The expression of angiogenic factors, such as the vascular endothelial growth factor (VEGF) subtypes and their receptors (VEGF-Rs), is upregulated on vascular endothelial cells during tumour angiogenesis. Previous studies demonstrated that these angiogenetic factors are major contributors to the growth in different malignant tumours (1). However, the role of these markers on the clinical follow-up and disease-prognosis in malignant melanoma (MM) remains unclear (1, 2). This study aimed to dissect the main associations between VEGF subtypes and receptors, patients’ characteristics and prognosis in a group of patients with MM.
METHODS
This was a subsequent analysis on a cohort of adult patients with primary or metastatic MM seen at the dermatology outpatient of Inselspital University Hospital of Bern (Switzerland) between 2003 and 2015, with a mean ± standard deviation (SD) follow-up of 6.5 ± 4.6 years. The methods and characteristics of the study population were described elsewhere (1). Patients with melano-cytic naevus or with uncomplete VEGF characterization or without complete follow-up were not included in this analysis. The study was done in agreement with the Declaration of Helsinki and was approved by the local Ethics Committee.
For analysis purposes, a selection of clinically relevant variables was considered. These included: demographics, tumour location and type, Breslow thickness, presence of ulceration and metastatic MM, sentinel lymph node (SLN) findings and VEGF subtypes detected by using next-generation tissue microarray (ngTMA) as described before (1). In addition, we included overall (OS) and disease-free survival (DFS) up to 15 years of follow-up.
Statistical Analysis
Associations among factors were investigated by using an algorithm that is able to display the best connections between variables taking into account other covariates in the system (3, 4). For analysis purposes, continuous variables were categorized by using clinically meaningful thresholds or, for VEGF subtypes, optimal cut-offs based on correlation with OS (1). Multiple generalized linear models (GLMs) with elastic net regularization were then fitted by taking each time, sequentially, a variable as the outcome and the others as covariates. Binomial and Poisson distributions were assumed for binary and survival outcomes, respectively. Relaxation of elastic net estimates was also used to get unbiased regression coefficients (5). Finally, in order to have comparable coefficients, log relative risks were converted to log odds ratios by using an approximation formula (6). System weights were then computed by using inverse exponential transformation on regression coefficients (3). The maximum spanning tree (MST) filter (7), was then applied to the matrix of weights and a semantic connectivity map was generated. In the map straight lines show the strongest connections while spatial proximity between variables indicate patterns of direct correlations. The analysis was performed with MATLAB v.9.2 (The MathWorks, Natick, MA, USA).
RESULTS
Overall 137 patients with MM were included in the study (60.0% men, mean age 62.3 ± 15.8 years) (Table SI1). Metastatic MM was present in 37.2% of patients. These patients were followed-up for a mean ± SD of 6.1 ± 6.6 vs. 6.8 ± 2.9 years for those without metastasis. Characteristics of VEGF subtypes are described in Table SII1. Regarding survival outcomes, cumulative OS and DFS were 67.5% and 50.0%, respectively, at 15 years.
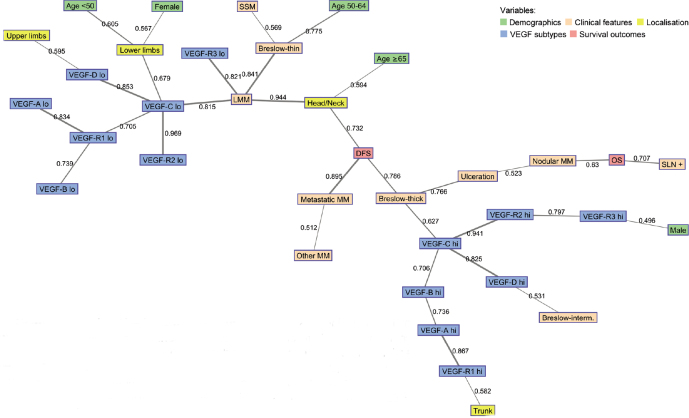
Low and high values of VEGF subtypes were distinguished in two clusters in the map (Fig. 1). In particular, high values of VEGF, especially VEGF-C, were directly associated with thick MM, which was linked to DFS and ulceration. DFS was also strongly connected to metastatic MM and head/neck localisation. OS was also in the same area and associated with positive SLN, nodular MM and ulceration. In addition, intermediate Breslow thickness, MM on the trunk and male sex were connected with high values of VEGF-D, VEGF-R1 and VEGF-R3. Looking at the connections among high values of VEGF, there was a close linking between VEGF-C, VEGF-R2 and VEGF-R3 and between VEGF-B, VEGF-A and VEGF-R1. VEGF-D was also strongly connected to VEGF-C and separated from other subtypes. Conversely, low values of VEGF, especially VEGF-C and VEGF-R3, were connected to LMM, thin MM and SSM. In addition, presence of MM on limbs, age < 50 years and females were also in this area and linked to VEGF-C and VEGF-D. When excluding patients with metastatic MM, high values of VEGF-C were still associated with decreased DFS (data not shown). However, it was not clear from our data whether this holds also for patients with thin MM.
