Fusarium head blight (FHB) caused by Fusarium graminearum is a destructive wheat (Triticum aestivum) disease worldwide (Bai et al., 2018). Previously, we cloned Fhb1 (TaHRC) encoding a histidine‐rich calcium‐binding protein and demonstrated that a large deletion near the start codon of TaHRC reduced FHB susceptibility (Su et al., 2019), which indicates that wheat carries susceptibility genes (S‐genes), and knockout of these S‐genes may enhance plant resistance. Recently, CRISPR/Cas9 genome editing system has been used to precisely knock out S‐genes to generate resistant mutants in crops (Chen et al., 2019); however, it requires transforming CRISPR/Cas9 and gRNA into plants using gene bombardment or Agrobacterium. Unfortunately, most wheat genotypes have extremely low callus induction and regeneration efficiency, which limits the application of genome editing in wheat breeding. Therefore, a new gRNA delivery system that bypasses the tissue culture is critical to the successful use of gene editing in wheat breeding.
Barley stripe mosaic virus (BSMV) has been engineered to deliver gRNAs into the leaves of wheat and maize (Hu et al., 2019). However, it remains unknown if the edited sequence changes are inheritable. Here, we developed a BSMV‐mediated gRNA delivery system to create inheritable mutations in TaHRC of FHB‐susceptible wheat cultivars.
We firstly overexpressed Cas9 (Cas9‐OE) in Bobwhite using particle bombardment transformation and then engineered a single guide RNA (sgRNA) into the downstream of the γb ORF in a BSMV RNA γ genome vector (Figure 1a). We constructed sgRNAs for two wheat genes, phytoene desaturase (TaPDS) for albinism and TaHRC for FHB resistance, using this vector and then agroinfiltrated four‐week‐old N. benthamiana leaves with mixed Agrobacterium cultures harbouring equal concentrations of the BSMVα, β and γ::TaPDS, or BSMVα, β and γ::TaHRC vectors. The inoculated tobacco leaves were checked for the presence of gRNA fragments using RT‐PCR at six days post‐inoculation (dpi), and then, the sap was used to inoculate Cas9‐OE Bobwhite wheat plants at the two‐leaf stage (Figure 1b). At 21 dpi, the mutation efficiency (indel%) was evaluated for TaPDS (58%) and TaHRC (49%) in the systemically infected wheat leaves using T7 endonuclease I (T7E1) mutation detection assays and further confirmed by Sanger sequencing (Figure 1c‐d). To evaluate the feasibility to use this system for multiplex gene editing, mixed Agrobacterium cultures harbouring BSMVγ::TaPDS and BSMVγ::TaHRC vectors were co‐agroinfiltrated into tobacco leaves, and then, the infected sap was used to inoculate the prepared Cas9‐OE Bobwhite plants mentioned above. The multiplex gene editing system effectively delivered gRNA and precisely edited the target wheat gene although the mutation efficiency was slightly lower (41% for TaPDS and 47% for TaHRC) than those for singleplex gene editing (Figure 1c, d, e).
Figure 1.
