Abstract
Psoriasis is a systemic, relapsing, inflammatory disease associated with serious comorbidities including mood problems and/or unhealthy lifestyle behaviours. Cutaneous and systemic abnormalities in innate and acquired immunity play a role in its pathogenesis. The exact pathogenetic mechanism remains elusive. Evidence is accumulating that TNF-alpha, IL-17 and IL-23 signalling are highly relevant as targeting these pathways reduces disease activity. Evidence suggests a strong link between psoriasis and depression in adults. The International Psoriasis Council (IPC) held a roundtable event, “Psoriasis and Mental Health”, in Barcelona, Spain which focused on the presence of depression and suicidality, plus the role of neuroinflammation in psoriasis, sleep disruption and the impact of depression on cardiovascular disease outcomes. We summarize here the expert presentations to provide additional insight into the understanding of psychiatric comorbidities of psoriasis and of the impact of chronic, systemic inflammation on neuro- and cardiovascular outcomes. the associations between psoriasis and other psychiatric comorbidities are still controversial and warrant further attention.
Key words: psoriasis, depression, cardiovascular mental health, sleep, neuroinflammation.
Psoriasis is a chronic inflammatory skin disorder with a broad and variable range of phenotypes, treatments, and associated comorbidities including metabolic syndrome (1), liver disease (2), respiratory disease (3), psoriatic arthritis (4), cardiovascular disease (5) and psychosocial disorders (6). It has been proposed that in psoriasis its cutaneous and specific systemic manifestations are driven by a shared pathogenic inflammatory mechanism.
SIGNIFICANCE
Psoriasis is a chronic, systemic disease which is marked by a significant psychosocial burden. In recent years, multiple lines of evidence support the strong association between psoriasis and psychosocial comorbidities. The role of neuroinflammation in psoriasis pathology, sleep disruption and the impact of depression on cardiovascular outcomes was explored in a workshop convened by the International Psoriasis Council. The workshop was assembled to increase the understanding of depression as an important comorbidity of psoriasis and elevate awareness of the impact of chronic, systemic inflammation on neuro- and cardiovascular outcomes. Studies discussed at this workshop suggest that depression is consistently associated with psoriasis, while suicidal ideation is not. Increased awareness of and concern about effective depression management in patients with psoriasis will undoubtedly improve overall disease management.
Psoriasis provides an ideal model within which to study relationships between chronic inflammation and depression and other psychiatric co-morbidities. For clinical practice, greater awareness of the association between psoriasis and depression is important (6).
The term ‘depression’ covers a range of experiences, from low mood symptoms that may be a transient reaction to adverse life events to the enduring and potentially life threatening disorder. The latter is more accurately called Depressive Disorder (International Classification of Diseases [ICD-10]) or Major Depressive Disorder (MDD; Diagnostic and Statistical Manual of Mental Disorders [DSM-5]) (7, 8). Although these disorders are not identical constructs, they overlap to a considerable extent and represent a common clinical syndrome characterized by sustained depressed mood and/or anhedonia (reduced motivation for or pleasure from previously enjoyable activities) and impairing somatic (sleep, energy, sensorimotor, appetites) and cognitive (self-esteem, concentration) functioning as well as the will to live (suicidal thinking and actions).
DEPRESSION AND NEUROINFLAMMATION
An influential current hypothesis of the neurobiology of MDD emphasizes a complex and interacting dysregulation of endocrine, immune, and monoamine neurotransmitter systems, often triggered initially by environmental stress but gradually becoming self-sustaining and resulting in impaired regional brain structure and function (9, 10).
Thus, chronic stress activates the hypothalamic-pituitary-adrenal (HPA) axis resulting in increased release of glucocorticoids from the adrenal cortex which, in turn, dysregulates amygdala function (Fig. 1)
Fig. 1.
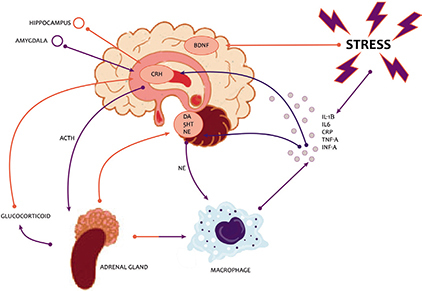
Hypothalamic-pituitary-adrenal (HPA) axis and depression. BDNF: brain-derived neurotrophic factor; CRH: corticotropin-releasing hormone; DA: dopamine; 5HT: serotonin; NE: noradrenaline.
(10). The elevated adrenal activity and associated increase in sympathetic tone causes the release of proinflammatory cytokines which, along with the increased glucocorticoids, increase the activity of the enzyme monoamine oxidase (MAO) which in turn causes reduced levels of the monoamine neurotransmitters serotonin (5-HT), noradrenaline, and dopamine. The elevated cytokines and glucocorticoids also depress neurotrophic factors such as brain-derived neurotrophic factor (BDNF) resulting in decreased neurogenesis in the hippocampus and reduced hippocampal volume. Amygdala and hippocampus dysregulation then maintains abnormal glucocorticoids, BDNF and cytokines, and increased proinflammatory cytokines give rise to physical illness symptoms and increased risk of inflammatory disorders such as cardiovascular disease (CVD).
