Abstract
Background
Few data are available on the prognosis of older patients who received corticosteroids for COVID-19. We aimed to compare the in-hospital mortality of geriatric patients hospitalized for COVID-19 who received corticosteroids or not.
Methods
We conducted a multicentric retrospective cohort study in 15 acute COVID-19 geriatric wards in the Paris area from March to April 2020 and November 2020 to May 2021. We included all consecutive patients aged 70 years and older who were hospitalized with confirmed COVID-19 in these wards. Propensity score and multivariate analyses were used.
Results
Of the 1 579 patients included (535 received corticosteroids), the median age was 86 (interquartile range 81–91) years, 56% of patients were female, the median Charlson Comorbidity Index (CCI) was 2.6 (interquartile range 1–4), and 64% of patients were frail (Clinical Frailty Score 5–9). The propensity score analysis paired 984 patients (492 with and without corticosteroids). The in-hospital mortality was 32.3% in the matched cohort. On multivariate analysis, the probability of in-hospital mortality was increased with corticosteroid use (odds ratio [OR] = 2.61 [95% confidence interval (CI) 1.63–4.20]). Other factors associated with in-hospital mortality were age (OR = 1.04 [1.01–1.07], CCI (OR = 1.18 [1.07–1.29], activities of daily living (OR = 0.85 [0.75–0.95], oxygen saturation < 90% on room air (OR = 2.15 [1.45–3.17], C-reactive protein level (OR = 2.06 [1.69–2.51], and lowest lymphocyte count (OR = 0.49 [0.38–0.63]). Among the 535 patients who received corticosteroids, 68.3% had at least one corticosteroid side effect, including delirium (32.9%), secondary infections (32.7%), and decompensated diabetes (14.4%).
Conclusions
In this multicentric matched-cohort study of geriatric patients hospitalized for COVID-19, the use of corticosteroids was significantly associated with in-hospital mortality.
Keywords: Older patients, SARS-CoV-2, Treatment
In 2020, the World Health Organization (WHO) declared the COVID-19 outbreak as a global pandemic. By March 2022, more than 470 million confirmed cases and more than 6 million deaths were reported (1). The population over 70 is the age group with the highest mortality with COVID-19 infection. Indeed, in-hospital mortality is 3.5 higher than for younger adults and ranges from 35% with age 70–79 years to 60% with age 80 years and older (2). This outcome could be explained by modifications induced by age on the immune system (3) and a higher rate of comorbidities in the geriatric population (4,5). All of these factors contribute to increased risk of critical illness, induced by an inappropriate immune response leading to a “cytokine storm,” lung damage, acute respiratory distress syndrome, and multiorgan failure (6).
Corticosteroids, with their well-known broad-spectrum anti-inflammatory and immunomodulatory effects, are emerging as a beneficial treatment. According to the results of the RECOVERY trial (7), a large randomized trial evaluating the effectiveness of dexamethasone in COVID-19, corticosteroids have emerged as the standard of care for severe and critical COVID-19 and have been recommended by the WHO since September 2020 (8).
However, only few data are available on the prognosis of older patients hospitalized for COVID-19 and receiving corticosteroids (9), and their results are heterogeneous. Two retrospective observational studies concluded a benefit of corticosteroids in COVID-19 for older patients (10,11), but subgroup analysis of RECOVERY trial data (7) did not find a significant difference in terms of mortality and ventilator-free days at 28 days between older adults patients (≥70 years old) who received corticosteroids and those who did not. Because of the high rate and severity of side effects of corticosteroids in the geriatric population (12,13), the risk/benefit balance could be even more questionable in the context of COVID-19 for this population.
The objective of this large multicentric observational study was to compare the in-hospital mortality rate of geriatric patients hospitalized for COVID-19 who received corticosteroids or not.
Materials and Methods
Ethical Support
This study was approved by the COVID-19 Assistance Publique-Hôpitaux de Paris (APHP) research committee and the ethics board of Sorbonne University on April 2021 (CER-2020-102). All included patients or their legal representative received an information letter specifying their rights and the terms of use of their medical data. Nonobjection was collected by the physicians in charge of the patients.
This report follows the STROBE recommendations (Supplementary Methods 1).
Study Design, Setting, and Participants
This was a multicentric retrospective cohort study including 15 acute COVID-19 geriatric wards in Paris, France. We included all consecutive patients hospitalized in these wards during 2 distinct periods corresponding to the first COVID-19 wave (March 13, 2020–April 15, 2020; GERICOVID cohort published by Zerah et al. (14)) and the second and third waves (November 1, 2020–May 31, 2021; GERICOCO cohort). Patients included had confirmed COVID-19, were aged 70 years and older, and COVID-19 was diagnosed by RT-PCR for severe acute respiratory coronavirus (SARS-CoV-2) or chest CT according to the WHO interim guidance (15). Patients were excluded if they refused the use of their medical data. The clinical outcome (ie, in-hospital mortality) was monitored up to May 7, 2020 for GERICOVID and up to June 31, 2021 for GERICOCO, the final date of follow-up (discharge of the last patient included). We had no missing data on death or destination at discharge.
Because of lack of recommendations before September 2020, several patients of the GERICOVID cohort received corticosteroids according to the local medical decision. For the GERICOCO cohort, according to the WHO recommendations (September 2020) (16), all patients with severe or critical COVID-19 (defined as oxygen saturation <90% on room air and/or signs of pneumonia and/or respiratory rate > 30 breaths per minute) received corticosteroids. The choice of corticosteroid type was left to the discretion of practitioners.
