Abstract
DNA damage and oxidative stress play a critical role in photoageing. Seborrhoeic keratosis (SK) affects sunlight-exposed sites in aged individuals. This study examined the mechanism of photoageing in SK. The guanine deaminase gene, which is involved in purine metabolism, was upregulated with uric acid levels and p21 in SK. Guanine deaminase was detectable in keratinocytes. Repeated exposure to ultraviolet (UV) increased levels of guanine deaminase, together with DNA damage, such as γ-H2AX and cyclobutane pyrimidine dimer formation, generation of reactive oxygen species, and keratinocyte senescence, which were reversed by guanine deaminase knockdown. However, guanine deaminase overexpression and H2O2 formed γ-H2AX, but not cyclobutane pyrimidine dimer. Loss-of-function guanine deaminase mutants reduced the metabolic end-product uric acid, which was increased by exposure to exogenous xanthine. Repeated exposure to UV increased levels of uric acid. Exogenous uric acid increased cellular senescence, reactive oxygen species, and γ-H2AX, similar to guanine deaminase. Overall, guanine deaminase upregulation increased UV-induced keratinocyte senescence in SK, via uric acid mediated by reactive oxygen species followed by DNA damage.
Key words: seborrhoeic keratosis, guanine deaminase, UV-induced keratinocyte senescence, uric acid, reactive oxygen species, DNA damage
Cumulative exposure to sunlight induces photoageing of skin. DNA damage and oxidative stress have been investigated to determine the molecular mechanism of photoageing (1, 2). Although DNA damage and oxidative stress cause skin ageing, these factors could be inter-related rather than independent. Purine is one of the components of DNA nucleotides. When adenosine or guanosine is metabolized, xanthine oxidase (XO) catalyses the conversion of xanthine to uric acid, an end-product of purine metabolism. Because XO donates electrons to molecular oxygen, it produces reactive oxygen species (ROS), and therefore, the purine degradation pathway has been suggested as a major source of ROS (3, 4). On the other hand, ROS frequently react with guanine, an intermediate guanosine metabolite, to generate 8-oxo-7,8-dihydroguanine (8-oxoG), a biomarker of oxidative DNA damage (5, 6) associated with skin ageing due to inadequate repair (2, 7).
SIGNIFICANCE.
Seborrhoeic keratosis develops mainly on sun-exposed areas of elderly individuals, suggesting that photoageing has a role in this condition. Based on evidence of purine degradation in skin following chronic exposure to sunlight, guanine deaminase was selected as a candidate gene related to purine metabolism. Although the expression of guanine deaminase in human keratinocytes has yet to be elucidated, this study demonstrated keratinocytes as the main source of guanine deaminase in skin. The role of guanine deaminase in ultraviolet (UV)-induced keratinocyte senescence was examined in seborrhoeic keratosis and primary cultured adult human keratinocytes. The results suggest that guanine deaminase upregulation in seborrhoeic keratosis mediates UV-induced keratinocyte senescence via the production of uric acid as an end-product, generating reactive oxygen species followed by DNA damage.
Concerning the association between purine degradation and ultraviolet (UV) irradiation, chronic exposure to sunlight has been shown to generate metabolic products, including xanthine and uric acid, following adenosine degradation (8). In contrast, the role of guanosine degradation in UV-induced uric acid production has yet to be identified, although guanosine is also metabolized into xanthine and uric acid. Uric acid has been considered as an anti-oxidant in human skin (9). The antioxidant activity of uric acid may account for potent antioxidant effect of caffeine in cosmetics (10). However, evidence suggests that uric acid induces oxidative stress, increasing the risk of cardiovascular diseases (3, 11). In addition, allopurinol, which reduces the serum levels of uric acid via inhibition of XO, acts as a free radical scavenger, improving cardiovascular dysfunction (12–14). Because oxidative stress occurs whenever ROS overwhelm the anti-oxidant capacity, the overall effect of purine metabolites on oxidative stress may depend on the role of uric acid in either the antioxidant or ROS source. However, the role of uric acid in oxidative stress has not been examined in skin disorders.
Seborrhoeic keratosis (SK) is the most common benign skin tumour involving epidermal keratinocytes. Studies investigating the pathogenesis of SK show a positive correlation between the expression of p16 or amyloid precursor protein and the prevalence of SK (15, 16). p16 is responsible for senescence, particularly in human keratinocytes (17). The levels of amyloid precursor protein increase in the UV-exposed epidermis of aged individuals (16). These results suggest a role of keratinocyte senescence, particularly UV-induced keratinocyte senescence, in development of SK. Epidemiological studies also suggest ageing and sun exposure as risk factors for SK (18, 19). Although the aetiology and pathogenesis of SK remain to be elucidated, these results indicate that cumulative exposure to sunlight and ageing are the main aetiological and risk factors for SK (20). Purine is degraded in skin that is chronically exposed to sunlight (8).
The aim of this study was therefore to investigate the association between purine degradation and UV-induced keratinocyte senescence in SK. Following the analysis of differentially expressed genes (DEGs) associated with purine metabolism and their clinical relevance in SK, the role of candidate DEGs in UV-induced keratinocyte senescence was examined. The results showed that guanine deaminase (GDA) is an upregulated DEG related to purine metabolism in SK. GDA plays a role in repeated UV-induced keratinocyte senescence via uric acid upregulation, resulting in ROS generation and DNA damage.
MATERIALS AND METHODS
Patients
A definitive diagnosis of SK may not be easy to establish, due to clinical variability and differences in histology (21). In order to avoid clinically atypical lesions, this study investigated homogenous light- to dark-brown, sharply demarcated, smooth-surfaced, flat papules, 5-8 mm in diameter. The histopathological findings of 5 lesions obtained from 5 patients also established the diagnosis. The study included 11 female and 3 male patients, ranging from 43 to 79 years ( mean 62.7 years). Lesions were located on the face (10), dorsa of hands (3) and shins (1). The Institutional Review Board of Dongguk University Ilsan Hospital approved this study, and the study was conducted according to the principles of the Declaration of Helsinki. After obtaining informed written consent from each patient, pairs of lesional and adjacent normal-looking skin specimens were obtained by biopsy for direct comparison. These specimens derived from 10 patients were used for real-time PCR, while those obtained from the other 4 patients were analysed immunohistochemically.
