Abstract
Superficial fungal infections have been known for hundreds of years. During the 20th century new diagnostic methods were developed and the taxonomy changed several times, which, unfortunately, resulted in many fungi having several names (synonyms). The taxonomy is important, as species-specific identification guides clinicians when choosing the most appropriate antifungal agent, and provides an indication of the source of infection (anthropophilic, zoophilic or geophilic). Traditional diagnostic tests (direct microscopy, culture and histopathology) are still widely used, but molecular-based methods, such as PCR, have many advantages, and increasingly supplement or replace conventional methods. Molecular-based methods provide detection of different genus/species spectra. This paper describes recent changes in dermatophyte taxonomy, and reviews the currently available diagnostics tools, focusing mainly on commercially available PCR test systems.
Key words: diagnostic, microscopy, PCR, dermatophytes, dermatomycoses
Superficial fungal infections have been known since the 5th century BC, when Hippocrates wrote about thrush in children. It took hundreds of years before the first scientific proof of infection was made by Agostino Bassi in 1835, who showed that the muscardine disease of the silkworm was caused by a fungus (1). In the following years Audouin from France suggested that some human diseases were caused by the same types of plant parasites (fungi). By the end of the 19th century important microbiological methods, such as obtaining pure cultures of the dermatophytes Trichophyton and Achorion schoenleinii, were introduced. A morphological classification was not established until 1910, when the famous mycologist R. Sabouraud published “Les teignes”, a monograph based on the standardization of test media and studies on clinical features of skin and hair infections and morphology in cultures (1). At the beginning of the 20th century different nomenclatural systems were suggested, based on clinical presentation and culture characteristics.
SIGNIFICANCE
Superficial fungal infections (e.g. ringworm, thrush and fungal nail infections) have been known for hundreds of years. It is crucial to diagnose the fungus correctly, in order to choose the correct anti-fungal medication, and to provide information about the source of infection. Traditionally, diagnosis is based on microscopy, culture and histopathology of the specimen (hair, skin, nails). More recent molecular-based methods have been developed, but there is no standardization as to which fungi they detect. This paper presents an update on fungal taxonomy and describes the diagnostic tools available.
The taxonomy of superficial fungal infections has changed several times since then, due to the development of new diagnostic methods. Unfortunately, this has resulted in many fungi having several names (synonyms). An attempt to simplify this, by giving “one fungus one name” has been initiated, and the development of molecular diagnostic methods has contributed to this process. This paper describes the latest changes in dermatophyte taxonomy and reviews currently available diagnostic tools.
TAXONOMY
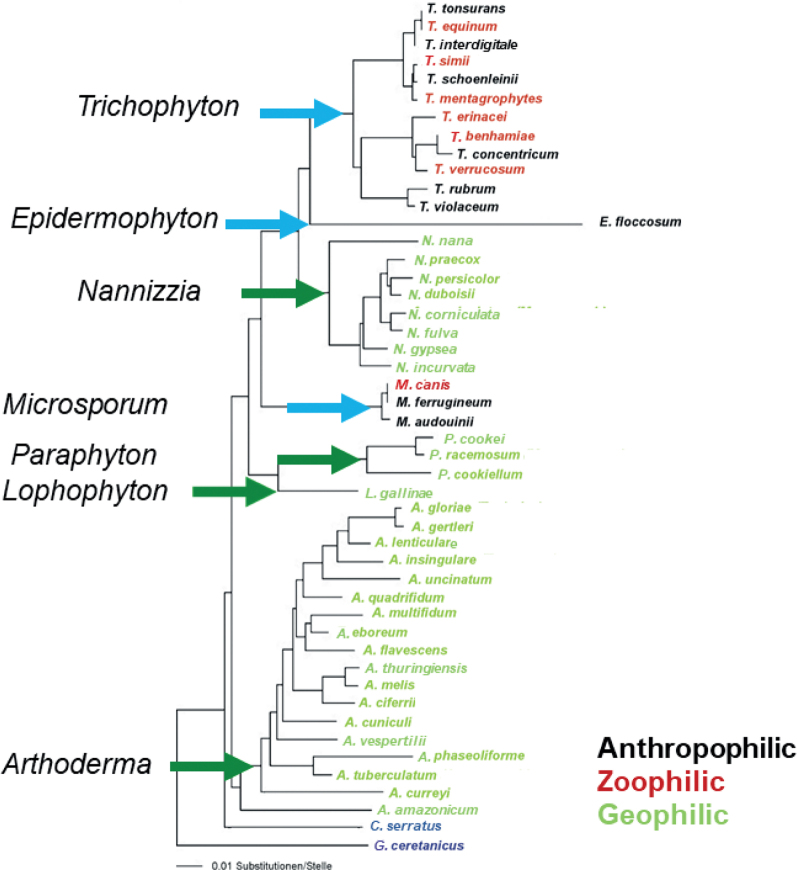
The taxonomy of dermatophytes changed most recently at the beginning of 2017 (2). The phylogenetic tree in Fig. 1, based on molecular data, shows the current valid nomenclature of the family Arthrodermataceae. C. serratus and G. ceretanicus were used as outgroups. Before that, the family of Arthrodermataceae, encompassing the dermatophyte fungi, included 3 anamorphic (fungi that have no sexual phase in their life cycle, also called imperfect fungi), Trichophyton, Microsporum, Epider mophyton, and one teleomorphic (fungi that have a sexual phase in their life cycle) genus (Arthroderma). As early as 2011, this dual nomenclature of fungi was abolished (3), mainly because the basis of taxonomy moved away from using morphological features towards molecular and phylogenetic data. On this basis, the teleomorphic genus in dermatophytes was abolished and 4 additional genera (Nannizzia, Lophophyton, Paraphyton and Arthroderma) were introduced to account for the former geophilic Microsporum and Trichophyton spp. according to the rules of the botanical code. In principle, a separate genus was established at all main clusters (tips of the arrows in Fig. 1) of the phylogenetic multilocus tree. Medical concerns were also addressed, i.e. the anthropophilic and zoophilic species names were retained in the well-known genera Microsporum, Trichophyton and Epidermophyton (2).
