Abstract
Recent studies have shown that heat shock proteins and trehalose synthesis are important factors in the thermotolerance of the fission yeast Schizosaccharomyces pombe. We examined the effects of trehalose-6-phosphate (trehalose-6P) synthase overexpression on resistance to several stresses in cells of S. pombe transformed with a plasmid bearing the tps1 gene, which codes for trehalose-6P synthase, under the control of the strong thiamine-repressible promoter. Upon induction of trehalose-6P synthase, the elevated levels of intracellular trehalose correlated not only with increased tolerance to heat shock but also with resistance to freezing and thawing, dehydration, osmostress, and toxic levels of ethanol, indicating that trehalose may be the stress metabolite underlying the overlap in induced tolerance to these stresses. Among the isogenic strains transformed with this construct, one in which the gene coding for the trehalose-hydrolyzing enzyme, neutral trehalase, was disrupted accumulated trehalose to a greater extent and was more resistant to the above stresses. Increased trehalose concentration is thus a major determinant of the general stress protection response in S. pombe.
Considerable evidence has accumulated in recent years to indicate that the intracellular level of trehalose may determine the survival response of yeasts under extreme environmental conditions (43, 45). The protective function of trehalose against stress can be interpreted in terms of the water replacement hypothesis or the glass transition hypothesis (5, 8). As predicted for such a role, trehalose shows remarkable stress protection properties in vitro (9) and accumulates in vivo when cells approach stationary phase as well as during sublethal heat treatment and by the action of other stressors (2, 20, 35, 43, 45). The homeostasis of trehalose in yeasts is controlled by the synthesizing enzyme complex trehalose-6-phosphate (trehalose-6P) synthase and the hydrolyzing enzyme(s) trehalase(s).
Most studies showing strong correlations between trehalose content and stress resistance have been performed by using the budding yeast Saccharomyces cerevisiae as a model (45). This system, however, has several important drawbacks. First, the analysis that can be performed in this yeast with mutants in which tps1 has been disrupted is limited. Cells unable to synthesize trehalose have a complex pleiotropic phenotype which includes lack of growth on readily fermentable sugars, loss of many regulatory responses, and deficient sporulation (42). Moreover, two distinct trehalases are present in S. cerevisiae (27), and their relative functions in trehalose metabolism are not clearly defined. The neutral (cytosolic) trehalase is generally considered to be involved in the intracellular degradation of trehalose (33), whereas the acid (vacuolar) trehalase appears to determine growth on exogenous trehalose (32). In addition, many studies on the role of trehalose in stress resistance include experimental strategies involving induced tolerance, by which cells become resistant to an otherwise lethal stress through adaptive treatment with a mild stress. Although this approach allows for the examination of particular changes accompanying tolerance acquisition, it also causes many different stress resistance determinants to be expressed and may mask the contribution from other stress-related factors. Indeed, several independent changes are known to occur during the adaptive period that induce tolerance to many types of stress (26, 34). These changes raise the question of whether an increase in trehalose content is merely a circumstantial event rather than a crucial factor. Others (1, 18) have found no obvious relationship between trehalose accumulation and tolerance and have questioned the relevance of trehalose as a determinant of resistance to some stresses.
In general, there is broad consensus that trehalose can serve as a stress protectant when yeast cells are challenged with high temperatures (2, 20). The correlation between trehalose accumulation and improved tolerance of stresses such as lyophilization (16), hyperosmotic shock (28), or freezing (19, 36) is much more limited. Fewer studies of trehalose as a resistance metabolite have been made with the fission yeast Schizosaccharomyces pombe than with S. cerevisiae. However, the S. pombe system has several advantages. Unlike S. cerevisiae, vegetative cells of this yeast contain only one trehalase (13), and mutants in which tps1 has been deleted do not present the growth limitations shown by their baker’s yeast counterparts (3). Recently, evidence has been obtained from mutants defective in trehalose-6P synthase function to suggest that trehalose synthesis is required for the in vivo acquisition of thermotolerance in S. pombe subjected to severe heat preconditioning (39).
