Abstract
Psoriasis is associated with multiple co-morbid medical conditions. The purpose of this study is to evaluate the relationships between psoriasis and cardiovascular disease, psoriatic arthritis, mental health conditions, and immune-mediated diseases, respectively. A literature search was performed during the study period January 1, 2015 to December 18, 2018. Of 2,499 records identified, 28 met our criteria selection and were included in this review. The relationships between psoriasis and these multiple comorbid disease conditions are discussed and are important to consider when developing the treatment plan and overall management of patients with psoriasis. Early recognition and treatment of comorbid disease conditions is important to help improve the quality of life for these patients.
Key words: psoriasis, cardiovascular disease, psoriatic arthritis, mental health conditions, depression, immune-mediated disease
Psoriasis is a chronic inflammatory skin disease that affects approximately 125 million individuals world-wide (1). Cardiovascular disease, psoriatic arthritis, and mood disorders are common comorbid disease conditions associated with psoriasis (2). Moreover, autoimmune diseases have been reported to be associated with psoriasis, which may suggest that the pathogenesis of psoriasis may involve autoimmune mechanisms. In this review, the relationships between psoriasis and cardiovascular disease, psoriatic arthritis, mental health conditions, and autoimmune diseases, respectively, will be evaluated.
METHODS
A literature search was performed to identify comorbid disease conditions associated with psoriasis. Psoriasis and the associated comorbid conditions of cardiovascular disease, psoriatic arthritis, psychiatric conditions, and autoimmune disorders were examined. Articles from the past 3 years, specifically January 1, 2015 to December 18, 2018, were searched via PubMed and Google Scholar with the following keywords: psoriasis, comorbid, cardiovascular, psoriatic arthritis, psychiatric disease, and autoimmune disorders. The available abstracts and literature that investigated the relationship between psoriasis and cardiovascular disease, major adverse cardiovascular events (MACE), psoriatic arthritis, depression, suicidal ideation, suicidal attempts, and immune-mediated disorders, respectively, were evaluated. We restricted the search results to English-only records. Case reports, case series, and studies including pediatric patients were excluded. A manual inspection of reference lists and relevant studies was also performed to identify any additional relevant studies.
RESULTS
A total of 2,499 records were identified. After application of criteria selection and removal of duplicates, 28 of these records were included in the review (Fig. 1). Citations within identified articles and relevant studies were also reviewed, and 18 articles that were not originally detected in database searches were also included.
Fig. 1.
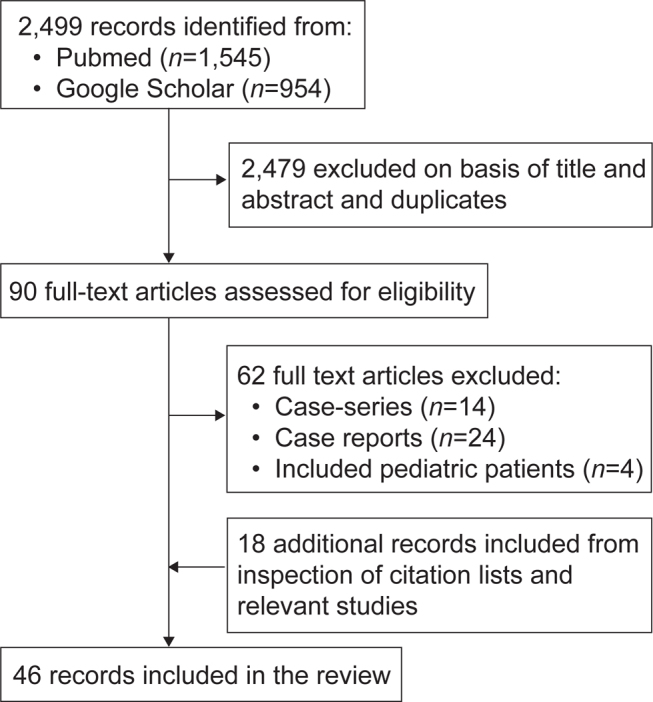
Study-flow diagram of the included studies.