Fig. 1.
Phylogenetic tree of the majority of species of the family Arthrodermataceae, based on the internal transcribed spacer region of the ribosomal DNA.
At the species level, the nomenclatural changes that affected the medically relevant dermatophytes of the aforementioned genera were minor at this time. Most of these taxonomic changes were proposed at the beginning of the 21st century. For example, the previous 50 anthropophilic and zoophilic Trichophyton species were reduced to 19 (4), and in 2017 they were reduced by a further 3 due to the disappearance of the teleomorphic genus. Here, corrections were carried out that mainly affected the classification of the dermatophytes into groups that encompassed their natural sources (anthropophilic, zoophilic, geophilic). For example, the anthropophilic and zoophilic strains of T. interdigitale, were separated once again, i.e. the zoophilic strains again received their own species name, T. mentagrophytes, whereas the anthropophilic strains were called T. interdigitale. Due to this name change, the previous species T. mentagrophytes, which was phylogenetically closely related to T. schoenleinii, had to be renamed. The name T. quinckeanum was used, because the originally described strains of T. mentagrophytes var. quinckeanum clustered here (Fig. 1). The skin fungus, Trichophyton sp. of Arthroderma benhamiae, which was isolated mainly from guinea pigs, was reduced to T. benhamiae. T. soudanense was removed from the T. rubrum complex and is now again listed as a separate species. New name combinations were also added, which were mostly geophilic species, due to the introduction of new genus names, e.g. Microsporum gypseum was renamed Nannizzia gypsea. The overall purpose of these changes was to base the new system on genetically robust determinants and to retain well-known dermatophyte names familiar to clinicians (2).
CLINICAL SIGNIFICANCE OF FUNGAL DIAGNOSTICS
Taxonomy may seem remote from everyday clinical practice, but it is important in many ways: first, an accurate diagnosis is important for choosing the correct antifungal treatment (5, 6). Species-specific diagnosis is sometimes also necessary, as different species may have diffierent antifungal susceptibility patterns (7). Secondly, the species name also informs clinicians about the source of the infection. By knowing the source of infection, it is possible to treat the index patient or animal in order to reduce the risk of further spread of disease. Thirdly, sub-species identification (strain typing) is useful in outbreaks as, for example, in India, where a specific T. mentagrophytes genotype VIII has been uniformly isolated as a causative agent of a countrywide spread of a chronic, relapsing dermatophyte epidemic (8). By thoroughly studying this sub-species new knowledge about virulence and resistance may become available. Finally, a negative fungal laboratory test is also important as a diagnosis of exclusion, when other dermatological diagnoses have also suspected. Even though identification to genus or species level is important it is not always performed in the clinical setting (9–11). Oral antifungal therapy should not be administered without a confirmed laboratory diagnosis, because up to 40% of the suspected diagnoses are wrong (9), and due to the possible side-effects and drug interaction, particularly in older patients who often have other underlying diseases and take additional medications. A third point is the potentially negative impact that unnecessary treatment may have on the human microbiome, and the increasing threat of drug resistance, which is well recognized with antibacterials, but can be equally applicable to antimycotics.
TRADITIONAL DIAGNOSTICS: DIRECT MICROSCOPY, HISTOLOGY AND CULTURE
Direct microscopy and culture have been used for the purpose of fungal identification over the last 100 years and are still used worldwide. The methods will be described in the following section.
Direct “non-specific” detection of fungal elements in clinical specimens
Direct microscopy is used for the primary identification of fungal elements in specimens after treating with sodium hydroxide or potassium hydroxide (KOH). Conventional light microscopy, without the benefit of any contrast with the background, is difficult to interpret, and stains, such as lactophenol cotton blue, Parker ink, chlorazol black E or Congo red, are therefore often added. Fluorescence microscopy after treatment of the specimen with optical brighteners, such as blankophor or calcoflour, can enhance the detection rate after microscopy (12, 13). Malassezia species show characteristic unipolar budding blastoconidia, but with the exception of this genus it is important to note that direct microscopic findings are neither genus- nor species-specific, even though it is possible to distinguish yeasts from hyphae and to detect pigmented fungal cells. Some very experienced technicians may be able to suggest a differentiation between other specific yeasts, dermatophytes and non-dermatophyte moulds, but without absolute certainty (14).
Direct microscopy of hair is important, as the growth pattern of the dermatophyte classifies it as either favus, endothrix (arthroconidia are present within the hair shaft) or ectothrix (where the fungus invades the hair shaft at mid-follicle and the arthrospores then grow out of the hair follicle and surround the surface of the hair shaft). The growth pattern, combined with the conidial size, can be used as a preliminary indication of the genus of the infecting dermatophyte (15–17). Histology is not used routinely in skin and hair infections, but is useful when Malassezia folliculitis is suspected, in order to rule out other causes of folliculitis. Some dermatologists use histology routinely for fungal identification in nails, as it may rule out contamination and is able to confirm the growth of the fungus directly in the specimen (11, 18–20). However, the prerequisite for this is an invasive biopsy.
Genus- and species-specific identification
Culture is highly dependent on growth media, e.g. some media are more dermatophyte-specific, while others are better for yeasts and non-dermatophyte moulds. Malassezia is lipid-dependent and, as a consequence, is often difficult to culture on normal laboratory media. Culture of nail material is challenging, as up to 30% of microscopy-positive nail specimens are culture-negative (21, 22). This may be due to the presence of non-viable material, either because of insufficient material from the proximal area of infection, or due to previous antifungal treatment.
Combination of the different techniques is usually practiced, as it enhances the chances of fungal detection and provides more clinically useful information. All traditional diagnostic methods are dependent on the skills of the laboratory technicians, whereas molecular diagnosis does not depend on the acquired skill sets of the laboratory staff, but may have other limitations, as described below.
