Abstract
Atopic dermatitis (AD) is a chronic, or chronically relapsing, inflammatory skin disease associated with asthma and allergic rhinitis, and is dominated by Th2 cells. The co-stimulatory T-cell receptor OX40 and its ligand, OX40L, play a central role in the pathogenesis of AD, as their interactions are crucial for the generation of TH2 memory cells. Using enzyme-linked immunoassay (ELISA) and flow cytometry on blood samples from patients with AD and healthy volunteers, this study shows that the serum level of soluble (s) OX40 is decreased in patients with AD, and the expression of OX40 by activated skin-homing CD4+ T cells is increased. This study further shows, using immunofluorescence on skin biopsies, that OX40+ and OX40L+ cells are co-located within the dermis, indicating local activity of OX40/OX40L. Serum levels of sOX40 were associated with atopic diseases and, together, these results support that the OX40 system is important for chronic inflammation in AD skin.
Key words: atopic dermatitis, OX40, OX40L
Atopic dermatitis (AD) is a chronic inflammatory skin disease that affects up to 20% of children and may persist into adulthood. The symptoms are eczema, intense pruritus, and dry skin, caused by a profound skin inflammation and epidermal barrier dysfunction. Up to 80% of patients with AD have elevated levels of IgE (1). Furthermore, development of memory T cells is believed to be central for chronicity in AD (2).
OX40 and its ligand, OX40L, are both members of the tumour necrosis factor superfamily and are important regulators of the immune response (3). The OX40 system is required for generating a long-term memory response and optimal T-cell activation (4–6). The OX40-OX40L axis is considered to be involved in the development of autoimmune and inflammatory conditions, including allergic asthma (7–9).
T-cell expression of OX40 and antigen-presenting cells (APC) expression of OX40L appear within 2–3 days after antigen-activation (6). The signal through OX40 enhances T-cell clonal expansion and, especially, survival of TH2 cells (10, 11). Apart from APC, OX40L is also expressed by mast cells (12). Both OX40 and OX40L also exist in soluble forms. Soluble OX40 (sOX40) has an anti-inflammatory effect when binding to the membrane-bound form of OX40L (13). sOX40 is able to mimic regulatory T-cell function, inhibit mast cell degranulation and thereby reduce the inflammatory response (13).
SIGNIFICANCE
Atopic dermatitis is a chronic, or chronically relapsing, inflammatory skin disease dominated by Th2 cells. The costimulatory T-cell receptor OX40 and its ligand, OX40L, are crucial for the generation of Th2 memory cells, and may thus play a central role in the pathogenesis and chronicity of atopic dermatitis. This study shows that OX40 expression is increased on skin-homing T cells in patients with atopic dermatitis, and that OX40 and OX40L cells are colocalized in the skin. These findings indicate that the OX40 system could play a pivotal role in the inflammatory reaction of atopic dermatitis in the skin.
Not only are mast cells implicated in the immediate hypersensitivity reactions, but also in the production of TH2 driving cytokines, such as interleukin (IL)-4 and IL-25, and hence central mediators of AD pathogenesis (14).
Furthermore, IgE-activated mast cells have been shown to interact with T cells through expression of OX40L (12, 15), suggesting a sophisticated interplay between these immune cells mediated by IgE and OX40L.
The aim of this study was to characterize the OX40/ OX40L axis in AD, including the OX40 expressing cells and the correlation between serum levels and disease-specific markers.
MATERIALS AND METHODS
Patient samples
Patients with AD were diagnosed using the Hanifin & Rajka criteria (16). None of the patients were receiving systemic immune suppressive therapy at the time of sample collection. All patients were assessed by use of the SCORing Atopic Dermatitis index (SCORAD) and measurement of total IgE serum level.