Microarray
Total RNA was extracted from the human skin, using the PureLink RNA Mini kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions, with 100 ng total RNA for labelling. Affymetrix GeneChip microarrays were prepared, hybridized, and scanned by the local, authorized Affymetrix service provider (BioCore, Seoul, South Korea). RNA was converted to cDNA and transcribed into cRNA in the presence of biotinylated ribonucleotides, according to standard Affymetrix protocols (Expression Analysis Technical Manual, 2001). Following fragmentation, 12 μg RNA fragments were hybridized for 16 h at 45°C on Affymetrix Primeview array GeneChips, followed by washing and staining in the Affymetrix Fluidics Station 450. GeneChips were scanned using the Affymetrix GeneChip Scanner 3000+7G. The data were analysed with Robust Multichip Analysis (RMA) using Affymetrix default analysis settings and global-scaling gas normalization method. The trimmed mean target intensity of each array was arbitrarily set to 100. The normalized, and log-transformed intensity values were analysed using GeneSpring GX 13.1.1 Agilent technologies. Fold change filters required the presence of unregulated genes in at least 200% of controls and downregulated genes in less than 50% of controls. Hierarchically clustered data were categorized into groups based on similarity across experiments, using Gene Spring GX 13.1.1, Agilent Technologies, CA, USA). The clustering algorithm was Euclidean distance, to obtain mean linkage.
Normal human epidermal cell culture
Adult skin specimens obtained from Caesarean sections and circumcisions were used to establish the cell cultures. Keratinocyte cultures were obtained by suspending individual epidermal cells derived according to published methods (22), in EpiLife Medium supplemented with bovine pituitary extract (BPE), bovine insulin (BI), hydrocortisone, human epidermal growth factor, and bovine transferrin (BT) (Invitrogen). Early passages from 3 to 4 were used for these experiments. Melanocyte cultures were obtained by suspending individual epidermal cells in Medium 254 supplemented with BPE, foetal bovine serum (FBS), BI, hydrocortisone, bFGF, BT, heparin, and phorbol 12-myristate 13-acetate (Invitrogen). Fibroblast cultures were obtained by suspending individual dermal cells lacking epidermis (23) in DMEM-supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Invitrogen). Cytokeratins 14 and 10 (mouse monoclonal; Santa Cruz Biotechnology, Dallas, TX, USA) were used as markers for keratinocytes; c-kit (mouse monoclonal; Santa Cruz Biotechnology) and tyrosinase-related protein 1 (goat polyclonal; Santa Cruz Biotechnology) were used as markers for melanocytes; and ER-TR7 (rat polyclonal; Santa Cruz Biotechnology) as a marker for fibroblasts (24–26).
UV irradiation
Each 6-well plate (2×105 keratinocytes/well) was irradiated with nonirritating doses of UVA or UVB, based on cell viability test, with doses showing less than 50% reduction in viable cell number (27), once or repeatedly on 3 consecutive days at a distance of 15 cm from the light source. The UVA and UVB sources included a TL-K 40W lamp (Royal Philips Amsterdam, Netherlands; 315-380 nm, peak 350 nm) and a TL 20W lamp (305-315 nm, peak 311 nm), respectively. The cells and the supernatants were harvested 24 h after the final irradiation, for subsequent analysis.
Guanine deaminase knockdown
GDA knockdown was achieved after the second UVB irradiation or the day after cell seeding by transfecting the cells with 500 nM siRNA against human GDA or a negative control (On-TARGETplus SMARTpool or Non-targeting siRNA; Invitrogen) using the TransIT-siQUEST transfection reagent (Mirus Bio, Madison, WI, USA) according to the manufacturer’s protocol.
Guanine deaminase and guanine deaminase mutant overexpression
For the construction of GDA, the amplified PCR products were inserted into the pCMV vector. GDA mutants with decreased GDA activity were constructed using a previously described method (28) with some modification. Briefly, the zinc-binding domain (amino acid residues, 76-84), collapsing-response mediator protein (CRMP) homology domain (amino acids, 350-402), or both were deleted. The mutants were named GDAΔ(76–84), GDAΔ(350-402), and GDAΔ(76-84 and 350-402). Because GDAΔ(350-402) was not expressed, other mutants were used for this study. For GDA and GDA mutant overexpression, transfection of cells was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Xanthine or uric acid treatment
One day after keratinocyte seeding (2×105 keratinocytes/well), the cells were treated with xanthine (10 and 50 μM) with or without allopurinol (10 μM) or uric acid (10 and 100 μM) (Sigma-Aldrich, St Louis, MO, USA) for 24–48 h. The cells or supernatants were harvested for subsequent analysis.
Real-time PCR
The cDNA was synthesized from the total RNA using the cDNA Synthesis Kit for RT-PCR (Promega, Fitchburg, WI, USA). The amount of target mRNA was quantified by real-time PCR using a Light Cycler real-time PCR machine (Roche, Penzberg, Germany). The relative amount of mRNA was calculated as the ratio of each target relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences used were as follows: GDA 5’-catagtgacaccacgtttttcc-3’(Forward) and 5’-cgattttcacttatatggctctga-3’ (Reverse); GAPDH 5’-tccactggcgtcttcacc-3’ (Forward) and 5’-ggcagagatgatgacccttt-3’ (Reverse).