Stress has been shown to trigger psoriasis onset and contribute to psoriasis flares in patients (6). The role of the HPA axis activity dysregulation and the link to the modulation of immune response in psoriasis have been explored (11, 12). Verhoeven et al. (13) demonstrated that flares of psoriasis are characterised by high activity of daily stressors and low cortisol levels. Furthermore, patients with persistently high levels of stressors have related lowered cortisol levels and may be particularly vulnerable to the influence of stressors on their psoriasis.
NEUROIMAGING
A number of changes in the HPA axis have been identified by neuroimaging. Molecular positron emission tomography (PET) studies during major depressive episodes (MDE), have found significantly elevated density of MAO-A - which could result in chronically low synaptic 5-HT levels through increased metabolism in a range of brain regions. Further evidence from PET studies demonstrates that 5-HT1A receptors (14) and 5-HT2A receptors (15, 16) are upregulated in MDD, suggesting an adaptive response to reduced 5-HT transmission, and that higher levels of 5-HT1A receptors predict higher suicidal ideation and attempts of greater lethality and intent (17).
Functional magnetic resonance imaging (fMRI) shows the amygdala is overly active in people with MDD when shown sad stimuli, but under-active when shown positive stimuli (18), suggesting a neural basis for the negative cognitive bias in MDD which is the therapeutic target of cognitive behavioural therapy.
Increasing evidence suggests involvement of inflammation in MDD in a proportion of individuals (19–22). Inflammatory disorders such as psoriasis are associated with a high prevalence of depression (23, 24). Similarly, symptoms of depression develop in a high proportion of patients administered pro-inflammatory cytokines for therapeutic reasons (25–27) and in healthy volunteers following peripheral immune challenges, such as vaccination or bacterial endotoxin injection, which result in a mild immune activation (28, 29). In MDD, several peripheral pro-inflammatory cytokines are elevated (30–33) and polymorphisms in inflammatory cytokine genes are associated with MDD and treatment response (34). Several PET studies have now investigated whether MDD is also associated with evidence for central neuroinflammation using tracers specific for the 18 kDA translocator protein (TSPO), a mitochondrial membrane protein which is overexpressed on activated microglia and, to a lesser extent, astrocytes. One study did not find elevated TSPO during a mild-to-moderate MDE (35). However, a subsequent study in more severely symptomatic patients (moderate-to-severe MDE) found significantly elevated TSPO, most prominent in prefrontal cortex, anterior cingulate cortex (ACC), and insula, and positively correlated with symptom severity (36). More recent data from a clinically and demographically comparable cohort of patients in a moderate-to-severe MDE replicates the TSPO elevation in the ACC, but suggests that microglial activation may be more strongly associated with the presence of suicidality than the diagnosis of MDD itself (37). These studies in working age adults with MDD are supported by the finding of elevated ACC TSPO in a recent small PET study in patients with late-life depression who had largely recovered from a depression episode but who had remaining evidence of peripheral inflammation (38). Thus, PET evidence for neuroinflammation in MDD, and potentially suicidality, seems to be converging on the ACC, a brain region playing an important role in regulating cognitive and emotional processing (39). Interestingly, no PET evidence for neuroinflammation was found in non-depressed patients with moderate-to-severe plaque psoriasis and raised peripheral cytokines (40). However, a comparable study in depressed psoriasis patients has not yet been conducted, and the neurobiological mechanism underlying the increased incidence of depression in psoriasis remains to be elucidated.
More recently increased TSPO binding has been shown in the brains of patients with MDD and post hoc analysis extended these findings by demonstrating that this abnormality is significant in unmedicated but not in medicated MDD subjects (41). Moreover, in drug-naive patients in a moderate-severe MDE, improvement in depressive symptoms following successful psychotherapy has been shown to be associated with a normalisation of elevated TSPO (42).
DEPRESSION AND PSORIASIS
Depending on the screening method used, depression prevalence estimates vary between 9 and 55% of people with psoriasis (43, 44). One meta-analysis and systematic review estimated rates of clinical depression at 28% using questionnaires while estimates were 12% using International Classification of Diseases codes, 19% using Diagnostic and Statistical Manual of Mental Disorders IV, and 9% for antidepressant use (24). Psoriasis patients in this study had significantly more depressive symptoms (standardized mean differences 1.16; 95% confidence interval (CI) 0.67–1.66), and population-based studies showed that they were at least one and a half times more likely to experience depression (odds ratio [OR] 1.57; 95% CI 1.40–1.76) and used more antidepressant medication than did controls (OR 4.24, 95% CI 1.53–11.76) (Table I). In a multicentre study (45), 13.8% of patients with psoriasis reported depression symptoms using the Hospital Anxiety and Depression scale (HADS) versus 4.3% in controls (OR 2.40, 95% CI 1.67–3.47)).
