Abstract
Background:
Mutations in isocitrate dehydrogenase 1/2 (IDH1/2) genes result in NADPH-dependent reduction of α-ketoglutarate and formation of 2-hydroxyglutarate, which blocks normal cellular differentiation and promotes leukemogenesis. Nearly 20% of AML cases carry IDH1/2 mutations. While multiple investigators have described the prognostic implications of IDH mutations in AML patients receiving chemotherapy, the impact of these mutations on outcomes after allogeneic hematopoietic cell transplantation (alloHCT) is unknown.
Patients:
We report on the clinical outcome of a cohort of AML patients, tested for IDH mutations and underwent alloHCT at City of Hope (2015–2017). Of a total of 317 screened patients, 99 underwent alloHCT, of which 23 carried and 76 did not carry IDH mutations (control).
Results:
No statistical significance was detected in patient’s overall survival (p=0.84). With a median follow up of 7.8 months, 1-year relapse rate of 29% and 13% was seen in IDH mutated and control group, respectively (p=0.033). IDH1/2 mutation status remained significantly associated with relapse (HR=2.8, p=0.046) after inclusion of pre-HCT disease status in a multivariable model.
Conclusions:
Our results, despite low patient numbers, indicates that IDH mutations are associated with higher relapse rate post-alloHCT. Further prospective studies investigating post-transplant IDH inhibition is required to improve outcomes in AML patients carrying IDH mutations.
Keywords: retrospective study, allogeneic HCT, IDH1/2 mutations, Survival, Relapse rate
MICRO ABSTRACT
The impact of IDH mutations on transplant outcomes is unknown. We retrospectively screened 99 AML patients who underwent alloHCT (2015–2017) of which 23 carried and 76 did not carry IDH mutations (control). Overall survival was not different among the two groups, but significantly higher rate of relapse was detected in IDH mutated group by univariate and multivariate analysis.
INTRODUCTION
Isocitrate dehydrogenase 1 (IDH1) and its mitochondrial homolog IDH2, are key component enzymes in the Krebs cycle, involved in conversion of isocitrate to α-ketoglutarate (α-KG). Somatic mutations in IDH1 or 2 are seen in approximately 15–30% of patients diagnosed with acute myeloid leukemia (AML), with an incidence of 6–16% for the IDH1 and 8–19% for the IDH2 mutation.1 While these mutations are less frequent in patients with MDS (5%)2 and MPN, the mutation frequency goes up to 20% with leukemic transformation.3 Mutant IDH enzymes catalyze NADPH-dependent reduction of α-KG to the oncometabolite R-2-hydroxygluterate (2HG).4 Upon translocation to the nucleus, 2HG competitively inhibits α-KG-dependent enzymes, including members of the TET protein family (TET1–3), 5-methylcytosine hydroxylases, and jumonji-C domain-containing group of histone lysine demethylases. Inhibition of these epigenetic regulators by 2-HG produces a hypermethylation with altered gene expression, leading to differentiation arrest in hematopoietic precursors.5,6 Therefore, somatic mutations in IDH1/2 impair normal myeloid differentiation, increase stem/progenitor cells, and promote leukemogenesis.2
Multiple studies have investigated the prognostic implications of IDH1/2 mutations in AML patients undergoing induction and consolidation chemotherapy with variable results, from a negative prognostic impact of IDH mutations with standard chemotherapy 7–9 to no adverse or even improved remission/survival after induction chemotherapy.10,11 Some studies show that co-occurrence of NPM1 alteration with either IDH1 or 2 mutation, results in an improved overall survival (OS),12–14 whereas other studies report lower OS in AML patients with IDH mutations who are otherwise cytogenetically normal (FLT3-ITDwt).9,15,16 In a report by Marcucci et al,10 patients with IDH1 mutation had shorter disease-free survival (DFS), whereas IDH2 mutated patients had a lower complete remission (CR) rates. The variability in outcomes across these studies might be due to heterogeneity in the location of hotspot mutations, other co-existing mutations, and serum levels of 2-HG, which also correlate with clinical outcome in AML patients carrying IDH1/2 mutations.17,18