Data Collection and Outcomes
Data were collected as part of routine care (no clinical or biological procedures added in the creation of the database) retrospectively by 2 physicians per ward from medical records and included sociodemographic data (age, sex, place of living), comorbidities and Charlson Comorbidity index (CCI) (17), number of medications (polypharmacy defined as ≥5 chronic medicines per day (18)), frailty (Clinical Frailty Score [CFS] (19): 1–3, fit; 4, vulnerable; 5–9, frail), and functional autonomy (activities of daily living [ADL] (20)). Also recorded were characteristics of COVID-19 such as the date of COVID-19 onset (defined as the day when the first symptoms were noticed), diagnosis (RT-PCR and/or chest CT anomalies), severity (fever, respiratory rate, oxygen saturation on room air, quick Sepsis-related Organ Failure Assessment [qSOFA] (21)). We also recorded biological data (C-reactive protein [CRP] level, lymphocyte count, presence of cytolysis, and cholestasis), and type of corticosteroids used. Finally, we recorded the status at the end of hospitalization in the acute geriatric ward (alive, dead, place of discharge). Side effects attributed to corticosteroids during the hospitalization were collected for only the GERICOCO cohort. Delirium (defined according to the Confusion Assessment Method (22)), behavioral disorder, gastrointestinal bleeding, acute cardiac failure, hypertension, diabetes decompensation, and secondary infections occurring during hospitalization for patients receiving corticosteroids were considered potential side effects.
The primary outcome was in-hospital mortality. The secondary outcome was corticosteroids side effects in the GERICOCO cohort during hospitalization.
Statistical Analysis
The statistical plan of the study was worked out before the study start (Supplementary Methods 2). The main objective of this study was to compare the in-hospital mortality rate of geriatric patients hospitalized for COVID-19 who received corticosteroids or not. In our previous publication, the in-hospital mortality rate was 31% (95% confidence interval [CI] 27–33) (14). To demonstrate a reduction of 6% in in-hospital mortality with corticosteroid use (7), we estimated that we needed 690 participants per group (power 80%, alpha risk .05).
Characteristics of patients are described as frequencies (percentages) for categorical variables and median (interquartile range [IQR]) for continuous variables. Categorical variables were compared by chi-square or Fisher’s exact test and continuous variables by Wilcoxon’s rank-sum test. Normality was assessed by a graphical representation of the data distribution.
In-hospital mortality was compared between patients with and without corticosteroid use by using the propensity score framework. The propensity-score approach aims at creating a new dataset in which the probability to receive corticosteroids or not is equally balanced among patient baseline characteristics (age, sex, CCI, depression, Parkinson disease, obesity, polypharmacy, institutionalization, CFS, and ADL score). Patients with or without corticosteroids were matched by using a 1:1 nearest-neighbor matching algorithm without replacement, with a caliper of 0.1 of the standard deviation of the propensity score on the logit scale (23). Covariate balance between the 2 groups was assessed after matching, and an absolute standardized difference < 0.1 was considered as evidence of balance (24). Then, within the matched data set, we created a logistic mixed model with a center effect as a random effect to assess independent variables associated with in-hospital mortality; adjusted odds ratios (ORs) and their 95% CIs were calculated. Variables included in the models were all variables with p < .10 on univariate analysis (stepwise selection). qSOFA score at COVID-19 onset was forced into the model because it was found significantly associated with in-hospital mortality in our first initial cohort (14). Multicollinearity bias was checked by using the variance inflation factor. We assessed missing values and their distribution in the 2 cohorts. Because missing values represented <2% of the data and were balanced between the 2 cohorts, no specific strategy was necessary.
Results
Characteristics of Patients at Admission to Geriatric Wards
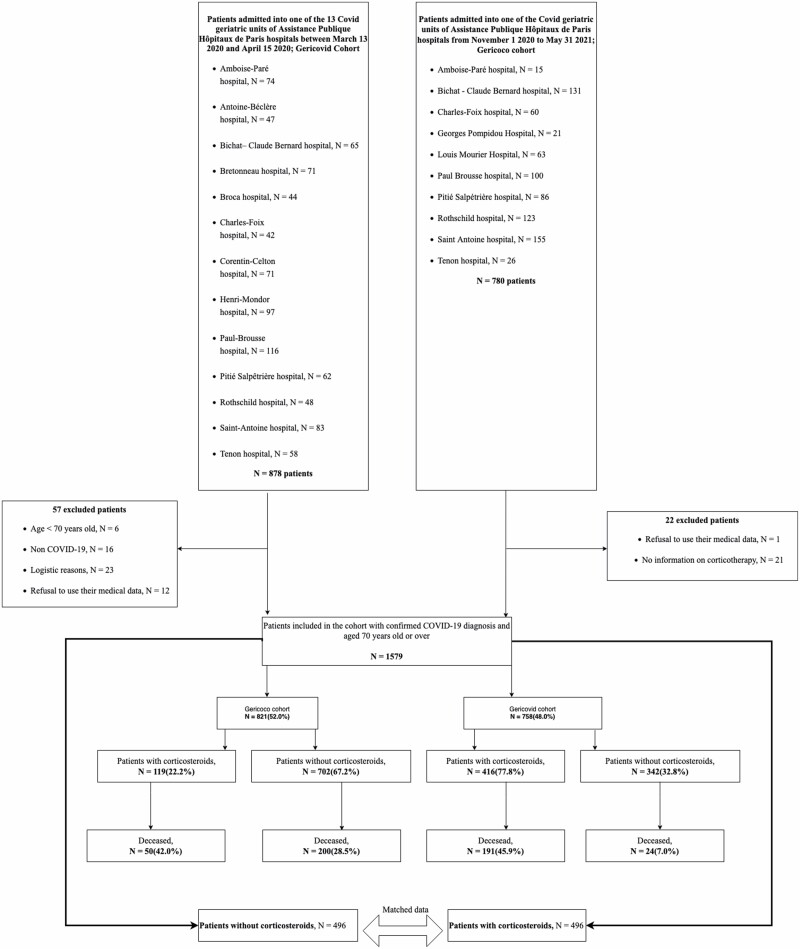
During the 2 screened periods, 1 579 patients were included (Figure 1): 535 (33.9%) received corticosteroids, 119 (22.2%) in the GERICOVID cohort and 416 (77.8%) in the GERICOCO cohort. In the prematched cohort, the median age was 86 (IQR 81–91) years, 56.3% patients were female, the median CCI was 2.6 (IQR 1–4), the median ADL score was 3.9 (IQR 2–6), 64.4% of patients were frail (CFS 5–9), and 21.3% of patients lived in a nursing home. Clinical characteristics are available in Supplementary Results 1. We could match 984 participants (492 in each group). Baseline characteristics before and after pairing are in Table 1.