Serum samples from 67 adults (age 30; range 24–42 years), and 60 children with AD (age 8; 3–14 years) were collected at the Department of Dermatology, University Hospital Schleswig Holstein, Campus Kiel, Germany. Clinical parameters were acquired at the time of blood collection: SCORAD, total serum IgE and the presence of asthma and/or rhinitis (Table I). Serum samples were also obtained from 31 healthy controls (HC) (age 39; 28–51 years) matched for age and sex with the adult AD group. The controls had no history of AD or other systemic inflammatory diseases. All adult patients with AD and HC gave their written informed consent. The parents provided written consent on behalf of their children with AD. For ethical reasons serum samples from healthy children were not included.
Table I.
Characteristics of patients with atopic dermatitis (AD)
| Group | Samples n | Age, years Median (range) | Sex% female (n) | SCORAD Median (range) | Total IgE (IU/ml) Median (range) | Asthma % (n) | Rhinitis % (n) | Asthma and rhinitis % (n) |
|---|---|---|---|---|---|---|---|---|
| ELISAa | ||||||||
| Children with AD | 60 | 8 (3–14) | 55.0 (33) | 37.0 (19.9–47.4) | 154.0 (36.50–839.0) | 28.3 (17) | 35.0 (21) | 20.0 (12) |
| Adults with AD | 67 | 30(24–42) | 52.2(35) | 32.0 (16.2–44.5) | 198.0 (61.7–802.0) | 35.8 (24) | 64.2 (43) | 35.0 (21) |
| Healthy controls | 31 | 39 (28 – 51) | 61.3 (19) | - | 40.0 (8.0–59.0) | - | - | - |
| Flow cytometryb | ||||||||
| Adults with AD | 11 | 29 (21.0–43.0) | 27.3 (3) | 54.6 (43.6–64.3) | 1,458 (357–4352) | 18.1 (2) | 27.3 (3) | 9.1 (1) |
| Healthy controls | 10 | 27 (24.0–32.5) | 40.0 (4) | - | - | - | - | - |
Serum samples of patients with AD analysed for levels of sOX40 and sOX40L by enzyme-linked immunoassay (ELISA).
Peripheral blood mononuclear cells from patients with AD analysed for membrane expression of OX40 and OX40L by flow cytometry.
Healthy controls were matched for age and sex to the patients with AD (for the adult group only).
SCORAD: Subjective SCORing Atopic Dermatitis.
Peripheral blood mononuclear cells (PBMCs) and plasma were collected from 11 adult patients with AD (age 29; 21–43 years) and 10 age- and sex-matched HC (age 27; 24–33 years) with no atopic diseases or systemic inflammatory diseases (Table I). The PBMCs were isolated by Ficoll-Paque (GE Healthcare Europe, Brøndby, Denmark) density gradient centrifugation and frozen at −135°C until analysis.
Paraffin-embedded biopsies were collected from involved and uninvolved skin from 5 patients with AD and skin biopsies were collected from 5 HC. Written consent was obtained in accordance with the guidelines of the Danish National Ethics Committee for Health Research and the principles of the Declaration of Helsinki.
Detection of sOX40 and sOX40L by ELISA
Soluble OX40 and sOX40L were measured in serum from patients with AD and from HC with a sOX40 ELISA kit (cat. no. BMS296TEN, eBioscience (eBioscience, San Diego, CA, USA)) and with an inhouse sOX40L ELISA, as described previously (7, 17). OD values were read by a Thermo Scientific Multiscan GO reader (Thermo Scientific, Waltham, MA, USA) at 450 nm, with 570 nm as a reference. The minimum detectable level of sOX40 and sOX40L was determined by the cut-off value of the standard curve. The lowest detectable levels of sOX40 and sOX40L were 1.9 and 0.039 ng/ml, respectively.
Flow cytometry
The expression of OX40 and OX40L on the cell surface were analysed by flow cytometry on PBMCs from patients with AD and HC.
The cells were thawed and blocked with 100 μg/ml mouse IgG (Jackson Immunoresearch Europe Ltd, (JIR), Cambridgeshire, UK) to prevent unspecific binding.