The simplest and most direct approach to evaluating the role of trehalose in protection against stress-induced injuries is to induce selective changes in trehalose content without simultaneously triggering significant changes in other cellular parameters. We addressed this problem by inducing overexpression, under normal physiological conditions, of the tps1 gene, which codes for trehalose-6P synthase, in S. pombe wild-type cells; cells in which tps1 had been deleted, which were unable to synthesize trehalose (3); and cells in which ntp1 had been deleted, which were devoid of neutral trehalase activity (6). The results indicate that modulation of tps1 function in S. pombe markedly affects tolerance not only of temperature upshifts but also of other stresses, including freezing and thawing, dehydration, and growth in the presence of normally toxic concentrations of ethanol and NaCl. Our procedure allows us to manipulate trehalose levels without prestressing the cells and demonstrates a central role for trehalose in fission yeast stress response.
MATERIALS AND METHODS
Yeast strains and culture conditions.
The S. pombe strains used in this study are shown in Table 1. Transformation of strains MM-1 (control), PBU13 (Δtps1), and MMT-3 (Δntp1) with plasmid pREP3X-tps1 was carried out by the lithium acetate method as described elsewhere (7). pREP3X-tps1 contains the gene encoding trehalose synthase in S. pombe under the control of the nmt1 thiamine-repressible promoter (7, 30). The overexpression phenotype in transformed strains was determined after 24 h of growth in the absence of thiamine. Cells were routinely grown with shaking (160 rpm) at 28°C in EMM2 with or without thiamine (5 mg/liter) (7). Culture media were supplemented with adenine and/or uracil (100 mg/liter) depending on the requirements of each particular strain.
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| MM-1 | h+ade6-M216 leu1-32 ura4-D18 | 6 |
| PBU13 | h+ade6-M216 leu1-32 ura4-D18 Δtps1::ura4+ | 3 |
| MMT-3 | h+ade6-M216 leu1-32 ura4-D18 Δntp1::ura4+ | 6 |
| SS10 | h+ade6-M216 leu1-32 ura4-D18 (pREP3X-tps1) | This work |
| SS1 | h+ade6-M216 leu1-32 ura4-D18 Δtps1::ura4+ (pREP3X-tps1) | This work |
| SS15 | h+ade6-M216 leu1-32 ura4-D18 Δntp1::ura4+ (pREP3X-tps1) | This work |
Heat shock treatment.
Cells were withdrawn from mid-exponential-phase cultures grown at 28°C for 24 h and transferred into fresh medium prewarmed at 48°C for the specified times. The heat-treated samples were then cooled in an ice bath. Afterwards, the cell suspensions were briefly vortexed, appropriately diluted, and spread in triplicate onto plates containing EMM2 solid medium with thiamine. The viability of the cells was measured by their ability to form colonies on EMM2 solid medium after incubation at 28°C for 8 days. The survival fraction was calculated, for each sample separately, as a percentage relative to the survival of control samples maintained without heat treatment.
Freezing and thawing conditions.
Cells from mid-log-phase cultures were obtained by centrifugation, washed twice with sterile distilled water, and resuspended so that the final cell concentration was similar in all samples within the same experiment. Freeze-thaw resistance was measured according to reference 25. Essentially, 100-μl aliquots of the cell suspensions were transferred to 1.5-ml microcentrifuge tubes that were submerged in liquid nitrogen for 5 min. The tubes were thawed for 5 min in a 25°C water bath. The number of survivors was determined by plating dilutions onto EMM2 agar with thiamine and incubating them at 28°C for 8 days before colony counting. The percentage of survival was determined relative to the survival of control samples that received no freeze-thaw treatment.
Dehydration resistance.
Cells were removed from log-phase cultures after 24 h of growth, collected by centrifugation, washed twice with sterile distilled water, and finally resuspended in 1/100 of the original volume in 200 mM glycerol. Suspensions were frozen in liquid nitrogen for 1 min and kept for 16 h at −75°C before lyophilization in a Christ (Osterode, Germany) Alpha 1-2 freeze-dryer. After dehydration, the cells were rehydrated with distilled water, diluted, and sampled in triplicate to determine viability by using the conventional spread-plate technique on EMM2 agar containing thiamine. The colony count after 8 days at 28°C was expressed as a percentage of the number of viable cells before dehydration.
Ethanol tolerance.
Ethanol (15%) was added to mid-exponential-phase cultures pregrown for 24 h. After 5 min of ethanol treatment, culture samples were removed and the cells were washed twice with distilled water and, after appropriate dilutions, spread on EMM2 agar plates supplemented with thiamine. In parallel experiments, aliquots of the original cell culture received spent medium instead of ethanol and were similarly spread. Viable colonies were counted in both cases after 8 days at 28°C, and viability was expressed as the relative percentage of survival.