Figure 1.
Flow chart.
Table 1.
Baseline Characteristics of Patients Without and With Corticosteroids Before and After Propensity-Score Matching
| Before Matching, N = 1579 | After Matching, N = 984 | ||||||
|---|---|---|---|---|---|---|---|
| Without Corticosteroids, N = 1 044 (66.1) | With Corticosteroids, N = 535 (33.9) | p Value | Without Corticosteroids, N = 492 (50) | With Corticosteroids, N = 492 (50) | p Value | SMD | |
| Age (y), median (IQR) | 86.4 (81–91) | 85.2 (80–90) | <.001 | 85.4 (81–90) | 85.2 (80–90) | .339 | 0.30 |
| Sex, female | 408 (30.1) | 282 (52.7) | <.001 | 250 (50.5) | 234 (47.3) | .309 | 0.053 |
| Charlson Comorbidity Index, median (IQR) | 2.6 (1–4) | 2.8 (1–4) | .267 | 2.7 (1.0–4.0) | 2.8 (1.0–4.0) | .965 | 0.023 |
| Missing values | 41 (3.9) | 17 (3.2) | |||||
| Depression | 312 (29.9) | 112 (20.9) | <.001 | 104 (21) | 105 (21.2) | .938 | 0.020 |
| Missing values | 1 (0.1) | 0 (0) | |||||
| Parkinson disease | 45 (4.3) | 22 (4.1) | .84 | 17 (3.4) | 22 (4.4) | .437 | 0.041 |
| Missing values | 2 (0.2) | 0 (0) | 1 (0.2) | 0 (0) | |||
| Obesity | 105 (10.1) | 72 (13.5) | .041 | 68 (13.7) | 65 (13.1) | .780 | <0.001 |
| Missing values | 11 (1.1) | 7 (1.3) | |||||
| Polypharmacy | 628 (60.2) | 366 (68.4) | .001 | 350 (70.7) | 340 (68.7) | .489 | 0.022 |
| Missing values | 3 (0.3) | 1 (0.2) | |||||
| CFS | <.001 | .990 | 0.009 | ||||
| CFS 1–3, fit | 195 (18.7) | 160 (29.9) | 146 (29.7) | 147 (29.9) | |||
| CFS 4, vulnerable | 115 (11) | 60 (11.2) | 55 (11.2) | 56 (11.4) | |||
| CFS 5–9, frail | 705 (67.5) | 312 (58.3) | 291 (59.1) | 289 (58.7) | |||
| Missing values | 29 (2.8) | 3 (0.6) | |||||
| ADL, median (IQR) | 3.7 (2–6) | 4.3 (3–6) | <.001 | 4.4 (3.0–6.0) | 4.4 (3.0–6.0) | .915 | 0.053 |
| Missing values | 32 (3.1) | 11 (2.1) | |||||
| Living in nursing home | 266 (25.5) | 70 (13.1) | <.001 | 62 (12.5) | 68 (13.7) | .572 | 0.012 |
| Missing values | 4 (0.4) | 0 (0) | |||||
Notes: ADL = activities of daily living; CFS = Clinical Frailty Score; IQR = interquartile range; SMD = standardized mean difference. Data are number (%) unless otherwise indicated. Propensity score was established on age, sex, ADL, living in a nursing home, polypharmacy, obesity, depression, and Charlson Comorbidity Index. Polypharmacy: ≥5 drugs. In case of no missing value, we kept the line empty.
In the matched cohort (Table 2), patients receiving corticosteroids had significantly less dementia and clinically more severe condition than patients without corticosteroids, and the qSOFA score at COVID-19 onset was significantly higher. Furthermore, the minimum lymphocyte count was lower, maximal CRP rate higher and length of hospital stay longer for patients receiving corticosteroids.
Table 2.