With advances in our understanding of the biology of AML, availability of DNA sequencing platforms and AML mutation testing, improved clinical outcomes and overall survival have been achieved for patients with relapsed AML. 19–21 Simultaneously, rates of non-relapse mortality (NRM) is decreased in patients with leukemia following alloHCT, mainly due to the modulation of conditioning regimen intensity, advancement in graft-versus-host disease (GVHD) prophylaxis and treatment, and improved donor selection and supportive care.22 However, relapse of the primary malignancy has remained the major cause of treatment failure and mortality for these patients. Based on most recent data from the Center for International Blood and Marrow Transplant Research, relapse of primary disease is the major cause of death after HLA matched (57%) and unrelated donor (46%) HCT at or beyond 100 days post-transplant. The survival outcomes of patients who relapse after alloHCT are uniformly poor, especially if the relapse occurs relatively early after transplant.23–25
The most recent European LeukemiaNet (ELN) guidelines classify AML with IDH mutations as an intermediate risk disease.26 Based on the current guidelines, allogeneic hematopoietic cell transplantation (alloHCT) is recommended for AML patients with intermediate risk disease in their first remission based on a post-induction chemotherapy relapse risk of 35–40%.27 Herein, we investigated if IDH mutations impact transplant outcomes in AML patients by comparing outcomes of patients carrying IDH mutations to those not carrying these mutations. To our knowledge, there are no reports on the impact of IDH1/2 mutations on the outcome of AML patients who underwent alloHCT.
METHODS
Study Population
We reviewed the medical records of AML patients who were screened for IDH1/2 mutations, using Next Generation Sequencing (NGS), from 2015 to 2017 at City of Hope (COH), and retrospectively analyzed the relapse rates, OS and other HCT-related outcomes. The NGS panel comprised of a comprehensive panel of somatic mutations and gene fusions commonly seen in hematologic malignancies. This study was approved by the institutional review board (IRB) of COH.
NGS Library Preparation and Bioinformatics Analysis
NGS libraries were prepared from genomic DNA (40 ng) using the SureSelect target enrichment system (Agilent Technologies Inc.) after transposase-based fragmentation and adapter ligation. The adapter-ligated library was amplified by polymerase chain reaction and quality control was performed for sizing and concentration. Target regions were captured using a customized SureSelect library (Agilent Technologies) for all coding exons plus ten flanking bases of 72 genes. After hybridization of 750 ng of adapter-ligated library with biotin-labeled probes that are specific to target regions, the dual-index tag were added during post-capture polymerase chain reaction amplification. The amplified captured libraries were quality-controlled using a high sensitivity DNA Bioanalyzer kit (Agilent Technologies Inc.) then pooled and sequenced using Miseq V2 Reagent Kit/300 cycles with 150 bp paired-end sequencing. Alignment of sequence reads to the human genome (GRCh37/hg19), variant calling and annotation was performed independently using two software applications – CLCBiomedical Workbench (CLC Bio, Aarhus, Denmark) and NextGENE (Softgenetics, State Collage, PA, USA). Annotated variants were processed using previously published criteria.28,29 Synonymous variants, variants located >2 bp outside protein-coding regions, polymorphisms present in >1% in population databases including ExaC, Exome Variant Server and the 1000 Genomes Project, and variants with <30X coverage were filtered. The remaining variants were evaluated using tumor-specific databases (COSMIC, cBioportal), information retrieved from literature, sequence conservation, and in silico prediction algorithms, including SIFT, Polyphen-2, and FATHMM, for clinical significance.
Flow Cytometry MRD Assay
The MRD flow cytometry assay was sent out to an outside laboratory, using an assay with detection sensitivity of more than 0.01% of white cells. This was achieved by validating the ability to add twice as many cells in order to more consistently collect one million white cell events. The 18 fluorchromes used in this assay were CD4, CD5, CD7, CD13, CD14, CD15, CD16, CD19, CD33, CD34, CD38, CD45, CD56, CD64, CD71, CD117, CD123, and HLA-DR. For a detailed description of this assay please refer to Wood et al30.
Statistical Analysis
Wilcoxon and chi-square or Fisher’s exact tests were used to compare the baseline characteristics between groups by IDH1/2 mutation status whenever appropriate. Kaplan-Meier curves and the log-rank test were used to evaluate OS and progression-free survival (PFS) from the date of HCT. Cumulative incidence curves and the Gray test were used to examine the differences in relapse rates and NRM.
