Abstract
Background
Hospital-acquired venous thromboembolism (VTE) is one of the leading preventable causes of in-hospital mortality. However, its risk assessment in medically ill inpatients is complicated due to the patients’ heterogeneity and complexity of currently available risk assessment models (RAMs). The simplified Geneva score provides simplicity but has not yet been prospectively validated. Immobility is an important predictor for VTE in RAMs, but its definition is inconsistent and based on subjective assessment by nurses or physicians. In this study, we aim to prospectively validate the simplified Geneva score and to examine the predictive performance of a novel and objective definition of in-hospital immobilization using accelerometry.
Methods and analysis
RISE is a multicenter prospective cohort study. The goal is to recruit 1350 adult inpatients admitted for medical illness in three Swiss tertiary care hospitals. We collect data on demographics, comorbidities, VTE risk and thromboprophylaxis. Mobility from admission to discharge is objectively measured using a wrist-worn accelerometer. Participants are followed for 90 days for the occurrence of symptomatic VTE (primary outcome). Secondary outcomes are the occurrence of clinically relevant bleeding, and mortality. The evolution of autonomy in the activities of daily living, the length of stay, and the occurrence of readmission are also recorded. Time-dependent area under the curve, sensitivity, specificity, and positive and negative predictive values are calculated for each RAM (i.e. the simplified and original Geneva score, Padua, and IMPROVE score) with and without the objective mobility measures to assess their accuracy in predicting hospital-acquired VTE at 90 days.
Ethics and expected impact
The ethics committee approved the protocol and the study was registered on ClinicalTrials.gov as NCT04439383. RISE has the potential to optimize VTE risk stratification, and thus to improve the quality of care of medically hospitalized patients.
Introduction
Hospital acquired venous thromboembolism (VTE), defined as pulmonary embolism (PE) or deep vein thrombosis (DVT), is one of the leading preventable causes of in-hospital mortality [1]. About 75% of all hospital-acquired VTE occur in hospitalized medical patients [2]. So much so that hospitalization for an acute medical illness is per se a risk factor for VTE [3].
Randomized-controlled trials (RCTs) conducted 15 to 20 years ago showed significant reductions in VTE with the use of heparin compared to placebo in selected medical inpatients [4–6]. However, pharmacological VTE prophylaxis increases the risk of bleeding [4]. Guidelines recommend providing pharmacological thromboprophylaxis (TPX) to hospitalized medical patients only if they are at increased risk of VTE during their hospital stay [7,8].
Assessing thromboembolic risk in medical inpatients is currently done empirically or using risk assessment models (RAMs) incorporating an array of demographic and clinical patient characteristics. Available validated RAMs, such as the original Geneva score [9], the Padua [10] or the IMPROVE score [11,12], have various shortcomings including a suboptimal sensitivity to identify high VTE risk patients (ranging from 73% to 90% among any of the RAMs) [13]. Furthermore, they have a large number of items score, some of which are not available at admission (e.g. ICU stay) [12]. The simplified Geneva score has recently been developed as a simpler and more usable RAM [13]. Prospective validation is needed before it can be implemented in everyday clinical practice. To that end, the first aim of this study is to externally validate this novel RAM.
Being an important risk factor for hospital-acquired VTE, immobilization is included in existing RAMs [9,10,12,14]. However, due to the lack of a standardized definition, its usefulness is limited [13,15]. In everyday practice, the degree of immobilization is estimated subjectively, based either on the physician’s own perception or on nursing assessment [16–21], with a questionable accuracy [21]. Patients and hospital staff also interpret physicians’ orders of mobilization with a substantial variation; for example ambulation orders “out of bed to chair” can lead to a daily step count of 0 to 1800 (0–1.3 km) [21].
Recent evidence suggests that objective measures of mobility using a wrist-worn tri-axis accelerometer improves the accuracy of mobility assessment in hospitalized patients [22–26]. Whether objective mobility measures could predict hospital-acquired VTE, and whether incorporation of these measures into VTE RAMs could improve their predictive ability has yet to be examined. Therefore, we aim to establish the predictive performance of a novel and objective definition of in-hospital immobilization using accelerometry.
Overall, risk assessment and prevention of hospital-acquired VTE remains a major challenge for hospital physicians, and expert societies have called for further research on this topic [8]. To that end, this prospective cohort study aims to improve VTE prevention in hospitalized medical patients.
Objectives and hypotheses
The primary objective is to prospectively validate the simplified Geneva score and to compare its prognostic performance with previously validated RAMs (i.e., the original Geneva, Padua, and IMPROVE scores). Therefore, we hypothesize that the novel, easier-to-use simplified Geneva score will be able to accurately detect medical inpatients at risk of hospital-acquired VTE and that it will be at least as accurate as previously validated RAMs.
Our second objective is to develop a new, objective, definition of inpatient immobilization using accelerometry and to compare its performance in predicting hospital-acquired VTE with that of the subjective measurement. Accordingly, we hypothesize that objective, accelerometry-assessed mobility will be more accurate in predicting the risk of hospital-acquired VTE than subjective physician perception and that its incorporation into the simplified Geneva score will improve its prognostic performance.
Materials and methods
Study design and setting
RISE (RIsk Stratification for hospital-acquired venous thromboEmbolism in medical patients) is a multicenter prospective cohort study including consecutive consenting adult patients admitted to the general internal medicine wards of three Swiss university hospitals (i.e., the Lausanne, Bern, and Geneva). The recruitment started June 22, 2020 and we expect that the last participant will finish the study in Spring 2022.
Patient selection
Consecutive adult patients with acute illness admitted for more than 24 hours to a general internal medicine ward are eligible. Exclusion criteria are the need for therapeutic anticoagulation (e.g., due to atrial fibrillation), estimated life expectancy of less than 30 days, insufficient proficiency of the German or French language, or prior enrolment in the cohort.
Importantly, patients with mental illness or cognitive impairment are not excluded from the study. Indeed, these disorders are frequently encountered in older patients, whose risk of VTE and immobilization are particularly high [27,28].