Unstimulated, blocked cells were stained using the following monoclonal antibodies, all titrated for optimal concentration: PE-Cy7-anti CD4 (cat. no. 560649, clone: RPA-T4), BV605-anti CD8 (cat. no. 564116, clone: SK1), BV510-anti CD14 (cat. no. 563079, clone: MΦP9), PerCP-Cy5.5-anti CD45RO (cat. no. 560607, clone: UCHL1), FITC-anti CD56 (cat. no. 562794, clone: B159), BV 421-anti CLA (cat. no. 563961, clone: HECA-452) (all from BD Biosciences, San Jose, CA, USA), APC-anti OX40 (cat. no. 17–1347, clone: ACT 35, eBioscience), PE-anti OX40L (cat. no. FAB10541p, clone: 159403,R&D Systems, Minneapolis, MN, USA) and LIVE/DEAD Near IR antibody (cat. no. L10119, Life Technologies, Carlsbad, CA, USA) for detection of dead cells. After addition of antibodies the cells were incubated for 30 min in the dark at 4°C and washed 3 times in wash buffer (phosphatebuffered saline (PBS), pH 7.4, supplemented with 0.5% bovine serum albumin (BSA) and 0.09% sodium azide). Cells were fixed in 0.9% formaldehyde and analysed within 24 h on a LSR Fortessa (BD Biosciences) flow cytometer with Diva software (version: BDFacs Diva 8.0 (BD Biosciences)). Data were analysed on FlowJo Software for MAC version 10.1 (Tree Star Inc., Ashland, USA). Fluorescence minus one (FMO) controls were used to gate OX40 and OX40L. Antibody-coated beads (OneComp, eBioscience) were used for compensation of spectral overlap.
Confocal microscopy
Formaldehyde-fixed, paraffin-embedded skin biopsies from involved and uninvolved skin from 5 adult patients with AD and 5 HC were cut into 5-μm sections using a microtome (version: HM 360, MICROM, Microm UK LTD, Bicester, UK). Slides were deparaffinized in xylene, rehydrated in an ethanol gradient, followed by heat-induced antigen retrieval in citrate-phosphate buffer (pH 6).
Slides were blocked with 10% donkey serum (cat. no. D9663, Sigma-Aldrich, St. Louis, MO, USA) prior to staining with polyclonal rabbit anti-OX40 (cat. no. 119904) and monoclonal mouse anti-OX40L (cat. no. Ab89896, clone: MM0505-8S23) both from Abcam, Cambridge, UK (16 μg/ml). Another set-up was made with monoclonal rabbit anti-tryptase (cat. no. ab134932, clone: EPR8476, Abcam) (7 μg/ml) and monoclonal mouse anti-OX40L, as mentioned above. All antibodies were analysed for optimal working concentration. Slides were incubated with primary antibodies overnight at 4°C. The slides were washed and donkey anti-mouse Alexa 488 (cat. no. 715-546-151, JIR) and donkey anti-rabbit Alexa 647 (cat. no. 711-606-152, JIR) secondary antibodies (both 7.5 μg/ml) were added together with DAPI (AppliChem GmbH, Darmstadt, Germany) (1 μg/ml) and incubated for 30 min. For negative control of unspecific binding, a mouse IgG1 isotype control (cat. no. X0931, DAKO, Glostrup, Denmark) was used. The slides were washed, all sections subsequently fixed with Mounting Medium (DAKO), and analysed by confocal microscopy (LSM710, Zeiss, Oberkochen, Germany).
Statistical analyses
Statistical analyses and graphs were done using Prism 6 (GraphPad Software, La Jolla CA USA). Unpaired data were analysed using the Mann-Whitney rank sum test, and paired data were analysed using the Wilcoxon signed-rank test. Correlations were tested using Spearman’s rho (ρ). Graphics are presented as medians with 10th to 90th percentiles and interquartile range (IQR), unless otherwise specified. A 2-sided p-value < 0.05 was considered statistically significant.