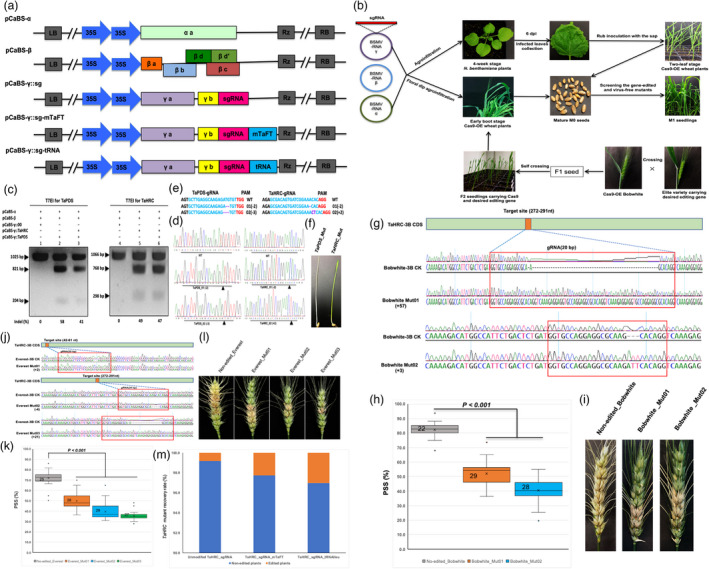
A BSMV‐mediated gene editing system for wheat. (a) BSMV vectors used in wheat gene editing. (b) Workflow for the BSMV‐mediated gene editing system. (c) T7EI assays detected TaPDS (1025 bp) and TaHRC (1066 bp) in BSMV infected Cas9‐overexpressed wheat leaves. Lanes 1 to 3 are the vector control and two mutants with TaPDS‐specific cleavage bands (821 and 204 bp). Lanes 4 and 6 are the vector control and two mutants with TaHRC‐specific cleavage bands (768 and 298 bp). (d) and (e) Edited In/del sequences at TaPDS and TaHRC target sites of the mutants. (f) Seedlings of a TaPDS albino mutant (left) and TaHRC mutant. (g) Sequence of edited and non‐edited TaHRC in Bobwhite showing insertions in Mut01 (57 bp) and Mut02 (3 bp). (h) and (i) FHB symptoms and percentages of symptomatic spikelets (PSS) between the mutants and control. (j) Sequences of edited and non‐edited TaHRC in Everest. (k) and (l) PSS and infected spikes of mutants and control. (m) Recovery rates of TaHRC mutants generated using TaHRC_sgRNA, TaHRC_sgRNA‐mTaFT and TaHRC_sgRNA‐tRNAIleu vectors. In (d) and (e), 01 = Mut01 and 02 = Mut02. In (e) and (j), + and − refer to inserted and deleted nucleotides in bp, respectively. In (g) and (j), orange bars are target sites of gRNAs. In (h) and (k), boxes, whiskers, center line, crosses and numbers represent 25th–75th percentile, ranges, medians, means and sample size. P‐values were from Student’s t‐tests.
To determine whether the edited sequences are heritable, we screened 187 M1 plants for TaPDS and 245 M1 plants for TaHRC and identified one TaPDS albino mutant and two TaHRC mutants (Figure 1f). To improve the edited mutant recovery rate, we used the floral‐dip agroinfiltration method (Zale et al., 2009) to treat the spikes of Cas9‐OE Bobwhite at preanthesis (Feekes 10.0), and obtained two mutants, Bobwhite_Mut01 and Bobwhite_Mut02 with 57‐ and 3‐nucleotide insertions at TaHRC, respectively (Figure 1c, e, g). These mutants were inoculated and phenotyped for FHB resistance as previously described (Su et al., 2019). The percentage of symptomatic spikelets (PSS) in a spike in the mutants was significantly lower than the non‐edited control at 14 dpi (Figure 1h‐i), demonstrating that the BSMV‐mediated gene editing is heritable and knockout of the TaHRC susceptible allele improved wheat FHB resistance.
To expand the utility of this editing system in other wheat cultivars with low transformation efficiency, we transferred the Cas9 gene into a locally adapted winter wheat cultivar 'Everest' by crossing Everest to a Cas9‐OE Bobwhite plant and selecting Cas9‐OE Everest F2 progeny that carried homozygous Cas9 and the target gene. The immature spikes of the selected Cas9‐OE Everest were agroinfiltrated with the mixed Agrobacterium cultures harbouring the BSMVα, β and γ::TaHRC vectors using the floral dip method. Sequencing 318 M1 plants at the target site identified three Everest‐TaHRC mutants with a 2 bp insertion (+2) in Everest_Mut01, a 4 bp deletion (−4) in Everest_Mut02 and a 21 bp insertion (+21) in Everest_Mut03 (Figure 1j). FHB phenotyping in a growth chamber showed a significantly lower PSS in the mutants than in the non‐edited Everest (Figure 1k‐l), confirming that loss‐of‐function mutations in TaHRC increased FHB resistance in the Everest derivatives.
