with long diagnostic delay. Fortunately, the same study established that the diagnostic delay has shortened during recent decades from 12 to 8 months (39). Correspondingly, the diagnostic delay in coeliac disease has become shorter (51).
SEROLOGICAL AND SMALL BOWEL MUCOSAL FINDINGS IN DERMATITIS HERPETIFORMIS
In DH, there are often circulating IgA-class autoantibodies against both transglutaminase isoenzymes, TG2 and TG3. TG2 is also the target for endomysial antibodies (EmA) (52), and ELISA-based TG2- and indirect IF-based EmA tests can equally be utilized in clinical practice (Table I). However, the evaluation of EmA is subjective and requires skilful laboratory personnel. TG2 antibodies have proven to be highly accurate in coeliac disease, but in DH these antibodies are mostly confined to those patients with small bowel mucosal villous atrophy, and hence a negative result does not exclude DH (53). However, together with a compatible clinical picture, TG2 antibodies are suggestive of DH, and further, indicative of small bowel mucosal damage. If elevated, TG2 antibody measurement can further be utilized in the follow-up of GFD adherence after the diagnosis. Circulating TG3 antibodies have been suggested to be DH-specific, but surprisingly, these antibodies occasionally also occur in the serum of coeliac disease patients without any detectable skin lesions (1, 54, 55). It has been shown, however, that in coeliac disease the affinity of the antibodies to TG3 is lower than in DH (1) and that TG3 reactivity increases with age in coeliac disease (55). Therefore, it can be speculated that skin symptom-free coeliac disease patients with TG3 reactivity are susceptible to future development of DH, especially if not compliant with a strict GFD. However, since the exact role and value of TG3 antibodies in DH and coeliac disease is, thus far, to some extent obscure, these antibodies are currently mostly used in research settings.
Small bowel mucosal biopsies obtained during upper gastrointestinal endoscopy are not necessary for DH diagnosis. It is widely recognized that the majority of the untreated DH patients have coeliac-type small bowel mucosal villous atrophy, but at least one-quarter of the patients evince normal villous architecture (53). However, virtually all subjects without evident small bowel mucosal damage evince intestinal coeliac-type inflammation and/or immune response. Characteristic for both DH and coeliac disease is increased densities of γδ+ intraepithelial lymphocytes in the small bowel mucosa (56), but even more specific finding is the presence of intestinal TG2-targeted autoantibody deposits (25, 26). However, both of these investigations require frozen small bowel mucosal samples, which are not available in every diagnostic centre. Importantly, even though small bowel mucosal changes vary from inflammatory changes to severe villous atrophy in DH, recent evidence has shown that the severity of mucosal damage at diagnosis does not have any effects on the long-term prognosis of DH (57, 58), which naturally strengthens the rationale behind the current policy of not obtaining routine small bowel biopsies when DH is diagnosed.
GLUTEN-FREE DIET AND DAPSONE TREATMENT IN DERMATITIS HERPETIFORMIS
The essential treatment for DH is a strict, life-long GFD. When adhering to a GFD, wheat, rye, barley and foods otherwise containing gluten are permanently excluded from the daily diet, but gluten-free oats (i.e. oats not contaminated by other cereals) are currently allowed in most countries and tolerated by the majority of patients with DH (59). Adherence to a GFD leads to healing of the small bowel mucosa and alleviation of the clinical symptoms, but total clearance of the DH rash may take several months or even a couple of years (17, 60). Therefore, at the beginning of GFD treatment the individuals with widespread, active rash need additional treatment with dapsone.
Dapsone is a sulfone drug with potent antimicrobial and anti-inflammatory properties, which relieves the DH rash and itch effectively, but has no effect on the enteropathy. The starting dose of dapsone should be 25–50 mg/day. If needed, the dose can be increased gradually up to 100 mg/day, and then, once the rash has disappeared, the dose should be slowly tapered and finally discontinued as the GFD alone controls the rash (60). Dapsone is usually well tolerated when recommended doses are used, but side-effects are possible, of which dose-dependent haemolysis is the most common and, for example, methaemoglobinaemia, agranulocytosis and hepatitis less frequent. Hence, clinical and laboratory monitoring during treatment is necessary. In Finland approximately 70% of patients with DH require dapsone treatment after being diagnosed, and when initiated, it is usually needed for 2–3 years (57, 60). In rare cases of DH, the rash continues despite long-lasting, strict, adherence to a GFD. Recently this condition, named refractory DH, was found to occur in less than 2% of patients with DH (61). The patients with refractory DH in that study had followed a strict GFD for a mean of 16 years, but dapsone was still essential due to the active DH rash. Interestingly, despite the ongoing clinical symptoms, the small bowel mucosa had recovered in all subjects, and none had developed lymphoma, which suggests that refractory DH probably diverges from refractory coeliac disease, in which the small bowel mucosa does not heal on a GFD and the risk of lymphoma is increased (62). However, since refractory DH seems to be very rare, in cases of non-responsive DH, intentional or accidental dietary lapses are a more common reason and have to be excluded by dietary consultation.