RESULTS
Serum levels of sOX40, but not of sOX40L, are decreased in atopic dermatitis
Serum levels of sOX40 and sOX40L were measured in patients with AD (67 adults and 60 children) and healthy adult volunteers (HC) (n = 31) (Table I). In adult patients with AD, sOX40 was significantly reduced (9.1; range 2.0–26.0 pg/ml) compared with HC (15.6; 3.7–32.3 pg/ ml) (p < 0.0001). In children with AD, sOX40 levels were comparable with adult AD levels (9.7; 2.0–24.6 pg/ml) (Fig. 1A). Regarding sOX40L, no significant differences were observed between the groups (Fig. 1B).
Fig. 1.
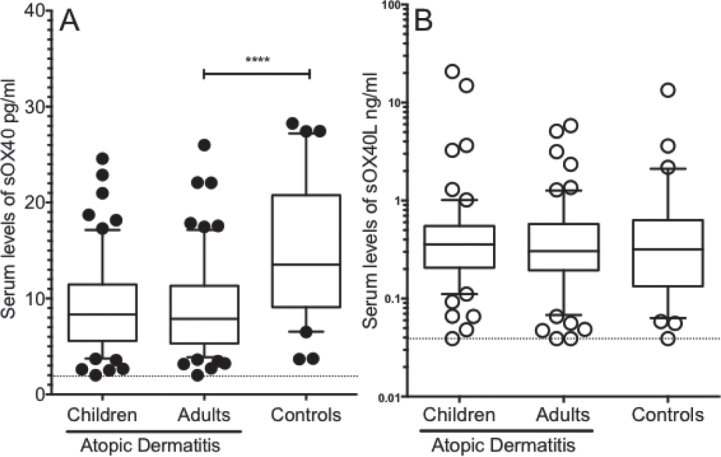
Decreased serum levels of sOX40, but not sOX40L, in atopic dermatitis (AD). Serum levels of soluble (s)OX40 and sOX40L were measured in patients with AD (adults n = 67 and children n = 60) and healthy controls (HC) (n = 31) matched for age and sex with AD adults, by enzyme-linked immunoassay (ELISA). (a) In adult patients with AD, serum levels of sOX40 were significantly decreased compared with HC. In children with AD, serum level of sOX40 showed similar levels as in adults with AD. (b) Serum levels of soluble OX40L showed no significant differences between the patients with AD and HC. Data are analysed by Mann-Whitney U test. Boxes indicate median and interquartile range (IQR) and whiskers indicate 10–90 percentiles. The dotted horizontal line indicates the ELISA cut-off value. ****p < 0.0001.
Serum levels of sOX40 is associated with atopic comorbidities and in children with disease activity
Significantly different levels of serum sOX40 were observed between the groups; therefore we examined the association between the individual serum sOX40 level, the SCORAD index, and other markers related to atopic disease activity. In the group of adults with AD, the patients with asthma had significant higher sOX40 serum levels (10.0; range 4.0–12.8 pg/ml), compared with those without asthma (7.0; 4.6–10.2 pg/ml) (p < 0.01). In children, sOX40 correlated with SCORAD (ρ = 0.404, p < 0.01) (Table II). Soluble OX40L showed no association with disease activity measurements, either in children or in adults.
Table II.
Correlation between baseline serum levels of sOX40 in atopic dermatitis and clinical parameters
| Adults | Children | |
|---|---|---|
| SCORAD | 0.096 | 0.404 ** |
| IgE | −0.036 | 0.198 |
Correlations between serum levels of sOX40 in atopic dermatitis and disease parameters subjective SCORing Atopic Dermatitis (SCORAD) and total serum immunoglobulin E (IgE) levels. Data are analysed by Spearman correlations and values are shown as Spearman’s rho (ρ).
Represent level of significance: *p < 0.05, **p < 0.01.