MOLECULAR-BASED DETECTION OF SUPERFICIAL FUNGAL INFECTIONS
Development of molecular-based methods for detection of dermatomycosis
With the introduction of molecular tools into the taxonomy of dermatophytes approximately 30 years ago, species-specific markers, such as the internal transcribed spacer (ITS) region of ribosomal DNA, were subsequently used for the diagnosis of this fungal group. In the mid-1990s, PCR methods were initially applied to cultured skin material. This included methods such as restriction fragment length polymorphism (RFLP) and random amplification of polymorphic DNA (RAPD) analyses, but also PCR fingerprinting (23–25). Later, so-called in-house PCR methods were developed, which were also able to identify the fungus directly in clinical specimens. These methods are generally based on amplification with a broad range and/or specific primers and, in a second stage, use hybridization with species-specific probes with or without a combination of high-resolution melting curve analysis. A distinction can be made between conventional and real-time PCR techniques. The former are more personnel-intensive because the hybridization step is performed separately and requires additional washing steps (enzyme-linked immunoassay (ELISA), blot or microarray technique) and are more susceptible to contamination because the amplified DNA is further processed manually. On the other hand, the thermal cyclers required are less expensive than real-time devices. However, the advantages of real-time PCR are that both the amplification and hybridization steps are performed in the same closed reaction tube without the risk of contamination. This also eliminates additional bench handling. However, it must be kept in mind that the number of probe hybridizations in conventional techniques is larger (e.g. 78 in the microarray format) than the number of colour labels (4–6), which are used to label different probes in real-time PCR technology. Thus, melting curve analysis is used to extend the spectrum of species to be detected. Nevertheless, these methods are not yet able to differentiate, at the same time, more than 20 clinically relevant dermatophyte species, including the few non-dermatophytes that can play a role in onychomycosis as infectious agents. Such an all-in-one detection test would replace protracted phenotypic diagnostics based on culture, which ultimately requires expert knowledge because morphological features in this fungal group are both polymorphic and partially overlap.
Commercial kits for direct detection of fungal infections on skin, hair and nail samples
Since 2008, commercial systems, that use the above-mentioned detection methods and cover different species spectra, have been available. The Dermatophyte PCR Kit was developed by the Statens Serum Institute (SSI), in Copenhagen Denmark in 2 versions; firstly, as a conventional PCR, and later as a real-time PCR that solely detects T. rubrum at species level, as it is the most common pathogen in onychomycosis and tinea pedis (Table I). Otherwise, the kit offers the possibility of detecting dermatophytes as a group (pan-dermatophyte), but this will include any non-pathogenic geophilic genera present. The conventional test system is based on a PCR with subsequent size analysis of the amplified DNA fragments in an agarose gel, whereas real-time PCR uses hybridization probes instead. Both test systems can be used for screening for dermatophytes, and this may be followed by subsequent species identification of non-rubrum species via culture or other molecular techniques, such as sequencing (26). In 2011, the FTD Dermatophyte test from Fast Track Diagnostics, was made available in Sliema, Malta. This test system, a 2-tube real-time PCR with probe hybridization, but without melting curve analysis, is able to detect 3 species (T. rubrum, T. violaceum, E. floccosum). The remaining detections are performed at species complex level, i.e. more than 1 species is detected here, but not differentiated from each other. This includes mainly dermatophytes species with different ecological niches: the T. tonsurans complex (no differentiation between T. equinum, zoophilic and T. tonsurans, anthropophilic), the T. interdigitale complex (T. interdigitale, T. schoenleinii antropophilic; T. mentagrophytes, T. quinckeanum zoophilic) and the M. canis complex (M. canis zoophilic, M. audouinii, M. ferrugineum anthropophilic). The kit does not have a detection option for the dermatophytes as a group. Biotype in Dresden, Germany launched the first version of the Mentype MycoDerm kit in 2013. These utilize 2 conventional PCR reactions, which can differentiate 2 species (E. floccosum and N. gypsea) on the basis of fragment size analyses. There is no differentiation between the T. tonsurans and the T. interdigitale complexes. T. rubrum is identified in a complex together with T. violaceum, as is M. canis complex. The second version of the test system (Mentype MycoDerm Lateral Flow) was available 2 years later with 3 PCR reactions. Further developments affect, on the one hand, the procedure, because fragment analysis was replaced by probe hybridization on a blot strip. On the other hand, the species spectrum to be detected has been enlarged. Now it is possible to detect T. rubrum, T. violaceum, M. audouinii at species level and M. canis in combination with M. ferrugineum. The T. tonsurans was separated from the T. interdigitale complexes. This was also the first kit that could detect T. benhamiae in a complex together with T. erinacei and T. verrucosum. Another concurrent test system, DermaGenius 1.0, from PathoNostics, produced in Maastrict, the Netherlands, based on a single multiplex real-time PCR with melting curve analysis, had the same identification gaps and a similar species spectrum as the FTD Kit. Neither kit included pan-dermatophyte detection. This changed with the 2nd version of the kit (DermaGenius 2.0), which became available in 2018. The species detection is the same as for Mentype MycoDerm Kit Lateral Flow, with 2 exceptions. N. gypsea is not included, but T. verrucosum is. T. benhamiae is clustered together with T. equinum (27). At the same time, the DERMADYN kit developed by DYN Diagnostics Ltd in Ha’eshel St., Israel became available. This test system is also based on a 2-tube multiplex PCR with a melting curve analysis and detects a similar spectrum to FTD dermatophytes (Table I). In addition N. gypsea is detected, but the T. simii complex is not included (28). In 2018, the last of the kits discussed here, Euroarray Dermatomycosis from Euroimmun, was launched in Lübeck, Germany. This is a multiplex PCR reaction with subsequent probe hybridization in the form of a “microarray”. This format enables the detection of all relevant (approximately 20) dermatophytes at the species level, including a pan-dermatophyte probe and 6 non-dermatophytes at the species level (Scopulariopsis brevicaulis, Fusarium and Candida spp.). Furthermore, there is species detection for rare pathogens, such as T. eriothrephon and T. bullosum. Only T. concentricum, a pathogen endemic to the Pacific Islands, is not separated from T. benhamiae, and T. soudanense is not differentiated from T. rubrum because the taxonomic change that separated them came after the development of the kit.