Matched Population Characteristics Without and With Corticosteroids
| Total | Without Corticosteroids | With Corticosteroids | p Value | |
|---|---|---|---|---|
| N = 984 | n = 492 (50) | n = 492 (50) | ||
| Comorbidities | ||||
| Dementia | 425 (43.2) | 228 (46.3) | 197 (40) | .0046 |
| Parkinson disease | 40 (4.1) | 18 (3.7) | 22 (4.5) | .518 |
| Hypertension | 692 (70.3) | 351 (71.3) | 341 (69.3) | .485 |
| Diabetes | 293 (29.8) | 141 (28.7) | 152 (30.9) | .443 |
| Atrial fibrillation | 289 (29.4) | 158 (32.1) | 131 (26.6) | .059 |
| Chronic heart failure | 252 (25.6) | 127 (25.8) | 125 (25.4) | .884 |
| Stroke | 207 (21.0) | 112 (22.8) | 95 (19.3) | .184 |
| Myocardial infraction | 241 (24.5) | 122 (24.8) | 119 (24.2) | .884 |
| Peripheral vascular disease | 143 (14.5) | 70 (12.2) | 73 (14.8) | .786 |
| COPD | 166 (16.9) | 83 (16.9) | 83 (16.9) | 1 |
| Connective tissue disease | 14 (1.4) | 6 (1.2) | 8 (1.6) | .590 |
| Liver disease | 1 (0.1) | 0 (0) | 1 (0.2) | 1 |
| Missing values | 3 (0.3) | 3 (0.6) | 0 (0) | |
| Peptic ulcer disease | 61 (6.2) | 36 (7.3) | 25 (5.1) | .146 |
| Hemiplegia | 35 (3.6) | 21 (4.4) | 14 (2.8) | .128 |
| Chronic kidney disease | 359 (36.5) | 189 (38.4) | 170 (34.6) | .208 |
| Solid tumor | 28 (2.8) | 10 (2.0) | 18 (3.7) | .125 |
| Leukemia/lymphoma | 21 (2.1) | 3 (0.6) | 18 (3.7) | <.001 |
| AIDS | 3 (0.3) | 2 (0.4) | 1 (0.2) | 1 |
| Symptoms | ||||
| Max temperature (°C), median (IQR) | 38.2 (37.7–39.0) | 38.0 (37.6–38.9) | 38.3 (37.8–39.0) | .004 |
| Missing values | 38 (3.9) | 23 (4.7) | 15 (3.0) | |
| Respiratory rate/min, median (IQR) | 30.7 (25–36) | 28.1 (24–32) | 33.0 (28–40) | <.001 |
| Missing values | 103 (10.5) | 67 (13.6) | 36 (7.3) | |
| Oxygen saturation < 90% on room air | 474 (48.2) | 162 (32.9) | 312 (63.4) | <.001 |
| Missing values | 2 (0.2) | 1 (0.2) | 1 (0.2) | |
| qSOFA ≥ 2 | 153 (15.5) | 57 (11.6) | 96 (19.5) | <.001 |
| Missing values | 133 (13.5) | 75 (15.2) | 58 (11.8) | |
| Biology | ||||
| Min lymphocyte count (G/L), median (IQR) | 0.7 (0.4–0.9) | 0.8 (0.5–1.0) | 0.6 (0.3–0.8) | <.001 |
| Missing values | 19 (1.9) | 14 (2.8) | 5 (1) | |
| C-reactive protein level (mg/L), median (IQR) | 132 (62–188) | 115 (43–164) | 149 (85–199) | <.001 |
| Missing values | 20 (2.0) | 15 (3.0) | 5 (1.0) | |
| Cytolysis | 310 (31.5) | 124 (25.2) | 186 (37.8) | <.001 |
| Missing values | 45 (4.6) | 30 (6.1) | 15 (3.0) | |
| Cholestasis | 270 (27.4) | 115 (23.4) | 155 (31.5) | .009 |
| Missing values | 46 (4.7) | 30 (6.1) | 16 (3.3) | |
| RT-PCR positive | 937 (95.2) | 455 (92.5) | 482 (98.0) | <.001 |
| Missing values | 11 (1.1) | 9 (1.8) | 2 (0.4) | |
| COVID-19 anomalies on chest CT | 615 (62.5) | 204 (41.5) | 411 (83.5) | <.001 |
| Missing values | 104 (10.6) | 92 (18.7) | 12 (2.4) | |
| Length of stay (d), median (IQR) | 12.3 (6–16) | 10.9 (6–14) | 13.7 (7–18) | <.001 |
| Missing values | 5 (0.5) | 1 (0.2) | 4 (0.8) | |
| Destination at discharge | <.001 | |||
| Return home | 180 (18.3) | 108 (22.0) | 72 (14.6) | |
| Transfer in rehabilitation unit | 401 (40.8) | 240 (48.8) | 161 (32.7) | |
| Death | 318 (32.3) | 100 (20.3) | 218 (44.3) | |
| Other* | 81 (8.2) | 41 (8.3) | 40 (8.1) |
Notes: AIDS = acquired immuno-deficiency syndrome; COPD = chronic obstructive pulmonary disease; CT = computed tomography; IQR = interquartile range. Data are number (%) unless otherwise indicated.
*Transfer to other units (intensive care unit, palliative care unit, other medical ward). In case of no missing value, we kept the line empty.