Ethical aspects
This study is conducted in accordance with the Declaration of Helsinki, the ICH-GCP guidelines, and all applicable legal/regulatory requirements. The Ethics Committee of the Canton of Berne (Kantonale Ethikkommission für die Forschung, Kanton Bern) authorized the RISE study on (Reference number: 2020–00606).
Baseline data collection and VTE risk assessment
For all eligible and consenting participants, study personnel prospectively collect demographic data (sex, year of birth, body weight, height, setting prior to admission), information on comorbidities (including all items of the Charlson Comorbidity Index [29,30]), medications at admission with a potential antithrombotic effect (aspirin, other antiplatelet therapy, nonsteroidal anti-inflammatory drugs), potential contraindications to pharmacological VTE prophylaxis (known hypersensitivity to heparin and history of heparin induced thrombocytopenia), and laboratory variables (thrombocytopenia, spontaneous international normalized ratio > 2 (INR), kidney failure and anemia) known to affect pharmacological TPX provision (Table 1).
Table 1. Baseline data collection.
|
Demographic characteristics Sex, year of birth, date of admission, date of study inclusion, body weight (kg), height (cm), setting prior to admission |
|
Items of the risk assessment models Previous VTE, hypercoagulable state/thrombophilia, active cancer, history of cancer within last 5 years, myeloproliferative syndrome, cardiac failure, respiratory failure, acute infection, rheumatologic disorder, immobilization (bed rest with bathroom privileges) ≥72 hours, estimated immobilization >7d, stroke (and date of event), myocardial infarction (and date of event), recent (≤1 month) trauma or surgery (and date of event), ongoing hormonal treatment, lower extremity paralysis/paresis, stay in the intensive care unit / intermediate care unit, nephrotic syndrome, recent travel (>6 hours), chronic venous insufficiency, pregnancy, dehydration |
|
Comorbidities [29] History of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, localized solid tumor, metastatic solid tumor, leukemia, lymphoma, AIDS, gastroduodenal ulcer, history of bleeding, inflammatory bowel disease; number of comorbidities |
|
Contraindications to pharmacological VTE prophylaxis Known hypersensitivity to heparin, history of heparin induced thrombocytopenia, liver failure, active non-major or major bleeding (and date of event), hemorrhagic transformation of acute ischemic stroke (and date of event) |
|
Laboratory findings Platelet count, international normalized ratio, serum creatinine, hemoglobin |
|
Medications at admission aspirin, other antiplatelet therapy (clopidogrel, prasugrel, ticagrelor), nonsteroidal anti-inflammatory drugs |
|
Autonomy Modified Barthel Index; Braden scale; location of eating, eliminating urine or stool, and washing |
Abbreviation: VTE, venous thromboembolism; d, days.
At baseline, all items of the simplified and original Geneva score, the IMPROVE score, and the Padua score are collected (Table 2), and the score for each RAM is calculated in order to categorize study participants into risk groups for VTE. All demographic, clinical, and laboratory data are collected from electronic health records (EHR) or at the patient’s bedside (from the patient and/or nurse in charge) by trained study personnel.
Table 2. VTE risk assessment models for risk stratification in hospitalized medical patients.
| Points | ||||
|---|---|---|---|---|
| Score Items | Simplified Geneva Score [13] | Original Geneva Score [9] | Padua Score [10] | IMPROVE Score [12,14] |
| Previous VTE | 3 | 2 | 3 | 3 |
| Hypercoagulable state a | 2 | 2 | 3 | 2 |
| Cancer b [9,10,31] | 2 | 2 | 3 | 2 |
| Myeloproliferative syndrome c | 2 | |||
| Cardiac failure d | 2 | 2 | 1 | |
| Respiratory failure e | 2 | |||
| Acute infection | 2 | 2 | 1 | |
| Acute rheumatologic disorder f | 2 | |||
| Immobilization | 2g | 1g | 1h | |
| Reduced mobility | 3i | |||
| Lower limb paralysis or paresis [31] | 2 | |||
| Age >60 years | 1 | 1 | 1 | |
| Age >70 years | 1 | |||
| Body mass index ≥30kg/m2 | 1 | 1 | 1 | |
| Recent stroke (≤ 3 months) [9] | 1 | 2 | 1 | |
| Recent myocardial infarction (≤ 1 month) [9] | 2 | |||
| Nephrotic syndrome | 2 | |||
| Hormonal treatment j | 1 | 1 | ||
| Travel within last 7 days (>6 hours) | 1 | |||
| Chronic venous insufficiency | 1 | |||
| Pregnancy | 1 | |||
| Dehydration | 1 | |||
| Recent trauma or surgery (<1 month) | 2 | |||
| Stay in intensive or coronary care unit | 1 | |||
| Cut-offs [8–10,12,13] | ||||
| Low VTE risk | 0–2 | 0–2 | 0–3 | 0–1 |
| High VTE risk | ≥3 | ≥3 | ≥4 | ≥2 |
Abbreviations: VTE, venous thromboembolism.
a anti-thrombin deficiency, APC resistance, protein C or protein S deficiency, factor V Leiden, G20210A prothrombin-mutation, antiphospholipid syndrome.
b metastatic cancer, or cancer treated with radiotherapy/chemotherapy/immunotherapy, or cancer surgery within last 6 months (also relates to myeloma or myelodysplastic syndrome), excluding non-melanoma skin cancer.
c essential thrombocytopenia, polycythemia vera, primary myelofibrosis, chronic myeloic leukemia.
d acute or chronic heart failure of any cause with a preserved or reduced ejection fraction.
e acute or chronic need for supplemental oxygen.
f rheumatoid arthritis, vasculitis, or connective tissue disease.
g immobilization was defined as complete bedrest or inability to walk for >30min per day for ≥3 days [9].
h immobilization was considered if the patient was being confined to bed or chair with or without bathroom privileges for ≥7 days immediately prior to and during hospital admission [31].
i reduced mobility was defined as anticipated bed rest with bathroom privileges for ≥3 days [10].
j contraception, post-menopausal hormone therapy, antitumor therapy containing estrogen, ethinylestradion, estradiol.