Western blot analysis
Each cell type was harvested 24 or 48 h after the seeding of cells (2×105 keratinocytes, melanocytes or fibroblasts/well) according to the experimental conditions. Equal amounts of extracted proteins (20 μg) were resolved and transferred to nitrocellulose membranes. The membranes were incubated with antibodies to GDA (mouse monoclonal; Santa Cruz Biotechnology), p16 (rabbit polyclonal; Bethyl Laboratories Inc., Montgomery, TX, USA), p21 (rabbit polyclonal; Santa Cruz Biotechnology), γ-H2AX (rabbit polyclonal; Abcam, Cambridge, UK), and β—actin (mouse monoclonal; Sigma-Aldrich). After incubation with appropriate anti-mouse or anti-rabbit horseradish peroxidase-conjugated antibodies (Thermo Fisher Scientific, Waltham, MA, USA), the samples were reacted with an enhanced chemiluminescence solution (Thermo Fisher Scientific) and the signals were captured on an image reader (LAS-3000; Fuji Photo Film, Tokyo, Japan). The protein bands were then analysed via densitometry.
Immunofluorescence staining
The biopsied skin specimens were fixed with 4% paraformaldehyde (Sigma-Aldrich) at room temperature for 24 h and embedded in paraffin wax and sectioned into 5-μm thick samples. After deparaffinization and rehydration, samples were preincubated with 3% bovine serum albumin. The cultured cells were fixed with 2% paraformaldehyde at room temperature for 20 min. The tissue samples or fixed cells were reacted with anti-GDA, anti-p16, anti-p21, anti-uric acid antibody (Abcam, Cambridge, UK) or anti-cyclobutane pyrimidine dimer (CPD) antibody (Cosmo Bio Co, Ltd., Tokyo, Japan) followed by 1:200 Alexa Fluor-labelled goat anti-mouse IgG (488; Molecular Probes, Eugene, OR, USA) or Alexa Fluor-labelled goat anti-rabbit (594; Molecular Probes). Nuclei were counterstained with Hoechst 33258 (Sigma-Aldrich). Staining intensity was evaluated using an image analysis system (Dp Manager 2.1; Olympus Optical Co., Tokyo, Japan) equipped with Wright Cell Imaging Facility (WCIF) ImageJ software (http://www.uhnresearch.ca/facilities/wcif/imagej).
Senescence-associated β-galactosidase staining
Cells were fixed for 15 min at room temperature with 2% formaldehyde, and washed with PBS followed by incubation at 37°C with freshly made senescence-associated (SA) β-Gal staining solution (Cell Signaling Technology, Beverly, MA, USA). Senescent cells displayed a perinuclear precipitation of blue dye, observed using a standard light microscope (DM LB microscope; Leica Microsystems, Wetzla, Germany).
Dihydroethidium staining
Dihydroethidium (DHE) staining is an indirect measure of ROS production. DHE exhibits blue fluorescence in its unoxidized form and red fluorescence when oxidized to 2-hydroxyethidium by the superoxide anion. As described previously (29), keratinocytes treated under different experimental conditions were incubated with 10 μM DHE fluorescent dye (Sigma-Aldrich) at 37°C for 30 min. After washing with PBS, the fluorescence intensities of the different groups were analysed immediately using a standard light microscope (DM LB microscope).
Xanthine and uric acid assay
The concentrations of xanthine and uric acid in harvested cells or supernatants were determined colorimetrically (λ = 570 nm) using a xanthine/hypoxanthine assay kit and a uric acid assay kit (Abcam), respectively, following the manufacturer’s instructions.
Cell viability test
Cells were stained with 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) for 4 h. The precipitated formazan was dissolved in dimethylsulphoxide and the optical density was measured using a spectrophotometer at 570 nm, with background subtraction at 630 nm. Cell viability was calculated as the ratio of cell growth under UV irradiation to that of unexposed cells.
Statistical analysis
The experimental data were analysed using Mann-Whitney U test with SPSS software (version 25.0.0, IBM, Chicago, IL, USA). The results were expressed as the means ± SDs. A p-value of less than 0.05 was considered significant.
RESULTS
Guanine deaminase is an upregulated differentially expressed genes related to purine metabolism in Seborrhoeic keratosis
Significant genes, showing fold changes higher than 2, or less than −2, involved in purine metabolism were identified based on the results of microarray analysis using non-lesional and lesional skin specimens derived from the 2 patients diagnosed with SK. Ten genes associated with purine metabolism were detected; however, GDA, which catalyses guanine to xanthine, was determined as an upregulated DEG (Table I).
TABLE I.
Microarray analysis of differentially expressed genes (DEGs) related to purine metabolism in seborrhoeic keratosis
| Description | Gene symbol | Probe set ID | Fold change (log ratio) | |
|---|---|---|---|---|
| Patient 1 | Patient 2 | |||
| Adenosine monophosphate deaminase 2 | AMPD2 | 11724224_a_at | 0.048 | -0.037 |
| 11730829_a_at | 0.008 | -0.227 | ||
| 11730830_x_at | -0.162 | -0.079 | ||
| 11745744_a_at | -0.037 | 0.114 | ||
| Adenosine monophosphate deaminase 1 | AMPD1 | 11730365_at | 0.177 | 0.190 |
| 5’-nucleotidase, cytosolic IA | NT5C1A | 11715302_s_at | 0.085 | -0.332 |
| 11759474_at | -0.072 | 0.168 | ||
| 5’-nucleotidase, cytosolic II | NT5C2 | 11722566_a_at | 0.320 | 0.077 |
| 11722567_at | -0.045 | 0.016 | ||
| 11722568_at | 0.026 | -0.227 | ||
| 11754974_x_at | 0.003 | 0.132 | ||
| 5’-nucleotidase, ecto (CD73) | NT5E | 11719174_a_at | 0.077 | -0.384 |
| 11744681_a_at | 0.109 | -0.425 | ||
| 11755207_a_at | -0.454 | -0.023 | ||
| Adenosine deaminase, RNA-specific, B1 | ADARB1 | 11718016_a_at | -0.149 | -0.281 |
| 11718017_a_at | -0.140 | -0.606 | ||
| 11718018_a_at | 0.162 | 0.047 | ||
| 11718019_x_at | 0.027 | 0.000 | ||
| 11751915_a_at | -0.230 | -0.041 | ||
| Adenosine deaminase, tRNA-specific 1 | ADAT1 | 11729264_x_at | -0.154 | 0.373 |
| 11751112_a_at | 0.183 | -0.044 | ||
| Adenosine deaminase | ADA | 11718788_a_at | -0.438 | -0.483 |
| 11758337_s_at | -0.197 | -0.266 | ||
| Purine nucleoside phosphorylase | PNP | 11718400_a_at | 0.204 | 0.006 |
| Guanine deaminase | GDA | 11720157_at | 1.844 | 2.814* |
| 11751115_a_at | 2.695* | 3.779* | ||
| 11756342_s_at | 2.878* | 4.786* | ||
Significant fold change.