In-hospital Mortality and Associated Factors
Within the unmatched cohort (n = 1 579), 465 (29.4%) patients died in hospital: 250 (30.3%) from the GERICOVID cohort and 215 (28.4%) from the GERICOCO cohort (Figure 1). Within the matched data set (N = 984), 318 (32.3%) patients died in hospital. On univariate analysis, in-hospital mortality was significantly higher with than without corticosteroid use (44.3% vs 20.3%; p < .001; Table 2). In the multivariate logistic mixed model (N = 821 patients), corticosteroid use was significantly associated with in-hospital mortality (OR = 2.61 [95% CI 1.63–4.20]; p < .001; Table 3). The other factors significantly associated with the outcome were minimal lymphocyte count, maximal CRP level, CCI, age, ADL, and oxygen saturation < 90% on room air.
Table 3.
Logistic Mixed Model of Factors Associated With In-hospital Mortality in the Matched Data Set
| Deceased | |||
|---|---|---|---|
| Predictors | OR | 95% CI | p Value |
| Max CRP level (mg/L) | 2.06 | 1.69–2.51 | <.001 |
| Min lymphocyte count (G/L) | 0.49 | 0.38–0.63 | <.001 |
| ADL | 0.85 | 0.75–0.95 | <.001 |
| Oxygen saturation < 90% on room air | |||
| No | Ref | Ref | |
| Yes | 2.15 | 1.45–3.17 | <.001 |
| Charlson Comorbidity Index | 1.18 | 1.07–1.29 | .001 |
| Corticosteroid use | .001 | ||
| No | Ref | Ref | |
| Yes | 2.61 | 1.63–4.20 | |
| Age | 1.04 | 1.01–1.07 | .013 |
| qSOFA ≥ 2 | 1.27 | 0.81–2.01 | .295 |
| CFS | |||
| CFS 1–3, fit | Ref | Ref | |
| CFS 4, vulnerable | 1.01 | 0.52–1.98 | .972 |
| CFS 5–9, frail | 1.28 | 0.75–2.18 | .363 |
| GERICOCO cohort | 0.75 | 0.45–1.24 | .267 |
| Random effects | |||
| ICC | 0.11 | ||
| NCenter | 15 | ||
| Observations | 821 | ||
| Marginal R2/conditional R2 | .413/.479 | ||
Notes: 95% CI = 95% confidence interval; ADL = activities of daily living; CRP = C-reactive protein; CFS = Clinical Frailty Score; ICC = intraclass correlation coefficient; OR = odds ratio; qSOFA = quick Sequential Organ Failure Assessment.
Table 4.
Side Effects Attributed to Corticosteroid Use During Hospitalization in the GERICOCO Cohort
| N | |
|---|---|
| Side effects (≥1) | 284 (68.3) |
| Gastrointestinal hemorrhage | 11 (2.8) |
| Decompensated diabetes | 60 (14.4) |
| Acute hypertension (>180/100 mmHg) | 51 (12.3) |
| Delirium | 137 (32.9) |
| Behavioral disorder | 59 (14.2) |
| Secondary infections | 136 (32.7) |
| Glucocorticoid disruption | 11 (3.4) |
Note: Data are n (%).
Corticosteroid Use and Side Effects
In the whole cohort, many patients (n = 401, 75.0%) received dexamethasone 6 mg/d. The other patients received methylprednisolone (n = 58, 10.8%), hydrocortisone (n = 28, 5.2%), prednisolone (n = 14, 2.6%), and methylprednisone (n = 5, 0.9%). The type of corticosteroid used was not reported for 23 patients.
Among the 416 patients who received some corticosteroids in the GERICOCO cohort, 284 (68.3%) experienced at least one corticosteroid-related side effect. Delirium (32.9%), secondary infections (32.7%), and decompensated diabetes (14.4%) were the most frequent. In 11 (3.4%) cases, corticosteroid use was disrupted because of the severity of side effect.
Discussion
Our cohort study evaluated the prognosis of geriatric patients receiving corticosteroids for COVID-19 in real life. The in-hospital mortality was 32.3% after propensity-score matching, and we identified several risk factors for death in this population. In particular, the in-hospital mortality was 2-fold increased with corticosteroid use (OR = 2.61 [95% CI 1.63–4.20]), and 68.3% of patients who received corticosteroids had side effects such as secondary infections and delirium.
The efficacy of corticosteroids for reducing mortality in COVID-19 in older patients is still controversial and could depend on the severity of disease, degree of inflammation or age (25,26). In the RECOVERY trial, a large randomized controlled trial (RCT) evaluating the benefit of dexamethasone in patients hospitalized for COVID-19, corticosteroid use significantly reduced 28-day mortality (OR = 0.83 [95% CI 0.75–0.93]; p < .001) (7), but the age-group analysis concluded that corticosteroid use was not associated with reduced mortality in patients over 70 years old (age 70–80: relative risk = 1.03 [95% CI 0.84–1.25] and over 80: relative risk = 0.89 [95% CI 0.75–1.05]) (7). The meta-analysis from the REACT Working group pooled data for 1703 patients from 7 RCTs including the RECOVERY trial and concluded that corticosteroid use was associated with significantly lower 28-day mortality rate (OR = 0.66 [95% CI 0.53–0.82]; p < .001) (27). The age-group analysis also concluded a reduction in 28-day mortality with corticosteroids and age over 60 years, but the cut-off age was low and patients were younger than in our study. van Paassen et al. published another meta-analysis including 20 197 patients from 44 studies (RCTs and observational) and also concluded an association of corticosteroid use with reduced short-term mortality (28 days, 30 days, and in-hospital OR = 0.72 [0.57–0.87]), but the authors did not perform an age analysis (28). However, a meta-analysis including 12 studies (15 754 patients) concluded an association of corticosteroid use with mortality (OR = 1.94 [95% CI 1.11-3.4]), but the authors did not analyze the age-group effect (29).