Treatments during hospital stay affecting the risk of hospital-acquired VTE or bleeding are recorded from the EHR, including type and duration of the pharmacological (low-molecular-weight heparin, unfractionated heparin, fondaparinux, other) and mechanical TPX (lower extremity compression stockings/bandages, intermittent pneumatic compression devices). In case therapeutic anticoagulation is initiated, the start date and the indication are documented. Furthermore, information on red blood cell transfusions, central venous catheter, and surgical procedures during hospitalization are recorded [32,33].
Autonomy in the activities of daily living (ADL) prior to hospitalization is assessed at admission using the modified Barthel Index [34]. For patients with cognitive impairment or confusion, the level of ADL autonomy is assessed by interviewing their relatives or caregivers. The modified Barthel Index has been reported as being the most accurate scale to assess activities of daily living (ADL) and has thus been widely used as a measure of autonomy [34]. The patient’s ability to perform different ADLs is rated as follows: fully independent, with minimal or moderate help, attempts task but putting him/herself at risk, or unable to perform. The maximum point score is 100; a total modified Barthel Index point score of 0–20 suggests total, 21–60 severe, 61–90 moderate, and 91–99 slight dependence. A point score of 100 indicates that the patient is independent of assistance from others.
Mobility assessment
Objective measurement of mobility is done with a wrist-worn tri-axis accelerometer (GENEActiv Original, ActivInsights Ltd, UK, https://www.activinsights.com/actigraphy/geneactiv-original/), parametrized at 50 Hz. The accelerometer is provided to patients immediately after inclusion. Patients are asked to wear the device continuously (day and night, including while showering) until hospital discharge or transfer to another department (e.g., intensive care, surgery unit, etc.). Accelerometry data is extracted and analyzed using the GGIR package for R (version 1.11 or later) [35]. A valid day of mobility measurement is defined as at least 10 hours of wearing the accelerometer during daytime, and at least 24 hours of valid data is required for analysis [36,37]. In the analysis, we consider the following measurements: minutes per day in different types of activities, no activity or sleep; total minutes during a day spent active/inactive; mean acceleration in miliG/vector. Physical activity raw data is further processed using the Verisense Step Count Algorithm for GGIR (https://github.com/ShimmerEngineering/Verisense-Toolbox/tree/master/Verisense_step_algorithm) and the open source GENEAclassify R-package (https://cran.r-project.org/web/packages/GENEAclassify/GENEAclassify.pdf), in order to obtain the number of steps taken per day. We estimate the percentage of time of a patient’s mobility, using a cut-off of <4 steps and ≥4 steps taken per minute to define periods of immobilization and mobilization, respectively, as previously reported in a study of medical inpatients [24].
For the subjective mobility measurement, we consider the patient’s, the nurse’s and the hospital treating physician’s mobility estimates. Patients are asked about their ability to walk, i.e. whether they are able to walk independently, with assistance from one or two people, with or without mobility aids, or if they are unable to walk at all. Furthermore, they are asked about the location of eating (bed, edge of bed, table), urinating and defecating (bed, chair next to bed, bathroom), and washing (bed, chair in front of sink, shower).
Nurses’ assessment of mobility is performed through items of the Braden scale [38]. The Braden scale has been developed and validated to identify hospitalized patients at risk of pressure sores. This scoring system includes six items with a total score ranging from 0 to 23. Patients with a score of nine or less are categorized as having a very high risk. Two items of this score are specifically dedicated to physical activity: “degree of physical activity” (patient is bedfast, chairfast, walks occasionally, walks often) and “ability to change and control position” (patient is completely immobile, very limited, slightly limited, or has no limitation in mobility). Therefore, nurses indirectly assess the mobility of patient.
On the second day of hospitalization, the physician is asked whether a corresponding patient fulfills the different immobilization criteria as defined in each RAM (Table 2). The physician is also asked to subjectively estimate the patient’s ability to ambulate in standardized terms (i.e., no ambulation, out of bed to chair, out of bed to ambulate once daily, twice or 3 times daily, or ambulate ad libitum) [21]. Hospital treating physicians are contacted on the second day of hospitalization rather than on admission because the decision to prescribe TPX is most likely already made and thus unlikely to be influenced by questions on the patient’s mobility status. Finally, information on physical therapy orders for mobilization are collected from EHR. The prescriptions of specific ambulation regimens or physical therapy are left at the discretion of the hospital treating physician.
Primary and secondary outcomes
The primary outcome is symptomatic objectively confirmed fatal and non-fatal VTE, defined as distal or proximal DVT or PE up to 90 days after hospital admission. As described in previous studies, the objective diagnostic of PE is based on available radiology (CT pulmonary angiography, pulmonary angiography, or ventilation-perfusion lung scan) or autopsy reports [39–42] (S4 File). Likewise, the objective diagnostic of DVT is based on compression ultrasonography or contrast venography [39,43]. As only symptomatic VTE events are recorded, patient need to present symptoms such as dyspnea, couch, acute chest pain or syncope for PE, and unilateral pain or swelling or erythema for DVT [44]. VTE events diagnosed during the first 48 hours of hospitalization are not considered as a primary outcome for this study in order to rule out pre-existing VTE that occurred prior to hospital admission [45]. In line with previous studies on VTE prophylaxis and given similarities in some risk factors and outcomes [31,46], symptomatic upper extremity DVT is also considered as a study outcome, although its incidence is expected to be low [46].
Secondary medical outcomes are the occurrence of major bleeding, clinically relevant non-major bleeding, and all-cause mortality during the follow-up period. As described in two previous Swiss VTE studies, SAFE-SSPE [40] and SWITCO65+ [43,47], major bleeding is defined as fatal bleeding, symptomatic bleeding at critical sites, bleeding with a reduction of hemoglobin of at least 20 g/L or bleeding leading to transfusion of 2 or more units of packed red blood cells [48]. Likewise, clinically relevant non-major bleeding is defined as overt bleeding that does not meet criteria for major bleeding but is associated with a medical intervention, unscheduled physician contact (visit or telephone call), or pain, or impairment of activities of daily life [49]. All-cause mortality is categorized as PE-related, bleeding-related, due to another cause or due to an undetermined cause according to already published criteria [40,50–53]. In addition, the evolution of the autonomy in the ADL during the follow-up period, using the modified Barthel Index, the length of hospital stay and rehospitalization for an acute medical illness up to 90 days are also considered as secondary outcomes.
