Abstract
We report the utility of rapid antigen tests (RAgT) in a cohort of US healthcare personnel with coronavirus disease 2019 (COVID-19) infection who met symptom criteria to return to work at day 5 or later of isolation. In total, 11.9% of initial RAgT were negative. RAgT can be helpful to guide return to work decisions.
Keywords: COVID-19, SARS-CoV-2
By early January 2022, the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) quickly became the dominant cause of coronavirus disease 2019 (COVID-19) infection in the United States [1]. An overwhelming number of infections within the healthcare workforce threatens the ability to provide care for all patients due to a steep increase in absenteeism among healthcare personnel (HCP). On 23 December 2021, the Centers for Disease Control and Prevention (CDC) provided updated guidance regarding the duration of isolation for HCP with COVID-19 [2], which included a contingency option for return-to-work at 5 days with or without a negative test. We report our initial results of rapid antigen testing (RAgT) at day ≥ 5 of COVID-19 infection among HCP, to maintain sufficient staffing while minimizing risk for nosocomial transmission.
METHODS
This retrospective cohort study included all Mayo Clinic HCP in Arizona, Florida, Minnesota, and Wisconsin who were diagnosed with COVID-19 following a positive test for SARS-CoV-2 between 3 January 2022 and 22 January 2022. All HCP (n = 1,661) who underwent RAgT for return-to-work purposes between 9 January and 28 January 2022 were included. This study was deemed exempt by the Mayo Clinic IRB.
Employee demographics and exposures of interest included sex, age, presence of prior SARS-CoV-2 infection, vaccination status, presence of symptoms at diagnosis, source of infection (if known), and healthcare worker category (job with or without patient contact). Vaccination timing and status was determined from Mayo Clinic occupational health records. Vaccine status at the time of infection was categorized as “up to date” when initial vaccination was completed at least 2 weeks prior to diagnosis and a booster dose has been received or is not yet due; “booster overdue” when initial vaccination was completed at least 2 weeks prior to diagnosis, and a booster dose is overdue; “partially vaccinated” when at least 1 dose of vaccine was received but initial vaccination series was not complete or was completed <2 weeks prior to diagnosis; and “unvaccinated” when no vaccine doses have been received.
The outcome of interest was the time to the first negative COVID-19 RAgT performed ≥ 5 days after an initial positive molecular test. Most of the antigen testing was performed using the FlowFlexTM COVID-19 Antigen Home Test (Acon Laboratories; San Diego, California, USA), which was distributed to HCP after their diagnostic COVID-19 molecular test. HCP could also submit documentation of RAgT results from alternative antigen test kits, which had been determined by the manufacturer to detect the Omicron variant. Employees were invited to undergo RAgT if they met all of the following criteria: (1) ≥ 5 days since symptom onset, or from an initial positive COVID-19 molecular test if asymptomatic at the time of diagnosis, (2) no fever for at least 24 hours without fever-reducing medications, (3) no current symptoms or mild residual symptoms that were improving, (4) not moderately or severely immunosuppressed or severe/critical SARS-CoV-2 infection, and (5) anticipated to work on campus. Employees with a positive RAgT at day ≥ 5 were not allowed to return to work on campus and were instructed to undergo repeat RAgT 24–72 hours later. A negative RAgT was required for return-to-work on campus earlier than day 10. Exposures and characteristics of employees with an initial negative return-to-work RAgT were compared to those with an initial positive return-to-work RAgT.
RESULTS
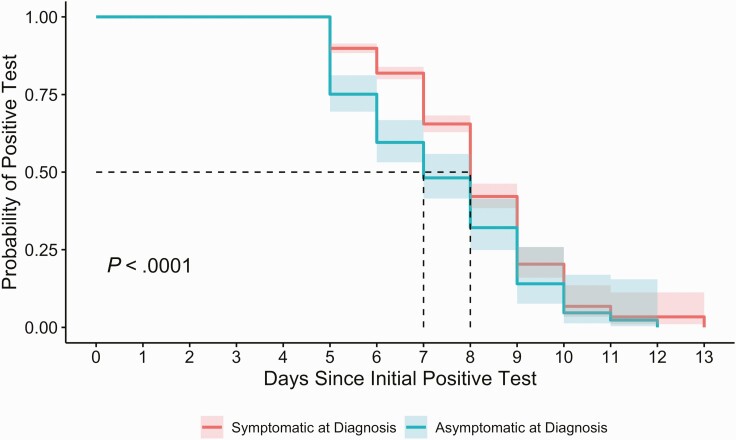
There were 1661 employees with a new positive SARS-CoV-2 diagnostic test between 3 January and 22 January 2022 who subsequently underwent RAgT a median of six days after the initial positive test (interquartile range [IQR] 5–7) (Supplementary Table 1). The initial RAgT was positive in 1076 (64.8%) individuals. Among those individuals, a second RAgT was performed in 585 (54.4%) individuals at a median of 7 days (IQR 7–7) after their initial diagnostic SARS-CoV-2 test. Sixty-four HCP completed > 2 RAgT between day 5 and 9 after initial positive test. Compared to employees with a negative RAgT, those with a positive RAgT were significantly more likely to have reported symptoms at the time of diagnosis (P < .01) (Figure 1). HCP with a positive RAgT were also more likely to hold patient-facing jobs and be up to date on COVID-19 vaccination (Supplementary Table 1). Compared with the standard isolation of 10 days for most HCP, RAgT reduced isolation time by 2 days per person on average among this cohort. A return-to-work test on day 5 was negative for 199 (11.9%) of the HCP.
