Summary
Sheath blight (ShB) significantly threatens rice yield production. However, the underlying mechanism of ShB defence in rice remains largely unknown. Here, we identified a highly ShB‐susceptible mutant Ds‐m which contained a mutation at the ammonium transporter 1;1 (AMT1;1) D358N. AMT1;1 D358N interacts with AMT1;1, AMT1;2 and AMT1;3 to inhibit the ammonium transport activity. The AMT1 RNAi was more susceptible and similar to the AMT1;1 D358N mutant; however, plants with higher NH4 + uptake activity were less susceptible to ShB. Glutamine synthetase 1;1 (GS1;1) mutant gs1;1 and overexpressors (GS1;1 OXs) were more and less susceptible to ShB respectively. Furthermore, AMT1;1 overexpressor (AMT1;1 OX)/gs1;1 and gs1;1 exhibited a similar response to ShB, suggesting that ammonium assimilation rather than accumulation controls the ShB defence. Genetic and physiological assays further demonstrated that plants with higher amino acid or chlorophyll content promoted rice resistance to ShB. Interestingly, the expression of ethylene‐related genes was higher in AMT1;1 OX and lower in RNAi mutants than in wild‐type. Also, ethylene signalling positively regulated rice resistance to ShB and NH4 + uptake, suggesting that ethylene signalling acts downstream of AMT and also NH4 + uptake is under feedback control. Taken together, our data demonstrated that the AMT1 promotes rice resistance to ShB via the regulation of diverse metabolic and signalling pathways.
Keywords: AMT1, sheath blight, resistance, nitrogen use efficiency, rice
Introduction
Rice sheath blight disease (ShB), caused by Rhizoctonia solani Kühn (R. solani), significantly threatens worldwide rice cultivation (Molla et al., 2020). It is estimated that the yield reduction caused by ShB ranged from 8 to 50%, based on disease severity, crop stage of disease infection and environmental conditions (Savary et al., 2000). All known examples of ShB resistance are due to a quantitative trait that is controlled by multiple genes in rice, namely QTLs (quantitative trait loci). Many QTLs have been identified based on resistance to R. solani in different rice cultivars, some of which have been mapped and functionally characterized (Li et al., 1995; Richa et al., 2016, 2017). The underlying molecular mechanisms of rice resistance to ShB have been extensively investigated. PR (pathogenesis‐related) genes are known to be significant contributors to plant defence. Specifically, the PR5 family gene OsOSM1 was confirmed to improve rice resistance against ShB (Xue et al., 2016). Similarly, overexpression of the ethylene (ET) biosynthetic gene OsACS2 results in enhanced ShB resistance (Helliwell et al., 2013). A recent genome‐wide association study (GWAS) demonstrated that the F‐box protein ZmFBL41 interacts and degrades ZmCAD (a lignin biosynthesis enzyme) to inhibit ShB resistance (Li et al., 2019). Previously, we identified that IDD14 and IDD13 activate PIN1a to promote rice resistance to ShB (Sun et al., 2019a, 2020) and DEP1 interacts with IDD14 to negatively regulate rice defence to ShB ( Liu et al., 2021a). Our previous study demonstrated that brassinosteroids (BRs) are negative regulators of ShB resistance in rice, whereas ET can enhance the resistance. RAVL1, a key transcription factor of BR signalling, directly activates BR and ET signalling‐related genes to modulate the rice immunity to ShB (Yuan et al., 2018). Previous studies demonstrated that transcription factors such as OsWRKY4, 13, 30 and 80 enhance ShB resistance in rice (John Lilly and Subramanian, 2019; Peng et al., 2012, 2016; Wang et al., 2015). Later, we proposed that OsWRKY53 functions as a negative regulator in rice resistance to ShB (Yuan et al., 2020). In a more recent study, we identified that rice sugar transporters SWEET11 and SWEET14 negatively and positively regulate the rice resistance to ShB, respectively (Gao et al., 2018; Kim et al., 2021). Also, DOF11 promotes rice resistance to ShB by direct activation of SWEET14 (Kim et al., 2021).
Previous studies have revealed that high doses of nitrogen (N) fertilizer can cause a significant increase in the occurrence of ShB (Molla et al., 2020). However, limited N supply will restrict growth and yield in plants. Therefore, it is of great significance to identify genes with high nitrogen use efficiency (NUE), high resistance and high yield under low N conditions. Paddy‐soil grown rice uses ammonium (NH4 +) as the primary nitrogen source (Britto et al., 2001). There are at least ten OsAMTs that mediate NH4 + uptake in the rice genome. Three polarly localized members OsAMT1;1, OsAMT1;2 and OsAMT1;3 of the AMT1 subfamily are the primary ammonium transporters, specifically under low NH4 + conditions. The three members are cooperatively responsible for NH4 + uptake in rice (Konishi and Ma, 2021). Overexpression of OsAMT1;1, a key transporter of NH4 + increases NUE, develops larger plants, increases yield under limited NH4 + and is involved in the rice defence response against pathogens (Pastor et al., 2014; Ranathunge et al., 2014), suggesting that NH4 + uptake plays important roles in the balance of rice growth and defence. However, the detailed underlying molecular mechanisms remain unknown.
Here, the role of AMT1‐mediated NH4 + uptake and subsequent assimilation in rice defence to ShB was investigated. The data suggest that N‐metabolites rather than NH4 + regulate rice defence. In addition, the genetic and physiological experiments demonstrated that chlorophyll and amino acids, but not γ‐ Amino acid butyric acid (GABA) metabolism, positively regulate ShB resistance. N‐dependent gene expression analysis in AMT1;1 OX and AMT1 RNAi identified that ethylene biosynthetic and signalling genes were under the control of AMT1. Interestingly, ethylene signalling controls the NH4 +‐dependent AMT1 induction via feedback regulation. Taken together, our analyses provide insight into the molecular mechanism of N transport and assimilation in ShB resistance in rice and identify the new signalling pathways by which rice modulates ShB defence under NH4 + fertilizer.
Results
AMT1;1 D358N mutant accumulates less NH4 + and is more susceptible to ShB
Previously, we have isolated ShB‐resistant and susceptible genes via Ds transposon tagging in rice mutants (Sun et al., 2019b). Among the lines, one more susceptible mutant (Ds‐m (m)) was identified in this study (Figure 1a,b). However, Southern blot analysis indicated that Ds‐m did not contain the Ds fragment (Figure 1c). Since Ds‐m leaves showed a pale green phenotype and accumulated less chlorophyll compared to wild‐type (WT) (Figure 1d,e), AMTs, GS/GOGAT, and chlorophyll biosynthetic and catabolic genes were sequenced (data not shown). Interestingly, the sequencing results identified that G1072of AMT1;1was changed to A, which results in amino acid replacement from aspartic acid (D)358 to asparagine (N) (Figure 1f). D358 is located at the transmembrane helix (Figure 1g), and AMT1;1 D358N mutants accumulated less NH4 + than did in WT plant roots (Figure 1h).
Figure 1.
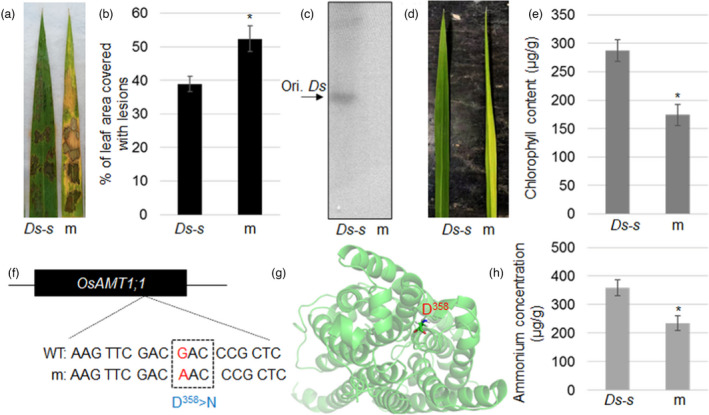
AMT1;1 D358N inhibits chlorophyll accumulation, NH4 + uptake and rice resistance to ShB. (a) Leaves of the starter line for regeneration (Ds‐s) and mutant (m) were inoculated with R. solani AG1‐IA and photographed after infection for 48 h. (b) The lesion area on the leaves shown in (a) was analysed. Data represent the means standard error (SE) (n > 10). (c) Ds insertion was examined by Southern blot analysis. GUS DNA fragment was used as a probe. Ori. Ds indicates the original Ds insertion site in Ds‐s. (d) Leaf morphology of Ds‐s and m plants. (e) Chlorophyll content from Ds‐s and m plants was determined. (f) The mutation site of in OsAMT1;1 coding region was identified. The D358 was replaced with N in m plants. (g) AMT1;1 structure display, and position of D358 at the TM helix. (h) The NH4 + content was calculated in Ds‐s and m plant roots. Significant differences at the P < 0.05 level are indicated by stars.