Guanine deaminase expression was increased with uric acid and p21 in seborrhoeic keratosis
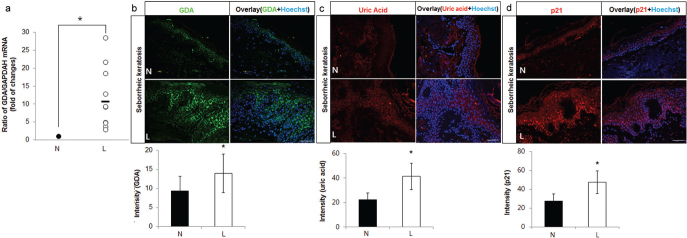
To validate the clinical association of GDA, real-time PCR and immunofluorescence staining were performed using skin specimens of SK and neighbouring non-lesional skin from 10 and 4 patients, respectively. Higher mRNA levels and stronger staining intensities against anti-GDA antibody were detected in the epidermis of SK (p < 0.05; Fig. 1a, b, respectively). Immunofluorescence studies revealed stronger staining intensities of guanine metabolic end-product using anti-uric acid antibody in the epidermis of SK with GDA upregulation (p < 0.05; Fig. 1c). Immunofluorescence staining using anti-p21 antibody for cellular senescence also showed stronger staining intensities in the epidermis of SK with GDA upregulation (p < 0.05; Fig. 1d).
Fig. 1.
Guanine deaminase (GDA) expression levels were increased with uric acid and p21 in seborrhoeic keratosis (SK). (a) Real-time PCR to determine the relative ratios of GDA mRNA in lesional skin (L) samples compared with non-lesional skin (N) samples obtained from 10 patients with seborrhoeic keratosis (SK). (b-d) Representative immunofluorescent staining using: (b) anti-GDA, (c) anti-uric acid, or (d) anti-p21 antibody, in lesional skin samples compared with that of non-lesional skin samples from 4 other patients with SK with increased GDA mRNA levels. Nuclei were counterstained with Hoechst 33258 (bar 0.05 mm). Intensities of immunofluorescence staining were measured using Wright Cell Imaging Facility (WCIF) ImageJ software. *p < 0.05 vs. control keratinocytes.
Repeated exposure to UV radiation increased guanine deaminase in keratinocytes and DNA damage, including anti-cyclobutane pyrimidine dimer formation undetected with guanine deaminase or reactive oxygen species alone
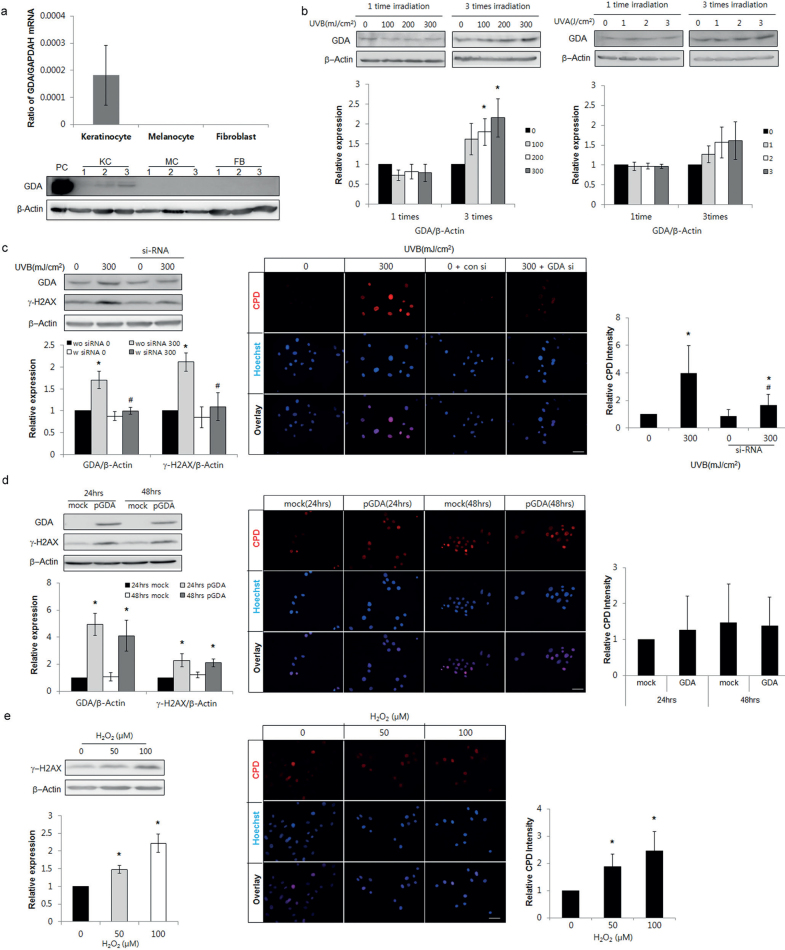
Based on the association between GDA and cellular senescence in SK (Fig. 1a, b, d), the role of GDA in skin photoageing or UV-induced cellular senescence was examined. Both GDA mRNA and protein were expressed in keratinocytes, but not in melanocytes or dermal fibroblasts (Fig. 2a). Single exposure to non-cytotoxic dosages of either UVA or UVB did not increase GDA levels, whereas repeated exposure to similar dosages increased the levels in keratinocytes (p < 0.05; Fig. 2b). Regardless of the type and irradiation levels of UV, GDA was not detected in melanocytes (Fig. S11). Based on these results, further studies were carried out in keratinocytes, using repeated UVB irradiation. To evaluate DNA damage, western blot analysis of γ-H2AX, a novel biomarker for DNA double-stranded breaks (30, 31), and immunofluorescence staining using anti-cyclobutane pyrimidine dimer (CPD) antibody were conducted (32). Repeated exposure to UV increased the levels of γ-H2AX protein and the number of CPD-positive cells, which were restored by GDA knockdown (p < 0.05; Fig. 2c). GDA overexpression also increased the levels of γ-H2AX protein; however, the overex-pression did not increase the number of CPD-positive cells (p < 0.05; Fig. 2d). Similar changes in γ-H2AX protein levels and CPD-positive cell number induced by GDA overexpression were detected by H2O2 treatment (p < 0.05; Fig. 2e).