Table I.
Psoriasis and Psycho-Comorbidity Studies
| Study | Depression estimates for psoriasis | Suicidal ideation estimates for psoriasis | Assessment tool | Sample Size | Type of study |
|---|---|---|---|---|---|
| Dowlatshahi et al. 2014 | 28% depressive symptoms | Questionnaires | 264,568 psoriasis patients and | Meta-analysis and systematic review | |
| (24) | 12% clinical depression | Classification of Diseases codes | 1,174,612 healthy controls were pooled. | ||
| 19% clinical depression | Diagnostic and Statistical Manual of Mental Disorders IV | ||||
| 9% antidepressant use SMD 1.16; 95% Cl 0.67–1.66) depressive symptoms OR 1.57; 95% Cl 1.53–1.76) odds of depression OR 4.24; 95% Cl 1.53–11.76 odds of use of antidepressants |
|||||
| Dalgard et al. 2015 (45) | 13.8% depression | 17.35% | Hamilton Anxiety and Depression scale | 626 psoriasis patients | Prospective cohort study |
| OR 3.02 (95% Cl 1.86–4.90) | OR = 1.94, 95% Cl 1.33–2.82 | ||||
| Jensen et al. 2016 (48) | IRR 1.08 (1.04–1.12) in mild and 1.36 (1.27–1.46) in severe psoriasis. | 35,001 patients with mild psoriasis and 7,510 with severe psoriasis | |||
| Wu et al. 2017 (52) | IRR: psoriasis, 1.14 [95% Cl, 1.11–1.17] | IRR 1.01; 95%CI 0.85–1.20 | 36,214 | Systematic review | |
| Chi et al. 2017 (53) | RR 1.13; 95% Cl 0.87–1.46), suicide attempt (RR 1.25; 95% Cl 0.89–1.75), or suicidaiity (RR 1.26; 95% Cl 0.97–1.64) among people with psoriasis | Systematic review | |||
| Lamb et al. 2017 (46) | 9.9% MDD | 35% of patients diagnosed with MDD | Patient Health Questionnaire Depression Scale | 607 psoriasis patients | |
| Singh et al. 2017 (54) | Psoriasis more likely to attempt and complete suicides (pooled OR,1.26; 95% Cl, 1.13–1.40) | 330,207 | Systematic review and meta analysis |
SMD: standard mean differences; OR: odds ratio; CI: confidence interval; IRR: incidence rate ratios; MDD: Major Depressive Disorder.
Furthermore, in a study including 607 patients with psoriasis, 9.9% (95% CI: 7.5–12.3%) screened positive for MDD, measured using the (Patient Health Questionnaire -9 items) PHQ-9 (46). Of these, 42% reported severe symptoms and 25% met criteria for urgent mental health specialist referral. Interestingly, in the 71% of patients from the cohort that identified with severe depression, this clinical finding was de novo. In the patient group diagnosed with MDD, 39% had a Dermatology Life Quality Index (DLQI) score <10 indicating low to medium impact of psoriasis on quality of life. Therefore, using the DLQI alone would potentially miss important depression symptoms that were detected by the PHQ-9.
Severity of psoriasis, as determined by a code for systemic medications, was found to be associated with higher risk of receiving a clinical diagnosis of depression, as evidenced by a hazard ratio of 1.72 (CI 1.57–1.88) in severe psoriasis compared to a hazard ratio of 1.38 (CI 1.35–1.40) in mild psoriasis (47). Depression may act as a trigger or exacerbating factor for psoriasis and may also be a consequence of the disease. Jensen et al. (48) examined the risk of new-onset depression in patients with psoriasis in a nationwide Danish cohort of 5 million people in the timeframe 2001–2011. In a total of 35,001 patients with mild psoriasis and 7,510 with severe psoriasis, incidence rates for depression were 20.0% (95% CI:19.9–20.0), 23.9% (23.1–24.7) and 31.6% (29.5–33.8) for the reference population, mild, and severe psoriasis, respectively. This finding indicates that in patients with severe disease new onset depression is more frequent.
In a Brazilian study, PASI (Psoriasis Area Severity Index), DLQI, State-Trait Anxiety Inventory (STAI) questionnaire, PHQ-9 and Holmes and Rahe Life Events scale were used to assess the total “load” of stressful life events in 119 psoriasis patients having experienced psoriasis for 3 years or more years and moderate severity (49). Salivary cortisol was assessed. The DLQI and stress questionnaire results demonstrated 20% of patients with depression, and nearly 20% of patients reported severe or very severe impact on QoL. The biometric study showed a positive correlation (r = 0.39, p < 0.001) between salivary cortisol values at bedtime and the PASI score.
The association of psoriasis with anxiety and psychological stress is well established. In order to evaluate psychological impact, it is important to use instruments such as the HADS and PHQ-9, which are validated measures of anxiety and depressive symptomatology. Studies have demonstrated that using the DLQI to measure impact on life is not sufficient to identify depression as about one in 3 patients with MDD or major anxiety reported low to medium DLQI scores (46).