Before investigating the function AMT1;1 D358N, we examined the conservation of plant AMT members and conserved residues via heat map analysis of amino acid similarity. The results showed that the AMTs were highly conserved (Figure 2a). Next, the protein sequence of OsAMT1;1 was used as the reference sequence to determine consensus sites, the conservation of the primary sequences of AMTs by creation of multiple sequence alignments (MSAs) and the conserved residues are presented in Table S1 and Figure 2b. Interestingly, D358 was included within the recognized conserved amino acids. The Δmep123 yeast strain that is defective in NH4 + transport was used to test the amino acid transport activity. The yeast growth assay indicated that AMT1;1 W166F, H199F, W245F, W248F, D358N, H366F and H410F failed to transport NH4 +, while the other conserved residue mutations did not affect AMT1;1 activity (Figure 2c). These results demonstrated that D358N mutation affected AMT1;1 activity.
Figure 2.
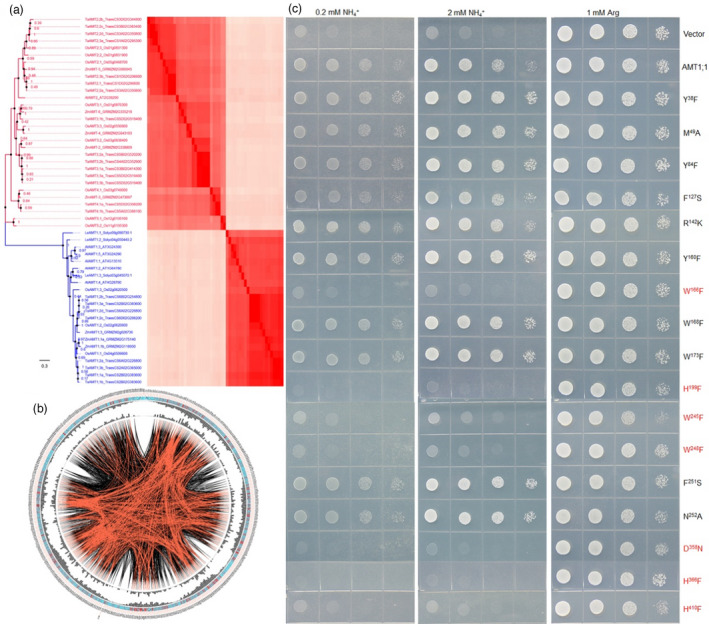
Function of the conserved residue of AMTs. (a) Phylogenetic tree for AMTs was generated using MEGA 7.0. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Heat map representation of protein sequence identities of the AMTs analysed by ClustalW. (b) Network analysis of conserved and coevolving residues from plant AMTs. The circular network shows the connectivity of coevolving residues. The coloured square boxes in the circle indicate MSA position conservation (highly conserved positions are shown in red and less conserved positions in blue). The second and third circles show the proximity mutual information (MI) and cumulative MI (cMI) values as histograms facing inward and outward respectively. In the centre of the circle, the edges that connect pairs of positions represent significant MI values (>6.5), with red lines indicating the highest MI scores (top 5%), black lines indicating midrange scores (between 70 and 95%) and grey lines indicating the lowest scores (the remaining 70%) as defined by MISTIC. (c) Except for AMT1;1 mutants Y38F, M49A, Y84F, F127S, R142K, Y160F, W168F, W173F, F251S and N252A, other mutations (W166F, H199F, W245F, W248F, D358N, H366F and H410F) in AMT1;1 led to loss of NH4 + transport activity. Empty vector (pDRf1) and AMT1;1 were used as the negative and positive controls respectively.
AMT1;1 D358N inhibits AMT1;1, AMT1;2 and AMT1;3 NH4 + transport activity to affect rice resistance to ShB
Since D358N affects AMT1;1 function and AMT1;1 D358N susceptible to ShB, AMT1;1 D358N overexpressors (OXs) and AMT1;1 RNAi plants were examined to test the defence response. Analysis by qRT‐PCR showed that AMT1;1 expression was higher in AMT1;1 D358N OXs (OX1, OX2) and significantly lower in AMT1;1 RNAi (Ri1, Ri2) plants compared to WT (Figure 3a). The methyl‐ammonium (MeA) uptake assay demonstrated that AMT1;1 D358N OXs and AMT1;1 RNAi plants (Li et al., 2016) were insensitive to 10 mM of toxic ammonium analog MeA compared with WT (Figure S1a, b). R. solani inoculation results showed that AMT1;1 D358N OXs were more susceptible than WT, while AMT1;1 RNAi exhibited a response similar to WT (Figure 3b). The percentage of leaf area covered with lesions was 41.3% in WT, 42.8% in AMT1;1 RNAi (Ri1) and 58.7% in AMT1;1 D358N OX1 plants (Figure 3c), suggesting that AMT1;1 D358N rather than AMT1;1 RNAi plants are more susceptible to ShB.
Figure 3.
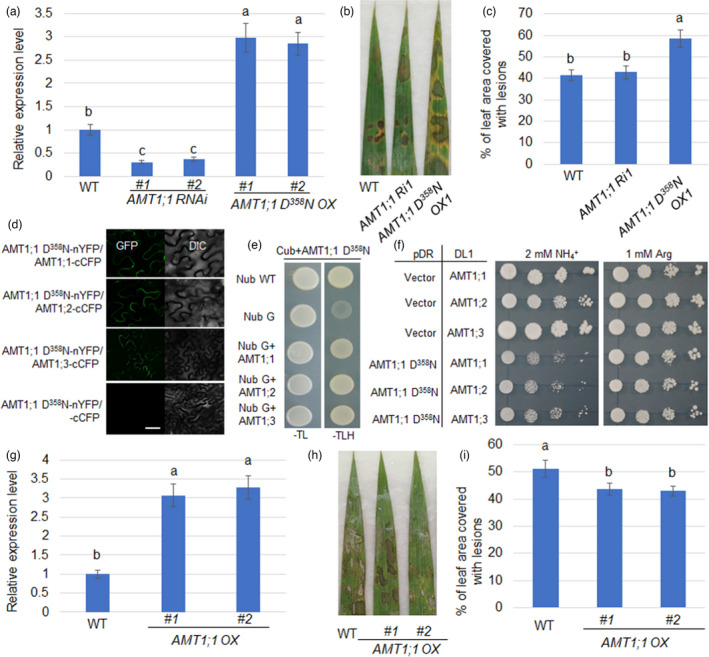
AMT1;1 D358N inhibits AMT1;1, AMT1;2 and AMT1;3 NH4 + transport activity to affect rice resistance to ShB. (a) qRT‐PCR was performed to analyse the expression level of AMT1;1 in AMT1;1 D358N OXs (OX1, OX2), AMT1;1 RNAi (Ri1, Ri2) and wild‐type. Sample mRNA levels were normalized to those of Ubiquitin mRNA. Error bars represent means ± SE (n = 3). (b)The leaves of wild‐type, AMT1;1 RNAi (Ri1) and AMT1;1 D358N OX1 were infected with R. solani AG1‐IA and photographed after 3 days of infection. Six leaves from each line were analysed, and the experiments were repeated three times. (c) The lesion area was calculated from the leaves shown in (b). Data represent means standard error (SE) (n > 10). (d) Reconstitution of the YFP fluorescence from AMT1;1 D358N‐nYFP‐AMT1;1‐cCFP, AMT1;1 D358N‐nYFP‐AMT1;2‐cCFP or AMT1;1 D358N‐nYFP‐AMT1;3‐cCFP (left, fluorescence channel; right, bright field). Co‐expression of AMT1;1 D358N‐nYFP‐cCFP was used as the negative control. Bars = 20 μm. (e) The interaction of AMT1;1 D358N and AMT1;1, AMT1;2 or AMT1;3 was tested via split‐ubiquitin yeast two‐hybrid assays. (f) Yeast growth assay was performed using Δmep123 strain to detect the NH4 + transport activity of AMT1;1, AMT1;2 or AMT1;3 with co‐expression of AMT1;1 D358N. Δmep123 was also used to generate the strain DL1 by integrating AMT1s into the Gap1 gene to generate ΔGap1::AMT1;1, ΔGap1::AMT1;2 and ΔGap1::AMT1;3. pDR‐f1 vector was used to express AMT1;1 D358N. The yeast cells were grown on solid yeast nitrogen‐based (YNB) medium at pH 5.2 containing 2% glucose, 2 mM ammonium chloride or 1 mM arginine as sole N source, at 28°C for 3 days. (g) The AMT1;1 expression level was identified in AMT1;1 OXs (OX1, OX2) by qRT‐PCR. Sample mRNA levels were normalized to those of Ubiquitin mRNA. Error bars represent means ± SE (n = 3). (h) The leaves of wild‐type and AMT1;1OX were infected with R. solani AG1‐IA and photographed after 3 days of infection. Six leaves from each line were analysed, and the experiments were repeated three times. (i) The lesion scales were analysed for the R. solani AG1‐IA‐infected leaves shown in (h) by determination of the lesion area on the leaf surface. Data represent means ± standard error (SE) (n > 10). Significant differences at the P < 0.05 level are indicated by different letters.