Growth with NaCl.
The growth of strain SS1 was determined on plates with solid medium supplemented with NaCl. Cells were grown first on liquid EMM2 with or without thiamine, and aliquots (5 μl) of serial dilutions of each culture were spotted on solid EMM2 containing different concentrations of NaCl in the presence or absence of thiamine.
Analytical determinations.
Intracellular trehalose was extracted and estimated as described in reference 14. Extraction of total RNA, Northern blot hybridization, and quantitative analysis of tps1 mRNA were performed as previously described (7, 15).
RESULTS
Trehalose content in cells overexpressing thiamine-controlled trehalose-6P synthase.
Trehalose levels were almost below the level of detection in cells from pREP-3X-tps1-transformed strains of S. pombe when the cells were growing exponentially on glucose in EMM2 medium supplemented with thiamine (Table 2). Under conditions repressive for the expression of the plasmidic tps1 gene, trehalose accumulated to measurable levels only in cells from strain SS15, which carries a disruption in the gene encoding neutral trehalase (6). This result supports the involvement of the trehalase enzyme in trehalose breakdown in vivo. The level of the intracellular pool of trehalose was significantly higher when these strains were cultured in the same medium lacking thiamine. Northern blot analysis of tps1 mRNA confirmed that tps1 overexpression occurred under negative control by thiamine (data not shown).
TABLE 2.
Trehalose concentration in exponentially growing pREP3X-tps1-transformed cells of S. pombe under conditions of thiamine repression or derepression before and after a heat shocka
| Strain | Thiamine addition | Trehalose concn
|
|
|---|---|---|---|
| At zero time | After heat shockc | ||
| SS10 (wt)b | + | <2 | 22 ± 3.0 |
| − | 16 ± 1.9 | 47 ± 3.8 | |
| SS1 (Δtps1) | + | <2 | <2 |
| − | 18 ± 0.9 | 40 ± 3.3 | |
| SS15 (Δntp1) | + | 4.2 ± 1.1 | 50 ± 3.1 |
| − | 27 ± 1.6 | 73 ± 5.2 | |
Cultures were grown in EMM2 at exponential phase for 24 h in the presence of thiamine (repression). These cells were used as inocula for media with or without thiamine, and the basal trehalose content was determined in nanomoles per 107 cells after a further 24 h of exponential growth (zero time) or following a heat stress. Values are the means from three independent determinations ± SD.
wt, wild type.
Defined as 48°C for 30 min.
Stress resistance to hyperthermic conditions in pREP3X-tps1-transformed cells.
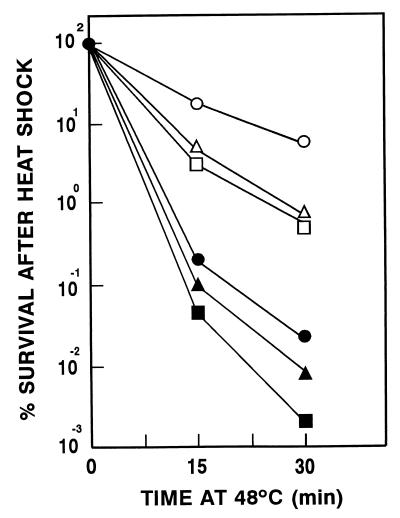
As expected, disruption of tps1 blocked trehalose synthesis (3) (Table 2) and also reduced the resistance of exponentially growing, glucose-repressed cells to the effects of a temperature upshift (Fig. 1). In contrast, overexpression of tps1 in different genetic backgrounds significantly increased trehalose concentration and cell survival during hyperthermia. For example, less than 0.01% of the cells in the wild-type strain SS10 survived a challenging heat shock (30 min at 48°C) when they were grown in a culture medium containing thiamine, which represses tps1 expression, but they were more resistant, by a factor of 2 to 3 log units, when grown in the absence of thiamine, which allows for tps1 overexpression (Fig. 1).
FIG. 1.