Fig. 2.
Repeated exposure to ultraviolet (UV) radiation increased guanine deaminase (GDA) in keratinocytes and DNA damage including anti-cyclobutane pyrimidine dimer (CPD) formation, which was undetected with GDA or reactive oxygen species (ROS). (a) Real-time PCR and western blot analysis of the relative ratios of GDA mRNA and protein levels in primary cultured normal human skin keratinocytes (KCs), melanocytes (MC), and fibroblasts (FB) obtained from 3 different subjects. KC overexpressing GDA were used as positive controls (PC). (b) Western blot of the relative ratios of GDA protein expression in KCs exposed to UVB or UVA irradiation once or 3 times. (c, d) Western blot analysis for the relative ratios of γ-H2AX levels and immunofluorescent staining using anti-CPD antibody in KCs with or without UVB irradiation (300 mJ/cm2, 3 times) and GDA knockdown (c) or GDA overexpression (d). (e) Western blot analysis of the relative ratios of γ-H2AX protein and immunofluorescent staining using anti-CPD antibody in KCs with or without H2O2 treatment. β-Actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as internal controls for western blot analysis and real-time PCR, respectively. During immunofluorescent staining, nuclei were counter-stained with Hoechst 33258 (bar 0.05 mm) and staining intensities were measured using Wright Cell Imaging Facility (WCIF) ImageJ software. Data represent means ± standard deviation (SD) of 3-4 independent experiments. *p < 0.05 vs. control keratinocytes, #p < 0.05 vs. UV-exposed keratinocytes.
Guanine deaminase mediated UV-induced keratinocyte senescence via reactive oxygen species production
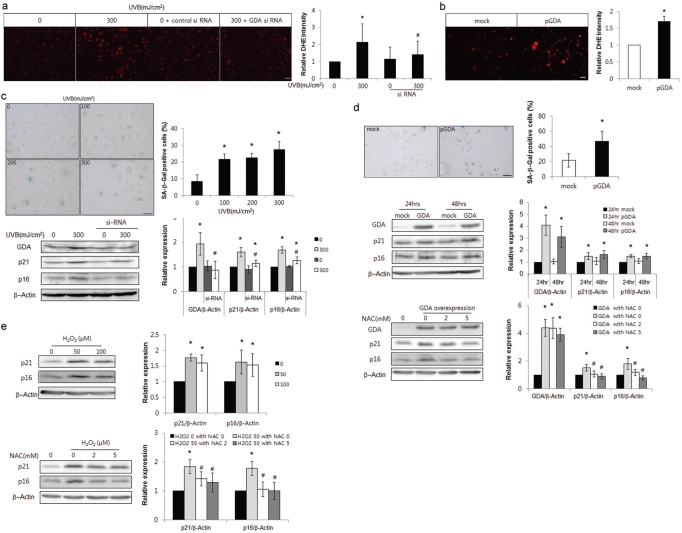
GDA catalysed UV-induced DNA damage (Fig. 2c); however, GDA-induced DNA damage differed from UV-induced changes, but was similar to ROS-induced oxidative damage (Fig. 2d, e). The current study investigated whether GDA was involved in UV-induced keratinocyte senescence by ROS generation. Repeated exposure to UV increased the number and intensity of dihydroethidium (DHE)-positive cells, which was reduced by GDA knockdown (p < 0.05; Fig. 3a). The number of DHE-stained keratinocytes was also increased by GDA overexpression (p < 0.05; Fig. 3b). The proportion of cells stained with senescence-associated β—Gal (SA β—Gal), a convenient biomarker of replicative senescence in keratinocytes (33, 34), and the levels of p16 and p21 proteins were increased in cultured keratinocytes under exposure to similar UV radiation, whereas the increased p16 and p21 levels upon repeated exposure to UV were reduced by GDA knockdown (p < 0.05; Fig. 3c). In addition to the proportion of SA β-Gal-positive cells, GDA overexpression increased the levels of p16 and p21, similar to H2O2 treatment, which were restored by N-acetyl-L-cysteine (NAC) pretreatment (p < 0.05; Fig. 3d, e, respectively).
Fig. 3.
Guanine deaminase (GDA) mediated ultraviolet (UV)-induced keratinocyte (KC) senescence via reactive oxygen species (ROS) production. (a, b) Dihydroethidium (DHE) staining in primary cultures of normal human skin keratinocytes with or without UVB irradiation (300 mJ/cm2, 3 times) and (a) GDA knockdown or (b) GDA overexpression. (c-e) Immunofluorescence staining with senescence-associated (SA) β-Gal and western blot analysis of the relative ratios of p16 and p21 protein levels in KCs with or without UVB irradiation and (c) GDA knockdown, (d) GDA overexpression and N-acetyl-L-cysteine (NAC) pretreatment, or (e) H2O2 treatment and NAC pretreatment. Staining intensities were measured using Wright Cell Imaging Facility (WCIF) ImageJ software. β-Actin was used as an internal control for western blot analysis. Data represent means±standard deviation (SD) of 3 or 4 independent experiments. *p < 0.05 vs. control KCs, #p < 0.05 vs. UV-exposed or H2O2-treated KCs.