A previous study demonstrated that AtAMT1;3 T464D mutant inactivates AtAMT1;1 and AtAMT1;3 function (Yuan et al., 2013). Therefore, the functional interaction between AMT1;1 D358N and AMT1;1, AMT1;2 or AMT1;3 was tested. Bimolecular fluorescence complementary (BiFC) and split‐ubiquitin yeast two‐hybrid assays showed that AMT1;1 D358N interacted with AMT1;1, AMT1;2 or AMT1;3 (Figure 3d,e). To test whether this interaction affects AMT1;1, AMT1;2 or AMT1;3 NH4 + transport activity, yeast growth assays were performed using the Δmep123 strain. The stable integration of AMT1;1, AMT1;2 or AMT1;3 into the yeast genome (Δgap1::AMT1;3) restored the growth defect of the yeast mutant DL1. However, episomal co‐expression of AMT1;1 D358N resulted in significant inhibition of yeast growth phenotypes that demonstrated the inhibition of NH4 + transport activity by AMT1;1, AMT1;2 or AMT1;3 (Figure 3f).
The above results suggest that AMT1;1 D358N is a dominant‐negative mutation that inhibits AMT1;1, AMT1;2 or AMT1;3 activity. The AMT1 RNAi (suppression of all AMT1;1, AMT1;2 and AMT1;3) plant (Kumar et al., 2020) and AMT1;1 overexpressors (OXs) response to ShB was examined. The AMT1;1 expression level was significantly higher in AMT1;1 OXs (OX1, OX2) (Figure 3g), and AMT1;1 OXs were more sensitive to MeA (Figure S1c,d). Inoculation with R. solani showed that AMT1;1 OXs were less susceptible to ShB compared to WT (Figure 3h). The percentage of leaf area covered with lesions was 40.6% in WT, 28.9% in AMT1;1 OX1, and 29.4% in AMT1;1 OX2 plants (Figure 3i). To avoid side effects from overexpression, AMT1;1 endogenous promoter was used to drive AtAMT1;3 T464D‐A141E Amtrac, a high‐capacity ammonium sensor, which has higher ammonium transport activity (De et al., 2013). Semi‐quantitative PCR detected heterologous expression of AtAMT1;3 T464D‐A141E in rice (Figure S2a). The AtAMT1;3 T464D‐A141E‐expressing plants accumulated more NH4 + than WT (Figure S2b). Inoculation with R. solani demonstrated that AtAMT1;3 T464D‐A141E‐expressing plants were less susceptible to ShB compared to WT (Figure S2c). The leaf area covered with lesions corresponded to 41.2% in WT, 30.4% in AtAMT1;3 T464D‐A141E‐1 and 31.3% in AtAMT1;3 T464D‐A141E‐2 plants (Figure S2d).
N‐metabolites, rather than NH4 + itself, regulate rice resistance to ShB
Since AMT1;1 promotes rice resistance to ShB, the role of the NH4 + and N‐metabolites in rice defence to ShB was further investigated. Glutamine synthetase 1;1 (GS1;1) is the key GS enzyme in rice (Tabuchi et al., 2005). The gs1;1 mutant was more susceptible, while GS1;1 OXs were less susceptible to ShB compared to WT (Figure 4a,b). Furthermore, GS1;1 expression was significantly higher in GS1;1 OXs than in WT (Figure S3a). To further identify whether AMT1;1‐mediated rice resistance to ShB requires GS1;1 activity, the genetic combination of AMT1;1 OX and gs1;1 was generated. Inoculation with R. solani revealed that AMT1;1 OX was less susceptible, while AMT1;1 OX/gs1;1 and gs1;1 were significantly more susceptible to ShB compared to WT plants (Figure 4c,d). In addition, the NH4 + content in all four genotypes (WT, gs1;1, AMT1;1 OX and AMT1;1 OX/gs1;1) was analysed. The results demonstrated that gs1;1 and AMT1;1 OX accumulated higher NH4 + than WT. The highest NH4 + content was detected in AMT1;1 OX/gs1;1 plants (Figure 4e), suggesting that N‐metabolites rather than NH4 + regulate ShB resistance in rice.
Figure 4.
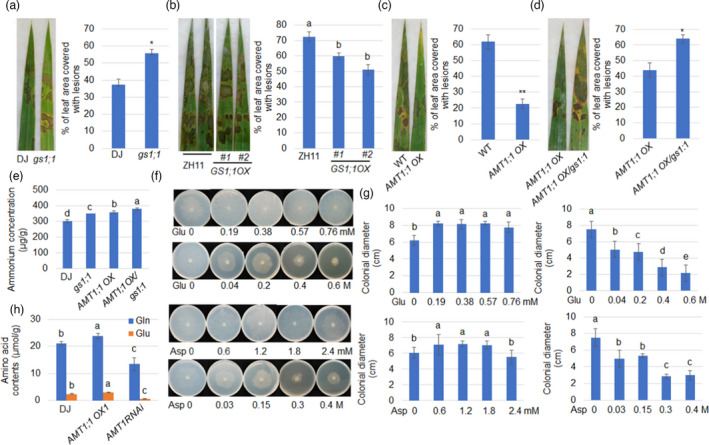
N‐metabolites rather than NH4 + regulate rice resistance to ShB. (a, b, c, d) Leaves from GS1;1 mutant (gs1;1), GS1;1 rice overexpression lines (OX1 and OX2), AMT1;1 OX1, AMT1;1 OX1/gs1:1 and together with their wild‐type plants were challenged with R. solani AG1‐IA. The corresponding statistical results of the lesion area were calculated. Six leaves from each line were analysed, and the experiments were repeated three times. Data represent the means ± standard error (SE) (n > 10). Significant differences at P < 0.05 are indicated by different letters. (e) Endogenous NH4 + levels in gs1;1, AMT1;1 OX1, AMT1;1 OX1/gs1;1 and wild‐type plants were measured in roots grown in 0.5 × MS for 3 days. (f, g) R. solani AG1‐IA was cultured on a Czapek–Dox medium with the addition of different concentrations of amino acids (glutamic acid and aspartic acid), and the colony diameter (with original cake diameter) was measured after 48 hours. The experiments were repeated at least ten times. Data represent means ± standard error (SE) (n > 10). Significant differences at P < 0.05 are indicated by different letters. (h) Glutamate and glutamine concentrations were measured from 10‐day‐old wild‐type, AMT1;1 OX1 and AMT RNAi plant leaves grown in hydroponics for 4 weeks with a spectrophotometric method. Data represent the means ± standard error (SE) (n > 10).
To explore how N metabolism affects the interaction between rice and R. solani, the function of amino acids during R. solani growth was examined. The results indicated that some common amino acids, such as glutamate (Glu), glutamine (Gln), aspartic acid (Asp), asparagine (Asn), phenylalanine (Phe), proline (Pro) and alanine (Ala), promoted the growth of hyphae in low N concentrations, while the growth of mycelia was inhibited in high N concentrations (Figure 4f,g and Figure S3). Also, amino acid concentration measurements demonstrated that AMT1;1 OX mutant contained more, but AMT1 RNAi contains less Glu and Gln than WT plants (Figure 4h). γ‐Amino Butyric Acid (GABA) is a non‐protein amino acid that is also an important product of N metabolism. It has been reported to play key roles in a variety of physiological processes in plants (Deng et al., 2020). However, overexpressing glutamate decarboxylase (GDCi‐OX) (Figure S4a,b), an enzyme that converts glutamate to GABA, increased the susceptibility of rice to ShB (Figure S4b,c). In addition, high concentrations of GABA (10 mM) promoted hyphae growth (Figure S4d,e), suggesting that amino acids and not GABA may regulate rice resistance to ShB.