PSORIASIS AND CARDIOVASCULAR DISEASE
Psoriasis and cardiovascular risk factors
Patients with psoriasis have a higher prevalence of traditional cardiovascular risk factors, including diabetes mellitus type 2, hypertension, dyslipidemia, and obesity (1, 3). Studies have shown that obesity is an independent risk factor for psoriasis (2). Specifically, studies have demonstrated a dose-dependent relationship between psoriasis severity and obesity (2). Moreover, independent of traditional risk factors, psoriasis is associated with a greater risk of diabetes. Recent evidence has suggested that the risk of diabetes, likelihood of insulin resistance, and diabetic complications increases with greater psoriasis severity, as defined by treatment patterns or BSA body surface area involved, independent of traditional risk factors (2). Studies have also demonstrated a higher prevalence of metabolic syndrome among patients with psoriasis compared to patients without psoriasis in adult and pediatric populations (2). The underlying mechanism of the association with psoriasis and these cardiovascular risk factors is not yet known, yet common inflammatory pathways, cellular mediators, and genetic susceptibility may contribute to these findings.
Association of psoriasis with vascular inflammation and cardiovascular events
Fluorodeoxyglucose F-18 positron emission tomography computed tomography (FDG PET/CT) is commonly used to measure aortic vascular inflammation and has been used as an indicator of cardiovascular risk and vascular disease (4, 5). Joshi et al. (5) demonstrated that patients with psoriasis with increased aortic vascular inflammation as evidenced by FDG PET/CT had significantly increased coronary artery disease indices, including total plaque burden, luminal stenosis, and high-risk plaques (5). Additionally, after adjustment for traditional cardiovascular risk factors, patients with severe psoriasis were at a higher risk for MACE compared to the general population (6). A prospective cohort study evaluated 115 psoriasis patients to determine the association between psoriasis disease severity and vascular inflammation as measured by FDG-PET/CT (7). At baseline, psoriasis severity was significantly associated with vascular inflammation. At one-year follow-up, improvement in psoriasis severity was associated with improvement in vascular inflammation, which maintained significance after accounting for traditional cardiovascular risk factors. Moreover, a significant 11% reduction in aortic vascular inflammation was observed for patients with greater than 75% reduction in psoriasis severity (7).
A study by Egeberg et al. (8) evaluated the impact of psoriasis duration on vascular disease and cardiovascular events. Among young patients with low cardiovascular risk by traditional risk scores and a high prevalence of cardiometabolic diseases, vascular inflammation as measured by FDG-PET/CT was significantly associated with disease duration. Moreover, duration of psoriasis demonstrated a strong association with MACE risk. Therefore, cumulative exposure to chronic inflammation among psoriasis patients may facilitate the development of vascular disease and MACE (8).
Patients with non-severe psoriasis also appear to be at an increased risk of developing cardiovascular events. Vascular indices of early arterial atherosclerosis (carotid intima-media thickness), endothelial dysfunction (flow-mediated dilation), and bioserum markers of oxidative stress (serum levels of advanced oxidation protein products) were assessed among patients with non-severe psoriasis. Compared to controls, patients with mild-to-moderate psoriasis without a history of any cardiovascular disease had significantly increased carotid intimamedia thickness, impaired flow-mediated dilation, and increased serum levels of advanced oxidation protein products (9). Additionally, a study utilizing echocardiographic evaluation demonstrated that left ventricular diastolic dysfunction was present among a greater number of young healthy patients with psoriasis compared with controls (10). Thus, patients with psoriasis appear to be at an increased risk of cardiovascular disease irrespective of underlying cardiovascular risk factors.
Impact of family history of cardiovascular disease
Family history of cardiovascular disease may help explain the increased risk of cardiovascular disease and MACE among patients with psoriasis. Egeberg et al. (11) compared the risk of incident MACE among patients with psoriasis with or without a family history of cardiovascular disease. Among patients with psoriasis and a family history of cardiovascular disease, the incidence ratios of MACE were 1.28 for mild and 1.62 for severe disease. No increased risk of MACE was detected among patients with psoriasis without a family history of cardiovascular disease (11). Therefore, it is important to determine the presence or absence of a family history of cardiovascular disease, as family history is likely a main contributor to MACE risk among patients with psoriasis.
Tumor necrosis factor inhibitor therapy and biomarkers of inflammation
C-reactive protein (CRP) is a biomarker of inflammation that has been utilized as a marker of cardiovascular risk (12). A retrospective cohort study evaluated the relationship between tumor necrosis factor (TNF) inhibitor therapy and changes in CRP among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis (13). At time of follow-up, mean change in CRP was lower among patients exposed to both a TNF inhibitor and methotrexate compared to methotrexate alone. For patients exposed to both a TNF inhibitor and methotrexate, the difference in mean CRP change was significantly lower compared to the methotrexate group after accounting for baseline CRP. Given that inflammation is a key contributing factor to the pathogenesis of cardiovascular disease (13), the results from this study suggest that exposure to TNF inhibitor therapy potentially reduces the risk of MACE among patients with chronic inflammatory conditions, including psoriasis. Thus, the results from this study further support the notion that TNF inhibitor therapy may offer a protective effect against developing MACE for patients with psoriasis.