Guanine deaminase metabolic products including uric acid enhanced keratinocyte senescence and generation of reactive oxygen species
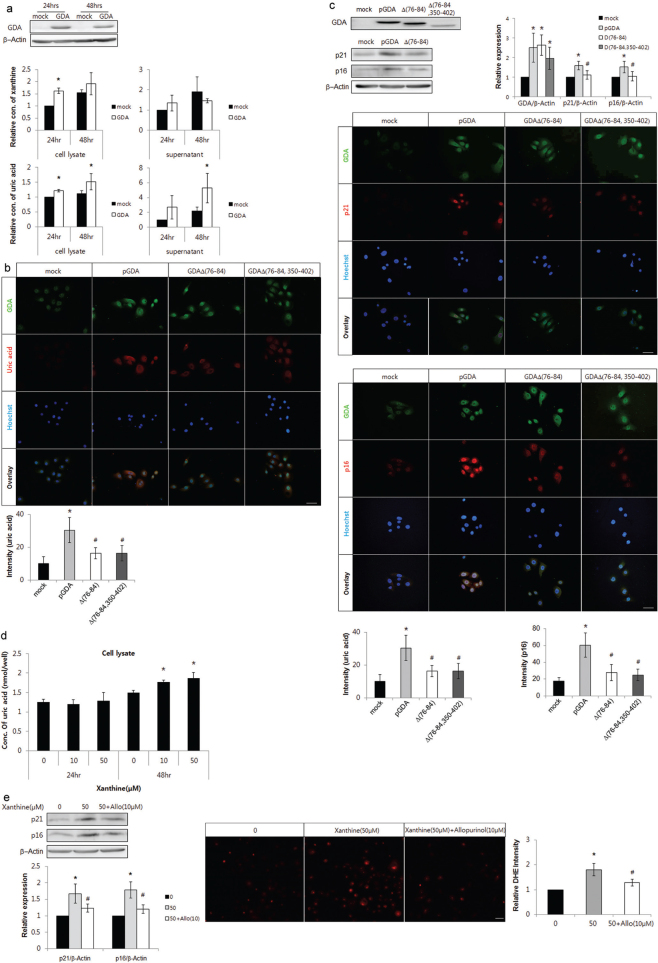
GDA overexpression increased the levels of xanthine and uric acid in keratinocytes (p < 0.05; Fig. 4a). Overexpression of loss-of-function mutants of GDA, GdAΔ(76–84) and GDAΔ(76-84 and 350-402), produced lower levels of uric acid compared with the wild-type overexpression (p < 0.05; Fig. 4b). GDAΔ(76–84) overexpression decreased the levels of p16 and p21 and the overexpression of both GDAΔ(76–84) and GDAΔ(76-84 and 350-402) reduced the staining intensities against anti-p16 or anti-p21 antibody compared with overexpressed wild-type, despite similar staining intensities against the anti-GDA antibody (p < 0.05; Fig. 4c). On the other hand, exogenous xanthine treatment increased uric acid levels in keratinocytes (p < 0.05; Fig. 4d). Exogenous xanthine also increased the levels of p21 and p16 proteins and ROS, which were reversed by allopurinol, an XO inhibitor (p < 0.05; Fig. 4e).
Fig. 4.
Guanine deaminase (GDA) metabolic products including uric acid enhanced keratinocyte senescence and reactive oxygen species (ROS) generation. (a) Western blot analysis showing the relative ratios of xanthine and uric acid levels in primary cultures of normal human skin keratinocytes with or without GDA overexpression. (b-c) Representative immunofluorescent staining using (b) anti-uric acid antibody, (c) western blot analysis showing the relative ratios of p16 and p21 and immunofluorescent staining using anti-GDA, anti-p16, or anti-p21 antibody in keratinocytes (KCs) overexpressing wild-type GDA or deletion mutants (Δ75-84 or Δ75-84 and 350-402) of GDA, in which GDA protein levels were identified by western blot analysis. (d-e) Assay for uric acid concentration (d) and western blot analysis showing the relative ratios of p16 and p21 protein levels and dihydroethidium (DHE) staining in KCs with or without xanthine and allopurinol (e). β-Actin was used as an internal control in western blot analysis. Nuclei were counter-stained with Hoechst 33258 (bar 0.05 mm) and staining intensities were measured using Wright Cell Imaging Facility (WCIF) ImageJ software. Data represent mean ± standard deviation of 3-4 independent experiments. *p < 0.05 vs. control KCs, #p < 0.05 vs. wild-type GDA-overexpressing KCs or KCs treated with xanthine.
Uric acid played the similar role as guanine deaminase in UV-induced keratinocyte senescence
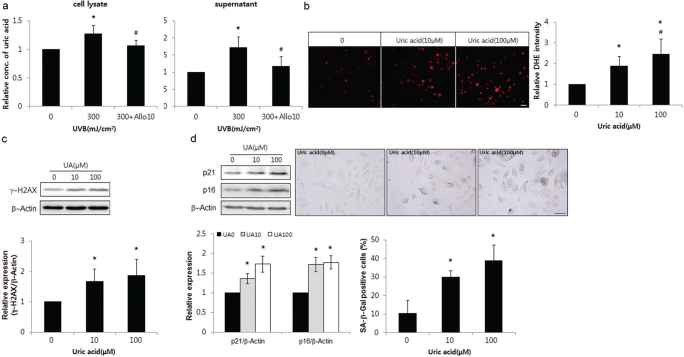
Uric acid levels were also increased in SK with GDA upregulation (Fig. 1c), as in GDA-overexpressing keratinocytes (Fig. 4a). However, uric acid is not the only product generated during GDA metabolism. It was necessary to examine the role of uric acid alone in UV-induced keratinocyte senescence. Repeated exposure to UV radiation increased the levels of uric acid in keratinocytes, which were reduced by allopurinol (p < 0.05; Fig. 5a). Exogenous uric acid increased ROS in a dose-dependent manner (p < 0.05; Fig. 5b). Uric acid treatment also increased the levels of γ-H2AX, p16, and p21 and the proportion of SA β-Gal-positive cells in a dose-dependent manner (p < 0.05; Fig. 5c, d, respectively).