Chlorophyll accumulation promotes rice defence in response to ShB
Nitrogen metabolism is involved in the synthesis of many nitrogen‐containing compounds (Baslam et al., 2020). A notable example is glutamate that forms 5‐aminolevulinic acid (ALA) through glutamyl tRNA reductase (GluTR) and glutamate‐1‐semialdehyde aminotransferase (GSA), functioning as the precursor to chlorophyll. Therefore, N is an important component of chlorophyll (Eckhardt et al., 2004). AM T1 RNAi and gs1;1 showed a pale green leaf phenotype and contain less chlorophyll, while AMT1;1 OX and GS1;1 OX accumulated more chlorophyll (Figure 5a,b,c). Also, our previous transcriptome data showed that R. solani inoculation altered N uptake, assimilation and chlorophyll synthesis (CHLD, CHLI, CHLM, PORB, DVR, CHLG and PORA) and catabolic gene (NOL, PAO, NYC3 and SGR) expression (Yuan et al., 2020) (Figure 5d). Inoculation of two rice mutants of chlorophyll synthesis‐related genes DVR (3,8‐divinyl protochlorophyllide a 8‐vinyl reductase) and YGL8 (yellow‐green leaf 8) (Kong et al., 2016; Nagata et al., 2005) with R. solani showed that dvr and ygl8 were more susceptible than WT plants (Figure 5e,f). Next, inoculation of RNAi and overexpression lines of chlorophyll degradation‐related gene NYC3 (α/β hydrolase‐fold family protein) (Cao et al., 2021) demonstrated that NYC3‐OX was more susceptible, while NYC3‐RNAi was less susceptible to ShB compared to WT plants (Figure 5g,h). These results are consistent with the recently published data showing that chlorophyll content is positively correlated with ShB defence in rice (Cao et al., 2021).
Figure 5.
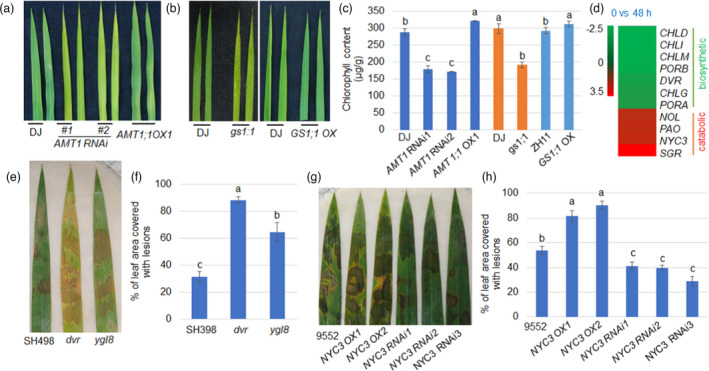
Chlorophyll accumulation promotes rice defence to ShB. (a) The leaves of AMT1 RNAi (#1 and #2), AMT1;1 OX and wild‐type were photographed. (b) Leaves from gs1;1, GS1;1 OX together with their corresponding wild‐type were photographed. (c) The chlorophyll contents of AMT1 RNAi, AMT1;1 OX and GS1;1 mutants as well as their corresponding wild‐type were determined. (d) R. solani AG1‐IA dependent (after 48 hours of inoculation) expression levels of chlorophyll biosynthesis and catabolic genes were shown in a heat map. (e) The response of chlorophyll synthesis‐related gene DVR and YGL8 mutants and wild‐type Shuhui498 (SH498) to R. solani. (f) The lesion scales were analysed for the R. solani AG1‐IA‐infected leaves shown in (e) by determination of the lesion area on the leaf surface. (n > 10). (g) The response of chlorophyll catabolic gene NYC3 RNAi and overexpression (OX) plants to R. solani. (h) The corresponding lesion scales were analysed by determination of the lesion area on the leaf surface. Data represent means ± standard error (SE) (n > 10). Significant differences at the P < 0.05 level are indicated by different letters.
AMT1;1‐mediated rice resistance to ShB depends on nitrogen levels
Since AMT1;1 is an ammonium transporter, the relationship between N availability and AMT1;1‐dependent rice resistance to ShB was examined under different N fertilization conditions. AMT1;1 OX, AMT1 RNAi and WT plants were cultured under high N (urea, HN 300 kg/ha), middle N (urea, MN 150 kg/ha) and low N concentrations (urea, LN 50 kg/ha) (Liu et al., 2021b). R. solani inoculation demonstrated that AMT1;1 OX plants were significantly more resistant, while AMT1 RNAi plants were more susceptible to ShB than WT under LN (Figure 6a,b) and MN (Figure 6c,d) conditions. However, the positive effect of AMT1;1 to rice defence against R. solani was eliminated under the HN fertilization conditions with no significant resistance differences among AMT1;1 OX, AMT1 RNAi and WT plants (Figure 6e,f). Total N content was also measured in AMT1;1 OX, AMT1 RNAi and WT plants that were grown under LN, MN or HN conditions. The results indicated that AMT1;1 OX accumulated higher, while AMT1 RNAi contained less N compared to WT under LN and MN conditions. Under HN conditions, AMT1 RNAi accumulated slightly less total N than AMT1;1 OX and WT plants, while AMT1;1 OX contained a similar level of total N compared to WT (Figure 6g,h).
Figure 6.
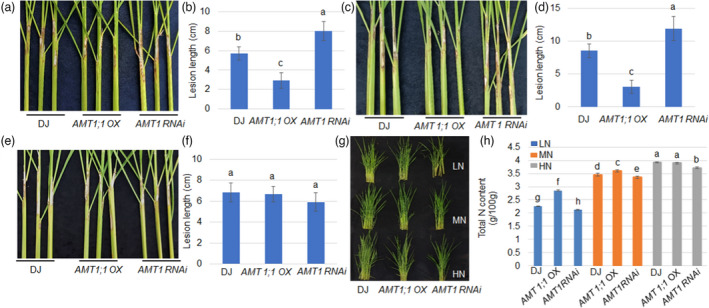
The effects of nitrogen fertilization level on AMT1;1‐mediated rice resistance to ShB. (a) Wild‐type (DJ), AMT1;1OX and AMT1RNAi plants cultured in LN supply condition (50 kg/ha) were inoculated with R. solani AG1‐IA for 14 d. (b) The lesion lengths shown in (a) were measured and statistically analysed. Data represent means ± standard error (SE) (n > 10). (c) Wild‐type (DJ), AMT1;1OX and AMT1RNAi plants cultured in LN supply condition (150 kg/ha) were inoculated with R. solani AG1‐IA for 14 d. (d) The lesion lengths shown in (c) were measured and statistically analysed. Data represent means ± standard error (SE) (n > 10). (e) Wild‐type (DJ), AMT1;1OX and AMT1RNAi plants cultured in HN supply condition (300 kg/ha) were inoculated with R. solani AG1‐IA for 14 d. (f) The lesion lengths shown in (C) were measured and statistically analysed. Data represent means ± standard error (SE) (n > 10). (g) Rice seedlings of wild‐type, AMT1;1 OX and AMT1RNAi plants grown under LN, MN and HN conditions for 14 days. (h) The total nitrogen from wild‐type, AMT1;1 OX and AMT1RNAi plants was determined by the Kjeldahl method. Data represent means ± standard error (SE) (n > 10). Significant differences at the P < 0.05 level are indicated by different letters.
Feedback activation of NH4 + uptake by ethylene signalling is important for rice resistance to ShB
Our previous transcriptome study identified that ET biosynthesis and signalling genes were up‐regulated after NH4 + treatment (Xuan et al., 2013). The qRT‐PCR results verified the transcriptome data where ACO2, ACO3, EIN2, EIL1 and ERFs were significantly induced with NH4 + treatment (Figure 7a). Previously, we identified that ET signalling positively regulates rice resistance to ShB (Yuan et al., 2018), suggesting that NH4 + assimilation may activate ET signalling to promote rice resistance to ShB. To test this hypothesis, ERS1, ETR2, EIL1, and EIL2 expression was examined in AMT1;1 OX, AMT1 RNAi and WT plants under LN conditions. qRT‐PCR results showed that two positive ET signalling regulators EIL1 (Mao et al., 2006) and EIL2 (Yang et al., 2015) expression levels were higher in AMT1;1 OX and lower in AMT1 RNAi than in WT. The expression of ETR2 (Wuriyanghan et al., 2009) and ERS1 (Ma et al., 2014), two negative ET signalling regulators, was suppressed and induced in AMT1;1 OX and AMT1 RNAi, respectively, than in WT under LN conditions (Figure 7b). However, EIL1 and EIL2 expression levels were lower, while ERS1 expression was higher in AMT1;1 OX than in WT plants under HN. Furthermore, the ETR2 expression level was similar in WT, AMT1;1 OX and AMT1;1 RNAi plants under HN (Figure S5). Interestingly, we found that NH4 +‐mediated induction of AMT1;1 and AMT1;2 was inhibited in eil1 mutants (Yuan et al., 2018) (Figure 7c) and that eil1 mutants accumulated less NH4 + than WT plants (Figure 7d).
Figure 7.