Impact of biologic therapy on cardiovascular risk for patients with psoriasis
Treatment of psoriasis with TNF inhibitor therapy has been linked with a reduced risk of MACE among patients with psoriasis (14–16). A retrospective cohort study assessed MACE risk among patients with psoriasis receiving TNF inhibitor therapy compared to oral/phototherapy and topical therapy, respectively (14). After adjustment for cardiovascular risk factors, patients with psoriasis on TNF inhibitor therapy experienced significantly lower MACE hazard rate (HR) compared with patients on topical therapy. The MACE HR for patients in the oral/phototherapy group was similar to the topical group (14). The results from this study suggest that TNF inhibitor therapy may offer a protective effect against risk of MACE for patients with psoriasis.
Cumulative exposure to TNF inhibitor therapy may help further lower the risk of MACE for patients with psoriasis. A retrospective study by Wu et al. (15) compared the risk of MACE among patients with psoriasis receiving methotrexate versus TNF inhibitor therapy. After 12 months, patients receiving TNF inhibitor therapy developed fewer MACE and had lower cardiovascular event hazards compared to patients receiving methotrexate. Specifically, the MACE HR was 45% lower for patients receiving TNF inhibitor therapy compared to methotrexate. By 24 months median follow-up, every additional 6 months of TNF inhibitor exposure was associated with an 11% reduction in MACE risk (15). Therefore, cumulative exposure to TNF inhibitor therapy may help lower the risk of MACE for patients with psoriasis (15). A retrospective cohort study assessed the risk of MACE in patients with psoriasis receiving a TNF inhibitor versus phototherapy (16). Compared to patients receiving phototherapy, patients on TNF inhibitor therapy had a reduced MACE HR. Furthermore, every 6-month incremental cumulative exposure to TNF inhibitors was associated with a statistically significant reduction in MACE risk over a median observation period of 15.4 months. Furthermore, the risk reduction in MACE with 6 months of cumulative exposure was 11.2% greater among patients receiving TNF inhibitor therapy compared to phototherapy (16). Thus, the results of this study further suggests that cumulative TNF inhibitor exposure may lower the risk of MACE for patients with psoriasis (16).
Biologic therapy may attenuate coronary artery disease progression in patients with severe psoriasis. A prospective, controlled clinical study by Hjuler et al. (17) evaluated the association of biologic therapy with changes in coronary artery disease progression. The study evaluated coronary CT angiography among patients with severe psoriasis without symptomatic coronary artery disease at baseline and after 13 months of receiving biologic therapy (adalimumab, etanercept, infliximab, and ustekinumab) compared to control group. Among the control group, the severity of luminal narrowing in diseased segments was increased at 13-month follow-up, yet in the intervention group this was unchanged. In addition, the non-contrast coronary artery calcium scores were stable in the intervention group and progressed in the control group. A likely explanation for this finding is that biologic therapy helps decrease systemic inflammation, thus preventing cardiovascular disease progression. A limitation of this study is the small sample size of 28, which should be taken into consideration when interpreting these results. Nevertheless, biologic therapy appears to be associated with reduced coronary artery disease progression in patients with severe psoriasis.
On the other hand, there are also studies with opposing data regarding the effect of TNF inhibitory therapy on vascular inflammation. A randomized multicenter study by Bissonnette et al. (18) evaluated the impact of the TNF inhibitor adalimumab on vascular inflammation in patients with psoriasis. Utilizing PET/CT, no difference in vascular inflammation was appreciated over 16 weeks in the adalimumab group compared to placebo. Moreover, a randomized clinical trial by Mehta et al. (19) compared vascular inflammation and levels of cardiovascular biomarkers among patients with moderate-to-severe psoriasis treated with adalimumab, phototherapy, or placebo. At week 12, there was no difference in change in vascular inflammation as measured by FDG PET/CT among the adalimumab group (change compared with placebo, 0.64%) or the phototherapy group (−1.60%). Biomarkers of inflammation, serum CRP and IL-6, were decreased in both the adalimumab and phototherapy groups. Therefore, while studies have demonstrated that TNF inhibitor therapy may lower the risk of vascular inflammation for patients with psoriasis, studies have also revealed evidence that is contradictory to these findings. For this reason, the exact impact of TNF inhibitor therapy on cardiovascular risk is currently still debated.