Thermotolerance of S. pombe SS10 (wild type) (▵), SS1 (Δtps1) (□), and SS15 (Δntp1) (○) strains containing pREP3X-tps1. Cells were grown for 24 h in EMM2 to mid-exponential phase in the presence (closed symbols) or absence (open symbols) of thiamine (5 mg/liter) and subjected to a heat shock at 48°C for the times indicated. Results shown are the means from three independent experiments with each strain. The minimum number of colonies counted for each of these determinations was 200 per plate. The standard deviations (SD) either did not exceed ±10% of the mean or were smaller than the symbols used.
Resistance to freeze-thaw stress as a function of tps1 expression.
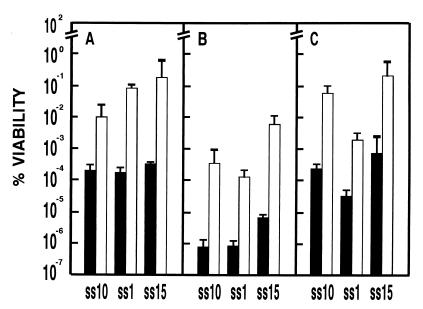
We compared the survival of cells overexpressing trehalose-6P synthase after a freeze-thaw cycle with the same cells under identical growth conditions but with plasmid-directed synthesis of trehalose repressed. While the viability of wild-type S. cerevisiae cells decreases by 99% when they are frozen directly (24), the S. pombe survival rate is even lower, less than 0.001% (Fig. 2A). Derepression of thiamine-controlled tps1 prior to freezing increased cell survival 20- to 200-fold, depending on the particular strain, demonstrating a role for trehalose in the adaptive process that confers protection against freezing (Fig. 2A). Incubation at 37°C for 1 h before freezing similarly increased cell survival in S. pombe wild-type and Δntp1 cells (data not shown). This type of thermal pretreatment induces an increase in trehalose concentration (12, 15, 39) (Table 2). Taken together, these results indicate that S. pombe strains overexpressing tps1 survive freezing better than control strains.
FIG. 2.
Colony survival of S. pombe SS10 (wild type), SS1 (Δtps1), and SS15 (Δntp1) strains containing pREP3X after different stresses. Before each treatment cells were cultured in the presence (■) or absence (□) of thiamine. (A) Freeze-thawing (B) dehydration; (C) 15% exogenous ethanol shock. Results are means from three independent experiments; error bars represent SD.
Effect of tps1 expression on resistance to dehydration stress.
We examined the effect of tps1 expression on the survival of exponential-phase cells subjected to lyophilization. Freeze-drying is a very drastic procedure that results in high mortality in vegetative cells of S. pombe (Fig. 2B). Viability after freezing and drying was extremely low in control cells that had been grown in the presence of thiamine but increased noticeably upon derepression of tps1. The magnitude of the difference in viability due to tps1 overexpression was 2 to 3 log units, depending on the strain. Differences in the extent of survival were due to changes in intracellular trehalose levels and not to other physiological changes, since all strains were examined at similar stages of growth. These results demonstrate that trehalose increases tolerance of dehydration, possibly by acting as a compatible solute during dehydration.
Correlation between intrinsic resistance to exogenous ethanol and trehalose accumulation.
Addition of 15% ethanol to exponentially growing S. pombe cultures drastically reduces viability (Fig. 2C). This reduction decreased if tps1 was induced, indicating that trehalose accumulation helps to alleviate ethanol toxicity. These results suggest that trehalose may have a common protective role in responses to diverse stress stimuli.
Trehalose content and osmostress resistance.
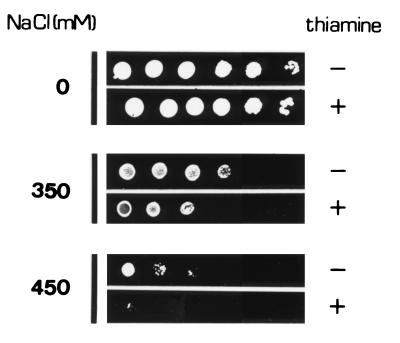
Although the tolerance for other stresses in cells overexpressing tps1 and accumulating trehalose increased by several orders of magnitude compared to that in controls (see above), salt tolerance in such cells showed a less impressive increase that was, nevertheless, consistent in all experiments (Fig. 3). These results are consistent with the hypothesis that trehalose accumulation contributes to osmotic adjustment and increases the survival and growth rates of S. pombe cells under severe osmotic stress.
FIG. 3.