All medical outcome events (hospital-acquired VTE, major and clinically relevant non-major bleeding, and death) are reviewed and adjudicated by a committee of three independent clinical experts. The final adjudication is based on the committee’s full consensus.
Study procedures
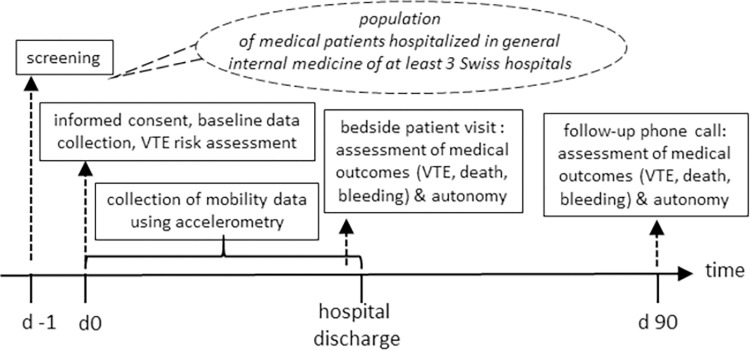
Study investigators screen consecutive patients newly admitted to general internal medicine of participating clinics for eligibility on weekdays (Figs 1 and 2). Eligible patients are informed about the study aims/procedures and asked to provide written informed consent. For patients who are unable to give informed consent due to mental illness or cognitive impairment, permission to participate in the study is obtained from a legally authorized representative. Participating patients are equipped with an accelerometer for collection of mobility data throughout the hospital stay, and trained study personnel collect patient baseline data on the day of enrolment (Fig 1).
Fig 1. Timeline of patient enrolment and schedule of data collection.
Adapted from the SPIRIT statement [54]. Abbreviations: d, day; RAM, risk assessment model; VTE, venous thromboembolism.
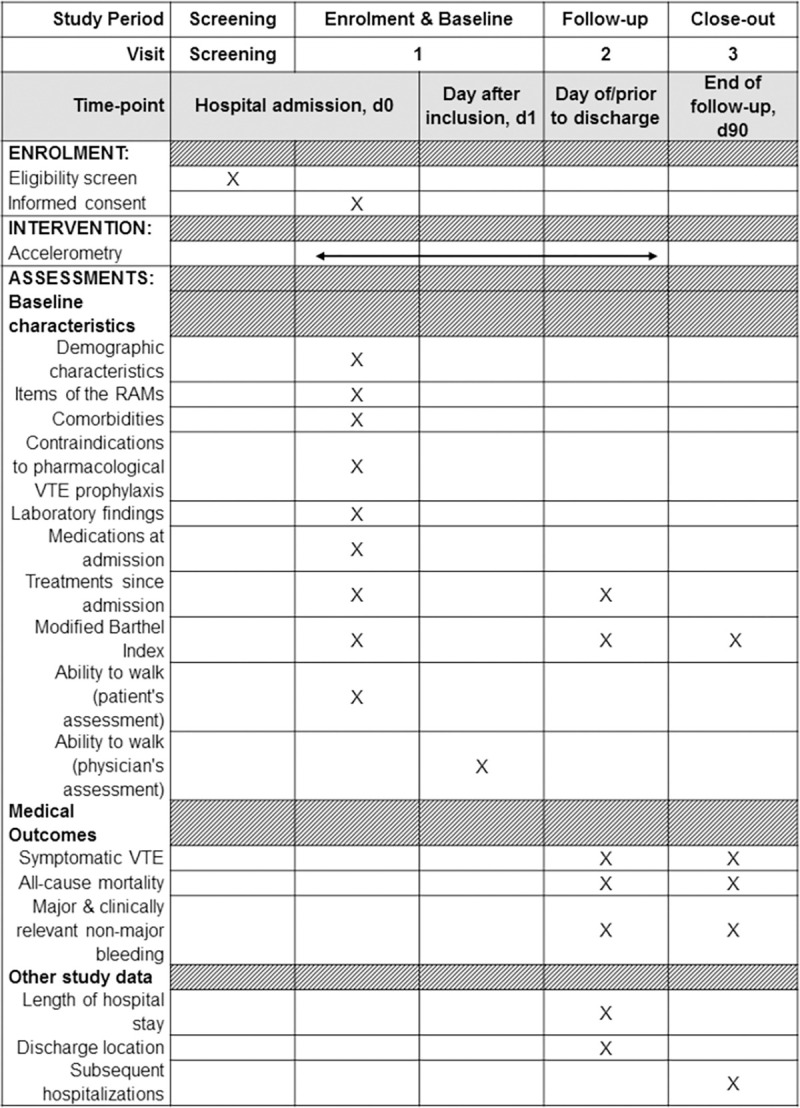
Fig 2. Study organization and follow-up.
A follow-up visit is conducted prior to discharge to collect information on discharge location, information on treatments since admission with a focus on pharmacological and mechanical TPX, patient autonomy, and clinical outcomes (Fig 1). Study investigators collect the accelerometer and upload accelerometry data to the database using the relevant software.
A follow-up phone call is performed at day 90 ± 5 after study inclusion by trained study personnel. In case of unavailability of the patient, their designated contact person or general practitioner is called instead. Information on outcomes is assessed. As initiation of therapeutic anticoagulation during follow-up affects the outcomes, we also collect information about the potential introduction of therapeutic anticoagulation since discharge (Fig 1). In case of the occurrence of a medical outcome event, study personnel collect all available documentation (e.g. medical reports, laboratory and imaging data) related to the event for the adjudication process.