Fig. 1.
Semantic map showing the best linking among selected variables in the study population. The numbers on connecting lines indicate normalized correlations (between 0 and 1). The line thickness corresponds to the strength of association (thin line <0.6; medium line 0.6-0.79; thick line >0.8). DFS: disease-free survival; hi: high; lo: low; LMM: lentigo malignant melanoma; MM: malignant melanoma; OS: overall survival; SLN: sentinel lymph node; SSM: superficial spreading melanoma; thick: thickness; VEGF: vascular endothelial growth factor.
DISCUSSION
In this study, a close linking between VEGF-C, VEGF-R2 and VEGF-R3 has been shown. Previous research has also shown that VEGF-C could be involved in lymphatic hyperplasia and metastatic lymph nodes spread by activating VEGFR-2 and VEGFR-3 (1, 8—10). Increasing evidence shows a specific role of VEGF-C and VEGF-R3 in tumour lymphangiogenesis and lymphatic metastasis in multiple malignancies (1, 8—10) Recent studies have revealed a positive association between VEGF-C expression and both progression stage and lymph node metastasis (1, 8, 11). In our study high values of VEGF-C were directly associated with thick MM, which was connected to many other prognostic factors. In fact, the VEGF-C/VEGFR-3 axis plays an important role in inducing enlargement of peritumoural lymphatic vessels and increasing lymph flow, which promotes lymphatic invasion and lymph node metastasis (1, 8, 11). Furthermore, VEGF-C participates in tumour cell chemotaxis, which is an independent, yet interlinked and important step in the process of cancer metastasis (1, 8, 9, 11). In similar studies VEGF-C expression was found significantly increased in metastatic MM compared to non-metastatic MM (1, 12—14).
One possible limitation of this study was a technical limit during the preparation of TMAs as described elsewhere (1). Furthermore, the analysis was mainly exploratory and specific hypotheses emerging from the map should be better tested in further ad hoc studies. Another limitation was the relatively low number of patients included in the analysis, which does not allow generalization of results.
Footnotes
REFERENCES
- 1.Seyed Jafari SM, Wiedmer C, Cazzaniga S, Frangez Z, Shafighi M, Beltraminelli H, et al. Correlation of vascular endothelial growth factor subtypes and their receptors with melanoma progression: A next-generation tissue microarray (ngTMA) automated analysis. PloS one 2018; 13: e0207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felcht M, Thomas M. Angiogenesis in malignant melanoma. J Dtsch Dermatol Ges 2015; 13: 125–136. [DOI] [PubMed] [Google Scholar]
- 3.Cazzaniga S, Apfelbacher C, Diepgen T, Ofenloch RF, Weisshaar E, Molin S, et al. Patterns of chronic hand eczema: a semantic map analysis of the CARPE registry data. Br J Dermatol 2018; 178: 229–237. [DOI] [PubMed] [Google Scholar]
- 4.Thomi R, Cazzaniga S, Seyed Jafari SM, Schlapbach C, Hunger RE. Association of hidradenitis suppurativa with T helper 1/T helper 17 phenotypes: a semantic map analysis. JAMA Dermatol 2018; 154: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinshausen N. Relaxed lasso. Computational Statistics & Data Analysis 2007; 52: 374–393. [Google Scholar]
- 6.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690–1691. [DOI] [PubMed] [Google Scholar]
- 7.Pemmaraju S, Skiena S. Computational Discrete Mathematics: Combinatorics and Graph Theory with Mathematica®. Cambridge university press; 2003. [Google Scholar]
- 8.Pepper MS, Skobe M. Lymphatic endothelium: morphological, molecular and functional properties. J Cell Biol 2003; 163: 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7: 192–198. [DOI] [PubMed] [Google Scholar]
- 10.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 1997; 16: 3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 2006; 66: 8065–8075. [DOI] [PubMed] [Google Scholar]
- 12.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 2003; 162: 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massi D, Puig S, Franchi A, Malvehy J, Vidal-Sicart S, González-Cao M, et al. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case-control study. J Clin Pathol 2006; 59: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 2005; 18: 1232–1242. [DOI] [PubMed] [Google Scholar]