Table I.
Species spectra detected by the commercially available test systems
| Species/KIT | DPK | FTD | MMD | MMD LF | DG 1.0 | DG 2.0 | DD | EADM |
|---|---|---|---|---|---|---|---|---|
| T. tonsurans | X | V | ||||||
| T. equinum | X | V | ||||||
| T. interdigitale | X | V | ||||||
| T. mentagrophytes | X | V | ||||||
| T. schoenleinii | X | X | V | |||||
| T. quinckeanum | X | X | V | |||||
| T. simii | X | nd | nd | nd | nd | V | ||
| T. erinacei | X | X | X | X | X | V | ||
| T. benhamiae | X | X | X | X | X | V | ||
| T. verrucosum | X | X | X | X | V | X | V | |
| T. bullosum | X | nd | nd | nd | X | X | nd | V |
| T. rubrum | V | V | V | V | V | V | V | |
| T. violaceum | X | V | V | V | V | V | V | |
| E. floccosum | X | V | V | V | V | V | V | V |
| M. audouinii | X | V | V | V | V | |||
| M. ferrugineum | X | V | ||||||
| M. canis | X | V | ||||||
| N. gypsea | X | X | V | V | X | X | V | V |
| N. fulva | X | X | X | X | X | X | X | V |
| N. incurvata | X | X | X | X | X | X | X | V |
| N. persicolor | X | X | X | X | X | X | X | V |
| pan Dermatophyte | V | X | X | V | X | X | X | V |
| non Dermatophyte | X | X | V | V | V | V | X | V |
Same coloured boxes refer to the detection of species complexes, but not individual species. V: detects, X: do not detect. The identically coloured boxes mark the species in the respective kit, which are detected together (as a complex), i.e. not separated from each other.
Nd: no data; DPK: Dermatophyte PCR Kit; FTD: Fast Track Dermatophytes; MMD: Mentype Mycoderm; MMD LF: Mentype <mycoderm Lateral Flow; DG 1.0: DermaGenius Version 1.0; DG 2.0: DermaGenius Version 2.0; DD: DermaDYN; EADM: Euroarray Dermatomycosis.
Overall, the clinician should be aware that there is a difference between what the commercial tests are able to detect (Table I). Most importantly, the majority of tests do not discriminate between zoophilic and anthropophilic species, which is a necessary step in order to find (and treat) the sources of infection. Another challenge is that many of the non-dermatophytes involved in the pathogenesis of onychomycosis are not detected in many of the kits. The broadest species-specific spectrum is offered by the Euroarray. The other test systems do not detect non-dermatophytes (SSI, FTD), apart from C. albicans (DermaGenius), or they provide detection of Scopulariopsis and Candida to genus level only (Mentype) (Table I).
Non-commercial molecular-based tests
A considerable number of in-house PCR techniques have been developed for the diagnosis of dermatophytes and other skin pathogenic fungi. We do not describe these developments in detail here, because they are not standardized, and in the vast majority of cases are used only by individual, or a few, laboratories involved in routine diagnostics. Of particular interest are the methods based on real-time PCR. Many of these approaches are able to identify up to 6 taxa and dermatophytes in general (29, 30). Ohst and colleagues (31) were able to detect 9 dermatophyte taxa by combining up to 10 PCRs in a sequential algorithm, and Bergmans and colleagues (32) could differentiate 11 species in a single-tube assay with probes and melting-curve analysis. Walser & Bosshard (33) report that using sloppy molecular beacons with species-specific melting temperature signatures allows the identification of 19 dermatophyte species. Until now it has been possible to detect a similar number of species only by applying post-PCR techniques, such as an oligonucleotide array (34). This may be a promising approach for a commercial test system, if it is possible to increase the sensitivity of the 2nd species-differentiating PCR reaction, which was negative in 76% of cases where PCR1 (pan dermatophytes) was positive.
Another molecular-based method, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF), is used to identify micro-organisms based on the characteristic protein spectrum of each species matched with a database. It has been applied to the identification of superficial fungal infection directly on culture material, both yeast, dermatophytes and moulds (35). This technique is fast and reproducible, but until now not applicable directly to clinical hair, skin or nail specimens (36–40). Protein spectra of 7 and 10 dermatophytes species (T. tonsurans, T. rubrum, T. interdigitale, T. mentagrophytes, T. verrucosum, T. violaceum, M. canis, M. audouinii, E. floccosum, N. gypsea) are included in the widely used Bruker and Biomerieux reference spectrum databases (41). So far, this method is not able to differentiate the phylogenetically closely related species, e.g. T. rubrum/soudanense, T. interdigitale/mentagrophytes, T. tonsurans/equinum or T. benhamiae/concentricum complex (42, 43). Identification can be improved by establishing an in-house database (44). It has also been used to detect antifungal resistance (45).
OTHER DIAGNOSTIC TOOLS
Wood’s light, filtered ultraviolet light, is often used as a bedside tool for differentiate tinea capitis caused by a Microsporum species (canis, audouinii and ferrugineum) from other dermatophyte infections, as it fluoresces greenish under Wood’s light, and endothrix infections are non-fluorescent (17).
Dermoscopy, reflectance confocal microscopy, optical coherence tomography and confocal laser scanning microscopy, all of which are non-invasive methods, can be used as add-on tools to differentiate tinea capitis and/or onychomycosis from other dermatological conditions (17, 20, 46–48).