OX40 and OX40L are highly expressed by skin-homing T cells and monocytes in atopic dermatitis
Considering the reduced sOX40 serum levels in patients with AD, we investigated the surface expression of OX40 and its ligand on PBMCs from adult patients with AD. Immunophenotyping of PBMCs from adult patients with AD (n = 11) and HC (n = 10) revealed that OX40 was mainly expressed by skin-homing memory-prone CLA+ T cells, in both AD patients and HC (Fig. 2A and B).
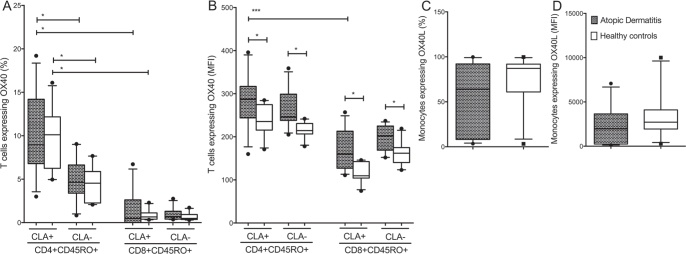
Fig. 2.
CD4+CD45RO+CLA+ T cells express high levels of OX40 in atopic dermatitis (AD), whereas OX40L is expressed primarily by monocytes. Membrane expression of OX40 and OX40L on peripheral blood mononuclear cells from patients with AD (n = 11) and healthy controls (HC) (n = 10), divided into CD4+CD45RO+ and CD8+CD45RO+ T cells with or without the expression of cutaneous lymphocyte-associated antigen (CLA). (a): OX40 was expressed primarily by the CD4+ CD45RO+ CLA+T cell subsets. (b) Median fluorescence intensity (MFI) of OX40 was significantly higher among patients with AD compared with HC. (c, d) OX40L+ monocytes, showed no difference between patients with AD and HC (both percentage and MFI). Data were analysed as non-paired data by Mann-Whitney U test. Boxes indicate median and interquartile range, and whiskers represent 10th–90th percentiles. *p < 0.05, ***p < 0.001.
In adult patients with AD, 8.9% (6.8–14.2%) of the CD4+CD45RO+CLA+ T cells expressed OX40 followed by 4.6% (3.4–6.6%) of the CD4+CD45RO+CLA− T cells. OX40 expression in AD patients was less pronounced on CD8+CD45RO+CLA+ T cells (0.5%; 0.0–2.6%) and CD8+CD45RO+CLA− T cells (0.7%; 0.4–1.3%)). No differences were observed compared with HC (Fig. 2A).
A similar pattern emerged when the results were evaluated using median fluorescence intensity (MFI), although with important differences: CD4+CD45RO+CLA+ T cells from patients with AD expressed the highest amount of OX40 per cell (MFI 287 (244–317)), and revealed a significantly higher level than HC (MFI 235; range 215–275) (p < 0.05). CD4+CD45RO+CLA− T cells expressed a lower amount of OX40 (MFI 246; range 238–299), but also significantly higher than HC (MFI 214 (206–231)) (p < 0.05) (Fig. 2B). A similar pattern was observed for the CD8+ T cells, but to a lesser degree than by CD4+ T cells. In general, monocytes expressed high levels of OX40L both in percentage (64.1%; range 8.1–92.1%) and MFI (1964; range 244–3649) (Fig. 2C and D), but no differences were observed between AD and HC (all p > 0.05).
Cellular expression of OX40 in patients with atopic dermatitis inversely correlates with SCORAD
To further evaluate OX40 in AD, the relative cell populations were correlated with disease activity (SCORAD) and serum IgE. The percentage of CD4+CD45RO+OX40+ CLA+/− T cells correlated inversely with disease activity measured by SCORAD (CLA+ (ρ = −0.809, p < 0.01) and CLA− (ρ = −0.782, p < 0.01)). Similarly, the MFI also correlated with SCORAD (CLA+ (ρ = −0.709, p < 0.1) and CLA- (ρ = −0.674, p < 0.05)), but not with serum IgE levels. No correlations with disease activity and serum IgE for OX40 expressed by CD8+CD45RO+ CLA+/− T cells or OX40L expressed by monocytes were observed. Individual plasma levels of sOX40 and sOX40L did not correlate with surface expression of OX40 and OX40L (data not shown).