RESULTS
A total 317 AML patients were screened for IDH1/2 mutations form 2009 to 2017. Of these, 99 patients underwent alloHCT with 23 patients carried either IDH1 or IDH2 mutation (IDH Mut). The remainder of patients (n=76) who underwent alloHCT but were negative for IDH mutations were used as the control group for this study. Patient demographics and alloHCT characteristics are summarized in Table 1. Briefly, the median ages at transplant for patients with IDH mutation and those without IDH mutations were 64 years (range: 36–73) and 54 years (range: 18–71), respectively (p=0.007). Thus, the majority of the IDH mutated patients (74%) received reduced-intensity conditioning regimens (vs. 59% in the control group). Disease status prior to HCT, donor type, graft source, donor/recipient CMV serostatus, and GvHD prophylaxis regimens were similar across both groups. No patient in IDH mutated group received maintenance therapy post alloHCT.
Table 1.
Patient and transplant characteristics.
| Variable | mIDH 1/2 (n=23) |
Wt IDH (n=76) |
Total (n=99) |
|---|---|---|---|
| Age at HCT | |||
| Median Range |
64.0 (36–73) |
54.0 (18–71) |
57.0 (18–73) |
|
| |||
| Sex | |||
| Male | 10(43.5%) | 36(47.4%) | 46(46.5%) |
| Female | 13(56.5%) | 40(52.6%) | 53(53.5%) |
|
| |||
| Disease status at HCT | |||
| CR-1 | 13(56.5% | 34(44.7%) | 47(47.5%) |
| 1’st Relapse | 2(8.7%) | 6(7.9%) | 8(8.1%) |
| CR-2 | 6(26.1%) | 11(14.5%) | 17(17.2%) |
| 2”nd Relapse | - | 1(1.3%) | 1(1%) |
| ≥3’rd CR | - | 3(3.9%) | 3(3%) |
| Induction Failure | 2(8.7%) | 21(27.6%) | 23(23.3%) |
|
| |||
| HLA Match Degree | |||
| HLA identical, Sibling | 7(30.4%) | 20(26.3%) | 27(27.3% |
| HLA matched, Unrelated | 4(17.4%) | 5(6.6%) | 9(9.1%) |
| HLA mismatched, Sibling | 0 | 1(1.3%) | 1(1%) |
| HLA mismatched, Unrelated | 9(39.1%) | 41(53.9%) | 50(50.5%) |
| Haploidentical | 3(13%) | 9(11.8%) | 12(12.1%) |
|
| |||
| Graft source | |||
| Bone marrow | 0(0%) | 1(1.3%) | 1(1%) |
| Cord blood | 0(0%) | 3(3.9%) | 3(3%) |
| Peripheral blood stem cells | 23(100%) | 72((94.7% | 95(96%) |
|
| |||
| CMV status | |||
| Negative | 1(4.3%) | 4(5.3%) | 5(5.1) |
| Positive | 22(95.7%) | 72((94.7%) | 94(94.9) |
|
| |||
| Regimen | |||
| RIC | 18(78.2%) | 50(65.7%) | 68(68.6%) |
| MAC | 5(21.7%) | 26(34.2%) | 31(31.3%) |
|
| |||
| GVHD prophylaxis | |||
| Tacrolimus/sirolimus | 18(78.2%) | 53(69.7%) | 71(71.7%) |
| Tacrolimus/Cytoxan | - | 10(13.1%) | 10 (10.1) |
| Tacrolimus/Cellcept | - | 4(5.2%) | 4 (4%) |
| Tacrolimus/MTX | 1(4.3%) | 5 (6.5%) | 6(6%) |
| Other | 4(17.3% | 4 (5.2%) | 8 (8%) |
Of the 23 patients carrying IDH mutations, 30% carried IDH1 (R132C and R132H were the most prevalent) and 70% carried IDH2 mutation (R140Q was the most prevalent). The mutation allele burden (mean) was 31.7% (range: 2–48) for IDH1 and 34.1% (range: 3–44) for IDH2 mutated patients. No patients in the IDH mutated or control group had antecedent hematologic disorder preceding AML diagnosis. Co-mutation was detected in 3 patients with FLT3-ITD or TKD, and NPM1 and DNMT3A mutations were noted in 4 and 1 patient, respectively. None of the patients carrying FLT3-ITD mutation were relapsed at their last follow-up. Cytogenetic analysis at diagnosis of AML revealed adverse risk in 21.7% of patients in the IDHmu group and 31.6% in the control group (p=0.46). Multicolor flow cytometry based-minimal residual disease (MRD) analysis was done pre-HCT in 14 patients in the IDH mutation group and 30 patients in the control groups. The MRD positivity rates were 14.3% and 16.7% in the IDH mutated and control group, respectively (p=1.0). Of the 23 patients who were positive for IDH mutations, 6 (26%) received IDH mutation-directed therapy on a clinical trial pre alloHCT. Of these, 3 patients entered MRD negative status pre-HCT, but the other 3 did not have MRD testing done on the pre-HCT marrow. Four patients remain leukemia-free post alloHCT and 2 have relapsed.