Dermatophyte screening test media, an agar medium containing a dermatophyte colour indicator can be used for dermatophyte screening. The anti-dermatophyte monoclonal antibody test, an immunochromatographic detection test, is able to confirm a dermatophyte infection, detectable at genus level. Some are known to give a false-positive reaction when non-dermatophytes are grown (22, 49–51).
CURRENT ROUTINE DIAGNOSTIC AND THEIR CHALLENGES
The use of phenotypic methods (microscopy and culture) for the detection of pathogens in tinea is still widespread. They are dependent on the skills of the laboratory technicians and culture is time-consuming. The culture method is still the only diagnostic method that is able to confirm the viability of the fungus, which is important for treatment assessment (11). Microbiological laboratories appreciate the automation possibilities in molecular diagnostics and often have already established similar methods and devices that can also be used for dermatophyte identification. Decisive factors in determining whether to set up a molecular mycology service are the number of samples, the availability of trained personnel for direct microscopy, culturing and cost-effectiveness, which depends strongly on whether and how molecular dermatophyte diagnostics are remunerated. Whether conventional diagnostics will still be used after the wider introduction of the molecular identification method depends primarily on the differentiated pathogen spectrum of the test system used. If not all relevant pathogens are covered, a pan-dermatophyte detection should be used in order not to miss a possible pathogen. However, even then, the detection of potential non-dermatophytes must be considered and, if not included, covered by diagnostics based on culture. It is, therefore, important to be aware of what fungi any locally available molecular test can or cannot detect.
Although molecular diagnostics are up to 30% more sensitive than culture diagnostics, the detection limit is more than one fungal cell (31). Therefore, the clinical specimens must be taken from the correct location and in sufficient quantity. In this respect, there is no difference from culture diagnostics. The detection of pathogenic dermatophytes, whether in culture or PCR, always requires antifungal therapy, because asymptomatic carriers also spread the fungi and can become symptomatic. Disadvantages of the available molecular tests, in general, are that the evolution of fungi can lead to (point) mutations, especially in species-specific sequences used in the primers and probe, so that they can no longer bind, and false-negative results may be generated.
This can be remedied by sequencing with broad-range or only dermatophyte-specific primers, which are more conserved and therefore less susceptible to mutations. Sequencing can then provide accurate species identification. Some laboratories already routinely use these methods for fungal diagnostics. However, the purchase of a sequencing device is expensive and, like an in-house PCR, the method has to be validated. Furthermore, there must be appropriately validated databases to enable correct identification.
CLINICAL AND LABORATORY INTERACTION CAN IMPROVE THE DIAGNOSTIC OUTCOME
It seems logical that there should be coherence between what the clinician suspects and what the mycology laboratory is able to detect. Nevertheless, in our experience this it often not the case. As described, different fungi have different needs for substrates in order to grow, and some molecular-based tests do not detect all relevant fungi. It is therefore important to inform the mycology laboratory, as a minimum, from which anatomical region (hair, skin or nail) the specimen is obtained, which dermatological disease, and fungal (dermatophyte, Candida, Malassezia or non-dermatophyte mould) genera is suspected (Table II). Furthermore, the attending physician should note on the referral form whether an animal contact is probable and whether, for example, a mycosis with a non-dermatophyte is considered in onychomycosis. The microbiologist needs this information in order to interpret the results of the molecular tests correctly, but also to decide whether a culture should be created in parallel if the kit has gaps in its repertoire.
Table II.
Helpful information for the clinician to differentiate between suspected fungal pathogens, which is needed for the laboratory for choosing the most appropriate diagnostic methods
| Anatomical region | Help for the clinician to differentiate between suspected fungal pathogens | Essential information Information helpful for for the microbiologist the microbiologist | |||
|---|---|---|---|---|---|
| Disease | Most common clinical signs | Age | Suspected pathogen | Exposure | |
| Scalp (hair region) | Tinea capitis | Broken hairs Kerion Favus Alopecia Scaling |
Children | Dermatophyte | Animal exposure Endemic contacts Woods light results Earlier treatment |
| Seborrhoeic dermatitis/Dandruff | Greasy skin scales on erythematous skin | Newborn Adults | Malassezia | ||
| Face | Tinea faciei | Area with raised erythematous border or red patch | All ages, but mostly children | Dermatophytes | Animal exposure Signs of tinea capitis |
| Seborrhoeic dermatitis | Greasy skin scales on erythematous skin primary centro-facial and eyebrows | Adults | Malassezia | ||
| Upper body | Pityriasis versicolor | Hypo-or hyperpigmented maculae | Young and adults | Malassezia | Immunosuppression |
| Malassezia folliculitis | Monomorphic pustules mainly located at seborrhoeic areas. No comedones | Young and adults | Malassezia | Immunosuppression | |
| Tinea corporis | Area with raised erythematous border or red patch. Skin scales | All ages | Dermatophyte | Animal exposure Other signs of tinea | |
| Hands | Tinea manuum | Area with raised erythematous border or red patches. Skin scales. Hyperkeratosis. | All ages | Other signs of tinea e.g. tinea pedis | |
| Groin & pubic area | Cutaneous candidiasis | Erythematous skin folds with satellite pustules (and skin scales) | All ages | Candida | Immunosuppression |
| Tinea cruris | Area with raised erythematous border or red patch. Skin scales | Adults | Dermatophytes | ||
| Seborrhoeic dermatitis | Greasy skin scales on erythematous skin | Adults | Malassezia | ||
| Feet | Cutaneous candidiasis | Interdigital maceration | All ages | Candida | Immunosuppression |
| Tinea pedis | Interdigital maceration, skin scales, raised erythematous boarder, ‘Moccasin foot’, thickening of the soles | All ages | Dermatophytes | ||
| Cutaneous non-D mould infection | Interdigital maceration | Mostly adults | Non-D moulds | Immunosuppression | |
| Nails | Candida onychomycosis | Paronychia Nail dystrophy | All ages | Candida | Immunosuppression Moist exposure |
| Tinea unguium | Hyperkeratosis, superficial white discoloration, yellow streaks. | All ages, but prevalence increases with age | Dermatophytes | Concomitant tinea pedis? | |
| Non-D onychomycosis | Hyperkeratosis, discoloration, paronychia/inflammation, nail dystrophy | All ages, but prevalence increases with age | Non-D moulds | ||
Non-D: non-dermatophyte.