OX40 and OX40L are co-localized in atopic dermatitis
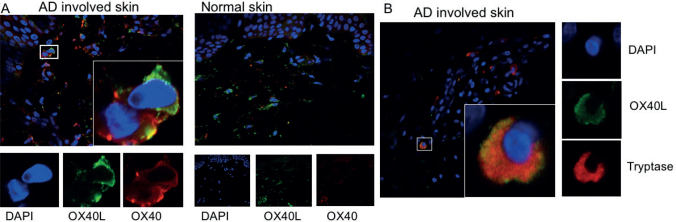
To confirm the involvement of OX40-OX40L interactions directly in the skin, immunofluorescence staining was carried out on involved and uninvolved skin biopsies from adult patients with AD and from HC. OX40- and OX40L-expressing cells were identified in both involved and uninvolved tissue (not shown) and found to be co-localized within the dermis (Fig. 3A). In AD skin, in particular, OX40-positive staining was present on the surface of OX40L-expressing cells (Fig. 3A). That these were indeed OX40L-expressing mast cells were confirmed by positive tryptase staining (Fig. 3B).
Fig. 3.
OX40-and OX40L-positive cells are present in atopic dermatitis (AD) skin. (a) Skin biopsies from involved (n = 5) and uninvolved skin (n = 5) from patients with AD were examined for the presence of OX40 (red) and OX40L (green) and compared with healthy controls (n = 5). Nuclear staining was carried out by DAPI (blue). OX40- and OX40L-positive cells were present in involved and uninvolved AD skin. (b) The presence of OX40L (green) co-expressed with tryptase (red) confirmed these to be mast cells, located to the dermis of patients with AD. Original magnification ×40.
DISCUSSION
The results of this study show that children and adults with AD have reduced serum levels of sOX40 compared with adult HC. Although the patients in the study cohort were included based on their presentation of AD, some patients also had allergic rhinitis and asthma. This subsequently made it possible to examine the association of serum levels of sOX40 and sOX40L and atopic comorbidities in patients with AD.
Previous studies have demonstrated elevated sOX40L levels in patients with active allergic asthma (8, 18). This was further supported by Farres et al., who showed that increased expression of OX40L on B cells is associated with the level of total IgE in allergic asthma patients (19). These findings are in line with murine asthma models, in which OX40L is highly expressed by a variety of cells within the lung during an induced allergic reaction (20, 21). Although, in general, decreased serum levels were observed in AD, adults with concomitant asthma had the highest levels of serum sOX40. As with all members of the TNF-super family, sOX40 can bind to its ligand expressed by different cell types. This may explain the decrease in serum levels of sOX40. However, in our experiments we observed similar levels of sOX40L in healthy volunteers and patients with AD and, thus, circulating sOX40L is not an appropriate marker of disease activity in AD.
More interestingly, focusing on circulating skin-homing CD4+ T cells from patients with AD we observed an increase in expression of OX40. As skin-homing T cells are able to upregulate OX40 expression, an increased number of pathogenic T cells can migrate to the inflammatory site of the skin and thereby enhance local inflammation.
In addition, this study was able to localize OX40-expressing cells to the skin of patients with AD. These cells were co-localized with mast cells, suggesting an interplay between mast cells and activated T cells through OX40-OX40L in the skin of patients with AD, and this may provide the background for part of the mechanism by which anti-OX40 therapy exerts its effect on both Eczema Area and Severity Index (EASI) score, biomarkers and skin thickness when used as treatment for AD (22).
Together, these findings indicate a close correlation between the OX40 system and atopic diseases.