Fig. 5.
Uric acid played the same role as guanine deaminase (GDA) in ultraviolet (UV)-induced keratinocyte (KC) senescence. (a) Assay for uric acid concentration in primary cultures of normal human skin keratinocytes with or without UVB irradiation and allopurinol. (b-d) dihydroethidium (DHE) staining (b) and western blot analysis showing the relative ratios of γ-H2AX (c), p16 and p21 levels and immunofluorescence staining with senescence-associated (SA) β-Gal (d) in KCs with or without exposure to different doses of uric acid. Staining intensities were measured using Wright Cell Imaging Facility (WCIF) ImageJ software. β-Actin was used as an internal control for western blot analysis. Data represent means ± standard deviation (SD) of 3-4 independent experiments. *p < 0.05 vs. control KCs, #p < 0.05 vs. UV-exposed keratinocytes.
DISCUSSION
Based on the analysis of DEGs associated with purine metabolism, GDA was selected as a candidate gene in SK (Table I). Higher levels of GDA mRNA and stronger GDA staining intensities with higher uric acid levels in patients diagnosed with SK (Fig. 1a–c) and enhanced levels of p21 protein, which is also a marker for cellular senescence along with p16 (35), in epidermal keratinocytes of SK with GDA upregulation (Fig. 1d) suggested the clinical significance of GDA and prompted investigation into the role of GDA in UV-induced keratinocyte senescence in SK.
Although the expression of GDA in human keratinocytes has yet to be elucidated, real-time PCR and western blot analysis revealed keratinocytes as the main source of GDA in the skin (Fig 2a). Repeated exposure to UV radiation (UVA or UVB) increased GDA levels, along with DNA damage, as shown by the increased γ-H2AX levels and CPD-positive cells, ROS production, and cellular senescence (Fig. 2b, c; Fig. 3a, c). These UV-induced changes were restored by GDA knockdown (Fig. 2c; Fig. 3a, c), suggesting that GDA was involved in UV-induced double-stranded breaks and CPD formation, ROS production, and cellular senescence in keratinocytes. However, the lack of significant effect of GDA overexpression on the number of CPD-positive keratinocytes (Fig. 2d) suggested that DNA damage induced by repeated UV irradiation differed from that of GDA upregulation. DNA may be damaged directly by UV radiation and indirectly by ROS production (2, 36). In this study, exposure to exogenous H2O2 did not alter the number of CPD-positive keratinocytes (Fig. 2e), although ROS were produced by both repeated UV and GDA overexpression (Fig. 3a, b, respectively). In fact, direct UV-induced DNA damage resulted in the formation of CPDs, unlike the indirect damage induced by ROS (37, 38). In addition, the increased levels of senescence protein induced by GDA overexpression and exogenous H2O2, and the senescence markers increased by GDA overexpression were restored by NAC (Fig. 3d, e), which establish an important role of oxidative stress in GDA-induced keratinocyte senescence. Overall, these results may suggest that upregulation of GDA by repeated UV irradiation as in SK exacerbated the DNA damage and keratinocyte senescence via ROS production.
GDA is an enzyme involved in conversion of guanine, which is formed by guanosine metabolism, into xanthine. Xanthine is further metabolized into uric acid by XO. This study also showed that the levels of xanthine and uric acid were increased by GDA overexpression in keratinocytes (Fig. 4a). Therefore, the foregoing GDA effect may be attributed to metabolic products generated by GDA enzymatic activity. The loss-of-function GDA mutant overexpression led to reduced production of uric acid, whereas treatment with exogenous xanthine increased the uric acid level, an end-product of guanine metabolism (Fig. 4b, d, respectively). Therefore, these conditions may be used to determine the role of metabolites generated by GDA. The contrasting effects of loss-of-function mutant overexpression and exogenous xanthine supplementation on keratinocyte senescence (Fig. 4c, e) indicate the role of metabolites including uric acid in GDA-induced keratinocyte senescence. Exogenous xanthine also increased ROS generation and senescence marker protein levels, which were restored by allopurinol (Fig. 4e), suggesting that ROS may be involved in keratinocyte senescence induced by GDA metabolites, as in the case of GDA. However, the effects induced by allopurinol may be attributed to both uric acid and XO, because allopurinol is an XO inhibitor and inhibits production of uric acid. XO donates electrons to molecular oxygen, and generates ROS (4, 12, 14). Although it is disputed whether uric acid acts like an oxidant or an anti-oxidant, the increase in ROS by the exogenous uric acid (Fig. 5b) suggests that uric acid exhibits an oxidant effect. Exposure to repeated UV radiation increased generation of uric acid (Fig. 5a) and the effect of uric acid on γ-H2AX and keratinocyte senescence was similar to that of GDA (Fig. 5c; Fig. 2d), indicating the role of GDA in UV-induced ROS production, γ-H2AX formation, and keratinocyte senescence via uric acid upregulation. Chronic sunlight exposure raised the levels of uric acid production (8). Although further studies investigating hyperuricaemia conditions, including gout, may elucidate the clinical significance of uric acid in skin photoageing, uric acid and keratinocyte senescence were increased in SK showing GDA upregulation (Fig. 1a–c). The findings may be interpreted as UV-induced keratinocyte senescence in SK mediated by uric acid.
In summary, GDA upregulation in SK mediates UV-induced keratinocyte senescence via production of uric acid as an end-product to generate ROS, resulting in DNA damage.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. NRF-2017R1A2A2A09069507, NRF-2018R1A5A2023127).