Effective biologic psoriasis treatments reduce signs and symptoms of psoriasis, lower disease impact (DLQI) and may also improve mental health outcomes (50, 51). Examination of depression through a prospective, longitudinal psoriasis registry (PSOLAR) demonstrated that treatment with biologics in moderate-to-severe psoriasis patients may reduce the risk of depressive symptoms (50). Similarly, a large cross-sectional study consisting of over 2 million patients with moderate-to-severe psoriasis demonstrated reduction of psychological stress and depression, in patients treated with biologics compared to those treated with oral therapies as measured by the Kessler Psychological Distress Scale-6 (K6) and Patient Health Questionnaire-2 (PHQ2) tools (51).
DEPRESSION AND SUICIDALITY IN PSORIASIS
The association between psoriasis, depression and suicidal ideation has been summarized in Table I. Depression and suicidal ideation tend to be more frequent in patients with severe disease. Although depression is consistently associated with psoriasis, suicidal ideation is not (52, 53). One cross-sectional multicentre study found that suicidal ideation was significantly associated with psoriasis (45) and some studies indicate that psoriasis is significantly linked to all 3 measures of suicidality (54), but the available data on suicide attempts and death by suicide is limited and less consistent. Younger patients and patients with more severe psoriasis are at significantly higher risk of suicidality (54). Higher levels of psoriasis-related stressors and reduced QoL were significantly associated with suicidality in people with psoriasis.
A large population-based study utilizing a primary care database in the United Kingdom with linked hospital and mortality records indicated that the prevalence of depression was raised in patients with psoriasis and this may lead to a greater risk of self-harm. Nevertheless, the risk of suicide was lower in patients with psoriasis compared with those without psoriasis, and it was postulated that increased contacts with healthcare professionals for treatment of skin and coexistent depression may have ameliorated suicide risk (55).
DEPRESSION AND ALEXITHYMIA IN PSORIASIS
Alexithymia, the difficulty in recognising and describing emotions as measured by the Toronto Alexithymia Scale (TAS-20), has been linked to psoriasis. The observed prevalence of alexithymia in patients with psoriasis ranges between approximately 15.6% and 33%, which is significantly higher than found among the general public (56–59). In a multicentre study which included 670 patients with psoriasis, the prevalence of alexithymia was 24.8% (95% CI: 21.7–28.2) (59). Some studies have shown that alexithymia is stable over time in the general population (60). However, it may be partly modifiable with therapeutic interventions, and reversal of alexithymia improves QoL, anxiety and depression (61, 62). It is likely that “trait alexithymia” and “state alexithymia” exist – the latter may be modified by therapeutic intervention or change in psychological status. It is important to identify alexithymic patients with psoriasis in clinical practice as they suffer from a higher disease burden, including significant impairment of QoL, higher levels of anxiety and depression, higher risk of alcohol dependency and impairment of work productivity, compared to patients without alexithymia (63).
SLEEP DISRUPTION, DEPRESSION AND PSORIASIS
Sleep is a complex behavioural and physiological process necessary for healthy functioning of the immune and metabolic systems, neuro-cognitive activity and emotional regulation. There is a large body of evidence demonstrating the vital role sleep has in maintaining health (64–66) and optimal physiological (67) and psychological functioning (68, 69). Persistent sleep disturbance is related to a number of medical illnesses including diabetes (70) and has established relationships with depression (71, 72).
Sleep disturbance is associated with a range of adverse health outcomes including elevated risk for a range of physical diseases, several of which people with psoriasis are already more susceptible to (e.g. diabetes (73), hypertension (74), CVD (75). Moreover, sleep disturbance increases risk of psychological illness (e.g. depression (72), anxiety (76)) and, over time, all-cause mortality (77).
Sleep disruption, including obstructive sleep apnoea (OSA), may contribute significantly to the health outcomes of psoriasis. A recent study (78) (186 respondents; mean age 39.2 years) assessed validated measures of sleep [Pittsburgh Sleep; Quality Index (PSQI), Berlin Questionnaire, Pre-Sleep Arousal Scale]; chronotype (Morningness-Eveningness Questionnaire); mood (Hospital Anxiety and Depression Scale); itch (5-D Itch Scale); and psoriasis severity (Simplified Psoriasis Index). The mean PSQI score was 9.2 ± 4.3, with 76.3% scoring above the threshold for poor sleep (≥ 6 on the PSQI) and 32.5% scoring ‘positive’ for probable OSA. Poor sleep and high likelihood of OSA were associated with more severe psoriasis (p < 0.05; η = 0.07; η2 = 0.005) (78). The precise mechanisms explaining the health outcomes and sleep link are not fully understood but are thought to include disrupted cortisol and cytokine production and changes in body temperature control all of which are implicated in psoriasis.
PSORIASIS, DEPRESSION AND CARDIOVASCULAR EVENTS
Not only is psoriasis accompanied by an increased risk of psychiatric comorbidities, but in particular when coupled with these comorbidities, such as depression, psoriasis promotes a greater risk of CVD events (79).