All these studies were not specific for older populations and did not consider comorbidities and functional status of patients, which are powerful prognostic factors in geriatric patients. Two retrospective cohort studies including 143 (10) and 267 (11) patients (mean age 85 years) concluded an association of corticosteroids with reduced mortality (hazards ratio = 0.61 [95% CI 0.41–0.93] for in-hospital mortality and 0.67 [95% CI 0.46–0.99] for 14-day mortality, respectively (10,11)). However, the size of the population was lower than in our study, and we performed a propensity-score analysis to balance baseline characteristics in the 2 groups before performing a logistic mixed analysis. Moreover, patients admitted in geriatric wards probably have a severity bias selection related to the need for management of complex multiorgan comorbidities in an acute condition and to limited access to intensive care units (ICUs) despite an indication. Thus, when compared with these previous studies, this population is characterized by multiorgan morbidity and functional dependency, with numerous patients with ICU profiles and close to ICU studies. In this ICU context, the COVIP study included 3 008 patients over 70 years old and concluded that corticosteroids remained associated with increased 30-day mortality (OR = 1.6 [95% CI 1.26–2.04]; p < .0001) (9).
We performed a large matched-cohort study considering comorbidities, polypharmacy, and functional status of real-life geriatric patients who were hospitalized for COVID-19. We found a significant association between corticosteroid use and in-hospital mortality. Several hypotheses could explain this result in the older population. The first is that corticosteroids affect the senescent immune system. Corticosteroids induce a transitory decrease in lymphocyte count, especially naive T cells (30), the T-cell compartment being the most affected by aging (31). This transitory effect could have a greater effect on older adults. Furthermore, lymphopenia is strongly associated with mortality in COVID-19 (32), which we confirmed in our cohort. This transitory worsening immunosuppression induced by corticosteroid use in older infected patients could extend the duration of viral clearance (33) but also increase the risk of secondary infections (34). The second hypothesis is that the risk of corticosteroids side effects could negatively affect the prognosis of comorbid patients, and we bring original and strong data on this point. The proportions of side effects occurring with corticosteroid use in the context of COVID-19 treatment are heterogenous, ranging from 11% to 13% for serious side effects and from 35% to 70% for overall side effects (35,36). In the geriatric population, this proportion ranges from 17% to 52% (10,11) to 68.3% in our cohort. Although the study design did not allow for a comparison with patients without corticosteroids, this prevalence appears important. Delirium, the most frequent complication reported in our cohort, is known to be associated with mortality in COVID-19 (37) and the severity of delirium increases with age and corticosteroid use (38). Secondary infections were reported in 7% of patients hospitalized for COVID-19 (39) but concerned 32.7% of our patients who received corticosteroids. This incidence suggests that secondary infections are more frequent with corticosteroid use (28) and that they could have a major prognostic impact because they are also associated with mortality.
In our study, age, comorbidities and functional autonomy were also associated with in-hospital mortality, which is congruent with other geriatric cohort studies of patients hospitalized for COVID-19 (40,41). Contrary to several studies (40,42), frailty was not significantly associated with in-hospital mortality in our cohort, but we found a trend (CFS 5–9, OR = 1.28 [95% CI 0.75–2.18]). One explanation could be the great proportion of frail patients included in our study (64% in the unmatched cohort and 59% in the matched cohort).
Limitations
The study included patients only within the Paris area, but this is the most affected area in France in terms of death from COVID-19 and the fourth in terms of COVID-19 incidence (43). Although this study was observational (with known limitations of retrospective data), it allowed us to include all consecutive hospitalized patients, with no restrictions on comorbidities, frailty, and autonomy. In addition, disease was more severe for patients with corticosteroid use than without corticosteroid use, but the propensity-score approach and the multivariate analysis, which included inclusion period and severity, limited this bias on baseline characteristics. Because of the retrospective design, some values were missing but concerned <2% of the overall data, which highlights the quality of data collection. Finally, our results may relate in part to certain characteristics of the French health care system and may not be extrapolated to other countries.
Conclusion
In this multicentric propensity matched-cohort study of 1 579 geriatric patients with confirmed COVID-19 in the Paris area, the hospital mortality was high (31.9%), and the use of corticosteroids for COVID-19 was associated with in-hospital mortality. RCTs are urgently needed to confirm these results in a geriatric population.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Supplementary Results 1. Characteristics of the unmatched cohort.
Supplementary Methods 1. STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Supplementary Methods 2. Original statistical analysis plan.
Acknowledgments
Special thanks to all authors of the GERICOVID Cohort (alphabetica order): Dr. Sophie Bastiani, Dr. Clémence Boully, Dr. Lauriane Bourdonnec, Dr. Delphine Casciari, Pr. Jean-Philippe David, Dr. Marie-Astrid Desoutter, Dr. Antoine Dureau, Dr. Virginie Fossey-Diaz, Dr. Anne-Sophie Grancher, Dr. Ariane Gross, Pr. Olivier Hanon, Dr. Flora Ketz, Dr. Sébastien Krypciak, Dr. Mathilde Lacrampe, Dr. Nadège Lemarié, Dr. Pauline de Malglaive, Dr. Alexandra Monti, Dr. Morgane Mary, Dr. Elise Mercadier, Pr. Bruno Riou, Dr. Mouna Romdhani, Dr. Swasti Roux, and Dr. Cédric de Villelongue.