FUTURE PERSPECTIVES
The advantages of molecular diagnostics for the initial diagnosis of dermatophytosis are beyond question. Afew studies have, so far, shown that the method can also be used for therapy monitoring (52, 53). Iwanaga et al., in particular, have demonstrated that the fungal load after 16 weeks of terbinafine therapy is significantly reduced (from 100% to 36%) (53). The patients’ culture were already negative at this time, but, microscopically, fungal elements could still be detected in the KOH preparation. The most plausible explanation for this is that resting fungal cells (e.g. in the form of arthroconidia) are still present and may potentially germinate again after discontinuation of therapy. The survival of dormant fungal cells inside the nail is supported by follow-up studies, which after 18 months show a complete cure in only 76% of elderly patients receiving 3-month terbinafine therapy (54). Dormant cells are missed in the culture. Therefore, therapy control with PCR procedures may be suitable in the future, not to mention the short time-span in which such a finding is available, in order to decide whether to continue the therapy. Only very special PCR procedures are able to discriminate between live and dead cells; however, it is not known how long dormant fungal cells survive in the nail, hair or skin of the human body (55).
To date, there has been no significant development of resistance in dermatophytes to the use of antimycotics. This has suddenly changed with the Indian epidemic, which has lasted for approximately 6 years, and goes hand in hand with the use of over-the-counter ointments containing antimycotics (e.g. terbinafine), antibiotics and steroids (e.g. clobetasol). Terbinafine resistance or partial resistance in T. mentagrophytes strains with genotype VIII and T. rubrum reach rates of more than 65% and 17%, respectively, in India and are spread globally (56). This means that T. mentagrophytes strains will have to be fine-typed by molecular genetics in order to determine the exact identity, or a susceptibility test has to be performed. The advantage of the latter is that breakpoints can be defined; the disadvantage is that the inoculum, due to the filamentous growth and the often poor conidia formation of the fungi, is challenging and therefore not done routinely. Molecular methods, in particular sequencing with detection of specific genetic mutations leading to antifungal resistance (e.g. squalene epoxidase gene mutation leading to terbinafine resistance), which are independent of the fungal growth could overcome this problem (8, 56–59).
CONCLUSION
The diagnosis of superficial fungal infections has evolved from the first microscopic description more than 100 years ago to current techniques that are able to detect a wide range of clinically relevant fungi using molecular-based techniques. Worldwide traditional diagnostic methods, such as direct microscopy and culture, are still used, as they are cheap and the equipment is already available. The development of molecular-based methods has already improved a lot during the last years, from only being able to detect fungi in cultures to now being able to detect fungi directly in clinical samples. The molecular species-specific fungal detection of clinically relevant fungi is possible, as well as detection of specific mutations causing antifungal resistance. If it were possible to combine all these tests, it would enable the clinician to obtain the correct species identification, the possible source of infection and the susceptibility pattern of the involved pathogen by sending a single sample. The evolution of the diagnosis of superficial fungal infections is not far from this goal.
ACKNOWLEDGEMENT
Professor Roderick Hay is acknowledged for his constructive comments and for reviewing the text.
Footnotes
Conflicts of interest: DMLS was paid as a consultant for advisory board meeting by AbbVie, Janssen, Sanofi and received speaker’s honoraria and/or received grants from the following companies: Abbvie, Galderma, Astellas, Novartis and Leo Pharma during the last 3 years. YG received speaker’s honoraria and grants from Euroimmun.
REFERENCES
- 1.Kane J, Summerbell R, Sigler L, Krajden S, Land G. Laboratory handbook of dermatophytes: a clinical guide and laboratory manual of dermatophytes and other filamentous fungi from skin, hair and nails. In: Kane J, editor. Belmont: Star Publishing Co.; 1997. [Google Scholar]
- 2.de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017; 182: 5–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, et al. The amsterdam declaration on fungal nomenclature. IMA Fungus 2011; 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gräser Y, Scott J, Summerbell R.. The new species concept in dermatophytes-a polyphasic approach. Mycopathologia 2008; 166: 239–256. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Jiang X, Yang M, González U, Lin X, Hua X, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev 2016; 5: CD004685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller LC, Barton RC, Mohd Mustapa MF, Proudfoot LE, Punjabi SP, Higgins EM. British Association of Dermatologists’ guidelines for the management of tinea capitis 2014. Br J Dermatol 2014; 171: 454–463. [DOI] [PubMed] [Google Scholar]
- 7.Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol 2017; 177: 47–62. [DOI] [PubMed] [Google Scholar]
- 8.Nenoff P, Verma SB, Vasani R, Burmester A, Hipler U, Wittig F, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes —a molecular study. Mycoses 2019; 62: 336–356. [DOI] [PubMed] [Google Scholar]