PSORIASIS AND PSORIATIC ARTHRITIS
Psoriatic arthritis is an inflammatory disease that involves the peripheral and axial joints, skin, nails, and entheses (20, 21). Psoriatic arthritis has been reported to affect approximately 6–42% of patients with psoriasis (2). The prevalence of psoriatic arthritis appears to increase with greater severity of skin disease and duration of psoriasis (2). Clinically, patients experience joint pain and swelling secondary to chronic joint inflammation that, if left untreated, can lead to long-term irreversible joint damage and disability (20, 21). Cutaneous lesions tend to precede joint involvement, which can develop years after being diagnosed with psoriasis (18, 19). Yet, according to a meta-analysis by Villani et al. (22), the prevalence of undiagnosed psoriatic arthritis in patients with psoriasis at time of seeking medical care is approximately 15.5%. Thus, all patients with psoriasis should be screened for psoriatic arthritis at every stage of their disease.
Screening for psoriatic arthritis
Multiple questionnaires are available to help diagnose psoriatic arthritis (21). These questionnaires include the Toronto Psoriatic Arthritis Screening Questionnaire (TOPAS), Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE), and the Psoriasis and Arthritis Screening Questionnaire (PASQ) (21). Despite the development of these screening questionnaires, the ability to differentiate psoriatic arthritis from other forms of arthritis remains difficult and the diagnosis of psoriatic arthritis is often delayed (23). In a large population-based survey (24), 37.6% of dermatologists indicated that their greatest challenge in managing patients with psoriatic arthritis is discerning psoriatic arthritis from other arthritic diseases, while 25% of rheumatologists indicated that delayed referral is one of their greatest challenges. Moreover, joint pain was reported among 51.8% of psoriasis patients without a diagnosis of psoriatic arthritis, however only 18.6% of dermatologists reported that their patients had joint pain (24). Based on these results, there could be a discrepancy in the interpretations of joint involvement between physicians and patients with psoriasis. Additionally, there may be a need for enhanced communication between dermatologists and rheumatologists as well as within rheumatologists using enhanced tools to differentiate psoriatic arthritis from other arthritic diseases (25, 26). Cohen et al. (23) offered a simple, concise screening tool that encompasses key characteristics of psoriatic arthritis. The tool consists of the mnemonic “PSA,” for which P stands for pain (joint pain), S stands for both stiffness (> 30 min after a period if inactivity) and sausage digit (dactylitis), and A stands for axial (axial joint involvement/back pain referring to stiffness that improves with activity) (23). Moreover, the Classification Criteria for Psoriatic Arthritis (CASPAR) incorporates clinical findings specific to psoriatic arthritis, including presence of psoriatic nail dystrophy, a negative rheumatoid factor test, dactylitis, and radiographic evidence of juxta-articular bone formation) (21). CASPAR is highly specific (99.1%), however has lower sensitivity for detecting early psoriatic arthritis (87.4%) (21). Thus, it serves better as a confirmatory test rather than a screening tool, and can help physicians differentiate psoriatic arthritis from other forms of arthritis.
Symptom and complications of psoriatic arthritis
Patients with psoriasis and comorbid psoriatic arthritis tend to experience more symptoms and complications with respect to physical functioning compared to psoriasis patients without psoriatic arthritis (27). Compared to psoriasis patients without psoriatic arthritis, patients with psoriasis and psoriatic arthritis appear to have significantly more comorbid conditions, including hypertension, diabetes mellitus, and hyperlipidemia (20). In addition, patients with psoriasis and psoriatic arthritis have higher health care utilization and costs compared to patients without comorbid psoriatic arthritis (20). A study by Edson-Heredia et al. (28) demonstrated that patients with moderate-to-severe psoriasis and comorbid psoriatic arthritis experienced a greater impact on quality of life and symptoms of itching, physical irritation, and pain compared to patients with moderate-to-severe psoriasis alone. Among psoriasis patients with comorbid psoriatic arthritis, a greater frequency of comorbid diseases was reported, including type 2 diabetes mellitus and hypertension, compared to patients with psoriasis alone (28). Thus, psoriasis patients with psoriatic arthritis may experience greater psoriasis-related disease burden compared to patients with psoriasis alone.