Footnotes
REFERENCES
- 1.Cavinato M, Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp Gerontol 2017; 94: 78–82. [DOI] [PubMed] [Google Scholar]
- 2.Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic Biol Med 2017; 107: 110–124. [DOI] [PubMed] [Google Scholar]
- 3.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des 2005; 11: 4145–4151. [DOI] [PubMed] [Google Scholar]
- 4.Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid Med Cell Longev 2016; 2016: 3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009; 27: 120–139. [DOI] [PubMed] [Google Scholar]
- 6.Dąbrowska N, Wiczkowski A. Analytics of oxidative stress markers in the early diagnosis of oxygen DNA damage. Adv Clin Exp Med 2017; 26: 155–166. [DOI] [PubMed] [Google Scholar]
- 7.Menck CF, Munford V. DNA repair diseases: What do they tell us about cancer and aging? Genet Mol Biol 2014; 37(1 Suppl): 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randhawa M, Sangar V, Tucker-Samaras S, Southall M. Metabolic signature of sun exposed skin suggests catabolic pathway overweighs anabolic pathway. PLoS One 2014; 9: e90367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules 2015; 5: 545–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman A, Herman AP. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol Physiol 2013; 26: 8–14. [DOI] [PubMed] [Google Scholar]
- 11.Puddu P, Puddu GM, Cravero E, Vizioli L, Muscari A. Relationships among hyperuricemia, endothelial dysfunction and cardiovascular disease: molecular mechanisms and clinical implications. J Cardiol 2012; 59: 235–242. [DOI] [PubMed] [Google Scholar]
- 12.George J, Struthers AD. The role of urate and xanthine oxidase inhibitors in cardiovascular disease. Cardiovasc Ther 2008; 26: 59–64. [DOI] [PubMed] [Google Scholar]
- 13.Okafor ON, Farrington K, Gorog DA. Allopurinol as a therapeutic option in cardiovascular disease. Pharmacol Ther 2017; 172: 139–150. [DOI] [PubMed] [Google Scholar]
- 14.Bredemeier M, Lopes LM, Eisenreich MA, Hickmann S, Bongiorno GK, d’Avila R, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2018; 18: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexandrova AK, Smolyannikova VA, Filatova VA, Alexandrova OK. Protein p16 role in seborrheic keratosis. Our Dermatol Online 2016; 7: 377–380. [Google Scholar]
- 16.Li Y, Wang Y, Zhang W, Jiang L, Zhou W, Liu Z, et al. Overexpression of amyloid precursor protein promotes the onset of seborrhoeic keratosis and is related to skin ageing. Acta Derm Venereol 2018; 98: 594–600. [DOI] [PubMed] [Google Scholar]
- 17.Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, et al. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol 2002; 22: 5157–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeatman JM, Kilkenny M, Marks R. The prevalence of seborrhoeic keratoses in an Australian population: does exposure to sunlight play a part in their frequency? Br J Dermatol 1997; 137: 411–414. [PubMed] [Google Scholar]
- 19.Kwon OS, Hwang EJ, Bae JH, Park HE, Lee JC, Youn JI, et al. Seborrheic keratosis in the Korean males: causative role of sunlight. Photodermatol Photoimmunol Photomed 2003; 19: 73–80. [DOI] [PubMed] [Google Scholar]
- 20.Wollina U. Seborrheic keratoses - the most common benign skin tumor of humans. Clinical presentation and an update on pathogenesis and treatment options. Open Access Maced J Med Sci 2018; 6: 2270–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wollina U. Recent advances in managing and understanding seborrheic keratosis. F1000 Res 2019; 28: doi: 10.12688/ f1000research.18983.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilchrest BA, Vrabel MA, Flynn E, Szabo G. Selective cultivation of human melanocytes from newborn and adult epidermis. J Invest Dermatol 1984: 83: 370–376. [DOI] [PubMed] [Google Scholar]
- 23.Keira SM, Ferreira LM, Gragnani A, Duarte IdS, Santos IANd. Experimental model for fibroblast culture. Acta Cir Bras 2004; 19 (Suppl 1): 11–16. [Google Scholar]
- 24.Wang F, Zieman A, Coulombe PA. Skin keratins. Methods Enzymol 2016; 568: 303–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grichnik JM, Ali WN, Burch JA, Byers JD, Garcia CA, Clark RE, et al. KIT Expression reveals a population of precursor melanocytes in human skin. J Invest Dermatol 1996; 106: 967–971. [DOI] [PubMed] [Google Scholar]
- 26.Kippenberger S, Loitsch S, Solano F, Bernd A, Kaufmann R. Quantification of tyrosinase, TRP-1, and TRP-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR - regulation by steroid hormones. J Invest Dermatol 1998; 110: 364–367. [DOI] [PubMed] [Google Scholar]
- 27.OECD . Guidelines for the Testing of Chemicals, In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. Paris: OECD publishing; 2015. [Google Scholar]
- 28.Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci 2004; 7: 145–152. [DOI] [PubMed] [Google Scholar]
- 29.Thum T, Fraccarollo D, Thum S, Schultheiss M, Daiber A, Wenzel P, et al. Differential effects of organic nitrates on endothelial progenitor cells are determined by oxidative stress. Arterioscler Thromb Vasc Biol 2007; 27: 748–754. [DOI] [PubMed] [Google Scholar]
- 30.Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo 2008; 22: 305–309. [PubMed] [Google Scholar]
- 31.Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol 2012; 920: 613–626. [DOI] [PubMed] [Google Scholar]
- 32.Cadet J, Douki T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem Photobiol Sci 2018; 17: 1816–1841. [DOI] [PubMed] [Google Scholar]
- 33.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92: 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, et al. Quantitative identification of senescent cells in aging and disease. Aging Cell 2017; 16: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandler H, Peters G. Stressing the cell cycle in senescence and aging. Curr Opin Cell Biol 2013; 25: 765–771. [DOI] [PubMed] [Google Scholar]
- 36.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, et al. UV-induced skin damage. Toxicology 2003; 189: 21–39. [DOI] [PubMed] [Google Scholar]
- 37.Moriwaki S, Takahashi Y. Photoaging and DNA repair. J Dermatol Sci 2008; 50: 169–176. [DOI] [PubMed] [Google Scholar]
- 38.Chang H, Oehrl W, Elsner P, Thiele JJ. The role of H2O2 as a mediator of UVB-induced apoptosis in keratinocytes. Free Radic Res 2003; 37: 655–663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.