Adaptation of the SS1 (Δtps1) strain of S. pombe to growth under continued osmotic stress. Cells lacking chromosomal tps1 and harboring pREP3X-tps1 were grown for 24 h in EMM2 in the absence of thiamine to overexpress tps1, plated on EMM-2 agar (without thiamine) which was supplemented with NaCl (0 to 0.45 M), and then incubated at 28°C for 8 days before being photographed. Control cells were processed in a similar manner but in the presence of thiamine (5 mg/liter). The numbers of cells spotted on solid medium containing NaCl were, from left to right, 5 × 104, 2 × 104, 104, 103, 102, and 10. One representative result is shown for each experiment.
DISCUSSION
Following exposure to a mild stress, yeast cells become resistant to a subsequent, more-severe stress that would be lethal in the absence of the conditioning pretreatment. Studies of S. cerevisiae have revealed that this adaptive response relies primarily on the increased synthesis of specialized stress proteins and/or organic solutes such as trehalose and glycerol (37). High concentrations of these small metabolites do not perturb enzyme structure or function, and these compounds can stabilize proteins in vitro (9, 14, 20). In this study, we measured the tolerance of S. pombe to stress as a function of tps1 expression and function. Unlike previous investigators (2, 12, 20), we altered the levels of intracellular trehalose by turning thiamine-controlled synthesis of trehalose-6P on and off, instead of inducing changes in the trehalose pool with stresses that could complicate the interpretation of the results. In so doing, we could separate the phenotypic effects of tps1 modulation on various stress responses from other stress-dependent changes that might simultaneously contribute to resistance. Overexpression of tps1 did not result in the accumulation of trehalose in excess of the level found in heat-stressed control cells (Table 2), suggesting the existence of some feedback control mechanism to prevent cells from exceeding a certain trehalose level under nonstressing conditions. Cells in which ntp1 had been disrupted showed the highest levels of accumulated trehalose (the SS15 strain; see Table 2).
Trehalose prevents proteins from denaturing at high temperatures and suppresses the aggregation of those already denatured, which favors their reactivation by molecular chaperones (41). Also, trehalose preserves membrane integrity during stress (11). Therefore, it was not surprising to find that strains of S. pombe overexpressing tps1 increased their thermal tolerance 100- to 1,000-fold when subjected to a temperature upshift. We used exponentially growing glucose-repressed cells of S. pombe for all experiments, and this strategy enabled us to maintain an elevated intracellular trehalose level when the trehalose pool is normally undetectable (3, 39) (Table 2).
More surprising were the results linking the trehalose content to resistance to freeze-thaw stress, a correlation not previously reported for fission yeast. Cells often acquire cross-resistance to different stresses when responding to a stress injury. In S. cerevisiae there is no inducible adaptation response to freeze-thaw stress following a cycle of freezing and thawing (36), but heat shock or osmotic stress do offer cross-protection against freeze-thawing (24, 36). Also, freeze-thaw-tolerant yeast strains have higher levels of trehalose than normal strains (19). Much of the damage to cells in a freeze-thaw stress results from freezing rather than thawing and is due primarily to physical factors such as ice crystal formation and dehydration (31, 36). To our knowledge, the effect of trehalose on ice nucleation is unclear, but its role as a substitute for water during dehydration is well documented (11). Regardless of the mechanism of action, our results indicate that in S. pombe, trehalose accumulation contributes to tolerance of freeze-thaw stress. This conclusion differs from that shown for S. cerevisiae, for which the induction of heat shock protein (hsp) is essential for the acquisition of resistance to freezing, while the presence of solutes such as trehalose or glycerol is of no benefit (25). Increased production of trehalose by S. pombe may be a cryoprotection mechanism (23) either complementary or alternative to hsp. In this case, since trehalose accumulates by means of trehalose-6P synthase, which behaves as an hsp (see below), it is difficult to distinguish between the requirement for the enzyme and a requirement for its product.