Contributor Information
Valentine Lidou-Renault, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Saint Antoine, Department of Geriatric Medicine, Paris, France.
Edouard Baudouin, Assistance Publique-Hôpitaux de Paris (APHP), University hospital of Paris-Saclay, Department of Geriatric Medicine, Paul Brousse Hospital, Villejuif, France; Université Paris-Saclay, INSERM 1178, CESP, Équipe MOODS, Le Kremlin-Bicêtre, France.
Pauline Courtois-Amiot, Assistance Publique-Hôpitaux de Paris (APHP), Université de Paris, Hôpital Bichat, Department of Geriatric Medicine, Paris, France.
Celine Bianco, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Saint Antoine, Department of Geriatric Medicine, Paris, France.
Hélène Esnault, Assistance Publique-Hôpitaux de Paris (APHP), Université de Paris, Hôpital Bichat, Department of Geriatric Medicine, Paris, France.
Audrey Rouet, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Tenon, Department of Geriatric Medicine, Paris, France.
Margaux Baque, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Saint Antoine, Department of Geriatric Medicine, Paris, France; Sorbonne Université, INSERM UMR1135, Centre d’immunologie et des Maladies Infectieuses, Paris, France.
Charlotte Tomeo, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Rothschild, Department of Geriatric Medicine, Paris, France.
Antonio Rainone, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Charles Foix, Department of Geriatric Medicine, Ivry Sur Seine, France.
Sara Thietart, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Pitié Salpêtrière, Department of Geriatric Medicine, Paris, France.
Romain Veber, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Rothschild, Department of Geriatric Medicine, Paris, France.
Clementine Ayache, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Rothschild, Department of Geriatric Medicine, Paris, France.
Marion Pepin, Assistance Publique-Hôpitaux de Paris (APHP), Hôpital Ambroise Paré, Department of Geriatric Medicine, Boulogne, Billancourt, France; Université de Versailles Saint-Quentin en Yvelines, Université Paris-Saclay, INSERM, CESP, Clinical Epidemiology, Villejuif, France.
Carmelo Lafuente-Lafuente, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Charles Foix, Department of Geriatric Medicine, Ivry Sur Seine, France.
Emmanuelle Duron, Assistance Publique-Hôpitaux de Paris (APHP), University hospital of Paris-Saclay, Department of Geriatric Medicine, Paul Brousse Hospital, Villejuif, France; Université Paris-Saclay, INSERM 1178, CESP, Équipe MOODS, Le Kremlin-Bicêtre, France.
Pierre-Emmanuel Cailleaux, Assistance Publique-Hôpitaux de Paris (APHP), Hôpital Louis Mourier, Department of Geriatric Medicine, Colombes, France.
Didier Haguenauer, Assistance Publique-Hôpitaux de Paris (APHP), Hôpital Louis Mourier, Department of Geriatric Medicine, Colombes, France.
Nadège Lemarié, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Tenon, Department of Geriatric Medicine, Paris, France.
Elena Paillaud, Assistance Publique-Hôpitaux de Paris (APHP), Université de Paris, Paris Cancer Institute CARPEM, Department of Geriatric Medicine, Hôpital Européen Georges Pompidou, Paris, France.
Agathe Raynaud-Simon, Assistance Publique-Hôpitaux de Paris (APHP), Université de Paris, Hôpital Bichat, Department of Geriatric Medicine, Paris, France.
Caroline Thomas, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Saint Antoine, Department of Geriatric Medicine, Paris, France.
Jacques Boddaert, Sorbonne Université, INSERM UMR1135, Centre d’immunologie et des Maladies Infectieuses, Paris, France; Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Pitié Salpêtrière, Department of Geriatric Medicine, Paris, France.
Lorène Zerah, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Pitié Salpêtrière, Department of Geriatric Medicine, Paris, France; Sorbonne Université, INSERM, Institut Pierre Louis d’Épidémiologie et de Santé Publique, IPLESP, Paris, France.
Hélène Vallet, Assistance Publique-Hôpitaux de Paris (APHP), Sorbonne Université, Hôpital Saint Antoine, Department of Geriatric Medicine, Paris, France; Sorbonne Université, INSERM UMR1135, Centre d’immunologie et des Maladies Infectieuses, Paris, France.
Funding
None declared.
Conflict of Interest
J.B. reported personal fees for lectures from VIFOR Pharma and Baxter companies outside the submitted work. All other authors declare no conflicts of interest.
Author Contributions
H.V. had full access to all the data in the study and takes responsibility for the integrity and the accuracy of the data. Study concept and design: H.V. and L.Z. Acquisition of the data: V.L.-R., E.B., P.C.-A., C.B., H.E., A.R., M.B., C.To., A.R., S.T., R.V., C.A., M.P., C.L.-L., E.D., P.E.C., D.H., N.L., E.P., A.R.S., C.Th., J.B. Statistical analysis: E.B. Drafting of the manuscript: V.L.-R., L.Z., and H.V. Critical revision of the manuscript for important intellectual content: all authors. English editing: Laura Smales from BioMedEditing (Toronto, Canada).
References
- 1. WHO Coronavirus (COVID-19) Dashboard. March 22. https://covid19.who.int. Accessed March 23, 2022.