Current recommendations are that treatment with a GFD should be life-long in DH, as in coeliac disease. However, there are some reports suggesting that a proportion of patients with DH following a GFD could later re-introduce gluten to their diet without developing symptoms or signs of DH (60, 63, 64). Three glutenchallenge studies have also investigated the possible redevelopment of gluten tolerance in DH. The first gluten-challenge study by Leonard et al. reported 11 out of 12 (92%) patients with DH relapsed with rash and 7 (64%) of these also with villous atrophy (65). However, when Bardella et al. later challenged 38 GFD-treated DH patients with gluten, they reported 7 (18%) who did not manifest any type of relapse in the skin or small bowel during the prolonged gluten challenge (66). Very recently, a 12-month gluten-challenge study was performed in 19 long-term GFD-treated DH patients in Finland (67). In this study, 18 (95%) of the patients relapsed in a mean of 6 months; 15 (79%) developed DH rash, 12 of whom also showed small bowel villous atrophy, and 3 patients showed progression of small bowel mucosal villous atrophy without skin symptoms or cutaneous IgA deposits. One patient, however, did not show any skin symptoms or IgA deposits, nor did he develop intestinal villous atrophy or inflammation. However, a long follow-up is needed before it can be concluded that gluten is truly tolerated by this patient, and at present, it seems that development of gluten tolerance in DH is rare or even non-existent, and life-long strict adherence to a GFD is still justified in all patients with DH.
Long-term prognosis on a gluten-free diet
Coeliac disease is known for increased all-cause and lymphoma mortality risk (68). Therefore it is interesting that, in a recent Finnish DH study, the all-cause mortality rate in DH was, in contrast, significantly decreased (standardized mortality rate 0.70), and the lymphoma mortality was increased during the first 5 years after diagnosis, but not thereafter (58). Similarly, a previous DH study from the UK found a slightly, but non-significantly, reduced mortality rate (hazard ratio 0.93) (69). In the Finnish study, 98% of patients with DH adhered to a GFD, which may explain their excellent prognosis, whereas in the study from the UK, data about dietary adherence was absent for one-third of patients (58, 69). Evidence clearly confirms that adherence to a GFD reduces the risk of lymphoma in DH, the risk of which has been shown to be similarly increased in DH and coeliac disease (70–72). In DH, the risk of gastrointestinal carcinomas has not been reported to be increased, which is in contrast to coeliac disease (69, 71, 72). Also, the increased bone fracture risk associated with coeliac disease seems not to be a complication of similar extent in DH, although bone complications have been very rarely studied in DH (69, 73).
Quality of life (QoL) aspects in coeliac disease have been widely studied, but only limited evidence of DH and QoL exist. However, according to current knowledge, the QoL of patients with DH seems to be reduced, but importantly, already after adherence to a GFD for 1 year, the QoL increases to the level of controls (35). The positive impact of GFD on DH patients’ QoL is also supported by another study, in which the Qol of long-term GFD-treated DH patients was equal to that of controls, and slightly better than that of long-term treated coeliac patients (74).
Similar to coeliac disease, DH has been associated with other autoimmune diseases, and the associations have mostly been explained by common genetic factors. In DH, the frequency of autoimmune thyroid disease has been reported to be as high as 4% and that of type 1 diabetes 1–2% (75–78). In addition, Sjögren’s syndrome, vitiligo and alopecia areata have been reported to associate with DH, although these associations are not well documented. Most of the associated autoimmune diseases have been reported to develop prior to the diagnosis of DH, but subsequent development is also a possibility. A recent Finnish register study demonstrated a rather interesting association of DH with bullous pemphigoid (79). In that study, patients with previously diagnosed DH had a 22-fold risk for the later development of bullous pemphigoid, with a mean of 3 years from diagnosis of DH to diagnosis of bullous pemphigoid. The authors speculated that a possible mechanism of this evolvement could be an epitope spreading phenomenon.