Cell membranes appear to be the primary target site of ethanol toxicity (22, 29, 38). Our data correlate increased trehalose levels with ethanol endurance and support the observations made by others that trehalose mitigates the ethanol-induced leakage of electrolytes from intact yeast cells and liposomes (22, 29). In addition to protein denaturation and membrane disordering, other changes induced by ethanol are the same as those resulting from heat stress (10, 44). Thus, cells preadapted to one type of stress might acquire tolerance to the other. We found that cells overexpressing tps1, which were resistant to heat shock, also were comparatively more ethanol tolerant, while no change in temperature sensitivity or ethanol tolerance was shown when tps1 was repressed. Thus, trehalose appears to be a key determinant of thermoresistance, tolerance to freezing, and ethanol endurance in S. pombe. This conclusion may apply to other stresses as well because induction of trehalose synthesis also confers additional tolerance to dehydration and more resistance to NaCl. Although glycerol appears to be the main osmolyte accumulated in salt-stressed yeasts and filamentous fungi (4), a possible role for trehalose in osmotolerance has recently been recognized (17, 21).
Our results are consistent with others obtained with S. cerevisiae that indicate that heat-shocked cells have enhanced tolerance to salt and freezing (25) and that salt-adapted cells are more tolerant to ethanol than control cells (40). Collectively, these data are consistent with the hypothesis that induction of defenses against some stressors may increase a cell’s resistance to other stresses. These data also suggest that trehalose synthesis is a crucial part of the general stress response that helps cells withstand extreme conditions. In this context, some effects of tps1 overexpression resemble those observed following ntp1 disruption (6), as expected from the opposite functions of trehalose-6P synthase and trehalase in trehalose metabolism.
We previously reported that the expression of tps1 and ntp1 is enhanced during hyperthermia and by the action of other stressors (15). Consequently, trehalose-6P synthase and neutral trehalase may be considered stress proteins. Recent work (41) has shown that trehalose stabilizes proteins during heat shock but interferes with refolding. This finding suggests a rapid hydrolysis of trehalose during recovery and provides an explanation for the apparent paradox of the simultaneous increase in both enzymes. We do not know if the only protective function of trehalose-6P synthase is to produce trehalose. Our present results are insufficient to determine whether enhanced transcription of tps1 or trehalose accumulation is the critical determinant of stress resistance. However, cells in which ntp1 has been disrupted, which overexpress tps1, always have higher levels of trehalose and are more stress tolerant than their ntp-containing counterparts. We interpret these data to mean that trehalose accumulation, rather than trehalose-6P synthase synthesis, is the primary mechanism of resistance. An alternative explanation is cross talk between trehalose synthesis and hsp synthesis in stress response signaling. Although previous work does not favor this possibility (39), future studies are needed to address this question definitively.
While we support the conclusion that trehalose is an important stress tolerance factor, it is probably only one of several determinants of stress resistance (37). In any case, our observations will be useful for the baking and brewing industries and for fuel production because yeast strains overexpressing tps1 can better survive temperature stress, freezing, dehydration, or the presence of high levels of salt or ethanol.
ACKNOWLEDGMENTS
We thank C. Gancedo (Madrid, Spain) for strain PBU-13, Y. Sánchez (Salamanca, Spain) for providing plasmids, and F. Garro for technical assistance.
J.F. and T.S. are recipients of a fellowship from Caja Murcia. This work was supported, in part, by grant PB97-1049 from DGICYT, Madrid, Spain.
REFERENCES
- 1.Alexandre H, Plourde L, Charpentier C, François J. Lack of correlation between trehalose accumulation, cell viability and intracellular acidification as induced by various stresses in Saccharomyces cerevisiae. Microbiology. 1998;144:1103–1111. doi: 10.1099/00221287-144-4-1103. [DOI] [PubMed] [Google Scholar]