Figure 1.
Probability of positive rapid antigen test over time following initial positive diagnostic test, by initial symptom status.
DISCUSSION
In this observational study of HCP with COVID-19 who met symptom criteria to return to work, there was a high frequency (54.2%; 1260/2326) of positive rapid antigen tests (RAgT) when performed ≥ 5 days after diagnosis of COVID-19 infection. Individuals who were symptomatic at diagnosis were significantly more likely to have an initial positive RAgT. Among HCP with an initial positive test, a small but statistically significant increase in the proportions of HCP with up-to-date vaccination status and patient-facing jobs may reflect confounding. Staff were able to test with a RAgT only when they met symptom resolution criteria; staff who were overdue for a booster may have had more significant symptoms, such that their initial test was deferred. Because RAgT became available to HCP during a time of high hospital census, HCP in patient-facing roles may have been motivated to test as soon as able to return to work and support staffing. Patient care staff did take their first RAgT slightly earlier than did nonpatient facing staff.
Our findings have significant implications for management of infected HCP. There are prior data that correlate positive RAgT with positive viral culture and the potential for transmission of SARS-CoV-2 infection [3, 4]. Based on our data, a significant percentage of infected HCP may continue to shed high concentrations of SARS-CoV-2 during days 5–10 after their initial diagnosis. This study demonstrates that RAgT can be used to guide return-to-work decisions safely and effectively for healthcare workers. Although these findings do not support a time-based return to work strategy shorter than 10 days, if RAgT resources are limited, it would be reasonable to initiate return-to-work testing later than day 5, especially for employees with symptomatic infection. Given the increased transmissibility of the Omicron variant as well as the at-risk populations cared for by healthcare workers, we believe addition of RAgT to other preventive measures [5, 6] is important to decrease the possibility of nosocomial transmission to patients and other HCP
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by internal funding at the Mayo Clinic.
Potential conflicts of interest. M. D. S. reports research support from Pfizer via Duke University for a COVID vaccine adverse event registry. M. J. B. reports personal fees from DiaSorin Molecular as an advisory board member, outside the submitted work; payments for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Roche Molecular, DiaSorin Molecular, and MedScape; and President, Pan American Society for Clinical Virology, unpaid. A. J. T. and E .F. B. report consulting fees (<5 k per year) from Up To Date, outside the submitted work. A. J. T. reports Musculoskeletal Infection Society Board, unpaid. L. E. B. reports retaining employment and talent after injury and illness (RETAIN) grant not related to the current manuscript. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Aaron J Tande, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Melanie D Swift, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA; Occupational Health Services, Mayo Clinic, Rochester, Minnesota, USA.
Douglas W Challener, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Elie F Berbari, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Christopher P Tommaso, Occupational Health Services, Mayo Clinic, Rochester, Minnesota, USA.
Darrin R Christopherson, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Matthew J Binnicker, Division of Clinical Microbiology, Mayo Clinic, Rochester, Minnesota, USA.
Laura E Breeher, Division of Public Health, Infectious Diseases and Occupational Medicine, Mayo Clinic, Rochester, Minnesota, USA; Occupational Health Services, Mayo Clinic, Rochester, Minnesota, USA.
REFERENCES
- 1. CDC. CDC COVID data tracker variant proportions. 2021. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Updated 11 January 2022. Accessed 10 June 2021.
- 2. CDC. Interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Updated 23 December 2021. Accessed 14 January 2022.
- 3. Korenkov M, Poopalasingam N, Madler M, et al. Evaluation of a rapid antigen test to detect SARS-CoV-2 infection and identify potentially infectious individuals. J Clin Microbiol 2021; 59:e0089621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pekosz A, Parvu V, Li M, et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis 2021; 73:e2861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swift MD, Breeher LE, Tande AJ, et al. Effectiveness of messenger RNA coronavirus disease 2019 (COVID-19) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a cohort of healthcare personnel. Clin Infect Dis 2021; 73:e1376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Habermann EB, Tande AJ, Pollock BD, Neville MR, Ting HH, Sampathkumar P.. Providing safe care for patients in the coronavirus disease 2019 (COVID-19) era: a case series evaluating risk for hospital-associated COVID-19. Infect Control Hosp Epidemiol 2021; 42:1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.