CONCLUSION
DH is a chronic, bullous skin disease, which is a skin manifestation of coeliac disease. It is suggested that long-lasting and undetected coeliac disease with TG2-directed immune response serves as a prerequisite for the development of DH and TG3 antibody response and, furthermore, that more accurate and active coeliac disease diagnostics has resulted in a declining incidence of DH (5, 6). The cutaneous symptoms of DH are troublesome and decrease the QoL of patients (35). It is therefore fortunate that the diagnostic delay has become shorter during recent decades (39). However, variable prevalence figures for DH in different countries, and delayed diagnosis in onethird of patients with DH in a high prevalence area (39) indicate that there is still a necessity for further improvement of DH diagnostics. Recognizing the cutaneous signs indicative of DH and IF examination of perilesional skin biopsy remain the cornerstones of DH diagnosis (Table I). Investigation of small bowel mucosal histology has no further value in routine diagnostics, and TG2 antibody testing has a supportive, but not exclusive, role in DH diagnosis. Future studies will presumably reveal whether measurement of TG3 antibody has additional value in DH diagnostics or in the identification of subjects at risk of development of DH. One future prospect is that TG3 antibody-based diagnosis of DH could be a possibility in the long run, which would facilitate the diagnosis of DH and enable diagnostics in centres without the possibility of IF examination. In coeliac disease, serologically-based diagnosis has been recommended in children since the year 2012 (80), and is also utilized in adults in some countries, such as Finland.
According to current knowledge, strict life-long adherence to GFD is justified in all patients with DH. The prognosis seems to be excellent in those individuals with DH who follow the diet rigorously, but other than adherence to a GFD, little is known about the factors that influence the development of complications or associated diseases of DH and mortality. Instead, it has been shown that the degree of villous atrophy has no effect on the above-mentioned outcomes of DH (57, 58). Factual non-responsiveness to GFD is rare in DH, but, in general, refractory DH seems to have better prognosis compared with refractory coeliac disease (61). However, current knowledge of refractory DH is scarce and more research evidence is needed in order to elaborate this entity more thoroughly. In addition, the differing mortality trends currently existing among DH and coeliac disease patients adhering to the same diet is an interesting topic for future studies.
ACKNOWLEDGEMENTS
The study was financially supported by the Academy of Finland and by the Competitive Research Funding of Tampere University Hospital (grants 9U053 and 9V059).
REFERENCES
- 1.Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 2002; 195: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieterich W, Schuppan D, Laag E, Bruckner-Tuderman L, Reunala T, Kárpáti S, et al. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Invest Dermatol 1999; 113: 133–136. [DOI] [PubMed] [Google Scholar]
- 3.Hall MA, Lanchbury JS, Bolsover WJ, Welsh KI, Ciclitira PJ. HLA association with dermatitis herpetiformis is accounted for by a cis or transassociated DQ heterodimer. Gut 1991; 32: 487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collin P, Salmi TT, Hervonen K, Kaukinen K, Reunala T. Dermatitis herpetiformis: a cutaneous manifestation of coeliac disease. Ann Med 2017; 49: 23–31. [DOI] [PubMed] [Google Scholar]
- 5.Salmi T, Hervonen K, Kautiainen H, Collin P, Reunala T. Prevalence and incidence of dermatitis herpetiformis: a 40-year prospective study from Finland. Br J Dermatol 2011; 165: 354–359. [DOI] [PubMed] [Google Scholar]
