Human papillomavirus (HPV) is a deoxyribonucleic acid virus from the papillomavirus family with over 200 genomically distinct strains that infect the epithelia of the skin or mucosa, and most commonly cause benign papillomas or warts (1, 2). Recalcitrant warts due to HPV infection may be disfiguring and impose considerable lifelong physical and psychological distress on patients (3). Despite the availability of many surgical and non-surgical therapeutic approaches, there is still a large demand for safe medications to treat recalcitrant warts due to HPV infection, especially in immunosuppressed patients.
HPV vaccinations are a successful preventive measure used to decrease HPV infection rates, and are primarily used to prevent the development of cervical cancer and other anogenital cancers (1, 2, 4). However, these vaccines may emerge as a promising alternative drug for the treatment of recalcitrant warts (1, 4, 5). Currently, there are 3 licensed HPV vaccines available in the USA: Gardasil® (a quadrivalent vaccine targeting HPVs 6/11/16/18), Cervarix® (a bivalent vaccine targeting serotypes 16/18) and Gardasil 9® (a nonavalent vaccine targeting HPVs 6/11/16/18/31/33/45/52/58) (1). Prophylactic vaccinations, as shown in several animal models of papillomavirus infection, are very successful in preventing natural or experimental infection of skin and mucosa (6, 7). Although HPV vaccines are commonly utilized prophylactically by eliciting a virus-neutralizing antibody response (thus blocking viral entry into host cells), little is known about their use as a treatment for existing HPV-related cutaneous and/or mucosal conditions (1). In the current case series, this study aimed to investigate treatment outcomes using a nonavalent HPV vaccine in immunosuppressed patients with recalcitrant warts.
METHODS
A cohort review of patients with recalcitrant warts, who were treated with a nonavalent human papillomavirus vaccination in the period December to June 2019, was performed in the Department of Dermatology, University Hospital Bern. Recalcitrant warts are defined in this study as warts that last longer than 2 years and which have not responded to conventional local therapies, such as cryotherapy, salicylic acid, dinitrochlorobenzene, imiquimod, intralesional bleomycin, or CO2-laser ablations. One immunocompetent and 4 immunosuppressed patients, who were not pregnant and who presented with recalcitrant warts, were included in this study. Patients without complete medical records or regular followups were excluded from the study. The included patients read and signed the informed consent form.
All patients received Gardasil® 9 (Human Papillomavirus 9-valent Vaccine, Recombinant, Merck & Co., Inc.,Whitehouse Station, NJ, USA) in 3 doses at 0, 2 and 6 months. The clinical efficacy of the treatment was documented as follows: photographs were taken before the first vaccination, one month after the 1st vaccination, one month after the 2nd vaccination and one month after the third Gardasil 9® vaccination. In addition, the Dermatology Life Quality Index (DLQI) was evaluated before and one month after the 3rd Gardasil 9® vaccination. The evaluation was performed by 2 dermatologists. The absence of all detectable lesions after the treatment was considered as complete resolution (CR). Disease regression (DR) was considered as a decrease in the size and/or number of more than 50% of target lesions.
RESULTS
We report here the cases of 5 patients (3 men, 2 women, age range: 19–65 years) with recalcitrant warts who received Gardasil 9® vaccine using a 3-dose schedule. The clinical data is summarized in Table SI1. The mean ± standard deviation therapy duration of previous warts treatment before Gardasil 9® administration was 7.8 ± 4.6 years.
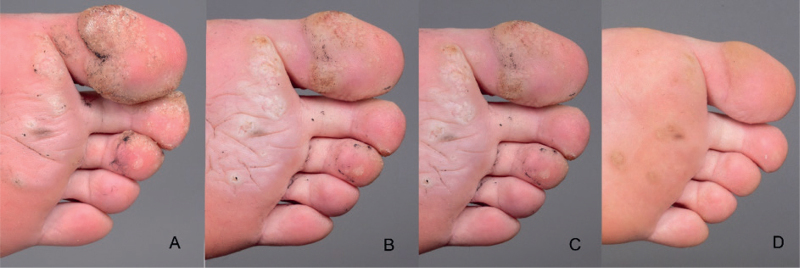
The 4th patient, a 19-year-old woman who showed an impressive response to the therapy, had recalcitrant warts for 5 years. Treatment with cryotherapy, fluorouracil/salicylic acid, diphenylcyclopropenone, and chloracetic acid failed to alleviate multiple warts lesions on her fingers and on the soles of her feet. However, improvement was noticed one month after the first Gardasil® vaccination, and nearly complete resolution was achieved 1 month after the 3rd Gardasil® dose (7 months after the first Gardasil® dose) (Fig. 1).
Fig. 1.
Response to Gardasil in patient 4 with plantar/finger warts. (A) Before Gardasil®. (B) One month after first dose of Gardasil®. (C) One month after 2nd dose of Gardasil®. (D) One month after 3rd dose of Gardasil®.