Sample size calculation
We performed the sample size calculation for the primary objective, i.e. the validation of the simplified Geneva score for the prediction of hospital-acquired VTE. Based on a 2010 Swiss cohort study [13], we assume that that 67% of patients are categorized as high risk and 33% as low risk based on the simplified Geneva score, and a 90-day incidence of hospital-acquired VTE with adequate thromboprophylaxis of 2.8% and 0.6%, in high risk and low risk patients, respectively, according to the simplified Geneva score [13]. Therefore, we will need 1308 patients to detect an absolute risk difference of 2.2% between high and low risk patients, with a power of 80% at a 2-sided alpha of 0.05. The numbers stated above correspond to a relative risk of 4.7, a sensitivity of 90%, and a specificity of 34% [13]. This sample size provides sufficient precision for the validation of the simplified Geneva score. Assuming an area under the curve (AUC) of 0.75, the normal-approximation 95% confidence interval (CI) ranges from 0.64 to 0.86. For sensitivity and specificity, the 95% Wilson CIs range from 72% to 96% and from 31% to 36%, respectively. We will recruit a total sample of 1350 patients to account for potential dropouts, which we expect to be few given the low follow-up burden [10].
For the second objective, namely the assessment of objective mobility measurement to predict the risk of VTE, the same measures of association and prognostic accuracy as described above are estimated. The sample size of 1350 patients provides comparable precision as stated above.
Planned statistical analyses
Once the 1350 patients will have completed the study, the following statistical analyses will be conducted. First, time to event analyses with competing risk methods will be used to assess the prognostic performance of the simplified Geneva score and the other RAMs (Table 2) and their association with hospital-acquired VTE, with non-VTE death representing the competing risk. We will use a subdistribution hazard model of Fine and Gray [55] to assess the association of the simplified Geneva score and the other RAMs with VTE, calculating subhazard ratios with 95% CIs. These analyses will be adjusted for the use of TPX and study site. Cumulative incidences of hospital-acquired VTE in low- and high-risk score patients will be assessed and graphically presented to assess calibration and compare different RAMs. The time-dependent AUC as well as sensitivity, specificity, and positive and negative predictive values will be calculated for each RAM to assess their accuracy to predict hospital-acquired VTE at 90 days using time-dependent receiver operating characteristic (ROC)-curve analysis, taking into account censored data and competing events.
Additionally, as some patients are treated with TPX, we will perform separate sensitivity analyses in patients with and without TPX. It is possible that during follow-up, a small number of patients will be started on therapeutic dose anticoagulation for reasons other than VTE (e.g., new onset atrial fibrillation); data of these patients will be censored in the main analysis.
Secondary time-to-event outcomes (major and clinically relevant non-major bleedings) will also be evaluated using competing risk regression. For all-cause mortality, we will use an ordinary Cox regression, for length of stay an accelerated failure time model. Binary outcomes (in-hospital VTE and readmission) will be evaluated using logistic regression. The Barthel-index will be assessed using linear regression. All models will be adjusted for the use of TPX and study site.
We will examine the association between subjective (physician’s perception) and objective (accelerometry-measured) mobility levels, as a continuous measure as well as divided into quartiles, and 90-day cumulative incidences of HA-VTE using competing risk regression (accounting for non-VTE related death as a competing event), unadjusted and adjusted for TPX and study site [37]. To define an optimal cutoff for objective immobility, we will assess sensitivity and specificity at different mobility levels using time-dependent ROC-curve analysis accounting for censored data and competing events. To compare the predictive performance of the simplified Geneva score using the standard subjectively-assessed definition of immobilization (i.e. physician perception, Table 2) versus using objective accelerometry-assessed mobility measures, we will use likelihood ratio tests and/or the Akaike information criterion (AIC) as well as the c-statistics in competing risk models; mobility measures will be used as single covariates, as well as incorporated in the simplified Geneva score. We will assess the net reclassification index to assess improvement in risk prediction if using the accelerometry-based mobility measure instead of the subjective immobility assessment for the simplified Geneva score.
Expected impact and strengths
RISE will provide the first prospective head-to-head comparison of validated VTE RAMs. Previous studies suggest that pharmacological VTE prophylaxis is inappropriately used in medical patients: an international cross-sectional study reported that only about 40% of medical patients at high risk of VTE received appropriate prophylaxis, while on the other hand, it was inappropriately prescribed in half of all low risk patients [9,56,57]. Multiple reasons have been postulated for inadequate use of VTE prophylaxis in hospitalized medical patients, including the challenge to assess thromboembolic risk [58]. Our results will provide a clearer guidance for physicians about optimal VTE risk assessment and thus have the potential to facilitate and improve VTE prevention and reduce hospital-acquired VTE and associated deaths in medical inpatients. The simplified identification of patients who may really benefit from TPX may thus not only result in improved quality of care, but also in cost-savings.
A prospective cohort design is the optimal study design and provides the highest quality data to meet the aim of this study. This design will also correct the inherent limitations of the already published retrospective head-to-head VTE RAM comparison. A longitudinal study design is necessary to investigate prognostic measures, and prospective data collection allows complete and standardized measurements of exposures prior to the occurrence of any outcomes; also, objective mobility measurement is only possible in a prospective manner. Moreover, given the broad eligibility criteria of RISE, the results of this study will be generalizable to the population of hospitalized medical patients at risk of hospital-acquired VTE, i.e. those without intake of therapeutic anticoagulation.
As reported in previous studies, the subjective evaluation of patient’s mobility is complex and unreliable [21,59,60]. In recent years, accelerometry-assessed mobility has become recognized as a valid and precise method to assess the mobility of inpatients [22–25]. A randomized Danish trial studying the effect of physical therapy on patient-reported outcomes after acute PE described several limitations using the incremental shuttle walk test as an objective mobility measure [59,61]. To this day, objective measures of mobility using accelerometry have only been assessed in studies with limited sample sizes [60,62]. RISE will be, to our knowledge, the first and the largest cohort studying VTE risk using accelerometry data.
Finally, the RISE cohort including 1350 general medical inpatients will be a valuable source for several secondary analyses, such as evaluating the association between TPX and bleeding, prospectively validating the IMPROVE bleeding risk score, and correlating nurse estimates of patients’ mobility, using the Braden score, with objective measurements.