A recent study demonstrated that edited mutant recovery rate can be increased by adding endogenous mobile RNA sequences such as Flowering Locus T (FT) and transferring RNA‐like sequences (tRNAs) to the 3‐end of sgRNAs (Ellison et al., 2020). Here, we fused a truncated wheat FT RNA sequence (mTaFT) with 135 bp of wheat Vrn3‐a allele and a tRNAIleu mobile sequence (isoleucine) to the 3′‐end of the sgRNA in the BSMV RNA γ genome vector that targeted the TaHRC in the Cas9‐OE wheat plants. We obtained a higher editing rate of TaHRC mutant lines from the M1 progeny (2.3% and 3.0%) using the modified TaHRC_sgRNA_mTaFT and TaHRC_sgRNA_tRNAIleu constructs than those (0.8%) using the TaHRC_sgRNA construct alone (Figure 1m). These results indicate that adding RNA mobility sequences in the virus vector increased the heritable sequence mutations in the edited genes and mutant recovery rate in wheat, concurring with Ellison et al. (2020), but disagreeing with Li et al. (2021). Different lengths or types of RNA mobile elements fused to the sgRNA in BSMV vector might contribute to the discrepancy.
In summary, we developed and optimized a BSMV‐mediated gRNA delivery system to edit TaHRC in Bobwhite and Everest, which has potential for application in routine wheat breeding to improve FHB resistance without genotype limitation.
Conflicts of interests
The authors declare no conflicts of interests.
Author contribution
HC and GB designed the project and wrote the manuscript. HC, ZS, BT, YL, YP, HT and VK performed experiments and approved the manuscript.
Acknowledgements
This is contribution number 22‐051‐J from the Kansas Agricultural Experiment Station. We thank Dr. Dawei Li for providing the BSMV clones. This project was partially supported by the US Wheat and Barley Scab Initiative and the USDA National Research Initiative Competitive Grants 2022‐68013‐36439 (WheatCAP).
Chen, H. , Su, Z. , Tian, B. , Liu, Y. , Pang, Y. , Kavetskyi, V. , Trick, H. N. and Bai, G. (2022) Development and optimization of a Barley stripe mosaic virus‐mediated gene editing system to improve Fusarium head blight resistance in wheat. Plant Biotechnol. J., 10.1111/pbi.13819
References
- Bai, G. , Su, Z. and Cai, J. (2018) Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 40, 336–346. [Google Scholar]
- Chen, K. , Wang, Y. , Zhang, R. , Zhang, H. and Gao, C. (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 70, 667–697. [DOI] [PubMed] [Google Scholar]
- Ellison, E. , Nagalakshmi, U. , Gamo, M. , Huang, P. , Dinesh‐Kumar, S. and Voytas, D. (2020) Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants, 6, 620–624. [DOI] [PubMed] [Google Scholar]
- Hu, J. , Li, S. , Li, Z. , Li, H. , Song, W. , Zhao, H. , Lai, J. et al. (2019) A Barley stripe mosaic virus‐based guide RNA delivery system for targeted mutagenesis in wheat and maize. Mol. Plant Pathol. 20, 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Hu, J. , Sun, Y. , Li, B. , Zhang, D. , Li, W. , Liu, J. et al. (2021) Highly efficient heritable genome editing in wheat using an RNA virus and bypassing tissue culture. Mol. Plant, 14, 1787–1798. [DOI] [PubMed] [Google Scholar]
- Su, Z. , Bernardo, A. , Tian, B. , Chen, H. , Wang, S. , Ma, H. , Cai, S. et al. (2019) A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 51, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Zale, J.M. , Agarwal, S. , Loar, S. and Steber, C. (2009) Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens . Plant Cell Rep. 28, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]