There were no significant differences in 12-month OS (71% vs. 68%; P=0.84) between patients carrying or not carrying IDH mutations (Figure 1a). With a median follow-up of 7.8 months (range: 1.0–52.2), 6 patients in the IDH mutated and 11 patients in the control group relapsed (1-year relapse rate of 29% [95% CI: 9.6–51.6%] and13% [95% CI: 5.5–24.2%], respectively), (p=0.033) (Figure 1b and Table 2). When disease status prior the alloHCT was included in a multivariable model (Table 2), the presence of IDH1/2 mutations was significantly associated with relapse (HR=2.78, 95% CI: 1.0–7.6, adjusted p value=0.046). None of the patients carrying IDH mutation with NPM1 or FLT3-ITD/TKD mutations relapsed post alloHCT, but one relapse was seen in an IDH2 mutated patient with DNMT3A mutation. No long-term survivors were noted in IDH mutated patients after relapse post HCT. NRM was similar in the control group and IDH mutated group (p=0.17). The incidence of acute (grades II-IV) and chronic GvHD were similar between the two groups (p=0.73 and p=0.63, respectively).
Figure 1.
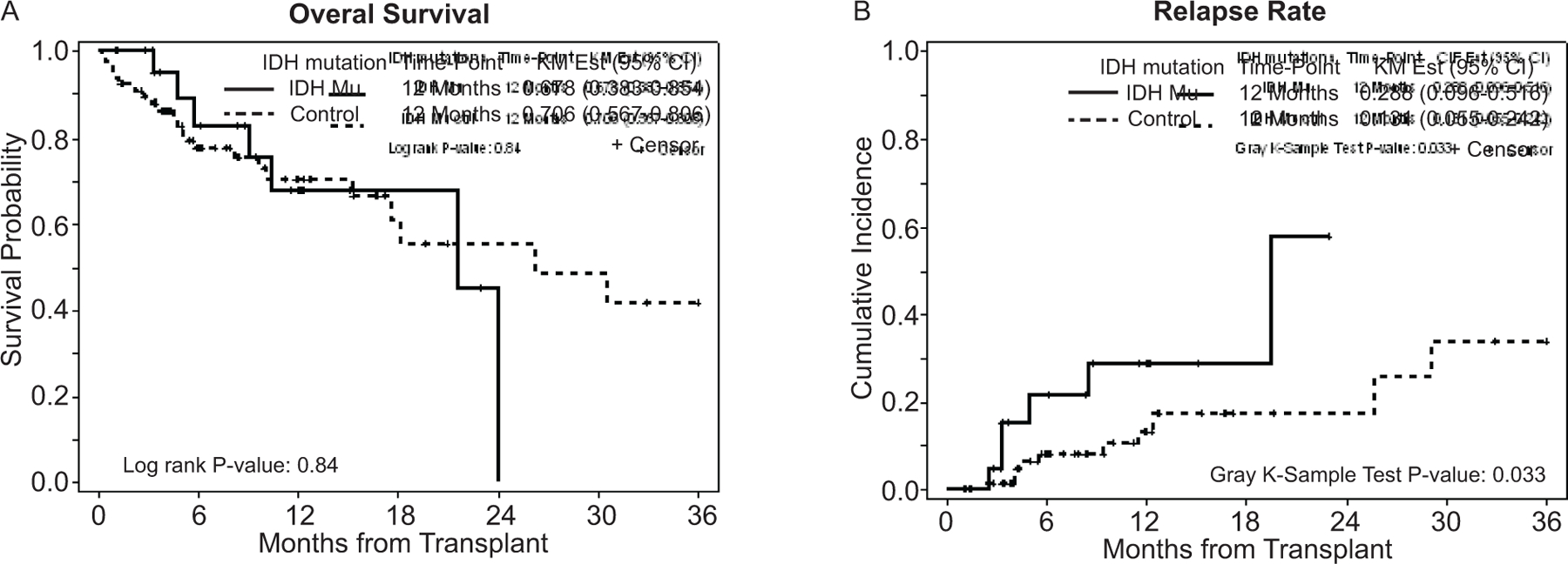
a) Overall survival in patients carrying and not carrying IDH mutations and b) Relapse rate in patients carrying and not carrying IDH mutations.