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 3. Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. SARS-CoV-2, COVID-19 and the ageing immune system. Nat Aging. 2021;1:769–782. doi: 10.1038/s43587-021-00114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 6. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO 1. Therapeutics and COVID-19: Living Guideline. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2021.3. Accessed November 13, 2021.
- 9. Jung C, Wernly B, Fjølner J, et al. Steroid use in elderly critically ill COVID-19 patients. Eur Respir J. 2021;58:2100979. doi: 10.1183/13993003.00979-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piniella-Ruiz E, Bellver-Álvarez MT, Mestre-Gómez B, et al. Impact of systemic corticosteroids on mortality in older adults with critical COVID-19 pneumonia. J Gerontol A Biol Sci Med Sci. 2021;76:e127–e132. doi: 10.1093/gerona/glab074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallay L, Tran V-T, Perrodeau E, et al. Fourteen-day survival among older adults with severe infection with severe acute respiratory syndrome coronavirus 2 treated with corticosteroid: a cohort study. Clin Microbiol Infect. 2021;27:1145–1150. doi: 10.1016/j.cmi.2021.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vanhaecke Collard C, Dramé M, Novella J-L, Blanchard F, Pennaforte J-L, Mahmoudi R. Functional manifestations associated to corticosteroid therapy among the elderly. Rev Med Inter. 2012;33:358–363. doi: 10.1016/j.revmed.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 13. McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20:131–137. doi: 10.1097/BOR.0b013e3282f51031 [DOI] [PubMed] [Google Scholar]
- 14. Zerah L, Baudouin E, Pépin M, et al. Clinical characteristics and outcomes of 821 older patients with SARS-Cov-2 infection admitted to acute care geriatric wards. J Gerontol A Biol Sci Med Sci. 2021;76:e4–e12. doi: 10.1093/gerona/glaa210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO 2. COVID-19 Clinical Management: Living Guidance. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Accessed November 13, 2021.
- 16. Rochwerg B, Agarwal A, Siemieniuk RA, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie C, RA. new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 18. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 19. Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x [DOI] [PubMed] [Google Scholar]
- 20. Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14:116–118. doi: 10.1097/00005650-197605001-00018 [DOI] [PubMed] [Google Scholar]
- 21. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941 [DOI] [PubMed] [Google Scholar]
- 23. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akter F, Araf Y, Hosen MJ. Corticosteroids for COVID-19: worth it or not? Mol Biol Rep. 2022;49:567–576. doi: 10.1007/s11033-021-06793-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. 2020;324:1292–1295. doi: 10.1001/jama.2020.16747 [DOI] [PubMed] [Google Scholar]
- 27. Sterne JAC, Murthy S, Diaz J, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J Med Virol. 2021;93:1538–1547. doi: 10.1002/jmv.26483 [DOI] [PubMed] [Google Scholar]
- 30. Olnes MJ, Kotliarov Y, Biancotto A, et al. Effects of systemically administered hydrocortisone on the human immunome. Sci Rep. 2016;6:23002. doi: 10.1038/srep23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fali T, Vallet H, Sauce D. Impact of stress on aged immune system compartments: overview from fundamental to clinical data. Exp Gerontol. 2018;105:19–26. doi: 10.1016/j.exger.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 32. Jafarzadeh A, Jafarzadeh S, Nozari P, Mokhtari P, Nemati M. Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms. Scand J Immunol. 2021;93:e12967. doi: 10.1111/sji.12967 [DOI] [PubMed] [Google Scholar]
- 33. Li J, Liao X, Zhou Y, et al. Association between glucocorticoids treatment and viral clearance delay in patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:1063. doi: 10.1186/s12879-021-06548-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo M, Gao M, Gao J, et al. Identifying risk factors for secondary infection post-SARS-CoV-2 infection in patients with severe and critical COVID-19. Front Immunol. 2021;12:715023. doi: 10.3389/fimmu.2021.715023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dupuis C, de Montmollin E, Buetti N, et al. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: a multicenter cohort study of the OUTCOMEREA network. PLoS One. 2021;16:e0255644. doi: 10.1371/journal.pone.0255644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Munch MW, Myatra SN, Vijayaraghavan BKT, et al. ; COVID STEROID 2 Trial Group. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hariyanto TI, Putri C, Hananto JE, Arisa J, Fransisca V Situmeang R, Kurniawan A. Delirium is a good predictor for poor outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression. J Psychiatr Res. 2021;142:361–368. doi: 10.1016/j.jpsychires.2021.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinotti G, Bonanni L, Barlati S, et al. Delirium in COVID-19 patients: a multicentric observational study in Italy. Neurol Sci. 2021;42:3981–3988. doi: 10.1007/s10072-021-05461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smits RAL, Trompet S, van der Linden CMJ, et al. Characteristics and outcomes of older patients hospitalised for COVID-19 in the first and second wave of the pandemic in The Netherlands: the COVID-OLD study. Age Ageing. 2022;51:afac048. doi: 10.1093/ageing/afac048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramos-Rincon J-M, Buonaiuto V, Ricci M, et al. Clinical characteristics and risk factors for mortality in very old patients hospitalized with COVID-19 in Spain. J Gerontol A Biol Sci Med Sci. 2021;76:e28–e37. doi: 10.1093/gerona/glaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jung C, Flaatten H, Fjølner J, et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021;25:149. doi: 10.1186/s13054-021-03551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Statistiques Coronavirus en France par région. Carte Coronavirus. https://www.carte-coronavirus.fr/regions.html. Accessed December 15, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.