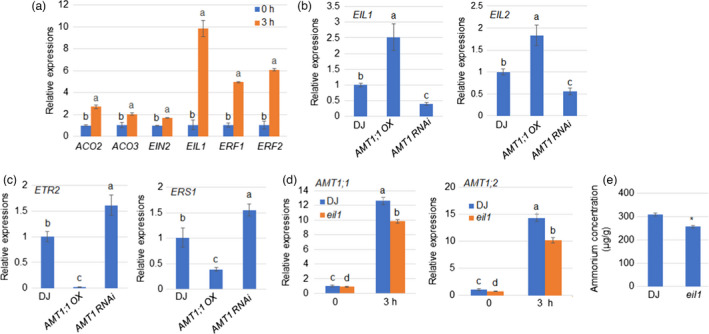
Feedback activation of NH4 + transport via ethylene signalling is important for rice resistance to ShB. (a) NH4 +‐dependent expression levels ACO2, ACO3, EIN2, EIL1 and ERFs were verified by qRT‐PCR with NH4 + treatment for 3 hours. (b) Positive ethylene signalling regulators EIL1 and EIL2 and (c) negative ethylene signalling regulators ERS1 and ETR2 expression levels were monitored in wild‐type (DJ), AMT1;1 OX and AMT1 RNAi plants grown in LN conditions for 14 days. (d) NH4 +‐induced AMT1;1 and AMT1;2 expression levels in WT and EIL1 mutant (eil1) was monitored. Sample mRNA levels were normalized to those of Ubiquitin mRNA. Error bars represent means ± SE (n = 3). (e) Endogenous NH4 + levels in WT and eil1 were measured in roots grown in 0.5 × MS for 3 days. Error bars represent means ± SE (n = 20). Significant differences at the P < 0.05 level are indicated by different letters.
AMT1;1 OX increases yield and resistance under LN conditions
Previous studies demonstrated that overexpression of AMT1;1 enhances NH4 + uptake and improves rice growth and yield at least under specialized N fertilization conditions (Ranathunge et al., 2014). Tillering is an important trait for grain yield in rice. Mature AMT1;1 RNAi plants developed significantly fewer tillers than in WT and AMT1;1 OX, while WT and AMT1;1 OX produced a similar number of tillers (Figure 8a,b). Furthermore, less filled grains per panicle and lower total grain yield per panicle were found in AMT1;1 RNAi than in WT and AMT1;1 OX. However, no differences were identified between WT and AMT1;1 OX (Figure 8c,d). The thousand‐grain weight was similar between WT and AMT1;1 RNAi, while AMT1;1 OX was higher than WT (Figure 8e).
Figure 8.
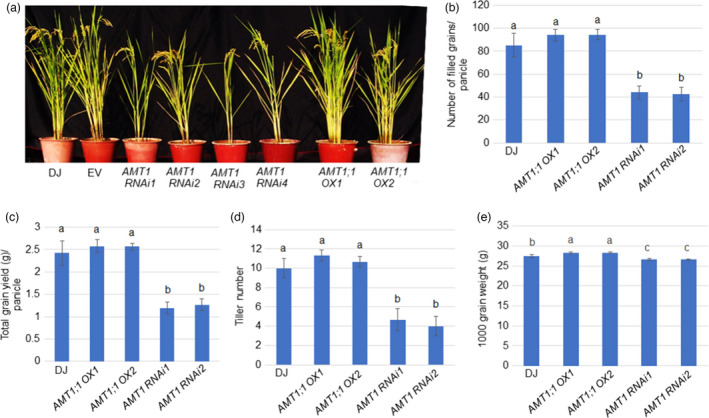
Comparison of yield index between AMT1;1 OX, AMT1 RNAi and WT plants grown in LN condition. (a) Mature plant morphology, (b) tiller number, (c) filled grains per panicle, (d) total grain yield and (e) 1000 grain weight were calculated. Data are means ± SD of 12 plants. Significant differences at the P < 0.05 level are indicated by different letters.
Discussion
ShB is one of the most important diseases, which severely affects the quality and quantity of production in rice. However, the underlying rice defence mechanisms remain largely unknown. In this study, the AMT1;1 function in rice defence to ShB was explored by analysing the roles of NH4 + and N‐metabolites as well as ET signalling during the defence process. The data illustrated that AMT1;1‐mediated NH4 + transport accelerated N metabolism and regulated subsequent NH4 +‐dependent ethylene‐related gene expression to promote rice resistance to ShB under limited N fertilizer conditions, suggesting that appropriate N uptake and assimilation are necessary for rice defence activation.
AMT1;1 D358N interacts with and inhibits AMT1;1, AMT1;2 and AMT1;3 to control rice defence
A pale green mutant Ds‐m was identified in the Ds‐tagging mutant pool, which was more susceptible to ShB than WT. However, Southern blot results verified that the Ds‐m phenotype was not caused by a Ds insertion. Since glutamate is the precursor of chlorophyll and forms ALA through GluTR and GSA (Eckhardt et al., 2004), AMT, GS/GOGAT, and chlorophyll biosynthetic and catabolic genes were sequenced in the Ds‐m mutant. Interestingly, Ds‐m contained a point mutation at G1072A, which resulted in the D358N change. Conserved residues and subsequent functional analysis revealed that D358 was highly conserved among plant AMTs and D358N replacement abolished AMT1;1 NH4 + activity. Furthermore, AMT1;1 D358N OX and AMT1 RNAi (suppression of AMT1;1, AMT1;2 and AMT1;3) plants accumulated less NH4 + and were more susceptible to ShB. However, suppression of a single AMT1;1 by RNAi did not inhibit rice resistance to ShB, suggesting that AMT1;1 D358N‐susceptible symptom may be caused by other mechanisms. A previous report demonstrated that AMT forms a homo‐ or hetero‐trimer and AtAMT1;3 T464D interacts and inhibits AtAMT1;1 NH4 + transport activity (Yuan et al., 2013). Our analysis indicated that AMT1;1 D358N interacted with AMT1;1, AMT1;2 and AMT1;3 and inhibited their NH4 + transport activity. AMT1;1, AMT1;2 and AMT1;3 are colocalized in the endodermis cell layer and are cooperatively responsible for the NH4 + transport in rice (Konishi and Ma, 2021), suggesting that AMT1;1 D358N‐mediated inhibition of AMT1;1, AMT1;2 and AMT1;3 activity can occur in planta. In other words, AMT1;1 D358N plants inhibit the function of AMT1;1, AMT1;2 and AMT1;3 to reduce rice resistance to ShB.
N‐metabolites, but not NH4 +, promote ShB resistance in rice
AMT1;1 D358N and AMT1 RNAi plants that contained less cellular NH4 + were more susceptible. However, AMT1;1 OX and pAMT1;1‐high‐capacity Amtrac plants that accumulated more cellular NH4 + were less susceptible to ShB, suggesting that cellular NH4 + is positively correlated with rice resistance. Next, the key glutamine synthetase gene mutant gs1;1 was more susceptible while GS1;1 OX was less susceptible to ShB, despite the increased and reduced cellular NH4 + in gs1;1 and GS1;1 OX respectively. These results suggest that NH4 + may not be the molecule responsible for the control of ShB resistance in rice. To definitively verify these results, AMT1;1 was overexpressed in the gs1;1 background. The results demonstrated that AMT1;1 OX/gs1;1 was similar to gs1;1 where increased cellular NH4 + was accumulated and the plants were more susceptible to ShB. Therefore, AMT1;1‐mediated rice resistance is required during the NH4 + assimilation process.
NH4 + is incorporated into the glutamine amide group by GS (Mur et al., 2017). A recent study reported that amino acid metabolism is an important process in the nitrogen‐mediated plant defence mechanism (Sun et al., 2020). Therefore, the direct role of amino acids on the growth of R. solani hyphae was investigated. The amino acids tested including Glu and Gln all inhibited R. solani growth at high concentrations. The AMT1;1 OX plants accumulated more Glu and Gln compared to WT. These data suggest that AMT1;1‐mediated defence may partially act via accumulation of amino acids to inhibit R. solani growth. However, GABA is a non‐protein amino acid that promoted R. solani growth even at high concentrations. Also, GABA biosynthetic gene overexpression plants GDCi OX were more susceptible to ShB, indicating that GABA negatively regulates rice resistance to ShB.
Glutamate is the precursor of chlorophyll (Eckhardt et al., 2004), and AMT1;1 D358N, AMT1 RNAi and gs1;1 accumulated less chlorophyll and were more susceptible to ShB. Our transcriptome results suggested that R. solani infection significantly suppressed chlorophyll biosynthesis gene expression while inducing chlorophyll catabolic gene expression, suggesting a potential function of chlorophyll in rice defence. A genetic study by testing the chlorophyll biosynthetic gene DVR and YGL8 mutants (Kong et al., 2016; Nagata et al., 2005), as well as chlorophyll catabolic gene NYC3 mutant (Cao et al., 2021), revealed that chlorophyll content was positively correlated with rice resistance to ShB (Cao et al., 2021). These results suggest that AMT1‐mediated NH4 + transport and assimilation promote chlorophyll synthesis by which rice partially increased resistance to ShB.