- 9.Effendy I, Lecha M, Feuilhade de CM, Di CN, Baran R. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol 2005; 19: 8–12. [DOI] [PubMed] [Google Scholar]
- 10.Narang T, Bishnoi A, Dogra S, Singh TD, Mahajan R, Kavita K. Disease burden and prescription patterns treating dermatophytosis in North India: salient findings from an online survey of 1041 dermatologists. J Eur Acad Dermatology Venereol 2019; 33: e391–e393. [DOI] [PubMed] [Google Scholar]
- 11.Saunte DML, Piraccini BM, Sergeev AY, Prohic A, Sigurgeirsson B, Rodríguez-Cerdeira C, et al. A survey among dermatologists: diagnostics of superficial fungal infections – what is used and what is needed to initiate therapy and assess efficacy? J Eur Acad Dermatol Venereol 2018; 33: 421–427; jdv. 15361. [DOI] [PubMed] [Google Scholar]
- 12.Monod M, Jaccoud S, Stirnimann R, Anex R, Villa F, Balmer S, et al. Economical microscope configuration for direct mycological examination with fluorescence in dermatology. Dermatology 2000; 201: 246–248. [DOI] [PubMed] [Google Scholar]
- 13.Ovrén E, Berglund L, Nordlind K, Rollman O.. Dermatophytosis: fluorostaining enhances speed and sensitivity in direct microscopy of skin, nail and hair specimens from dermatology outpatients. Mycoses 2016; 59: 436–441. [DOI] [PubMed] [Google Scholar]
- 14.Pihet M, Le Govic Y. Reappraisal of conventional diagnosis for dermatophytes. Mycopathologia 2017; 182: 169–180. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Hofstader SL, Adam P, Summerbell RC. Tinea capitis: an overview with emphasis on management. Pediatr Dermatol 1999; 16: 171–189. [DOI] [PubMed] [Google Scholar]
- 16.Morar N, Dlova NC, Gupta AK, Aboobaker J. Tinea capitis in Kwa-Zulu Natal, South Africa. Pediatr Dermatol 2004; 21: 444–447. [DOI] [PubMed] [Google Scholar]
- 17.Hay RJ. Tinea Capitis: current status. Mycopathologia 2017; 182: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaman BF, Açikalin A, Ünal I, Aksungur VL. Diagnostic values of KOH examination, histological examination, and culture for onychomycosis: a latent class analysis. Int J Dermatol 2019; 58: 319–324. [DOI] [PubMed] [Google Scholar]
- 19.Velasquez-Agudelo V, Cardona-Arias JA. Meta-analysis of the utility of culture, biopsy, and direct KOH examination for the diagnosis of onychomycosis. BMC Infect Dis 2017; 17: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipner SR, Scher RK. Onychomycosis. J Am Acad Dermatol 2019; 80: 835–851. [DOI] [PubMed] [Google Scholar]
- 21.Monod M, Bontems O, Zaugg C, Lechenne B, Fratti M, Panizzon R. Fast and reliable PCR/sequencing/RFLP assay for identification of fungi in onychomycoses. J Med Microbiol 2006; 55: 1211–1216. [DOI] [PubMed] [Google Scholar]
- 22.Monod M, Méhul B.. Recent findings in onychomycosis and their application for appropriate treatment. J Fungi 2019; 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki M, Aoki M, Ishizaki H, Nishimura K, Miyaji M. Phylogeny of Epidermophyton floccosum and other dermatophytes. Mycopathologia 1996; 134: 121–128. [DOI] [PubMed] [Google Scholar]
- 24.Mochizuki T, Sugie N, Uehara M. Random amplification of polymorphic DNA is useful for the differentiation of several anthropophilic dermatophytes. Mycoses 1997; 40: 405–409. [DOI] [PubMed] [Google Scholar]
- 25.Graser Y, El Fari M, Presber W, Sterry W, Tietz HJ. Identification of common dermatophytes (trichophyton, microsporum, epidermophyton) using polymerase chain reactions. Br J Dermatol 1998; 138: 576–582. [DOI] [PubMed] [Google Scholar]
- 26.Gräser Y, Czaika V, Ohst T.. Diagnostic PCR of dermatophytes – an overview. JDDG J der Dtsch Dermatologischen Gesellschaft 2012; 10: 721–725. [DOI] [PubMed] [Google Scholar]
- 27.Uhrlaß S, Wittig F, Koch D, Krüger C, Harder M, Gaajetaan G, et al. Halten die neuen molekularen Teste – Microarray und Realtime-Polymerasekettenreaktion – zum Dermatophytennachweis das, was sie versprechen? Hautarzt 2019; 70: 618–626. [DOI] [PubMed] [Google Scholar]
- 28.Sherman S, Goshen M, Treigerman O, Ben-Zion K, Carp M-J, Maisler N, et al. Evaluation of multiplex real-time PCR for identifying dermatophytes in clinical samples – a multicentre study. Mycoses 2018; 61: 119–126. [DOI] [PubMed] [Google Scholar]
- 29.Wisselink GJ, van Zanten E, Kooistra-Smid AMD. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods 2011; 85: 62–66. [DOI] [PubMed] [Google Scholar]
- 30.Alexander CL, Shankland GS, Carman W, Williams C. Introduction of a dermatophyte polymerase chain reaction assay to the diagnostic mycology service in Scotland. Br J Dermatol 2011; 164: 966–972. [DOI] [PubMed] [Google Scholar]
- 31.Ohst T, Kupsch C, Gräser Y.. Detection of common dermatophytes in clinical specimens using a simple quantitative real-time TaqMan polymerase chain reaction assay. Br J Dermatol 2016; 174: 602–609. [DOI] [PubMed] [Google Scholar]
- 32.Bergmans AMC, van der Ent M, Klaassen A, Böhm N, Andriesse GI, Wintermans RGF. Evaluation of a single-tube real-time PCR for detection and identification of 11 dermatophyte species in clinical material. Clin Microbiol Infect 2010; 16: 704–710. [DOI] [PubMed] [Google Scholar]
- 33.Walser M, Bosshard PP. Development and evaluation of a pan-dermatophyte polymerase chain reaction with species-level identification using sloppy molecular beacon probes. Br J Dermatol 2019; 180: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 34.Li HC, Bouchara J-P, Hsu MM-L, Barton R, Chang TC. Identification of dermatophytes by an oligonucleotide array. J Clin Microbiol 2007; 45: 3160–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel R. A Moldy Application of MALDI: MALDI-ToF mass spectrometry for fungal identification. J Fungi 2019; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denis J, Machouart M, Morio F, Sabou M, Kauffmann-LaCroix C, Contet-Audonneau N, et al. Performance of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identifying clinical Malassezia isolates. J Clin Microbiol 2017; 55: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassagne C, Normand A-C, L’Ollivier C, Ranque S, Piarroux R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses 2016; 59: 678–690. [DOI] [PubMed] [Google Scholar]