Table 2.
Multivariate Analysis
| Event/Total | 1-Year Relapse (95% CI) | HR (95% CI)* | P-value* | HR (95% CI)† | P-value† | |
|---|---|---|---|---|---|---|
| IDH1/2 | 0.033 | 0.046 | ||||
| Control | 11/76 | 0.131 (0.055–0.242) | Reference | Reference | ||
| Mutation | 6/23 | 0.288 (0.096–0.516) | 2.77 (1.04–7.32) | 2.78 (1.02–7.60) |
Based on Gray test in univariate analysis
Based on Fine and Gray model adjusted for disease status in multivariable analysis
Among the 218 AML patients who did not proceed to alloHCT, mainly due to advanced age or refractory leukemia, IDH1 or 2 mutations were detected in 30 patients. Of these patients, 15 were enrolled on IDH-targeted therapies and 5 patients (16.6%) were alive at the last follow-up. The remaining 15 patients received best available therapy with 3 (10%) being alive at the last follow-up.
DISCUSSION AND CONCLUSION
This retrospective review shows for the first time to our knowledge that patients carrying the IDHmu remain at a high risk of post allogeneic HCT relapse compared to IDHwt patients. This opens the possibility of using IDH inhibitors as post HCT maintenance therapy. This strategy of post alloHCT maintenance therapy to reduce the incidence of relapse are well-established in treatment of acute leukemia. Addition of Tyrosine Kinase Inhibitors with induction chemotherapy and post-transplant maintenance in patients with Philadelphia chromosome positive ALL has shown improvement in overall, relapse free and event free survival.31 32 Similarly, in patients with AML with FLT3-ITD mutations, sorafenib maintenance has resulted in better overall and event-free survival.33,34 Results from the randomized, SORMIN study has shown improvement in RFS in patients who received post-HCT sorafenib maintenance for 24 months.35 Hypomethylating agents have been used to decrease relapse rates post alloHCT. Addition of azacitidine after alloHCT in high risk populations improved EFS and OS, indicating the efficacy of this strategy.36 Similarly, decitabine at low dosage has been used as post HCT maintenance strategy and showed effective in reducing relapse rates.37 Multiple clinical trials are ongoing to reduce relapse rate in AML patients after standard frontline chemotherapy using novel strategies such as checkpoint inhibitors such as Nivolumab (NCT02275533), SL-401 (NCT02270463), and lenalidomide and azacitidine (NCT01743859). Results of these trials will help with prolonging remission in older, unfit patients who are unable to go through alloHCT.
Although our study is limited by the relatively small number of patients, short follow-up duration, and heterogeneity of the cohorts, it is the first to describe the outcome of AML patients with IDH mutations who underwent alloHCT. In multivariate analysis, IDH mutation was the only predictor of relapse post-HCT. Multivariate analysis in our cohort of patients using conventional risk factors such as age, cytogenetics and pre-HCT MRD status did not predict for higher relapse rates post HCT. The lack of OS benefit in our study may be related to the short follow-up duration and small patient numbers. However, despite the small sample size, this study gives estimates of relapse rates in patients with IDH mutations undergoing alloHCT which can be informative in planning a larger observational studies or designing interventional studies post HCT. Additional multicenter studies are warranted in patients carrying IDH mutations to better understand the role of IDH mutations in patients with AML/MDS and improve leukemia free survival.