- 6.West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol 2014; 109: 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall RP, Clark RE, Ward FE. Dermatitis herpetiformis in two American blacks: HLA type and clinical characteristics. J Am Acad Dermatol 1990; 22: 436–439. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Yang B, Lin Y, Chen S, Zhou G, Wang G, et al. Dermatitis herpetiformis in China: a report of 22 cases. J Eur Acad Dermatol Venereol 2012; 26: 903–907. [DOI] [PubMed] [Google Scholar]
- 9.Kang J, Kang A, Green A, Gwee K, Ho K. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther 2013; 38: 226–245. [DOI] [PubMed] [Google Scholar]
- 10.Buckley DB, English J, Molloy W, Doyle CT, Whelton MJ. Dermatitis herpetiformis: a review of 119 cases. Clin Exp Dermatol 1983; 8: 477–487. [DOI] [PubMed] [Google Scholar]
- 11.Hervonen K, Salmi T, Kurppa K, Kaukinen K, Collin P, Reunala T. Dermatitis herpetiformis in children: a long-term follow-up study. Br J Dermatol 2014; 171: 1242–1243. [DOI] [PubMed] [Google Scholar]
- 12.Dahlbom I, Korponay-Szabo IR, Kovács JB, Szalai Z, Mäki M, Hansson T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J Pediatr Gastroenterol Nutr 2010; 50: 140–146. [DOI] [PubMed] [Google Scholar]
- 13.Antiga E, Verdelli A, Calabrò A, Fabbri P, Caproni M. Clinical and immunopathological features of 159 patients with dermatitis herpetiformis: an Italian experience. G Ital Dermatol Venereol 2013; 148: 163–169. [PubMed] [Google Scholar]
- 14.Duhring LA. Dermatitis herpetiformis. JAMA 1884; 3: 225–229. [DOI] [PubMed] [Google Scholar]
- 15.Gee S. On the coeliac disease. St Bart Hosp Rep 1888; 24: 17–20. [Google Scholar]
- 16.Marks J, Shuster S, Watson AJ. Small-bowel changes in dermatitis herpetiformis. Lancet 1966; 2: 1280–1282. [DOI] [PubMed] [Google Scholar]
- 17.Fry L, McMinn R, Cowan JD, Hoffbrand A. Gluten-free diet and reintroduction of gluten in dermatitis herpetiformis. Arch Dermatol 1969; 100: 129–135. [PubMed] [Google Scholar]
- 18.Fry L, Riches D, Seah P, Hoffbrand A. Clearance of skin lesions in dermatitis herpetiformis after gluten withdrawal. Lancet 1973; 301: 288–291. [DOI] [PubMed] [Google Scholar]
- 19.Hervonen K, Hakanen M, Kaukinen K, Collin P, Reunala T. First-degree relatives are frequently affected in coeliac disease and dermatitis herpetiformis. Scand J Gastroenterol 2002; 37: 51–55. [DOI] [PubMed] [Google Scholar]
- 20.Hervonen K, Karell K, Holopainen P, Collin P, Partanen J, Reunala T. Concordance of dermatitis herpetiformis and celiac disease in monozygous twins. J Invest Dermatol 2000; 115: 990–993. [DOI] [PubMed] [Google Scholar]
- 21.Balas A, Vicario J, Zambrano A, Acuna D, Garcfa-Novo D. Absolute linkage of celiac disease and dermatitis herpetiformis to HLA-DQ. Tissue Antigens 1997; 50: 52–56. [DOI] [PubMed] [Google Scholar]
- 22.Salmi T, Hervonen K, Kurppa K, Collin P, Kaukinen K, Reunala T. Celiac disease evolving into dermatitis herpetiformis in patients adhering to normal or gluten-free diet. Scand J Gastroenterol 2015; 50: 387–392. [DOI] [PubMed] [Google Scholar]
- 23.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997; 3: 797–801. [DOI] [PubMed] [Google Scholar]
- 24.Sulkanen S, Halttunen T, Laurila K, Kolho KL, Korponay-Szabó IR, Sarnesto A, et al. Tissue transglutaminase antibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998; 115: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 25.Korponay-Szabó IR, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs J, et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004; 53: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmi TT, Hervonen K, Laurila K, Collin P, Mäki M, Koskinen O, et al. Small bowel transglutaminase 2-specific IgA deposits in dermatitis herpetiformis. Acta Derm Venereol 2014; 94: 393–397. [DOI] [PubMed] [Google Scholar]