Importance of recognition and treatment of psoriatic arthritis
Early recognition of psoriatic arthritis is imperative because improved control of inflammation can prevent joint destruction and improve quality of life. According to a study by Haroon et al. (29), a diagnostic delay of over 6 months from time of symptom onset to visit with a rheumatologist contributed to the development of joint erosions and worse functional disability as evidenced by Health Assessment Questionnaire (HAQ) scores for patients with psoriatic arthritis. Yet, a delayed diagnosis of psoriatic arthritis over one year was not associated with a significant difference in HAQ scores. A large United Kingdom multicenter study that evaluated factors contributing to work disability among patients with psoriatic arthritis found that worse physical function was associated with unemployment (30). Moreover, among participants that were employed, greater disease activity and worse physical function were associated with higher levels of productivity loss. Thus, among patients with psoriatic arthritis, productivity loss could potentially be prevented with treatment of psoriatic arthritis (30). Rahman et al. (31) also found that treatment of psoriatic arthritis was associated with improvements in physical function and health-related quality of life. Additionally, a study by Kirkham et al. (32) demonstrated that treatment of psoriatic arthritis was associated with improved quality of life as measured by patient reported outcomes (specifically, EuroQol-5D scores). Moreover, patients with shorter disease duration exhibited significantly greater improvements in disease activity and patient reported outcomes of joint pain and quality of life (32). The results from this study suggest that early intervention may have a more prominent impact on patient-reported outcomes of disease activity and quality of life. Moreover, treatment options for psoriasis, including biologics, can have different efficacy on cutaneous disease versus joint disease, which is important to consider when choosing appropriate therapy, which should ultimately be tailored for the individual patient (33).
PSORIASIS AND MENTAL HEALTH CONDITIONS
Patients with psoriasis are at an increased risk of depression compared to the general population (34). Moreover, depression in psoriasis patients may increase risk of other comorbidities. In a prospective cohort study, patients with psoriasis and major depressive disorder (MDD) were found to be at a significantly increased risk of developing psoriatic arthritis compared to psoriasis patients without MDD (35). Additionally, a study by Egeberg et al. (36) found patients with psoriasis and comorbid depression to be at an increased risk of myocardial infarction, stroke, and cardiovascular death. Moreover, Aberra et al. (37) demonstrated that vascular inflammation (as measured by FDG PET/CT) and total and non-calcified coronary plaque burden (as measured by coronary CT angiography) were significantly higher among patients with psoriasis and self-reported depression versus patients with psoriasis alone. After adjustment for traditional cardiovascular disease risk factors, vascular inflammation, total plaque burden, and non-calcified burden were significantly associated with self-reported depression (37). The reported findings may be due to the chronic inflammation present in psoriasis, which potentially increases the risk of developing cardiovascular events. Furthermore, psoriasis patients with comorbid depression are at an even greater cardiovascular risk due to the reported association between depression and cardiovascular events, subclinical atherosclerosis, and all-cause mortality, independent of traditional cardiovascular risk factors (37). Therefore, patients with psoriasis and comorbid depression appear to be at a greater risk of inflammation-induced atherosclerotic plaque development, ultimately increasing the risk of developing cardiovascular events. Comorbid depression could also result in reduced adherence to treatment for psoriasis as well as utilization of healthcare resources, consequently interfering with cardiovascular risk factor management (36, 38). Thus, detecting and treating comorbid depression may prevent the development of complications for these patients.
Psoriasis is also associated with anxiety and suicidal ideation (39, 40). A systematic review and meta-analysis by Singh et al. (41) assessed the relationship between psoriasis and suicidality. Compared to patients without psoriasis, patients with psoriasis were twice as likely to exhibit suicidal ideation and suicidal behaviors (combined attempted and completed suicides; pooled (41). Thus, it is important to detect and treat comorbid mental health conditions in patients with psoriasis.