- 2.Attfield P V. Trehalose accumulates in Saccharomyces cerevisiae during exposure to agents that induce heat shock response. FEBS Lett. 1987;225:259–263. doi: 10.1016/0014-5793(87)81170-5. [DOI] [PubMed] [Google Scholar]
- 3.Blázquez M A, Stucka R, Feldmann H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomberg A, Adler L. Physiology of osmotolerance in fungi. Adv Microb Physiol. 1992;33:145–212. doi: 10.1016/s0065-2911(08)60217-9. [DOI] [PubMed] [Google Scholar]
- 5.Burke M J. The glassy state and survival of anhydrous biological systems. In: Leopold A C, editor. Membranes, metabolism and dry organisms. Ithaca, N.Y: Cornell University Press; 1985. pp. 358–363. [Google Scholar]
- 6.Cansado J, Soto T, Fernández J, Vicente-Soler J, Gacto M. Characterization of mutants devoid of neutral trehalase activity in the fission yeast Schizosaccharomyces pombe: partial protection from heat shock and high-salt stress. J Bacteriol. 1998;180:1342–1345. doi: 10.1128/jb.180.5.1342-1345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cansado J, Vicente-Soler J, Soto T, Fernández J, Gacto M. Trehalose-6P synthase is essential for trehalase activation triggered by glucose, nitrogen source or heat shock, but not by osmostress, in Schizosaccharomyces pombe. Biochim Biophys Acta. 1998;1381:271–278. doi: 10.1016/s0304-4165(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 8.Clegg J S. The physical properties and metallic status of Artemia cysts at low water contents. The “water replacement hypothesis,”. In: Leopold A C, editor. Membranes, metabolism and dry organisms. Ithaca, N.Y: Cornell University Press; 1985. pp. 169–187. [Google Scholar]
- 9.Colaço C, Sen S, Thangavelu M, Pinder S, Roser B. Extraordinary stability of enzymes dried in trehalose: simplified molecular biology. Bio/Technology. 1992;10:1007–1011. doi: 10.1038/nbt0992-1007. [DOI] [PubMed] [Google Scholar]
- 10.Coote P J, Cole M B, Jones M V. Induction of increased thermotolerance in Saccharomyces cerevisiae may be triggered by a mechanism involving intracellular pH. J Gen Microbiol. 1991;137:1701–1708. doi: 10.1099/00221287-137-7-1701. [DOI] [PubMed] [Google Scholar]
- 11.Crowe J H, Crowe L M, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 12.De Virgilio C, Simmen U, Hottiger T, Boller T, Wiemken A. Heat shock induces enzymes of trehalose metabolism, trehalose accumulation and thermotolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett. 1990;273:107–110. doi: 10.1016/0014-5793(90)81062-s. [DOI] [PubMed] [Google Scholar]
- 13.De Virgilio C, Muller J, Boller T, Wiemken A. A constitutive, heat shock-activated neutral trehalase occurs in Schizosaccharomyces pombe in addition to the sporulation-specific acid trehalase. FEMS Microbiol Lett. 1991;84:85–90. doi: 10.1016/0378-1097(91)90400-5. [DOI] [PubMed] [Google Scholar]
- 14.Fernández J, Soto T, Vicente-Soler J, Cansado J, Gacto M. Increased thermal stability of the enzyme content in permeabilized whole cells from the fission yeast Schizosaccharomyces pombe by exogenous trehalose and other compounds. Can J Microbiol. 1995;41:936–941. doi: 10.1139/m95-129. [DOI] [PubMed] [Google Scholar]
- 15.Fernández J, Soto T, Vicente-Soler J, Cansado J, Gacto M. Heat shock response in Schizosaccharomyces pombe cells lacking cyclic AMP-dependent phosphorylation. Curr Genet. 1997;31:112–118. doi: 10.1007/s002940050183. [DOI] [PubMed] [Google Scholar]
- 16.Gadd G M, Chalmers K, Reed R H. The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol Lett. 1987;48:249–254. [Google Scholar]
- 17.García M J, Rios G, Ali R, Bellés J M, Serrano R. Comparative physiology of salt tolerance in Candida tropicalis and Saccharomyces cerevisiae. Microbiology. 1997;143:1125–1131. doi: 10.1099/00221287-143-4-1125. [DOI] [PubMed] [Google Scholar]
- 18.Gross C, Watson K. Heat shock protein synthesis and trehalose accumulation are not required for induced thermotolerance in derepressed Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1996;220:766–772. doi: 10.1006/bbrc.1996.0478. [DOI] [PubMed] [Google Scholar]
- 19.Hino A, Mihara K, Nakasima K, Takano H. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl Environ Microbiol. 1990;56:1386–1391. doi: 10.1128/aem.56.5.1386-1391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hottiger T, Boller T, Wiemken A. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 1987;220:113–115. doi: 10.1016/0014-5793(87)80886-4. [DOI] [PubMed] [Google Scholar]
- 21.Hounsa C G, Brant E V, Thevelein J, Hohmann S, Prior B A. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology. 1998;144:671–680. doi: 10.1099/00221287-144-3-671. [DOI] [PubMed] [Google Scholar]