- 27.Kivelä L, Kaukinen K, Lähdeaho M-L, Huhtala H, Ashorn M, Ruuska T, et al. Presentation of celiac disease in Finnish children is no longer changing: a 50-year perspective. J Pediatr 2015; 167: 1109–1115.e1. [DOI] [PubMed] [Google Scholar]
- 28.Virta LJ, Kaukinen K, Collin P. Incidence and prevalence of diagnosed coeliac disease in Finland: results of effective case finding in adults. Scand J Gastroenterol 2009; 44: 933–938. [DOI] [PubMed] [Google Scholar]
- 29.Zone JJ, Schmidt LA, Taylor TB, Hull CM, Sotiriou MC, Jaskowski TD, et al. Dermatitis herpetiformis sera or goat anti–transglutaminase-3 transferred to human skin-grafted mice mimics dermatitis herpetiformis immunopathology. J Immunol 2011; 186: 4474–4480. [DOI] [PubMed] [Google Scholar]
- 30.Aine L, Mäki M, Reunala T. Coeliac-type dental enamel defects in patients with dermatitis herpetiformis. Acta Derm Venereol 1992; 72: 25–27. [PubMed] [Google Scholar]
- 31.Karpati S, Torok E, Kosnai I. Discrete palmar and plantar symptoms in children with dermatitis herpetiformis Duhring. Cutis 1986; 37: 184–187. [PubMed] [Google Scholar]
- 32.Tu H, Parmentier L, Stieger M, Spanou Z, Horn M, Beltraminelli H, et al. Acral purpura as leading clinical manifestation of dermatitis herpetiformis: report of two adult cases with a review of the literature. Dermatology 2013; 227: 1–4. [DOI] [PubMed] [Google Scholar]
- 33.Zaghi D, Witheiler D, Menter AM. Petechial eruption on fingers. Dermatitis herpetiformis. JAMA Dermatol 2014; 150: 1353–1354. [DOI] [PubMed] [Google Scholar]
- 34.Alakoski A, Salmi T, Hervonen K, Kautiainen H, Salo M, Kaukinen K, et al. Chronic gastritis in dermatitis herpetiformis: a controlled study. Clin Dev Immunol 2012; 2012: 640630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasternack C, Kaukinen K, Kurppa K, Mäki M, Collin P, Hervonen K, et al. Gastrointestinal symptoms increase the burden of illness in dermatitis herpetiformis: a prospective study. Acta Derm Venereol 2017; 97: 58–62. [DOI] [PubMed] [Google Scholar]
- 36.Visakorpi JK, Mäki M. Changing clinical features of coeliac disease. Acta Pediatr Suppl 1994; 83: 10–13. [DOI] [PubMed] [Google Scholar]
- 37.Tapsas D, Hollen E, Stenhammar L, Fält-Magnusson K. The clinical presentation of coeliac disease in 1030 Swedish children: changing features over the past four decades. Dig Liver Dis 2016; 48: 16–22. [DOI] [PubMed] [Google Scholar]
- 38.Spijkerman M, Tan IJ, Kolkman JJ, Withoff S, Wijmenga C, Wisschedijk MC, et al. A large variety of clinical features and concomitant disorders in celiac disease – a cohort study in the Netherlands. Dig Liver Dis 2016; 48: 499–505. [DOI] [PubMed] [Google Scholar]
- 39.Mansikka E, Salmi T, Kaukinen K, Collin P, Huhtala H, Reunala T, et al. Diagnostic delay in dermatitis herpetiformis in highprevalence area. Acta Derm Venereol 2018; 98: 195–199. [DOI] [PubMed] [Google Scholar]
- 40.Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis: part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol 2011; 64: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 41.Zone J, Meyer L, Petersen M. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol 1996; 132: 912–918. [PubMed] [Google Scholar]
- 42.Hietikko M, Hervonen K, Salmi T, Ilus T, Zone J, Kaukinen K, et al. Disappearance of epidermal transglutaminase and IgA deposits from the papillary dermis of patients with dermatitis herpetiformis after a long-term gluten-free diet. Br J Dermatol 2018; 178: 198–201. [DOI] [PubMed] [Google Scholar]
- 43.Cannistraci C, Lesnoni La Parola I, Cardinali G, Bolasco G, Aspite N, Stigliano V, et al. Co-localization of IgA and TG3 in healthy skin of coeliac patients. J Eur Acad Dermatol Venereol 2007; 21: 509–514. [DOI] [PubMed] [Google Scholar]