Thus, RISE has the potential to generate important knowledge about VTE prevention and risk stratification and to improve the quality of care of medical hospitalized patients.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors want to thank all patients that have already agreed to participate in this study. We would also like to thank the study personnel that have recruited all the participants so far and the medical experts that have adjudicated all the already reported medical outcomes.
Data Availability
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion. Deidentified research data will be made publicly available when the study is completed and published.
Funding Statement
The RISE cohort is funded by several non-profit foundations (SGAIM Foundation, Novartis Biomedical Research Foundation, Swiss Heart Foundation, Chuard Schmidt Foundation, Gottfried und Julia Bangerter Foundation). The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement. 2nd ed Rockville, MD: Agency for Healthcare Research and Quality, August 2016. AHRQ Publication No 16-0001-EF; accessed at: https://wwwahrqgov/sites/default/files/publications/files/vteguidepdf July 15, 2019. [Google Scholar]
- 2.Goldhaber SZ, Dunn K, MacDougall RC. New onset of venous thromboembolism among hospitalized patients at Brigham and Women’s Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118(6):1680–4. Epub 2000/12/15. doi: 10.1378/chest.118.6.1680 . [DOI] [PubMed] [Google Scholar]
- 3.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–15. Epub 2000/03/29. doi: 10.1001/archinte.160.6.809 . [DOI] [PubMed] [Google Scholar]
- 4.Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;(5):CD003747. Epub 2014/05/09. doi: 10.1002/14651858.CD003747.pub4 ; PubMed Central PMCID: PMC6491079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakkar AK, Cimminiello C, Goldhaber SZ, Parakh R, Wang C, Bergmann JF. Low-molecular-weight heparin and mortality in acutely ill medical patients. The New England journal of medicine. 2011;365(26):2463–72. Epub 2011/12/30. doi: 10.1056/NEJMoa1111288 . [DOI] [PubMed] [Google Scholar]
- 6.Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. The New England journal of medicine. 1999;341(11):793–800. Epub 1999/09/09. doi: 10.1056/NEJM199909093411103 . [DOI] [PubMed] [Google Scholar]
- 7.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–e226S. Epub 2012/02/15. doi: 10.1378/chest.11-2296 ; PubMed Central PMCID: PMC3278052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schunemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer-Westendorf J, Spencer FA, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–225. Epub 2018/11/30. doi: 10.1182/bloodadvances.2018022954 ; PubMed Central PMCID: PMC6258910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nendaz M, Spirk D, Kucher N, Aujesky D, Hayoz D, Beer JH, et al. Multicentre validation of the Geneva Risk Score for hospitalised medical patients at risk of venous thromboembolism. Explicit ASsessment of Thromboembolic RIsk and Prophylaxis for Medical PATients in SwitzErland (ESTIMATE). Thromb Haemost. 2014;111(3):531–8. Epub 2013/11/15. doi: 10.1160/TH13-05-0427 . [DOI] [PubMed] [Google Scholar]
- 10.Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–7. Epub 2010/08/27. doi: 10.1111/j.1538-7836.2010.04044.x . [DOI] [PubMed] [Google Scholar]
- 11.Spyropoulos AC, Anderson FA Jr., FitzGerald G, Decousus H, Pini M, Chong BH, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–14. Epub 2011/03/26. doi: 10.1378/chest.10-1944 . [DOI] [PubMed] [Google Scholar]
- 12.Spyropoulos AC, Anderson FA Jr., FitzGerald G, Decousus H, Pini M, Chong BH, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–14. Epub 2011/03/26. doi: 10.1378/chest.10-1944 . [DOI] [PubMed] [Google Scholar]
- 13.Blondon M, Spirk D, Kucher N, Aujesky D, Hayoz D, Beer JH, et al. Comparative Performance of Clinical Risk Assessment Models for Hospital-Acquired Venous Thromboembolism in Medical Patients. Thromb Haemost. 2018;118(1):82–9. Epub 2018/01/06. doi: 10.1160/TH17-06-0403 . [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg D, Eichorn A, Alarcon M, McCullagh L, McGinn T, Spyropoulos AC. External validation of the risk assessment model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for medical patients in a tertiary health system. Journal of the American Heart Association. 2014;3(6):e001152. Epub 2014/11/19. doi: 10.1161/JAHA.114.001152 ; PubMed Central PMCID: PMC4338701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull RD. Relevance of immobility and importance of risk assessment management for medically ill patients. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2013;19(3):268–76. doi: 10.1177/1076029612452781 . [DOI] [PubMed] [Google Scholar]
- 16.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. Journal of the American Geriatrics Society. 2004;52(8):1263–70. doi: 10.1111/j.1532-5415.2004.52354.x . [DOI] [PubMed] [Google Scholar]
- 17.Callen BL, Mahoney JE, Grieves CB, Wells TJ, Enloe M. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatric nursing. 2004;25(4):212–7. doi: 10.1016/j.gerinurse.2004.06.016 . [DOI] [PubMed] [Google Scholar]
- 18.Lazarus BA, Murphy JB, Coletta EM, McQuade WH, Culpepper L. The provision of physical activity to hospitalized elderly patients. Arch Intern Med. 1991;151(12):2452–6. . [PubMed] [Google Scholar]