Due to the complex interaction of psoriasis and comorbidities, evidence is accumulating that psoriasis may trigger the metabolic syndrome and cardiovascular comorbidities. This relationship may be multifactorial, comprising lifestyles and systematic inflammation. Recently it was shown that blockade of IL12/23 reduced aortic vascular inflammation (80).
On the other hand these comorbidities may aggravate psoriasis. Recently, a Mendelian randomization study showed a causal relationship between body mass index (BMI) and psoriasis, whereas there was little support to a possible causal effect of psoriasis genetic risk (81).
The link between psoriasis, depression and CVD was investigated in a nationwide Danish cohort of patients with psoriasis (n = 29,406). Incidence rates were calculated, and incidence rate ratios (IRRs) adjusted for age, gender, socio-economic status, medication and comorbidity were estimated by Poisson regression models. Risk of myocardial infarction (MI) (IRR 1.57, 95% confidence interval (95% CI) 1.07–2.29), stroke (IRR 1.95, 95% CI 1.43–2.66), and death from CVD (IRR 2.24, 95% CI 1.53–3.26) were increased significantly during acute depression, and risk of stroke (IRR 1.51, 95% CI 1.19–1.90) was increased significantly in chronic depression. During remission from depression, only the risk of stroke was increased. In patients with psoriasis, depression is associated with increased risk of MI, stroke and death from CVD, especially during acute depression (82). These findings suggest that the added burden of comorbid depression in patients with psoriasis confers a greater risk of major adverse CVD events.
To further understand whether the impact of psoriasis and comorbid depression occurred prior to CVD events, 36 patients with psoriasis and reported comorbid depression and 36 age- and sex-matched patients with psoriasis but no reported history of psychiatric illness were enrolled in a study for quantifying vascular inflammation and coronary CT angiography using 18-FDG PET/CT (83). Patients with psoriasis and comorbid depression experienced both greater vascular inflammation and greater total and non-calcified coronary plaque burden when compared to psoriasis patients without comorbid psychiatric illness. These differences remained significant after adjustment for age, sex and Framingham risk score, suggesting that comorbid depression in psoriasis contributes to CVD risk beyond what can be attributed to traditional cardiovascular risk factors.
Additionally, a prospective cohort study demonstrated that chronic psychological stress increases neural activity, as measured by amygdala activity, in patients with severe psoriasis disease compared to healthy volunteers (84). Elevated neural activity was found to significantly contribute to the risk of subclinical CVD in patients with psoriasis. Skin clearance after one year of treatment corresponded with reduction of amygdala activity and subclinical CVD factors. Taken together, these studies highlight the strong link between psychological stress and CVD morbidity in psoriasis.
Unfortunately, so far no predictive models nor biomarkers are available to predict the course of the disease and in particular the development of comorbidities. Several studies however suggest that predictive models may become available in the near future. For example, a genome-wide analysis of the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) revealed that HLA-C*06:02-negative patients responded better to adalimumab, whereas HLA-C*06:02-positive patients responded better to treatment with ustekinumab (85). Another analysis of this registry indicated that various baseline clinical characteristics predict response to treatment with biologics (86).
CONCLUSIONS
Psoriasis, depression and CVD are associated and are probably connected through systemic inflammation; neuroimaging has provided evidence for the common inflammatory pathway connecting these conditions. The interaction of psychological stress with the inflammatory state of psoriasis and depression is complex and poorly understood. Stressful life events can trigger onset, exacerbate and initiate flares of psoriasis. In addition, social interactions, economic challenges, and negative behaviours (alcohol and smoking) also contribute to the complex interactions of these conditions.
The association between psoriasis and distress is broader than depression alone. Psychological stress and anxiety are also well established features in many patients with psoriasis and are aggravating or precipitating factors of depression. Alexithymia, which is also linked to depression, warrants further investigation. Of note, the association between psoriasis and death by suicide may be a matter of debate but suicidal ideation is more frequently associated with psoriasis.
The impairment of patient QoL by psoriasis is common knowledge and is of major importance in the assessment of treatment outcomes. However, awareness of the association of psoriasis with depression, although established in epidemiological studies, is less well-recognised and addressed in the clinic. Early diagnosis of depression through validated tools and insight into related psychological/psychiatric disorders should form important targets in the treatment of patients with psoriasis.
Increased awareness of and concern about effective depression management in patients with psoriasis will undoubtedly improve overall disease management. Quick and easy to perform screening tools for depression and suicidal ideation are necessary if we hope to add depression screening to our clinical repertoire for managing psoriasis patients. For example, The PHQ-2 is a validated measure that screens for depressed mood and anhedonia over the past 2 weeks and includes the first two questions of the PHQ-9 and may prove useful for mood assessment. Many dermatologists report feeling ill-equipped to address psychological/psychiatric comorbidity in the clinic so implementation of resources to increase the accessibility of psychological support and psychiatric referral in dermatologic practices, and especially in the setting of psoriasis dedicated units in referral centers is key. Further research is needed in order to understand how targeting specific pro-inflammatory cytokines which underlie the chronic inflammation of psoriasis affects both CVD events and depression.