PSORIASIS AND IMMUNE-MEDIATED DISORDERS
An increased frequency of immune-mediated disorders has been reported among patients with psoriasis (42–46). However, a definite relationship between psoriasis and immune-mediated diseases remains unclear (42). Compared to patients with mild psoriasis, patients with severe psoriasis demonstrated significantly higher diagnosis rates of rheumatoid arthritis, lupus, and Crohn’s disease (43). In addition, the presence of autoreactive T cells have been demonstrated in the pathogenesis of psoriasis, which suggests that psoriasis may be autoimmune in nature (44–46). A nationwide, population-based, cross-sectional study evaluated the association between psoriasis and various immune-mediated rheumatic diseases among 267,230 patients with psoriasis and 267,230 controls without psoriasis (47). Psoriasis was significantly associated with ankylosing spondylitis (AS), rheumatoid arthritis (RA), Behçet disease, systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and dermatomyositis/polymyositis (DM/PM). Moreover, male patients with psoriasis exhibited higher associations with AS, RA, SLE, SSc, and DM/PM compared to female patients with psoriasis (47).
A case-control study evaluating 287 patients with bullous pemphigoid (BP) and 1,373 matched controls found that the prevalence rate of psoriasis was greater among patients with BP versus controls (48). Moreover, psoriasis preceded the diagnosis of BP, by a mean duration of 25.2 years (48). Additionally, a cross-sectional study detected a significant association between psoriasis and Hashimoto’s thyroiditis that sustained after adjusting for confounding variables, including sex, age, psoriatic arthritis, and use of systemic anti-psoriasis agents (odds ratio 2.49) (49). Chronic inflammation and subsequent damage to the basement membrane has been suggested as a possible mechanism for the reported association between BP and psoriasis (42). Another theory is that treatment for psoriasis may worsen subclinical bullous pemphigoid (42). Further studies would help determine the exact association between psoriasis and BP, as well as other autoimmune disorders.
CONCLUSION
The association between psoriasis and comorbid disease conditions is important to consider when developing the treatment plan and overall management of patients with psoriasis. Psoriasis is associated with cardiovascular disease, and chronic inflammation likely plays a major role in this relationship. Treatment of psoriasis improves underlying inflammation and TNF inhibitor therapy may provide a protective effect against risk of MACE for patients with psoriasis, which would ultimately promote better health outcomes for these patients. Moreover, psoriatic arthritis is a common comorbid condition associated with psoriasis that can lead to permanent disability. Early treatment is imperative to help prevent complications of psoriatic arthritis and improve quality of life for these patients. Furthermore, it is important to address and treat comorbid psychiatric conditions among patients with psoriasis, including depression, suicidal behavior, and suicidal ideation. Future clinical trials would help better assess the role of biologic therapy on improving health outcomes, wellness, and quality-of-life for patients with psoriasis. Certain immune-mediated disorders have been reported to be associated with psoriasis. Further research will help better assess these associations as well as the autoimmune aspects of the underlying pathogenesis of psoriasis.
ACKNOWLEDGEMENT
Disclosure of interest: MA and EBL have no conflicts of interest to declare. T-FT is an investigator for Abbvie, Boehringer Ingelheim, Celgene, Eli-Lilly, Galderma, Janssen-Cilag, Novartis International AG, Pfizer Inc.; a consultant for Abbvie, Boehringer Ingelheim, Celgene, Eli-Lilly, Janssen-Cilag, Novartis International AG, Pfizer Inc. JJW is an investigator for AbbVie, Amgen, Eli Lilly, Janssen, Novartis; a consultant for AbbVie, Almirall, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Dr. Reddy’s Laboratories, Eli Lilly, Janssen, LEO Pharma, Novartis, Promius Pharma, Regeneron, Sun Pharmaceutical, and UCB, Valeant Pharmaceuticals North America LLC; and a speaker for AbbVie, Celgene, Novartis, Regeneron, Sanofi Genzyme, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America LLC.
REFERENCES
- 1.National Psoriasis Foundation . Available from: https://www.psoriasis.org/content/statistics Accessed January 14, 2019.