In our cohort of patients carrying IDH mutations, pre-alloHCT treatment with IDH mutation-directed therapy (n=6, 26%), resulted in MRD negative status in 50% of patients whereas the rest did not have MRD testing done on the pre-HCT marrow, thus their MRD status was unknown. More than half of patients who received IDH mutation-directed therapy (n=4, 66.7%) remained leukemia-free post alloHCT. However, given the small number of patients, it is hard to conclude if prior exposure to IDH inhibitors will influence relapse rates post alloHCT.
Lastly, in a phase I/II trial, patients with mutant-IDH2 and advanced myeloid malignancies were treated with oral IDH2 inhibitor therapy (Enasidenib), and an overall response rate of 40.3% (20% CR) with median duration of response of 5.8 months was achieved.38 Dinardo et al,39 also published the results of a phase I dose escalation/expansion study, using IDH1 inhibitor, Ivosidenib, in patients with relapsed/refractory AML with IDH1 mutation. In this study, the overall response rate was 41.6% (95% CI: 32.9–50.8%) with CR rate of 21.6 %(95% CI: 14–7-29.8). Based on these data and our results, a prospective trial to evaluate Enasidenib for IDH2 mutated AML patients and Ivosidenib for IDH1 mutated patients as post-alloHCT maintenance is warranted.
CLINICAL PRACTICE POINTS
Based on published literature, the role of IDH mutations in patients with newly diagnosed AML is not clear. These patients are currently classified into the intermediate risk group, and allogeneic HCT is recommended for their treatment. However, data on the outcomes of AML patients carrying IDH mutations, who subsequently undergo allogeneic HCT is scares. Our retrospective study for the first time has shown an increased risk of post allogeneic HCT relapse in AML patients carrying IDH mutations, indicating that these patients need to be followed closely post-transplant. This increased relapse rates was independent of the traditional risk factors for relapse such as intensity of conditioning regimen, poor risk cytogenetic markers, co-mutations and pre-HCT MRD status. FDA approval of IDH inhibitors in relapsed and refractory AML patients with IDH 1/2 mutations provides the possibility of using these agents for AML maintenance therapy and improving leukemia free survival.
AKNOWLEDGMENTS
Authors thank City of Hope staff and nurses, as well as the patients and their families, without whom this work would not be possible. This study was partially supported by NIH P30 CA033572 (Biostatistics Core).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
A. Salhotra is a consultant for Kadmon Corporation (non-paid), A. Stein is on speakers’ bureau for Celgene. Other authors declare no conflict of interest.
References:
- 1.Im AP, Sehgal AR, Carroll MP, et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia 2014;28(9):1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010;18(6):553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardanani A, Lasho TL, Finke CM, Mai M, McClure RF, Tefferi A. IDH1 and IDH2 mutation analysis in chronic- and blast-phase myeloproliferative neoplasms. Leukemia 2010;24(6):1146–1151. [DOI] [PubMed] [Google Scholar]
- 4.Prensner JR, Chinnaiyan AM. Metabolism unhinged: IDH mutations in cancer. Nat Med 2011;17(3):291–293. [DOI] [PubMed] [Google Scholar]
- 5.Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst 2010;102(13):932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise DR, Ward PS, Shay JE, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A 2011;108(49):19611–19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng JH, Guo XP, Chen YY, Wang ZJ, Cheng YP, Tang YM. Prognostic significance of IDH1 mutations in acute myeloid leukemia: a meta-analysis. Am J Blood Res 2012;2(4):254–264. [PMC free article] [PubMed] [Google Scholar]
- 8.Aref S, Kamel Areida el S, Abdel Aaal MF, et al. Prevalence and Clinical Effect of IDH1 and IDH2 Mutations Among Cytogenetically Normal Acute Myeloid Leukemia Patients. Clin Lymphoma Myeloma Leuk 2015;15(9):550–555. [DOI] [PubMed] [Google Scholar]
- 9.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol 2010;28(22):3636–3643. [DOI] [PubMed] [Google Scholar]
- 10.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 2010;28(14):2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel KP, Barkoh BA, Chen Z, et al. Diagnostic testing for IDH1 and IDH2 variants in acute myeloid leukemia an algorithmic approach using high-resolution melting curve analysis. J Mol Diagn 2011;13(6):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thol F, Damm F, Wagner K, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood 2010;116(4):614–616. [DOI] [PubMed] [Google Scholar]