ACKNOWLEDGEMENTS
Disclosures: CEK has received grants and/or honoraria or consultation and/or research support and/or participated in clinical trials sponsored by Abbvie, Almirall, Celgene, Janssen, Leo-Pharma, Lilly, Merck-Serono, Novartis, Pfizer, UCB. CEK is supported by the NIHR Manchester Biomedical Research Centre. PST has received consulting fees from Galen Limited; Sunovion Pharmaceuticals Europe Ltd; myTomorrows; LivaNova UK Ltd. TA has no conflicts of interest to declare. NNM is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc, and Novartis receiving grants and/ or research funding; and as a principal investigator for the National Institute of Health receiving grants and/or research funding. FS has acted as a consultant for Abbvie, Eli Lilly, Janssen, Pierre Fabre. PvdK received compensation for consultancy services and presenteations from Celgene, Allmirall, Abbvie, Lilly, Novartis, Janssen, Leo, Bristol Mayer Squibb, Dermavant and UCB. ABKl has received compensation as a consultant and an investigator for Novartis, Abbvie, UCB, Lilly, and Janssen and has received fellowship funding from Janssen and Abbvie. CB has received funding for research or honoraria from Abbvie; Almiral; Celgene; Janssen; Lilly; Novartis; Pfizer. CEMG has received grants and/or honoraria or consultation fees from Abbvie, Almirall, BMS, Celgene, Galderma, GSK, Janssen, LEO-Pharma, Lilly, Novartis, Pfizer and UCB Pharma. CEMG is funded in part by the NIHR Manchester Biomedical Research Centre and by MRC grant MR/1011808/1and is an NIHR Emeritus Senior Investigator. FV has received grants and/or honoraria or consultation fees and/or research support and/ or participated in clinical trials sponsored by Abbvie, Amgen, Janssen, Leo-Pharma, Lilly, Novartis, Pfizer. JMvdW has no conflicts of interest to declare. DA has received research grants from Abbvie, Almirall, Celgene, Eli Lilly, Novartis, UCB and the Leo Foundation. LP has received grants and/or honoraria or consultation fees and/or research support and/or participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, Leo-Pharma, Lilly, Merck-Serono, MSD, Novartis, Pfizer, Regeneron, Roche, Sandoz, Sanofi, UCB and has participated in speaker’s bureau for Celgene, Janssen, Lilly, MSD, Novartis, Pfizer.
Funding: AbbVie provided an educational grant to the International Psoriasis Council to support the symposium. This sponsor had no influence on the content and viewpoints in this manuscript.
REFERENCES
- 1.Gisondi P, Fostini AC, Fossa I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol 2018; 36: 21–28. [DOI] [PubMed] [Google Scholar]
- 2.Fiore M, Leone S, Maraolo AE, Berti E, Damiani G. Liver illness and psoriatic patients. Biomed Res Int 2018; 2018: 3140983.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damiani G, Radaeli A, Olivini A, Calvara-Pinton P, Malerba M. Increased airway inflammation in patients with psoriasis. Br J Dermatol 2016; 175: 797–799. [DOI] [PubMed] [Google Scholar]
- 4.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019; 80: 251–265. [DOI] [PubMed] [Google Scholar]
- 5.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Implications for management. J Am Acad Dermatol 2017; 76: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz J, Evers AW, Bundy C, Kimball AB. Getting under the skin: report from the International Psoriasis Council Workshop on the role of stress in psoriasis. Front Psychol 2016; 7: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 9.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 2016; 17: 497–511. [DOI] [PubMed] [Google Scholar]
- 10.Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract 2007; 61: 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JM, Cruser D, Podawiltz A, Mummert DI, Jones H, Mummert ME. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis. Dermatol Res Pract 2012; 2012: 403908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor CJ, Liu V, Fiedorowicz JG. Exploring the physiological link between psoriasis and mood disorders. Dermatol Res Pract 2015; 2015: 409637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhoeven EW, Kraaimaat FW, Jong EM, Schalkwijk J, van de Kerkhof PC, Evers AW. Effect of daily stressors on psoriasis: a prospective study. J Invest Dermatol 2009; 129: 2075–2077. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in major depressive disorder. Eur Neuropsy-chopharmacol 2016; 26: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry 2003; 160: 90–99. [DOI] [PubMed] [Google Scholar]
- 16.Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry 2006; 163: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 17.Oquendo MA, Galfalvy H, Sullivan GM, Miller JM, Milak MM, Sublette ME, et al. Positron emission tomographic imaging of the serotonergic system and prediction of risk and lethality of future suicidal behavior. JAMA Psychiatry 2016; 73: 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnone D, McKie S, Elliott R, Thomas EJ, Downey D, Juhasz G, et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry 2012; 169: 841–850. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci 2013; 14: 135–151. [DOI] [PubMed] [Google Scholar]