- 2.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol 2017; 76: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-Armenteros JM, Gómez-Arbonés X, Buti-Soler M, Betriu-Bars A, Sanmartin-Novell V, Ortega-Bravo M, et al. Psoriasis, metabolic syndrome and cardiovascular risk factors. A population-based study. J Eur Acad Dermatol Venereol 2018; 33: 128–135. [DOI] [PubMed] [Google Scholar]
- 4.Teague HL, Ahlman MA, Alavi A, Wagner DD, Lichtman AH, Nahrendorf M, et al. Unraveling vascular inflammation: from immunology to imaging. J Am Coll Cardiol 2017; 70: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol 2018; 3: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a populationbased cohort study. Ann Rheum Dis 2015; 74: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol 2017; 2: 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeberg A, Skov L, Joshi AA, Mallbris L, Gislason GH, Wu JJ, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J Am Acad Dermatol 2017; 77: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberka M, Bańska-Kisiel K, Bergler-Czop B, Biedroń M, Brzezińska-Wcisło L, Okopień B, et al. Mild to moderate psoriasis is associated with oxidative stress, subclinical atherosclerosis, and endothelial dysfunction. Pol Arch Intern Med 2018; 128: 434–439. [DOI] [PubMed] [Google Scholar]
- 10.Gorga E, Scodro M, Valentini F, D’Ortona R, Arisi M, Sciatti E, et al. Echocardiographic evaluation of diastolic dysfunction in young and healthy patients with psoriasis: A case-control study. Monaldi Arch Chest Dis 2018; 88: 15–19. [DOI] [PubMed] [Google Scholar]
- 11.Egeberg A, Bruun LE, Mallbris L, Gislason GH, Skov L, Wu JJ, et al. Family history predicts major adverse cardiovascular events (MACE) in young adults with psoriasis. J Am Acad Dermatol 2016; 75: 340–346. [DOI] [PubMed] [Google Scholar]
- 12.Shrivastava AK, Singh HV, Raizada A, Singh SK. C–reactive protein, inflammation and coronary heart disease. Egypt Heart J 2015; 67: 89–97. [Google Scholar]
- 13.Wu JJ, Rowan CG, Bebchuk JD, Anthony MS. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis. J Am Acad Dermatol 2015; 72: 917–919. [DOI] [PubMed] [Google Scholar]
- 14.Wu JJ, Joshi AA, Reddy SP, Batech M, Egeberg A, Ahlehoff O, et al. Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol 2018; 32: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 15.Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol 2017; 76: 81–90. [DOI] [PubMed] [Google Scholar]
- 16.Wu JJ, Sundaram M, Cloutier M, Gauthier-Loiselle M, Guérin A, Singh R, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor–α inhibitors versus phototherapy: An observational cohort study. J Am Acad Dermatol 2018; 79: 60–68. [DOI] [PubMed] [Google Scholar]
- 17.Hjuler KF, Bøttcher M, Vestergaard C, Bøtker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol 2016; 152: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 18.Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol 2017; 137: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 19.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging 2018; 11: e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman SR, Zhao Y, Shi L, Tran MH, Lu J. Economic and comorbidity burden among moderate-to-severe psoriasis patients with comorbid psoriatic arthritis. Arthritis Care Res (Hoboken) 2015; 67: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: Early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 2017; 76: 21–37. [DOI] [PubMed] [Google Scholar]
- 22.Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Ricard MA, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta–analysis. J Am Acad Dermatol 2015; 73: 242–248. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JM, Husni ME, Qureshi AA, Merola JF. Psoriatic arthritis: It’s as easy as “PSA”. J Am Acad Dermatol 2015; 72: 905–906. [DOI] [PubMed] [Google Scholar]
- 24.Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population–based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Am J Clin Dermatol 2016; 17: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGonagle D, Hermann KG, Tan AL. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology 2014; 54: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorter S, Van der Heijde DM, Van der Linden S, Houben H, Rethans JJ, Scherpbier AJ, et al. Psoriatic arthritis: performance of rheumatologists in daily practice. Ann Rheum Dis 2002; 61: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pariser D, Schenkel B, Carter C, Farahi K, Brown TM, Ellis CN; Psoriasis Patient Interview Study Group . A multicenter, noninterventional study to evaluate patient–reported experiences of living with psoriasis. J Dermatolog Treat 2016; 27: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edson-Heredia E, Zhu B, Guo J, Maeda-Chubachi T, Lebwohl M. Disease burden and quality of life in psoriasis patients with and without comorbid psoriatic arthritis: results from National Psoriasis Foundation panel surveys. Cutis 2015; 95: 173–178. [PubMed] [Google Scholar]