- 22.Ingram L O. Microbial tolerance to ethanol. Role of cell membrane. Trends Biotechnol. 1986;4:40–44. [Google Scholar]
- 23.Iwahashi H, Obuchi K, Fujii S, Komatsu Y. The correlative evidence suggesting that trehalose stabilizes membrane structure in the yeast Saccharomyces cerevisiae. Cell Mol Biol. 1995;41:763–769. [PubMed] [Google Scholar]
- 24.Kaul S C, Obuchi K, Iwahashi H, Komatsu Y. Cryoprotection provided by heat shock treatment in Saccharomyces cerevisiae. Cell Mol Biol. 1992;38:135–143. [PubMed] [Google Scholar]
- 25.Lewis J G, Learmonth R P, Watson K. Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology. 1995;141:687–694. doi: 10.1099/13500872-141-3-687. [DOI] [PubMed] [Google Scholar]
- 26.Lindquist S L. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 27.Londesborough J, Varimo K. Characterization of two trehalases in baker’s yeast. Biochem J. 1984;219:511–518. doi: 10.1042/bj2190511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie K F, Singh K K, Brown A D. Water stress plate hypersensitivity of yeasts: protective role of trehalose in Saccharomyces cerevisiae. J Gen Microbiol. 1988;134:1661–1666. doi: 10.1099/00221287-134-6-1661. [DOI] [PubMed] [Google Scholar]
- 29.Mansure J J C, Panek A D, Crowe L M, Crowe J H. Trehalose inhibits ethanol effects on intact yeast cells and liposomes. Biochim Biophys Acta. 1994;1191:309–316. doi: 10.1016/0005-2736(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 30.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 31.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 32.Nwaka S, Mechler B, Holzer H. Deletion of the ATH1 gene in Saccharomyces cerevisiae prevents growth on trehalose. FEBS Lett. 1996;386:235–238. doi: 10.1016/0014-5793(96)00450-4. [DOI] [PubMed] [Google Scholar]
- 33.Nwaka S, Holzer H. Molecular biology of trehalose and trehalases in the yeast Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol. 1997;58:197–237. doi: 10.1016/s0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- 34.Panaretou B, Piper P W. The plasma membrane of yeast acquires a novel heat-shock protein (hsp30) and displays a decline in proton-pumping ATPase levels in response to both heat shock and entry to stationary phase. Eur J Biochem. 1992;206:635–640. doi: 10.1111/j.1432-1033.1992.tb16968.x. [DOI] [PubMed] [Google Scholar]
- 35.Panek A D. Trehalose metabolism—new horizons in technological applications. Braz J Med Biol Res. 1995;28:169–181. [PubMed] [Google Scholar]
- 36.Park J-I, Grant C M, Attfield P V, Dawes I W. The freeze-thaw stress response of the yeast Saccharomyces cerevisiae is growth phase specific and is controlled by nutritional state via the RAS-cyclic AMP signal transduction pathway. Appl Environ Microbiol. 1997;63:3818–3824. doi: 10.1128/aem.63.10.3818-3824.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piper P W. Molecular events associated with the acquisition of heat tolerance in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;11:339–356. doi: 10.1111/j.1574-6976.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 38.Piper P W, Talreja K, Panaretou B, Moradas-Ferreira P, Byrne K, Praekelt U M, Meacock P, Récnacq M, Boucherie H. Induction of major heat-shock proteins of Saccharomyces cerevisiae, including plasma membrane Hsp30, by ethanol levels above a critical threshold. Microbiology. 1994;140:3031–3038. doi: 10.1099/13500872-140-11-3031. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro M J S, Reinders A, Boller T, Wiemken A, De Virgilio C. Trehalose synthesis is important for the acquisition of thermotolerance in Schizosaccharomyces pombe. Mol Microbiol. 1997;25:571–581. doi: 10.1046/j.1365-2958.1997.4961856.x. [DOI] [PubMed] [Google Scholar]
- 40.Sharma S C. A possible role of trehalose in osmotolerance and ethanol tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1997;152:11–15. doi: 10.1111/j.1574-6968.1997.tb10402.x. [DOI] [PubMed] [Google Scholar]
- 41.Singer M A, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 42.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 43.Van Laere A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 44.Van Uden N. Effects of ethanol on the temperature relations of viability and growth in yeast. Crit Rev Biotechnol. 1984;1:263–272. [Google Scholar]
- 45.Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek. 1990;58:209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]