In conclusion, OX40 expression is increased on skinhoming T cells and, together with our finding that OX40 and OX40L are co-localized in the skin, the OX40-OX40L system may play an important role in the recruitment to and activation of these cells in the skin. This finding is supported by the association of disease activity and atopic co-morbidities in atopic dermatitis, and we hypothesize that the OX40 system plays a pivotal role in the inflammatory reaction in the skin. It is therefore of interest to follow the development of AD treatments targeting the OX40 system.
ACKNOWLEDGEMENTS
JSHE was supported by grants from the Aage Bang’s Foundation and Aarhus University Scholarship. The authors thank Karin Skovgaard for providing technical support.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest 2004; 113: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009; 9: 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 2001; 15: 445–455. [DOI] [PubMed] [Google Scholar]
- 5.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol 2000; 165: 3043–3050. [DOI] [PubMed] [Google Scholar]
- 6.Ishii N, Takahashi T, Soroosh P, Sugamura K. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol 2010; 105: 63–98. [DOI] [PubMed] [Google Scholar]
- 7.Laustsen JK, Rasmussen TK, Stengaard-Pedersen K, Hørslev-Petersen K, Hetland ML, Østergaard M, et al. Soluble OX40L is associated with presence of autoantibodies in early rheumatoid arthritis. Arthritis Res Ther 2014; 16: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei W, Zhu CH, Zeng da X, Wang Q, Zhang XQ, Chen YB, et al. SOX40L: an important inflammatory mediator in adult bronchial asthma. Ann Acad Med Singapore 2012; 41: 200–204. [PubMed] [Google Scholar]
- 9.Foks AC, van Puijvelde GH, Bot I, ter Borg MN, Habets KL, Johnson JL, et al. Interruption of the OX40-OX40 ligand pathway in LDL receptor-deficient mice causes regression of atherosclerosis. J Immunol 2013; 191: 4573–4580. [DOI] [PubMed] [Google Scholar]
- 10.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol 2010; 28: 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino A, Tanaka Y, Akiba H, Mita Y, Sakurai T, Takaoka A, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol 2003; 33: 861–869. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol 2004; 173: 5247–5257. [DOI] [PubMed] [Google Scholar]
- 13.Sibilano R, Gri G, Frossi B, Tripodo C, Suzuki R, Rivera J, et al. Technical advance: soluble OX40 molecule mimics regulatory T cell modulatory activity on FcepsilonRI-dependent mast cell degranulation. J Leukoc Biol 2011; 90: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood 2003; 101: 3594–3596. [DOI] [PubMed] [Google Scholar]
- 15.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 2006; 176: 2238–2248. [DOI] [PubMed] [Google Scholar]
- 16.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980; Suppl 92: 44–47. [Google Scholar]
- 17.Kragstrup TW, Vorup-Jensen T, Deleuran B, Hvid M. A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients. Springerplus 2013; 2: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzat MH, Imam SS, Shaheen KY, Elbrhami EM. Serum OX40 ligand levels in asthmatic children: a potential biomarker of severity and persistence. Allergy Asthma Proc 2011; 32: 313–318. [DOI] [PubMed] [Google Scholar]
- 19.Farres MN, Sabry MK, Ahmed EE, Elkady HM, Mohamed NA. OX40 ligand: a potential costimulatory molecule in atopic asthma. J Asthma 2014; 51: 573–577. [DOI] [PubMed] [Google Scholar]
- 20.Lei W, Zeng DX, Zhu CH, Liu GQ, Zhang XQ, Wang CG, et al. The upregulated expression of OX40/OX40L and their promotion of T cells proliferation in the murine model of asthma. J Thorac Dis 2014; 6: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei W, Zeng D, Liu G, Zhang XQ, Wang CG, Wang Q, Huang JA. Crucial role of OX40/OX40L signaling in a murine model of asthma. Mol Med Rep 2018; 17: 4213–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E, Pavel AB, Estrada Y, Zhou L, Estrada YD, Zhang N, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 144: 482–493. [DOI] [PubMed] [Google Scholar]