- 44.Bonciolini V, Antiga E, Bianchi B, DelBianco E, Ninci A, Maio V, et al. Granular IgA deposits in the skin of patients with coeliac disease: is it always dermatitis herpetiformis? Acta Derm Venereol 2019; 99: 78–83. [DOI] [PubMed] [Google Scholar]
- 45.Ko CJ, Colegio OR, Moss JE, McNiff JM. Fibrillar IgA deposition in dermatitis herpetiformis- and underreported pattern with potential clinical significance. J Cutan Pathol 2010; 37: 475–477. [DOI] [PubMed] [Google Scholar]
- 46.Lilo MT, Yan S, Chapman MS, Linos K. A case of dermatitis herpetiformis with fibrillar immunoglobulin A deposition: a rare pattern not to be missed. Am J Dermatopathol 2019; 41: 511–513. [DOI] [PubMed] [Google Scholar]
- 47.Miraflor AP, Paul J, Yan S, LeBlanc RE. Dermatitis herpetiformis with fibrillar IgA deposition and unusual histologic findings. JAAD Case Rep 2017; 3: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohata C, Ishii N, Hamada T, Shimomura Y, Niizeki H, Dainichi T, et al. Distinct characteristics in Japanese dermatitis herpetiformis: a review of all 91 Japanese patients over the last 35 years. Clin Dev Immunol 2012; 2012: 562168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohata C, Ishii N, Niizeki H, Shimomura Y, Furumura M, Inoko H, et al. Unique characteristics in Japanese dermatitis herpetiformis. Br J Dermatol 2016; 174: 180–183. [DOI] [PubMed] [Google Scholar]
- 50.Pierard J, Whimster I. The histological diagnosis of dermatitis herpetiformis, bullous pemphigoid and erythema multiforme. Br J Dermatol 1961; 73: 253–266. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs V, Kurppa K, Huhtala H, Collin P, Mäki M, Kaukinen K. Factors associated with long diagnostic delay in celiac disease. Scand J Gastroenterol 2014; 49: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 52.Korponay-Szabó IR, Laurila K, Szondy Z, Halttunen T, Szalai Z, Dahlbom I, et al. Missing endomysial and reticulin binding of coeliac antibodies in translutaminase 2 knockout tissues. Gut 2003; 52: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansikka E, Hervonen K, Salmi TT, Kautiainen H, Kaukinen K, Collin P, et al. The decreasing prevalence of severe villous atrophy in dermatitis herpetiformis: a 45-year experience in 393 patients. J Clin Gastroenterol 2017; 51: 235–239. [DOI] [PubMed] [Google Scholar]
- 54.Hull CM, Liddle M, Hansen N, Meyer L, Schmidt L, Taylor T, et al. Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol 2008; 159: 120–124. [DOI] [PubMed] [Google Scholar]
- 55.Salmi T, Kurppa K, Hervonen K, Laurila K, Collin P, Huhtala H, et al. Serum transglutaminase 3 antibodies correlate with age at celiac disease diagnosis. Dig Liver Dis 2016; 48: 632–637. [DOI] [PubMed] [Google Scholar]
- 56.Järvinen TT, Kaukinen K, Laurila K, Kyrönpalo S, Rasmussen M, Mäki M, et al. Intraepithelial lymphocytes in celiac disease. Am J Gastroenterol 2003; 98: 1332–1337. [DOI] [PubMed] [Google Scholar]
- 57.Mansikka E, Hervonen K, Kaukinen K, Collin P, Huhtala H, Reunala T. Prognosis of dermatitis herpetiformis patients with and without villous atrophy at diagnosis. Nutrients 2018; 10. piiE641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hervonen K, Alakoski A, Salmi T, Helakorpi S, Kautiainen H, Kaukinen K, et al. Reduced mortality in dermatitis herpetiformis: a population-based study of 476 patients. Br J Dermatol 2012; 167: 1331–1337. [DOI] [PubMed] [Google Scholar]
- 59.Reunala T, Collin P, Holm K, Pikkarainen P, Miettinen A, Vuolteenaho N, et al. Tolerance to oats in dermatitis herpetiformis. Gut 1998; 42: 490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garioch JJ, Lewis HM, Gargent SA, Leonard JN, Fry L. 25 years’ experience of a gluten-free diet in the treatment of dermatitis herpetiformis. Br J Dermatol 1994; 131: 541–545. [DOI] [PubMed] [Google Scholar]