Ethylene signalling activates NH4 + uptake via feedback regulation to promote rice resistance to ShB
Cellular NH4 + is not only used to synthesize amino acids but also functions as a signal molecule to regulate global gene expression (Patterson et al., 2010). We further investigated whether other signalling pathways regulate AMT1;1‐mediated rice resistance, aside from N metabolism. Plant hormone signalling is tightly associated with rice defence to ShB (Molla et al., 2020). We previously identified that NH4 + treatment regulates the expression of auxin signalling genes (Xuan et al., 2018) and demonstrated that auxin signalling activation via exogenous IAA application improves rice resistance to ShB (Sun et al., 2019a), implying that NH4 + supply may modulate auxin signalling to regulate rice resistance to ShB. In addition, our previous studies identified that ET biosynthesis and signalling genes were induced by NH4 + treatment (Xuan et al., 2013) and that ET signalling promotes rice resistance to ShB (Yuan et al., 2018), suggesting that NH4 + signalling may activate ET signalling to promote rice resistance. Our analyses identified that EIL1 and EIL2 which activate ethylene signalling were positively regulated while ETR2 and ERS1, two negative regulators of ethylene signalling, were suppressed by AMT1;1 under the LN conditions. However, under the HN conditions, EIL1 and EIL2 expression levels were significantly lower while ETR2 and ERS1 levels were higher in AMT1;1 OX than in WT. These results suggest that ET signalling may be sensitive to the cellular N levels and may be associated with rice resistance to ShB. Furthermore, we identified that NH4 +‐mediated induction of AMT1 genes was inhibited in the key ET signalling gene eil1 mutant. The eil1 mutant accumulated less NH4 +, suggesting that ethylene signalling controls NH4 + transport via feedback regulation to fine‐tune the cellular N transport and assimilation, which may be important for rice defence and growth.
AMT1;1 OX increases rice resistance and NUE under limited N fertilizer
Nitrogen fertilizers supplied to rice crops are partially lost via various mechanisms including ammonia volatilization, denitrification and leaching, causing environmental concerns by polluting the atmosphere, aquatic systems and groundwater (Choudhury and Kennedy, 2005). Therefore, limiting the amounts of NH4 + applied to the fields without loss of crop yield is an important agricultural strategy in rice. Our results demonstrated that AMT1;1 OX plants significantly promoted rice resistance to ShB under the LN conditions. Under HN conditions, AMT1;1 OX accumulated similar NH4 + content compared to WT and also exhibited a similar ShB response to WT plants. As previously reported, AMT1;1 OX uptake more NH4 + and significantly increase yield production at least under specialized N fertilization conditions (Ranathunge et al., 2014). Our data also confirmed that AMT1;1 OX produced a relatively higher yield, suggesting that AMT1;1 OX increased NUE and ShB resistance in rice.
In this study, the data demonstrated that AMT1;1‐mediated increase in rice resistance was via N‐metabolite activation and ethylene signalling. This study demonstrates the precise use of nitrogen based on the underlying molecular mechanisms of N metabolism to improve yield production and immunity against ShB and other pathogens in rice.
Materials and methods
Plant growth and R. solani AG1‐IA inoculation
All of the rice plants treated with R. solani were cultured in the Shenyang Agriculture University greenhouse at 23–30°C, 80% relative humidity (RH) and 12‐h light/12‐h dark photoperiod. Nicotiana benthamiana plants were grown in environmental chambers at 22–24°C, 80% RH and 16‐h light/8‐h dark photoperiod for 4 weeks before use. The R. solani strain AG1‐IA was cultured on solid PDA (Potato Dextrose Agar) medium at 28°C in an incubator. Rice was inoculated according to previously reported methods (Cao et al., 2021).
Molecular phylogenetic analysis using maximum likelihood
The amino acid sequences of AMT proteins in rice, maize, Arabidopsis, wheat and potato were used as bait for searching in the Uniprot database (http://www.uniprot.org/) using BLASTp. MSAs of these protein sequences were conducted using the Clustal Omega program (Larkin et al., 2007). Phylogenetic relationships were inferred using the maximum likelihood (ML) methods with 1,000 bootstrap iterations (Kumar et al., 2016).
Ammonium uptake assay in yeast
The yeast strain 31019b (Δmep123), which is defective in NH4 + absorption (Marini et al., 1997), was used to test the NH4 + transport activity of AMT1;1 and AMT1;1 mutants. Δmep123 was also used to construct the strain DL1 by introducing AMT1s into the Gap1 gene locus to generate ΔGap1::AMT1;1, ΔGap1::AMT1;2 and ΔGap1::AMT1;3. The pDR‐f1 vector was used to express AMT1;1 D358N, following previously reported methods (Yuan et al., 2013). The yeast transformants were grown on solid yeast nitrogen‐based (YNB) medium at pH 5.2 containing 2% glucose (w/v), 2 mM ammonium chloride or 1 mM arginine as the sole nitrogen source.
Vector construction and transgenic plant generation
AMT1;1 promoter was fused to an Amtrac high‐capacity gene ORF in the pGA1611 binary vector. The primers used for plasmid construction are listed in Table S3. pAMT1;1:Amtrac high capacity was transformed into Japonica rice cultivar Dongjin (DJ) calli via Agrobacterium‐mediated transformation method (Hiei et al., 1994). The gs1;1 mutant (PFG_3A‐09512) was obtained from a rice T‐DNA mutants collection (http://signal.salk.edu/cgi‐bin/RiceGE/) (An et al., 2003). The overexpression of GS1;1 and GDCi was generated from the rice cultivar Zhonghua 11 (ZH11). The modified pCAMBIA1381‐Ubi vector was used to construct GS1;1 and GDCi overexpression vectors at HindIII/KpnI and HindIII/HpaI respectively. The primers used for the GS1;1 and GDCi overexpression vector constructions are listed in Table S4.
Analysis of amino acid effects on R. solani growth
R. solani AG1‐IA was cultured on a Czapek–Dox medium with the addition of different concentrations of amino acids. Colonized PDA plugs (7 mm in diameter) were excised using a hole borer and transferred to the centre of the fresh media surface. These petri dishes were then cultured in a 37°C incubator for 42 hours, and the diameters of the colonies were measured. The assays were conducted repeatedly at least eight times.
Determination of NH4 + and total N content
The NH4 + content in roots and shoots of 7‐day‐old rice seedlings was measured using an F‐kit (Roche) according to the manufacturer’s instructions (Oliveira et al., 2002). The total N content in rice plants was determined by the Kjeldahl method using the Hanon k1160 Automatic Kjeldahl nitrogen determinator (Shandong, China).
RNA extraction and qRT‐PCR analysis
Total RNA was extracted from the one‐month‐old leaves from tested rice plants using TRIzol reagent (Takara, Dalian, Liaoning, China). Elimination of genomic DNA and reverse transcription reactions were performed according to the manufacturer’s instructions using the commercial kit (Takara, Dalian, Liaoning, China). qRT‐PCR analysis was performed using the CFX96 real‐time PCR system (Bio‐Rad, Hercules, CA, USA) and ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, Jiangsu, China). Gene expression values were normalized against Ubiquitin values in the same samples. Two technical and three biological replicates were used for each analysis. The primers used for qRT‐PCR are listed in the supplemental Table S2.
Determination of chlorophyll content
The chlorophyll content in leaves of one‐month‐old plants was determined using the ultraviolet spectrophotometer following a previously reported method (Lichtenthaler, 1987).
Amino acid measurement
Gln and Glu content was measured using an L‐Glu analysis kit (Yamasa, Tokyo, Japan) following the manufacturer’s instructions (Hirano et al., 2008).
Split‐ubiquitin yeast two‐hybrid assay
AMT1;1, AMT1;2 and AMT1;3 were fused to the N‐terminus of Ubiquitin through Nub vector pXN25_GW and AMT1;1 D358N was fused to the C‐terminus of Ubiquitin through Cub vector pMETYC_GW based on standard GATEWAY cloning protocol (Invitrogen, CA, USA). Yeast two‐hybrid assays were performed according to a previously published method (Lalonde et al., 2010).
BiFC and southern blotting assays
AMT1;1 D358N was cloned into a YFPN vector, while AMT1;1, AMT1;2 and AMT1;3 were cloned into CFPC plasmids. The constructs were co‐transformed into tobacco leaves using Agrobacterium strain GV3101 (Kim et al., 2009). The YFP fluorescence signals were observed under a confocal microscope (Olympus FV1000, Japan) 36 to 48 hours after infiltration. Southern Blotting assay of Ds insertion was carried out with reference to the method described by a previous study (Xuan et al., 2016).