- 13.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol 2015;90(8):732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas S, Lugthart S, Kavelaars FG, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood 2010;116(12):2122–2126. [DOI] [PubMed] [Google Scholar]
- 16.Nomdedeu J, Hoyos M, Carricondo M, et al. Adverse impact of IDH1 and IDH2 mutations in primary AML: experience of the Spanish CETLAM group. Leuk Res 2012;36(8):990–997. [DOI] [PubMed] [Google Scholar]
- 17.Green CL, Evans CM, Zhao L, et al. The prognostic significance of IDH2 mutations in AML depends on the location of the mutation. Blood 2011;118(2):409–412. [DOI] [PubMed] [Google Scholar]
- 18.Wang JH, Chen WL, Li JM, et al. Prognostic significance of 2-hydroxyglutarate levels in acute myeloid leukemia in China. Proc Natl Acad Sci U S A. 2013;110(42):17017–17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarjian HM, DiNardo CD, Nogueras-Gonzalez GM, et al. Results of second salvage therapy in 673 adults with acute myelogenous leukemia treated at a single institution since 2000. Cancer 2018;124(12):2534–2540. [DOI] [PubMed] [Google Scholar]
- 20.Khan N, Hantel A, Knoebel RW, et al. Efficacy of single-agent decitabine in relapsed and refractory acute myeloid leukemia. Leuk Lymphoma 2017;58(9):1–7. [DOI] [PubMed] [Google Scholar]
- 21.Pemmaraju N, Kantarjian H, Garcia-Manero G, et al. Improving outcomes for patients with acute myeloid leukemia in first relapse: a single center experience. Am J Hematol 2015;90(1):27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015;21(3):454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orti G, Sanz J, Garcia-Cadenas I, et al. Analysis of relapse after transplantation in acute leukemia: A comparative on second allogeneic hematopoietic cell transplantation and donor lymphocyte infusions. Exp Hematol 2018;62:24–32. [DOI] [PubMed] [Google Scholar]
- 25.Schneidawind C, Hagmaier V, Faul C, Kanz L, Bethge W, Schneidawind D. Second allogeneic hematopoietic cell transplantation enables long-term disease-free survival in relapsed acute leukemia. Ann Hematol 2018;97(12):2491–2500. [DOI] [PubMed] [Google Scholar]
- 26.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majhail NS, Farnia SH, Carpenter PA, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation 2015;21(11):1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MM, Datto M, Duncavage EJ, et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 2017;19(1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol 2013;999:123–136. [DOI] [PubMed] [Google Scholar]
- 31.Giebel S, Czyz A, Ottmann O, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A position statement of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer 2016;122(19):2941–2951. [DOI] [PubMed] [Google Scholar]
- 32.Ravandi F, Othus M, O’Brien SM, et al. US Intergroup Study of Chemotherapy Plus Dasatinib and Allogeneic Stem Cell Transplant in Philadelphia Chromosome Positive ALL. Blood Adv 2016;1(3):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battipaglia G, Ruggeri A, Massoud R, et al. Efficacy and feasibility of sorafenib as a maintenance agent after allogeneic hematopoietic stem cell transplantation for Fms-like tyrosine kinase 3-mutated acute myeloid leukemia. Cancer 2017;123(15):2867–2874. [DOI] [PubMed] [Google Scholar]
- 34.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med 2017;377(5):454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burchert A, Bug G, Finke J, et al. Sorafenib As Maintenance Therapy Post Allogeneic Stem Cell Transplantation for FLT3-ITD Positive AML: Results from the Randomized, Double-Blind, Placebo-Controlled Multicentre Sormain Trial. Blood 2018;132(Suppl 1):661–661. [Google Scholar]
- 36.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer 2010;116(23):5420–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pusic I, Choi J, Fiala MA, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant 2015;21(10):1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130(6):722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiNardo CD, Stein EM, de Botton S, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med 2018;378(25):2386–2398. [DOI] [PubMed] [Google Scholar]