- 19.Hoyer EH, Young DL, Friedman LA, Brotman DJ, Klein LM, Friedman M, et al. Routine Inpatient Mobility Assessment and Hospital Discharge Planning. JAMA internal medicine. 2019;179(1):118–20. doi: 10.1001/jamainternmed.2018.5145 ; PubMed Central PMCID: PMC6583395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinvani L, Kozikowski A, Patel V, Mulvany C, Smilios C, Qiu G, et al. Measuring Functional Status in Hospitalized Older Adults Through Electronic Health Record Documentation. Southern medical journal. 2018;111(4):220–5. doi: 10.14423/SMJ.0000000000000788 . [DOI] [PubMed] [Google Scholar]
- 21.Daskivich TJ, Houman J, Lopez M, Luu M, Fleshner P, Zaghiyan K, et al. Association of Wearable Activity Monitors With Assessment of Daily Ambulation and Length of Stay Among Patients Undergoing Major Surgery. JAMA network open. 2019;2(2):e187673. doi: 10.1001/jamanetworkopen.2018.7673 ; PubMed Central PMCID: PMC6484591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CJ, Roth DL, Allman RM. Validation of use of wireless monitors to measure levels of mobility during hospitalization. Journal of rehabilitation research and development. 2008;45(4):551–8. doi: 10.1682/jrrd.2007.06.0086 . [DOI] [PubMed] [Google Scholar]
- 23.Hartley P, Keevil VL, Westgate K, White T, Brage S, Romero-Ortuno R, et al. Using Accelerometers to Measure Physical Activity in Older Patients Admitted to Hospital. Current gerontology and geriatrics research. 2018;2018:3280240. doi: 10.1155/2018/3280240 ; PubMed Central PMCID: PMC6211152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim SER, Dodds R, Bacon D, Sayer AA, Roberts HC. Physical activity among hospitalised older people: insights from upper and lower limb accelerometry. Aging clinical and experimental research. 2018;30(11):1363–9. doi: 10.1007/s40520-018-0930-0 ; PubMed Central PMCID: PMC6208771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowlands AV, Olds TS, Hillsdon M, Pulsford R, Hurst TL, Eston RG, et al. Assessing sedentary behavior with the GENEActiv: introducing the sedentary sphere. Medicine and science in sports and exercise. 2014;46(6):1235–47. doi: 10.1249/MSS.0000000000000224 . [DOI] [PubMed] [Google Scholar]
- 26.Lim SER, Ibrahim K, Sayer AA, Roberts HC. Assessment of Physical Activity of Hospitalised Older Adults: A Systematic Review. The journal of nutrition, health & aging. 2018;22(3):377–86. doi: 10.1007/s12603-017-0931-2 . [DOI] [PubMed] [Google Scholar]
- 27.Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164(9):963–8. doi: 10.1001/archinte.164.9.963 . [DOI] [PubMed] [Google Scholar]
- 28.Laporte S, Mismetti P, Decousus H, Uresandi F, Otero R, Lobo JL, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117(13):1711–6. doi: 10.1161/CIRCULATIONAHA.107.726232 . [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 . [DOI] [PubMed] [Google Scholar]
- 30.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173(6):676–82. Epub 2011/02/19. doi: 10.1093/aje/kwq433 . [DOI] [PubMed] [Google Scholar]
- 31.Spyropoulos AC, Ageno W, Albers GW, Elliott CG, Halperin JL, Hiatt WR, et al. Rivaroxaban for Thromboprophylaxis after Hospitalization for Medical Illness. The New England journal of medicine. 2018;379(12):1118–27. Epub 2018/08/28. doi: 10.1056/NEJMoa1805090 . [DOI] [PubMed] [Google Scholar]
- 32.Decousus H, Tapson VF, Bergmann JF, Chong BH, Froehlich JB, Kakkar AK, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69–79. Epub 2010/05/11. doi: 10.1378/chest.09-3081 . [DOI] [PubMed] [Google Scholar]
- 33.Hostler DC, Marx ES, Moores LK, Petteys SK, Hostler JM, Mitchell JD, et al. Validation of the International Medical Prevention Registry on Venous Thromboembolism Bleeding Risk Score. Chest. 2016;149(2):372–9. Epub 2016/02/13. doi: 10.1378/chest.14-2842 . [DOI] [PubMed] [Google Scholar]
- 34.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. Journal of clinical epidemiology. 1989;42(8):703–9. doi: 10.1016/0895-4356(89)90065-6 [DOI] [PubMed] [Google Scholar]
- 35.Rowlands AV, Yates T, Davies M, Khunti K, Edwardson CL. Raw accelerometer data analysis with GGIR R-package: does accelerometer brand matter? Medicine and science in sports and exercise. 2016;48(10):1935–41. Epub 2016/05/18. doi: 10.1249/MSS.0000000000000978 . [DOI] [PubMed] [Google Scholar]
- 36.Dillon CB, Fitzgerald AP, Kearney PM, Perry IJ, Rennie KL, Kozarski R, et al. Number of Days Required to Estimate Habitual Activity Using Wrist-Worn GENEActiv Accelerometer: A Cross-Sectional Study. PloS one. 2016;11(5):e0109913. Epub 2016/05/07. doi: 10.1371/journal.pone.0109913 ; PubMed Central PMCID: PMC4858250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tasheva P, Vollenweider P, Kraege V, Roulet G, Lamy O, Marques-Vidal P, et al. Association Between Physical Activity Levels in the Hospital Setting and Hospital-Acquired Functional Decline in Elderly Patients. JAMA Network Open. 2020;3(1):e1920185–e. doi: 10.1001/jamanetworkopen.2019.20185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res. 1987;36(4):205–10. Epub 1987/07/01. . [PubMed] [Google Scholar]
- 39.Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, Gent M, et al. Idraparinux versus standard therapy for venous thromboembolic disease. The New England journal of medicine. 2007;357(11):1094–104. Epub 2007/09/15. doi: 10.1056/NEJMoa064247 . [DOI] [PubMed] [Google Scholar]
- 40.Baumgartner C, Klok FA, Carrier M, Limacher A, Moor J, Righini M, et al. Clinical Surveillance vs. Anticoagulation For low-risk patiEnts with isolated SubSegmental Pulmonary Embolism: protocol for a multicentre randomised placebo-controlled non-inferiority trial (SAFE-SSPE). BMJ Open. 2020;10(11):e040151. doi: 10.1136/bmjopen-2020-040151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Investigators TC. Low-Molecular-Weight Heparin in the Treatment of Patients with Venous Thromboembolism. New England Journal of Medicine. 1997;337(10):657–62. doi: 10.1056/NEJM199709043371001 . [DOI] [PubMed] [Google Scholar]