- 21.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol 2002; 5: 389–399. [DOI] [PubMed] [Google Scholar]
- 22.Krishnadas R, Cavanagh J. Depression: an inflammatory illness?. J Neurol Neurosurg Psychiatry 2012; 83: 495–502. [DOI] [PubMed] [Google Scholar]
- 23.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry 2005; 58: 175–189. [DOI] [PubMed] [Google Scholar]
- 24.Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol 2014; 134: 1542–1551. [DOI] [PubMed] [Google Scholar]
- 25.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsy-chiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs 2005; 19: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002; 26: 643–652. [DOI] [PubMed] [Google Scholar]
- 27.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol 2002; 22: 86–90. [DOI] [PubMed] [Google Scholar]
- 28.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 2001; 58: 445–452. [DOI] [PubMed] [Google Scholar]
- 30.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 2001; 15: 199–226. [DOI] [PubMed] [Google Scholar]
- 31.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186. [DOI] [PubMed] [Google Scholar]
- 32.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 33.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav Immun 2013; 31: 31–47. [DOI] [PubMed] [Google Scholar]
- 35.Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav Immun 2013; 33: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015; 72: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry 2018; 83: 61–69. [DOI] [PubMed] [Google Scholar]
- 38.Su L, Faluyi YO, Hong YT, Fryer TD, Mak E, Gabel S, et al. Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. Br J Psychiatry 2016; 209: 525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talbot PS, Cooper SJ. Anterior cingulate and subgenual prefrontal blood flow changes following tryptophan depletion in healthy males. Neuropsychopharmacology 2006; 31: 1757–1767. [DOI] [PubMed] [Google Scholar]
- 40.Hunter HJ, Hinz R, Gerhard A, Talbot PS, Su Z, Holland G, et al. Brain inflammation and psoriasis: a [(11) C]-(R)-PK11195 positron emission tomography study. Br J Dermatol 2016; 175: 1082–1084. [DOI] [PubMed] [Google Scholar]
- 41.Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res 2018; 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Sagar AP, Keri S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry 2018; 83: 1–7. [DOI] [PubMed] [Google Scholar]
- 43.Dommasch ED, Li T, Okereke OI, Li Y, Qureshi AA, Cho E. Risk of depression in women with psoriasis: a cohort study. Br J Dermatol 2015; 173: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol 2017; 31: 1999–2009. [DOI] [PubMed] [Google Scholar]
- 45.Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, Jemec GB, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol 2015; 135: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamb RC, Matcham F, Turner MA, Rayner L, Simpson A, Hotopf M, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol 2017; 176: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 47.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010; 146: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen P, Ahlehoff O, Egeberg A, Gislason G, Hansen PR, Skov L. Psoriasis and new-onset depression: a Danish nationwide cohort study. Acta Derm Venereol 2016; 96: 39–42. [DOI] [PubMed] [Google Scholar]
- 49.Brunoni AR, Santos IS, Sabbag C, Lotufo PA, Bensenor IM. Psoriasis severity and hypothalamic-pituitary-adrenal axis function: results from the CALIPSO study. Braz J Med Biol Res 2014; 47: 1102–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strober B, Gooderham M, de Jong E, Kimball AB, Langley RG, Lakdawala N, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol 2018; 78: 70–80. [DOI] [PubMed] [Google Scholar]
- 51.Salame N, Ehsani-Chimeh N, Armstrong AW. Comparison of mental health outcomes among adults with psoriasis on biologic versus oral therapies: a population-based study. J Dermatolog Treat 2019; 30: 135–140. [DOI] [PubMed] [Google Scholar]
- 52.Wu JJ, Penfold RB, Primatesta P, Fox TK, Stewart C, Reddy SP, et al. The risk of depression, suicidal ideation and suicide attempt in patients with psoriasis, psoriatic arthritis or ankylosing spondylitis. J Eur Acad Dermatol Venereol 2017; 31: 1168–1175. [DOI] [PubMed] [Google Scholar]
- 53.Chi CC, Chen TH, Wang SH, Tung TH. Risk of suicidality in people with psoriasis: a systematic review and meta-analysis of cohort studies. Am J Clin Dermatol 2017; 18: 621–627. [DOI] [PubMed] [Google Scholar]
- 54.Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and suicidality: A systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 425–440. [DOI] [PubMed] [Google Scholar]
- 55.Parisi R, Webb RT, Kleyn CE, Carr MJ, Kapur N, Griffiths CEM, et al. Psychiatric morbidity and suicidal behaviour in psoriasis: a primary care cohort study. Br J Dermatol 2019; 180: 108–115. [DOI] [PubMed] [Google Scholar]
- 56.Allegranti I, Gon T, Magaton-Rizzi G, Aguglia E. Prevalence of alexithymic characteristics in psoriatic patients. Acta Derm Venereol 1994; Suppl 186: 146–147. [PubMed] [Google Scholar]