Statistical analyses
Statistical analyses were conducted using Prism 5.0 software (GraphPad, San Diego, CA, USA) with a one‐way analysis of variance (ANOVA) for comparison of significant differences between multiple groups. Also, Student’s t‐test was used to compare the differences between the two groups. Differences between the groups were considered significant with at least P < 0.05.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
XXW and YHX planned and designed the research. XXW, HC, DPY, VK and SMK performed most of the experiments. XXW, BLJ and DPY analysed data. XXW, BLJ and YHX wrote the manuscript. XXW, HC, DPY and VK contributed equally to this work.
Supporting information
Table S1. Gateway primers used in this study.
Table S2. qRT‐PCR and RT‐PCR primers used in this study.
Table S3. Primers used in this study for the construction of plants expressing AtAMT1;3 T464D‐A141E driven by AMT1;1 endogenous promoter.
Table S4. Primers used in this study for the construction of the overexpression vector.
Figure S1. Sensitivity test of AMT1;1 RNAi and overexpression plants to methyl‐ammonium (MeA).
Figure S2. AtAMT1;3 T464D‐A141E expression promotes rice resistance to ShB.
Figure S3. Verification of the effects of amino acids on R. solani growth.
Figure S4. Identification of the effect of GABA on rice resistance against ShB.
Figure S5. Expression levels of ethylene signalling genes under HN conditions in wild‐type, AMT1;1 OX and AMT1;1 RNAi plants were quantified by qRT‐PCR.
Acknowledgements
This work was supported by the Nature Science Foundation of Liaoning (2020‐YQ‐05) and the National Natural Science Foundation of China (32072406). We appreciate very much Prof. Chang‐deok Han from Gyeongsang National University for providing AMT1 RNAi, AMT1;1 OX and gs1;1 mutant seeds, Prof. Wolf Frommer from Carnegie Institution for Science for providing the Amtrac high‐capacity plasmids, and Prof. Shimin Zuo from Yangzhou University for providing chlorophyll synthetic and catabolic gene mutant seeds.
Wu, X. X. , Yuan, D. P. , Chen, H. , Kumar, V. , Kang, S. M. , Jia, B. and Xuan, Y. H. (2022) Ammonium transporter 1 increases rice resistance to sheath blight by promoting nitrogen assimilation and ethylene signalling. Plant Biotechnol. J., 10.1111/pbi.13789
Contributor Information
Baolei Jia, Email: baoleijia@cau.ac.kr.
Yuan Hu Xuan, Email: xuanyuanhu115@syau.edu.cn.
References
- An, S. , Park, S. , Jeong, D.‐H. , Lee, D.‐Y. , Kang, H.‐G. , Yu, J.‐H. , Hur, J. et al. (2003) Generation and analysis of end sequence database for T‐DNA tagging lines in rice. Plant Physiol. 133, 2040–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslam, M. , Mitsui, T. , Sueyoshi, K. and Ohyama, T. (2020) Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 22, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, W. , Zhang, H. , Zhou, Y. , Zhao, J. , Lu, S. , Wang, X. , Chen, X. et al. (2021) Suppressing chlorophyll degradation by silencing OsNYC3 improves rice resistance to Rhizoctonia solani, the causal agent of sheath blight. Plant Biotechnol J, 20, 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, A.T.M.A. and Kennedy, I.R. (2005) Nitrogen fertilizer losses from rice soils and control of environmental pollution problems. Commun. Soil Sci. Plant Anal. 36, 1625–1639. [Google Scholar]
- De Michele, R. , Ast, C. , Loqué, D. , Ho, C.‐H. , Andrade, S.L.A. , Lanquar, V. , Grossmann, G. et al. (2013) Fluorescent sensors reporting the activity of ammonium transceptors in live cells. Elife, 2, e00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X. , Xu, X. , Liu, Y. , Zhang, Y. , Yang, L. , Zhang, S. and Xu, J. (2020) Induction of γ‐aminobutyric acid plays a positive role to Arabidopsis resistance against Pseudomonas syringae . J. Integrat. Plant Bio. 62, 1797–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt, U. , Grimm, B. and Hörtensteiner, S. (2004) Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 56, 1–14. [DOI] [PubMed] [Google Scholar]
- Gao, Y. , Zhang, C. , Han, X. , Wang, Z.Y. , Ma, L. , Yuan, P. , Wu, J.N. et al. (2018) Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease. Mol. Plant Pathol. 19, 2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell, E.E. , Wang, Q. and Yang, Y. (2013) Transgenic rice with inducible ethylene production exhibits broad‐spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol. J. 11, 33–42. [DOI] [PubMed] [Google Scholar]
- Hiei, Y. , Ohta, S. , Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Hirano, T. , Satoh, Y. , Ohki, A. , Takada, R. , Arai, T. and Michiyama, H. (2008) Inhibition of ammonium assimilation restores elongation of seminal rice roots repressed by high levels of exogenous ammonium. Physiol. Plant, 134, 183–190. [DOI] [PubMed] [Google Scholar]
- John, L.J. and Subramanian, B. (2019) Gene network mediated by WRKY13 to regulate resistance against sheath infecting fungi in rice (Oryza sativa L.). Plant Sci. 280, 269–282. [DOI] [PubMed] [Google Scholar]
- Kim, J.G. , Li, X. , Roden, J.A. , Taylor, K.W. , Aakre, C.D. , Su, B. , Lalonde, S. et al. (2009) Xanthomonas T3S effector XopN suppresses PAMP‐triggered immunity and interacts with a tomato atypical receptor‐like kinase and TFT1. Plant Cell, 21, 1305–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, P. , Xue, C.Y. , Song, H.D. , Gao, Y. , Feng, L. , Li, Y.H. and Xuan, Y.H. (2021) Tissue‐specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol. J. 19, 409–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, W. , Yu, X. , Chen, H. , Liu, L. , Xiao, Y. , Wang, Y. , Wang, C. et al. (2016) The catalytic subunit of magnesium‐protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice. Plant Mol. Biol. 92, 177–191. [DOI] [PubMed] [Google Scholar]
- Konishi, N. and Ma, J.F. (2021) Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol. 232, 1778–1792. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. and Tamura, K. (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V. , Kim, S.H. , Priatama, R.A. , Jeong, J.H. , Adnan, M.R. , Saputra, B.A. , Kim, C.M. et al. (2020) NH4+ suppresses NO3–‐dependent lateral root growth and alters gene expression and gravity response in OsAMT1 RNAi mutants of rice (Oryza sativa). J. Plant Biol. 63, 391–407. [Google Scholar]
- Lalonde, S. , Sero, A. , Pratelli, R. , Pilot, G. , Chen, J. , Sardi, M.I. , Parsa, S.A. et al. (2010) A membrane protein/signaling protein interaction network for Arabidopsis version AMPv2. Front. Physiol., 1, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, M.A. , Blackshields, G. , Brown, N.P. , Chenna, R. , McGettigan, P.A. , McWilliam, H. , Valentin, F. et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948. [DOI] [PubMed] [Google Scholar]
- Li, C. , Tang, Z. , Wei, J. , Qu, H. , Xie, Y. and Xu, G. (2016) The OsAMT1.1 gene functions in ammonium uptake and ammonium‐potassium homeostasis over low and high ammonium concentration ranges. J. Genet. Genomics, 43, 639–649. [DOI] [PubMed] [Google Scholar]
- Li, N. , Lin, B. , Wang, H. , Li, X. , Yang, F. , Ding, X. , Yan, J. et al. (2019) Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat. Genet. 51, 1540–1548. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Pinson, S.R.M. , Marchetti, M.A. , Stansel, J.W. and Park, W.D.J.T. (1995) Characterization of quantitative trait loci (QTLs) in cultivated rice contributing to field resistance to sheath blight (Rhizoctonia solani). Theoretical Appl. Genet., 91, 382–388. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler, H.K. (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 148, 350–382. [Google Scholar]
- Liu, J.M. , Mei, Q. , Xue, C.Y. , Wang, Z.Y. , Li, D.P. , Zhang, Y.X. and Xuan, Y.H. (2021a) Mutation of G‐protein γ subunit DEP1 increases planting density and resistance to sheath blight disease in rice. Plant Biotechnol. J. 19, 418–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Wang, H. , Jiang, Z. , Wang, W. , Xu, R. , Wang, Q. , Zhang, Z. et al. (2021b) Genomic basis of geographical adaptation to soil nitrogen in rice. Nature, 590, 600–605. [DOI] [PubMed] [Google Scholar]