- 42.Newton DH, Monreal Bosch M, Amendola M, Wolfe L, Perez Ductor C, Lecumberri R, et al. Analysis of noncatheter-associated upper extremity deep venous thrombosis from the RIETE registry. J Vasc Surg Venous Lymphat Disord. 2017;5(1):18–24.e1. Epub 2016/12/19. doi: 10.1016/j.jvsv.2016.08.002 . [DOI] [PubMed] [Google Scholar]
- 43.Mean M, Righini M, Jaeger K, Beer HJ, Frauchiger B, Osterwalder J, et al. The Swiss cohort of elderly patients with venous thromboembolism (SWITCO65+): rationale and methodology. Journal of thrombosis and thrombolysis. 2013;36(4):475–83. doi: 10.1007/s11239-013-0875-2 . [DOI] [PubMed] [Google Scholar]
- 44.Ploton G, Pistorius M-A, Raimbeau A, Denis Le Seve J, Bergère G, Ngohou C, et al. A STROBE cohort study of 755 deep and superficial upper-extremity vein thrombosis. Medicine. 2020;99(6):e18996. doi: 10.1097/MD.0000000000018996 00005792-202002070-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy PM, Rachas A, Meyer G, Le Gal G, Durieux P, El Kouri D, et al. Multifaceted Intervention to Prevent Venous Thromboembolism in Patients Hospitalized for Acute Medical Illness: A Multicenter Cluster-Randomized Trial. PloS one. 2016;11(5):e0154832. Epub 2016/05/27. doi: 10.1371/journal.pone.0154832 ; PubMed Central PMCID: PMC4881951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cote LP, Greenberg S, Caprini JA, Tafur A, Choi C, Munoz FJ, et al. Comparisons Between Upper and Lower Extremity Deep Vein Thrombosis: A Review of the RIETE Registry. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2017;23(7):748–54. Epub 2016/08/31. doi: 10.1177/1076029616663847 . [DOI] [PubMed] [Google Scholar]
- 47.Frei AN, Stalder O, Limacher A, Méan M, Baumgartner C, Rodondi N, et al. Comparison of Bleeding Risk Scores in Elderly Patients Receiving Extended Anticoagulation with Vitamin K Antagonists for Venous Thromboembolism. Thromb Haemost. (EFirst). Epub 30.04.2021. doi: 10.1055/s-0041-1726345 [DOI] [PubMed] [Google Scholar]
- 48.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. Epub 2005/04/22. doi: 10.1111/j.1538-7836.2005.01204.x . [DOI] [PubMed] [Google Scholar]
- 49.Raskob GE, Spyropoulos AC, Zrubek J, Ageno W, Albers G, Elliott CG, et al. The MARINER trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. Thromb Haemost. 2016;115(6):1240–8. doi: 10.1160/TH15-09-0756 . [DOI] [PubMed] [Google Scholar]
- 50.Tritschler T, Kraaijpoel N, Girard P, Büller HR, Langlois N, Righini M, et al. Definition of pulmonary embolism-related death and classification of the cause of death in venous thromboembolism studies: Communication from the SSC of the ISTH. J Thromb Haemost. 2020;18(6):1495–500. Epub 2020/06/05. doi: 10.1111/jth.14769 . [DOI] [PubMed] [Google Scholar]
- 51.Tritschler T, Kraaijpoel N, Langlois N, Girard P, Schulman S, Buller HR, et al. Development of a standardized definition of pulmonary embolism-related death: a cross-sectional survey of international thrombosis experts. J Thromb Haemost. 2020. doi: 10.1111/jth.14775 . [DOI] [PubMed] [Google Scholar]
- 52.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Es G-Av, et al. Clinical End Points in Coronary Stent Trials. Circulation. 2007;115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. Manual of the international statistical classification of diseases, injury, and causes of death. Geneva. 1977. [Google Scholar]
- 54.SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Annals of Internal Medicine. 2013;158(3):200–7. doi: 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 56.Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet (London, England). 2008;371(9610):387–94. Epub 2008/02/05. doi: 10.1016/S0140-6736(08)60202-0 . [DOI] [PubMed] [Google Scholar]
- 57.Spirk D, Nendaz M, Aujesky D, Hayoz D, Beer JH, Husmann M, et al. Predictors of thromboprophylaxis in hospitalised medical patients. Explicit ASsessment of Thromboembolic RIsk and Prophylaxis for Medical PATients in SwitzErland (ESTIMATE). Thromb Haemost. 2015;113(5):1127–34. Epub 2015/01/23. doi: 10.1160/TH14-06-0525 . [DOI] [PubMed] [Google Scholar]
- 58.Theriault T, Touchette M, Goupil V, Echenberg D, Lanthier L. Thromboprophylaxis adherence to the ninth edition of American college of chest physicians antithrombotic guidelines in a tertiary care centre: a cross-sectional study. Journal of evaluation in clinical practice. 2016;22(6):952–7. doi: 10.1111/jep.12569 . [DOI] [PubMed] [Google Scholar]
- 59.Rolving N, Brocki BC, Bloch-Nielsen JR, Larsen TB, Jensen FL, Mikkelsen HR, et al. Effect of a Physiotherapist-Guided Home-Based Exercise Intervention on Physical Capacity and Patient-Reported Outcomes Among Patients With Acute Pulmonary Embolism: A Randomized Clinical Trial. JAMA Network Open. 2020;3(2):e200064–e. doi: 10.1001/jamanetworkopen.2020.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pavon JM, Sloane RJ, Pieper CF, Colón-Emeric CS, Gallagher D, Cohen HJ, et al. Physical Activity in the Hospital: Documentation and Influence on Venous Thromboembolism Prophylaxis. Journal of Aging and Physical Activity. 2020;28(2):306–10. doi: 10.1123/japa.2018-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47(12):1019–24. Epub 1992/12/01. doi: 10.1136/thx.47.12.1019 ; PubMed Central PMCID: PMC1021093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamilton AC, Lee N, Stilphen M, Hu B, Schramm S, Frost F, et al. Increasing Mobility via In-hospital Ambulation Protocol Delivered by Mobility Technicians: A Pilot Randomized Controlled Trial. J Hosp Med. 2019;14(5):272–7. Epub 2019/02/23. doi: 10.12788/jhm.3153 . [DOI] [PubMed] [Google Scholar]