All patients experienced a decrease in the number of lesions after the 3rd dose of Gardasil® (1 complete resolution (CR), 4 disease regression (DR)). No adverse reactions were noted in any of the patients during the 3-dose treatment schedule. Aside from the optimal clinical response, the patients declared that they were able to cope better with their disease after vaccination, as shown by the DLQI, which improved significantly from 11.4 ± 3.4 to 3.0 ± 4.5 (p = 0.0114).
DISCUSSION
The results of this study showed the efficacy of a nonavalent HPV vaccine for the management of immunosuppressed patients with skin warts that responded poorly to other treatment strategies. This vaccine is recommended to prevent anogenital warts and cancers caused by certain types of HPV (8).
Immunosuppressed individuals have a greater susceptibility to HPV infection, typically resulting in the development of multiple benign tumours, such as papillomas (warts) of the skin and mucous membranes. Warts in immunocompetent individuals can spontaneously regress within 2 years, but in immunocompromised individuals, warts often persist and even spread, resulting in recalcitrant warts (1, 9, 10). Therefore, there is still a large demand for safe medications to manage skin warts. HPV vaccinations might emerge as a promising alternative drug treatment for this disease, since HPV types 1-4, 7, 8, 10, 27 and 57 are the most frequent strains of common skin warts, including plane warts (1, 4, 5).
Although HPV vaccination is used as a primary preventative strategy, a few cases have evaluated HPV vaccination as a treatment (the quadrivalent, bivalent, or nonavalent vaccine) for patients with cutaneous warts, (1, 3, 11–15). Similar to our study, a recent meta-analysis showed that 76.6% of patients experienced a significant decrease in the number of lesions post-vaccine (1). Most of the HPV types in common skin warts are not targeted by the vaccine. However, its mechanism of action is still unclear. There is a significant homology of L1 protein capsids between various HPV types, which presumably results in cross-protection of the vaccine (3, 11).
Our findings demonstrate a positive clinical outcome of the administration of a nonavalent HPV vaccine in the treatment of recalcitrant warts. Regarding financial aspects, this vaccine may be beneficial as an alternative treatment to treat or at least stabilize recalcitrant warts, especially in immunosuppressed patients. The patients with local therapies usually have to be treated for years and require many medical consultations. We assume that vaccination as a therapy can be a much cheaper and less time-consuming alternative, with a sustainable benefit.
These results therefore support the need for randomized controlled trials of therapeutic HPV vaccination for cutaneous warts, in order to assess the beneficial effects of HPV vaccines on skin warts in more detail, together with cost-benefit assessments, and possibly the creation of a vaccine specifically for skin warts.
Footnotes
REFERENCES
- 1.Pham CT, Juhasz M, Sung C, Mesinkovska NA. The human papillomavirus vaccine as a treatment for HPV-related dysplastic and neoplastic conditions: a literature review. J Am Acad Dermatol 2020; 82: 202–212. [DOI] [PubMed] [Google Scholar]
- 2.Zur Hausen H. Papillomavirus infections – a major cause of human cancers. Biochim Biophys Acta 1996; 1288: F55–F78. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson SB, Gallo ES. Nonavalent human papillomavirus vaccination as a treatment for warts in an immunosuppressed adult. JAAD Case Rep 2017; 3: 367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccine Information Statements (VISs). HPV (Human Papillomavirus) VIS. Current Edition Date 2019 Oct 30. Available from: https://www.cdc.gov/vaccines/hcp/vis/visstatements/hpv.html.
- 5.Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet 2017; 390: 2143–2159. [DOI] [PubMed] [Google Scholar]
- 6.Kirnbauer R, Chandrachud LM, O’Neil BW, Wagner ER, Grindlay GJ, Armstrong A, et al. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 1996; 219: 37–44. [DOI] [PubMed] [Google Scholar]
- 7.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol 1995; 69: 3959–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 89–99. [DOI] [PubMed] [Google Scholar]
- 9.Kwok CS, Gibbs S, Bennett C, Holland R, Abbott R. Topical treatments for cutaneous warts. Cochrane Database Syst Rev 2012 Sep 12:CD001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen J, Korta DZ, Chapman LW, Kelly KM. Laser treatment of nongenital verrucae: a systematic review. JAMA Dermatol 2016; 152: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 11.Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol 2013; 149: 370–372. [DOI] [PubMed] [Google Scholar]
- 12.Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol 2010; 146: 475–477. [DOI] [PubMed] [Google Scholar]
- 13.Landis MN, Lookingbill DP, Sluzevich JC. Recalcitrant plantar warts treated with recombinant quadrivalent human papillomavirus vaccine. J Am Acad Dermatol 2012; 67: e73–e74. [DOI] [PubMed] [Google Scholar]
- 14.Abeck D, Folster-Holst R. Quadrivalent human papillomavirus vaccination: a promising treatment for recalcitrant cutaneous warts in children. Acta Derm Venereol 2015; 95: 1017–1019. [DOI] [PubMed] [Google Scholar]
- 15.Landini MM, Borgogna C, Peretti A, Doorbar J, Griffin H, Mignone F, et al. Identification of the skin virome in a boy with widespread human papillomavirus-2-positive warts that completely regressed after administration of tetravalent human papillomavirus vaccine. Br J Dermatol 2015; 173: 597–600. [DOI] [PubMed] [Google Scholar]