- Ma, B. , Yin, C.C. , He, S.J. , Lu, X. , Zhang, W.K. , Lu, T.G. , Chen, S.Y. et al. (2014) Ethylene‐induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet, 10, e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, C. , Wang, S. , Jia, Q. and Wu, P. (2006) OsEIL1, a rice homolog of the Arabidopsis EIN3 regulates the ethylene response as a positive component. Plant Mol. Biol. 61, 141–152. [DOI] [PubMed] [Google Scholar]
- Marini, A.M. , Soussi‐Boudekou, S. , Vissers, S. and Andre, B. (1997) A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell Biol. 17, 4282–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla, K.A. , Karmakar, S. , Molla, J. , Bajaj, P. , Varshney, R.K. , Datta, S.K. and Datta, K. (2020) Understanding sheath blight resistance in rice: the road behind and the road ahead. Plant Biotechnol J. 18, 895–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur, L.A.J. , Simpson, C. , Kumari, A. , Gupta, A.K. and Gupta, K.J. (2017) Moving nitrogen to the centre of plant defence against pathogens. Ann Bot. 119, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata, N. , Tanaka, R. , Satoh, S. and Tanaka, A. (2005) Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell, 17, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, I.C. , Brears, T. , Knight, T.J. , Clark, A. and Coruzzi, G.M. (2002) Overexpression of cytosolic glutamine synthetase. relation to nitrogen, light, and photorespiration. Plant Physiol. 129, 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor, V. , Gamir, J. , Camanes, G. , Cerezo, M. , Sanchez‐Bel, P. and Flors, V. (2014) Disruption of the ammonium transporter AMT1.1 alters basal defenses generating resistance against Pseudomonas syringae and Plectosphaerella cucumerina . Front. Plant Sci., 5, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, K. , Cakmak, T. , Cooper, A. , Lager, I. , Rasmusson, A.G. and Escobar, M.A. (2010) Distinct signalling pathways and transcriptome response signatures differentiate ammonium‐and nitrate‐supplied plants. Plant Cell Environ., 33, 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, X. , Hu, Y. , Tang, X. , Zhou, P. , Deng, X. , Wang, H. and Guo, Z. (2012) Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta, 236, 1485–1498. [DOI] [PubMed] [Google Scholar]
- Peng, X. , Wang, H. , Jang, J.C. , Xiao, T. , He, H. , Jiang, D. and Tang, X. (2016) OsWRKY80‐OsWRKY4 module as a positive regulatory circuit in rice resistance against Rhizoctonia solani . Rice, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge, K. , El‐Kereamy, A. , Gidda, S. , Bi, Y.M. and Rothstein, S.J. (2014) AMT1;1 transgenic rice plants with enhanced NH4(+) permeability show superior growth and higher yield under optimal and suboptimal NH4(+) conditions. J. Exp. Bot. 65, 965–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richa, K. , Tiwari, I.M. , Kumari, M. , Devanna, B.N. , Sonah, H. , Kumari, A. , Nagar, R. et al. (2016) Functional characterization of novel chitinase genes present in the sheath blight resistance QTL: qSBR11‐1 in rice line tetep. Front. Plant Sci. 7, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richa, K. , Tiwari, I.M. , Devanna, B.N. , Botella, J.R. , Sharma, V. and Sharma, T.R. (2017) Novel chitinase Gene LOC_Os11g47510 from Indica rice tetep provides enhanced resistance against sheath blight pathogen Rhizoctonia solani in rice. Front. Plant Sci. 8, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary, S. , Willocquet, L. , Elazegui, F.A. , Castilla, N.P. and Teng, P.S. (2000) Rice pest constraints in tropical Asia: quantification of yield losses due to rice pests in a range of production situations. Plant Dis. 84, 357–369. [DOI] [PubMed] [Google Scholar]
- Sun, Q. , Li, D.D. , Chu, J. , Yuan, P. , Li, S. , Zhong, L.J. , Han, X. and et al.(2020) Indeterminate domain proteins regulate rice defense to sheath blight disease. Rice, 13, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Li, T.Y. , Li, D.D. , Wang, Z.Y. , Li, S. , Li, D.P. , Han, X. et al. (2019a) Overexpression of loose plant Architecture 1 increases planting density and resistance to sheath blight disease via activation of PIN‐FORMED 1a in rice. Plant Biotechnol. J. 17, 855–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Q. , Liu, Y. , Wang, Z.Y. , Li, S. , Ye, L. , Xie, J.X. , Zhao, G.Q. et al. (2019b) Isolation and characterization of genes related to sheath blight resistance via the tagging of mutants in rice. Plant Gene, 19, 100200. [Google Scholar]
- Tabuchi, M. , Sugiyama, K. , Ishiyama, K. , Inoue, E. , Sato, T. , Takahashi, H. and Yamaya, T. (2005) Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. Plant J. 42, 641–651. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Meng, J. , Peng, X. , Tang, X. , Zhou, P. , Xiang, J. and Deng, X. (2015) Rice WRKY4 acts as a transcriptional activator mediating defense responses toward Rhizoctonia solani, the causing agent of rice sheath blight. Plant Mol. Biol. 89, 157–171. [DOI] [PubMed] [Google Scholar]
- Wuriyanghan, H. , Zhang, B. , Cao, W.H. , Ma, B. , Lei, G. , Liu, Y.F. , Wei, W. et al. (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell, 21, 1473–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan, Y.H. , Kumar, V. , Zhu, X.F. , Je, B.I. , Kim, C.M. , Huang, J. , Cho, J.H. et al. (2018) IDD10 is Involved in the Interaction between NH4 + and Auxin Signaling in Rice Roots. J. Plant Biol. 61, 1–8. [Google Scholar]
- Xuan, Y.H. , Peterson, T. and Han, C.D. (2016) Generation and analysis of transposon Ac/Ds‐induced chromosomal rearrangements in rice plants. Meth. Mol. Biol. 1469, 49–61. [DOI] [PubMed] [Google Scholar]
- Xuan, Y.H. , Priatama, R.A. , Huang, J. , Je, B.I. , Liu, J.M. , Park, S.J. , Piao, H.L. et al. (2013) Indeterminate domain 10 regulates ammonium‐mediated gene expression in rice roots. New Phytol. 197, 791–804. [DOI] [PubMed] [Google Scholar]
- Xue, X. , Cao, Z.X. , Zhang, X.T. , Wang, Y. , Zhang, Y.F. , Chen, Z.X. , Pan, X.B. et al. (2016) Overexpression of OsOSM1 enhances resistance to rice sheath blight. Plant Dis. 100, 1634–1642. [DOI] [PubMed] [Google Scholar]
- Yang, C. , Ma, B. , He, S.J. , Xiong, Q. , Duan, K.X. , Yin, C.C. , Chen, H. et al. (2015) MAOHUZI6/ETHYLENE INSENSITIVE3‐LIKE1 and ETHYLENE INSENSITIVE3‐LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol. 169, 148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, D.P. , Hong, W.J. , Wang, S.T. , Jia, X.T. , Liu, Y. , Li, S. , Li, Z.M. et al. (2020) Transcriptome analysis of rice leaves in response to Rhizoctonia solani infection and reveals a novel regulatory mechanism. Plant Biotechnol. Rep. 14, 559–573. [Google Scholar]
- Yuan, L. , Gu, R. , Xuan, Y. , Smith‐Valle, E. , Loque, D. , Frommer, W.B. and von Wiren, N. (2013) Allosteric regulation of transport activity by heterotrimerization of Arabidopsis ammonium transporter complexes in vivo. Plant Cell, 25, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, P. , Zhang, C. , Wang, Z.Y. , Zhu, X.F. and Xuan, Y.H. (2018) RAVL1 activates brassinosteroids and ethylene signaling to modulate response to sheath blight disease in rice. Phytopathology, 108, 1104–1113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gateway primers used in this study.
Table S2. qRT‐PCR and RT‐PCR primers used in this study.
Table S3. Primers used in this study for the construction of plants expressing AtAMT1;3 T464D‐A141E driven by AMT1;1 endogenous promoter.
Table S4. Primers used in this study for the construction of the overexpression vector.
Figure S1. Sensitivity test of AMT1;1 RNAi and overexpression plants to methyl‐ammonium (MeA).
Figure S2. AtAMT1;3 T464D‐A141E expression promotes rice resistance to ShB.
Figure S3. Verification of the effects of amino acids on R. solani growth.
Figure S4. Identification of the effect of GABA on rice resistance against ShB.
Figure S5. Expression levels of ethylene signalling genes under HN conditions in wild‐type, AMT1;1 OX and AMT1;1 RNAi plants were quantified by qRT‐PCR.