- 61.Hervonen K, Salmi T, Ilus T, Paasikivi K, Vornanen M, Laurila K, et al. Dermatitis herpetiformis refractory togluten free dietary treatment. Acta Derm Venereol 2016; 96: 82–86. [DOI] [PubMed] [Google Scholar]
- 62.Malamut G, Cellier C. Refractory celiac disease: epidemiology and clinical manifestations. Dig Dis 2015; 33: 221–226. [DOI] [PubMed] [Google Scholar]
- 63.Gawkrodger D, Blackwell J, Gilmour H, Rifkind E, Heading R, Barnetson R. Dermatitis herpetiformis: diagnosis, diet and demography. Gut 1984; 25: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paek SY, Steinberg SM, Katz SI. Remission in dermatitis herpetiformis: a cohort study. Arch Dermatol 2011; 147: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leonard J, Haffenden G, Tucker W, Unsworth J, Swain F, McMinn R, et al. Gluten challenge in dermatitis herpetiformis. N Engl J Med 1983; 308: 816–819. [DOI] [PubMed] [Google Scholar]
- 66.Bardella M, Fredella C, Trovato C, Ermacora E, Cavalli R, Saladino V, et al. Long-term remission in patients with dermatitis herpetiformis on a normal diet. Br J Dermatol 2003; 149: 968–971. [DOI] [PubMed] [Google Scholar]
- 67.Mansikka E, Hervonen K, Kaukinen K, Ilus T, Oksanen P, Lindfors K, et al. Gluten challenge induces skin and small bowel relapse in long-term gluten-free diet treated dermatitis herpetiformis. J Invest Dermatol 2019; 139: 2108–2114. [DOI] [PubMed] [Google Scholar]
- 68.Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther 2012; 35: 540–551. [DOI] [PubMed] [Google Scholar]
- 69.Lewis N, Logan R, Hubbard R, West J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther 2008; 27: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 70.Lewis H, Reunala T, Garioch J, Leonard J, Fry J, Collin P, et al. Protective effect of gluten-free diet against development of lymphoma in dermatitis herpetiformis. Br J Dermatol 1996; 135: 363–367. [PubMed] [Google Scholar]
- 71.Sigurgeisson B, Agnarsson BA, Lindelof B. Risk of lymphoma in patients with dermatitis herpetiformis. BMJ 1994; 308: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year populationbased study. Dig Liver Dis 2006; 38: 374–380. [DOI] [PubMed] [Google Scholar]
- 73.Heikkilä K, Pearce J, Mäki M, Kaukinen K. Celiac disease and bone fractures: a systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100: 25–43. [DOI] [PubMed] [Google Scholar]
- 74.Pasternack C, Kaukinen K, Kurppa K, Mäki M, Collin P, Reunala T, et al. Quality of life and gastrointestinal symptoms in long-term treated dermatitis herpetiformis patients: a cross-sectional study in Finland. Am J Clin Dermatol 2015; 16: 545–552. [DOI] [PubMed] [Google Scholar]
- 75.Gaspari AA, Huang C-M, Davey RJ, Bondy C, Lawley TJ, Katz S. Prevalence of thyroid abnormalities in patients with dermatitis herpetiformis and in control subjects with HLAB8/-DR3. Am J Med 1990; 88: 145–150. [DOI] [PubMed] [Google Scholar]
- 76.Reijonen H, Ilonen J, Knip M, Reunala T, Reijonen H. Insulindependent diabetes mellitus associated with dermatitis herpetiformis: evidence for heterogeneity of HLA-associated genes. Tissue Antigens 1991; 37: 94–96. [DOI] [PubMed] [Google Scholar]
- 77.Reunala T, Collin P. Diseases associated with dermatitis herpetiformis. Br J Dermatol 1997; 136: 315–318. [PubMed] [Google Scholar]
- 78.Hervonen K, Viljamaa M, Collin P, Knip M, Reunala T. The occurrence of type 1 diabetes in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol 2004; 150: 136–138. [DOI] [PubMed] [Google Scholar]
- 79.Varpuluoma O, Jokelainen J, Försti AK, Timonen M, Huilaja L, Tasanen K. Dermatitis herpetiformis and celiac disease increase the risk of bullous pemphigoid. J Invest Dermatol 2019; 139: 600–604. [DOI] [PubMed] [Google Scholar]
- 80.Husby S, Koletzko S, Korponay-Szabó I, Mearin M, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012; 54: 136–160. [DOI] [PubMed] [Google Scholar]