- 57.Richards HL, Fortune DG, Griffiths CE, Main CJ. Alexithymia in patients with psoriasis: clinical correlates and psychometric properties of the Toronto Alexithymia Scale-20. J Psychosom Res 2005; 58: 89–96. [DOI] [PubMed] [Google Scholar]
- 58.Korkoliakou P, Christodoulou C, Kouris A, Porichi E, Efstathiou V, Kaloudi E, et al. Alexithymia, anxiety and depression in patients with psoriasis: a case-control study. Ann Gen Psychiatry 2014; 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sampogna F, Puig L, Spuls P, Girolomoni G, Radtke MA, Kirby B, et al. Prevalence of alexithymia in patients with psoriasis and its association with disease burden: a multicentre observational study. Br J Dermatol 2017; 176: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 60.Tolmunen T, Heliste M, Lehto SM, Hintikka J, Honkalampi K, Kauhanen J. Stability of alexithymia in the general population: an 11-year follow-up. Compr Psychiatry 2011; 52: 536–541. [DOI] [PubMed] [Google Scholar]
- 61.Cameron K, Ogrodniczuk J, Hadjipavlou G. Changes in alexithymia following psychological intervention: a review. Harv Rev Psychiatry 2014; 22: 162–178. [DOI] [PubMed] [Google Scholar]
- 62.Sampogna F, Puig L, Spuls P, Girolomoni G, Radtke MA, Kirby B, et al. Reversibility of alexithymia with effective treatment of moderate to severe psoriasis: longitudinal data from EPI-DEPSO. Br J Dermatol 2019; 180: 397–403. [DOI] [PubMed] [Google Scholar]
- 63.Kokkonen P, Karvonen JT, Veijola J, Laksy K, Jokelainen J, Jarvelin MR, et al. Prevalence and sociodemographic correlates of alexithymia in a population sample of young adults. Compr Psychiatry 2001; 42: 471–476. [DOI] [PubMed] [Google Scholar]
- 64.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med 2007; 11: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simpson N, Dinges DF. Sleep and inflammation. Nutrition reviews 2007; 65: S244–S252. [DOI] [PubMed] [Google Scholar]
- 66.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA 1989; 262: 1479–1484. [DOI] [PubMed] [Google Scholar]
- 67.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun 2002; 16: 503–512. [DOI] [PubMed] [Google Scholar]
- 68.Beattie L, Kyle SD, Espie CA, Biello SM. Social interactions, emotion and sleep: A systematic review and research agenda. Sleep Medicine Reviews 2015; 24: 83–100. [DOI] [PubMed] [Google Scholar]
- 69.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol 2005; 25: 117–129. [DOI] [PubMed] [Google Scholar]
- 70.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Int Med 2005; 165: 863–867. [DOI] [PubMed] [Google Scholar]
- 71.Morin CM, Ware JC. Sleep and psychopathology. Appl Prev Psychol 1996; 5: 211–224. [Google Scholar]
- 72.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Effect Diss 2011; 135: 10–19. [DOI] [PubMed] [Google Scholar]
- 73.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 2005; 99: 2008–2019. [DOI] [PubMed] [Google Scholar]
- 74.Palagini L, Maria Bruno R, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Phar Des 2013; 19: 2409–2419. [DOI] [PubMed] [Google Scholar]
- 75.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis 2009; 51: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep 2007; 30: 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010; 33: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henry AL, Kyle SD, Chisholm A, Griffiths CEM, Bundy C. A cross-sectional survey of the nature and correlates of sleep disturbance in people with psoriasis. Br J Dermatol 2017; 177: 1052–1059. [DOI] [PubMed] [Google Scholar]
- 79.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol 2017; 76: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A Phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial). J Invest Dermatol 2019. Jul 19. pii: S0022-202X(19)32537–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Budu-Aggrey A, Brumpton B, Tyrrell J, Watkins S, Modalsli EH, Celis-Moralis C, et al. Evidence of a causal relationship between body mass index and psoriasis: A mendelian randomization study. PLoS Med 2019; 16: e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Impact of depression on risk of myocardial infarction, stroke and cardiovascular death in patients with psoriasis: a Danish nationwide study. Acta Derm Venereol 2016; 96: 218–221. [DOI] [PubMed] [Google Scholar]
- 83.Aberra TM, Joshi AA, Lerman JB, Rodante JA, Dahiya AK, Teague HL, et al. Self-reported depression in psoriasis is associated with subclinical vascular diseases. Atherosclerosis 2016; 251: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goyal A, Dey AK, Chaturvedi A, Elnabawi YA, Aberra TM, Chung JH, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis: a prospective cohort study. JACC Cardiovasc Imaging 2018. Nov 8. pii: S1936-878X(18)30920–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dand N, Duckworth M, Baudry D, et al. HLA-C*06: 02 genotype is a predictive biomarker of biologic treatment response in psoriasis. J Allergy Clin Immunol 2019: 143: 2120–2130. [DOI] [PubMed] [Google Scholar]
- 86.Warren RB, Marsden A, Tomenson B, et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol 2019: 180: 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]