- 29.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015; 74: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 30.Tillett W, Shaddick G, Askari A, Cooper A, Creamer P, Clunie G, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology 2015; 54: 157–162. [DOI] [PubMed] [Google Scholar]
- 31.Rahman P, Puig L, Gottlieb AB, Kavanaugh A, McInnes IB, Ritchlin C, et al. Ustekinumab treatment and improvement of physical function and health-related quality of life in patients with psoriatic arthritis. Arthritis Care Res 2016; 68: 1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkham B, Li W, Boggs R, Mallbris L, Nab HW, Tarallo M. Early treatment of psoriatic arthritis is associated with improved patient-reported outcomes: findings from the etanercept PRESTA trial. Clin Exp Rheumatol 2015; 33: 11–19. [PubMed] [Google Scholar]
- 33.Amin M, No DJ, Egeberg A, Wu JJ. Choosing first-line biologic treatment for moderate-to-severe psoriasis: what does the evidence say? Am J Clin Dermatol 2018; 19: 1–3. [DOI] [PubMed] [Google Scholar]
- 34.Wu JJ, Penfold RB, Primatesta P, Fox TK, Stewart C, Reddy SP, et al. The risk of depression, suicidal ideation and suicide attempt in patients with psoriasis, psoriatic arthritis or ankylosing spondylitis. J Eur Acad Dermatol Venereol 2017; 31: 1168–1175. [DOI] [PubMed] [Google Scholar]
- 35.Lewinson RT, Vallerand IA, Lowerison MW, Parsons LM, Frolkis AD, Kaplan GG, et al. Depression is associated with an increased risk of psoriatic arthritis among patients with psoriasis: a population-based study. J Invest Dermatol 2017; 137: 828–835. [DOI] [PubMed] [Google Scholar]
- 36.Egeberg A, Khalid U, Hilmar Gislason G, Mallbris L, Skov L, Riis Hansen P. Impact of depression on risk of myocardial infarction, stroke and cardiovascular death in patients with psoriasis: a Danish Nationwide Study. Acta Derm Venereol 2016; 96: 218–222. [DOI] [PubMed] [Google Scholar]
- 37.Aberra TM, Joshi AA, Lerman JB, Rodante JA, Dahiya AK, Teague HL, et al. Self-reported depression in psoriasis is associated with subclinical vascular diseases. Atherosclerosis 2016; 251: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulkarni AS, Balkrishnan R, Camacho FT, Anderson RT, Feldman SR. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol 2004; 3: 661–666. [PubMed] [Google Scholar]
- 39.Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol 2015; 90: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu JJ, Feldman SR, Koo J, Marangell LB. Epidemiology of mental health comorbidity in psoriasis. J Dermatolog Treat 2018; 29: 487–495. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and suicidality: A systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 425–440. [DOI] [PubMed] [Google Scholar]
- 42.Sticherling M. Psoriasis and autoimmunity. Autoimmunity reviews 2016;15: 1167–1170. [DOI] [PubMed] [Google Scholar]
- 43.Edson-Heredia E, Zhu B, Lefevre C, Wang M, Barrett A, Bushe CJ, et al. Prevalence and incidence rates of cardiovascular, autoimmune, and other diseases in patients with psoriatic or psoriatic arthritis: a retrospective study using Clinical Practice Research Datalink. J Eur Acad Dermatol Venereol 2015; 29: 955–963. [DOI] [PubMed] [Google Scholar]
- 44.Furue K, Ito T, Tsuji G, Kadono T, Nakahara T, Furue M. Autoimmunity and autoimmune co-morbidities in psoriasis. Immunology 2018; 154: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prinz JC. Human leukocyte antigens and the autoreactive t cell response in psoriasis pathogenesis. Front Immunol 2018; 9: 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prinz JC. Melanocytes: Target cells of an HLA-C* 06: 02–restricted autoimmune response in psoriasis. J Invest Dermatol 2017; 137: 2053–2058. [DOI] [PubMed] [Google Scholar]
- 47.Ju HJ, Kim KJ, Kim DS, Lee JH, Kim GM, Park CJ, et al. Increased risks of autoimmune rheumatic diseases in patients with psoriasis: A nationwide population-based study. J Am Acad Dermatol 2018; 79: 778–781. [DOI] [PubMed] [Google Scholar]
- 48.Kridin K, Bergman R. Association between bullous pemphigoid and psoriasis: a case–control study. J Am Acad Dermatol 2017; 77: 370–372. [DOI] [PubMed] [Google Scholar]
- 49.Kiguradze T, Bruins FM, Guido N, Bhattacharya T, Rademaker A, Florek AG, et al. Evidence for the association of Hashimoto’s thyroiditis with psoriasis: a cross-sectional retrospective study. Int J Dermatol 2017; 56: 553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]