- 38.Karabiçak N, Karatuna O, Ilkit M, Akyar I. Evaluation of the Bruker matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) system for the identification of clinically important dermatophyte species. Mycopathologia 2015;180: 165–171. [DOI] [PubMed] [Google Scholar]
- 39.Hedayati MT, Ansari S, Ahmadi B, Taghizadeh Armaki M, Shokohi T, Abastabar M, et al. Identification of clinical dermatophyte isolates obtained from Iran by matrix-assisted laser desorption/ionization time-offlight mass spectrometry. Curr Med Mycol 2019; 5: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Intra J, Sarto C, Tiberti N, Besana S, Savarino C, Brambilla P. Genus-level identification of dermatophytes by MALDI-TOF MS after 2 days of colony growth. Lett Appl Microbiol 2018; 67: 136–143. [DOI] [PubMed] [Google Scholar]
- 41.Normand A-C, Cassagne C, Gautier M, Becker P, Ranque S, Hendrickx M, et al. Decision criteria for MALDI-TOF MS-based identification of filamentous fungi using commercial and in-house reference databases. BMC Microbiol 2017; 17: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su H, Packeu A, Ahmed SA, Al-Hatmi AMS, Blechert O, Ilkit M, et al. Species distinction in the Trichophyton rubrum complex. J Clin Microbiol 2019; 57. pii: e00352-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suh S-O, Grosso KM, Carrion ME. Multilocus phylogeny of the Trichophyton mentagrophytes species complex and the application of matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry for the rapid identification of dermatophytes. Mycologia 2019; 110: 118–130. [DOI] [PubMed] [Google Scholar]
- 44.da Cunha KC, Riat A, Normand A-C, Bosshard PP, de Almeida MTG, Piarroux R, et al. Fast identification of dermatophytes by MALDI-TOF/MS using direct transfer of fungal cells on ground steel target plates. Mycoses 2018; 61: 691–697. [DOI] [PubMed] [Google Scholar]
- 45.Delavy M, Dos Santos AR, Heiman CM, Coste AT. Investigating antifungal susceptibility in Candida species with MALDI-TOF MS-based assays. Front Cell Infect Microbiol 2019; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veasey JV., Meneses OMS, da Silva FO. Reflectance confocal microscopy of tinea capitis: comparing images with results of dermoscopy and mycological exams. Int J Dermatol 2019; 58: 849–851. [DOI] [PubMed] [Google Scholar]
- 47.Pharaon M, Gari-Toussaint M, Khemis A, Zorzi K, Petit L, Martel P, et al. Diagnosis and treatment monitoring of toenail onychomycosis by reflectance confocal microscopy: Prospective cohort study in 58 patients. J Am Acad Dermatol 2014; 71: 56–61. [DOI] [PubMed] [Google Scholar]
- 48.Rothmund G, Sattler EC, Kaestle R, Fischer C, Haas CJ, Starz H, et al. Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosis – comparison of six diagnostic methods. Mycoses 2013; 56: 47–55. [DOI] [PubMed] [Google Scholar]
- 49.Li X-F, Shen Y-N, Chen W, Chen H, LV G-X, Liu W-D. A new medium for diagnosis of dermatophyte infection. Eur J Dermatology 2009; 19: 34–37. [DOI] [PubMed] [Google Scholar]
- 50.Rahman MA, Chowdhury OA, Debnath MR, Ahmed SM, Das S, Choudhury R, et al. Comparison among different culture media for the detection of dermatophytes. Mymensingh Med J 2018; 27: 626–630. [PubMed] [Google Scholar]
- 51.Tsunemi Y, Hiruma M. Clinical study of Dermatophyte Test Strip, an immunochromatographic method, to detect tinea unguium dermatophytes. J Dermatol 2016; 43: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 52.Kupsch C, Czaika V-A, Deutsch C, Gräser Y. Trichophyton mentagrophytes – a new genotype of zoophilic dermatophyte causes sexually transmitted infections. J Dtsch Dermatol Ges 2019; 17: 493–501. [DOI] [PubMed] [Google Scholar]
- 53.Iwanaga T, Ushigami T, Anzawa K, Mochizuki T. Pathogenic dermatophytes survive in nail lesions during oral terbinafine treatment for tinea unguium. Mycopathologia 2017; 182: 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loo DS. Onychomycosis in the elderly: drug treatment options. Drugs Aging 2007; 24: 293–302. [DOI] [PubMed] [Google Scholar]
- 55.Soejima T, Minami J-I, Xiao J-Z, Abe F.. Innovative use of platinum compounds to selectively detect live microorganisms by polymerase chain reaction. Biotechnol Bioeng 2016; 113: 301–310. [DOI] [PubMed] [Google Scholar]
- 56.Süß A, Uhrlaß S, Ludes A, Verma SB, Monod M, Krüger C, et al. Ausgeprägte Tinea corporis durch ein Terbinafin-resistentes Trichophyton-mentagrophytes-Isolat vom indischen Genotyp bei einem Säugling aus Bahrain in Deutschland. Hautarzt 2019; 70: 888–896. [DOI] [PubMed] [Google Scholar]
- 57.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 2018;61: 477–484. [DOI] [PubMed] [Google Scholar]
- 58.Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, Gupta A, et al. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother 2018; 62: e01038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saunte DML, Hare RK, Jørgensen KM, Jørgensen R, Deleuran M, Zachariae CO, et al. Emerging terbinafine resistance in trichophyton: clinical characteristics, squalene epoxidase gene mutations and a reliable EUCAST method for detection. Antimicrob Agents Chemother 2019; 63. pii: e01126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]