Abstract
Background
Recent reports indicated declines in hepatitis C virus (HCV) testing during the first half of 2020 in the United States due to coronavirus disease 2019 (COVID-19), but the longer-term impact on HCV testing and treatment is unclear.
Methods
We obtained monthly state-level volumes of HCV antibody, RNA and genotype testing, and HCV treatment initiation, stratified by age and gender, spanning January 2019 until December 2020 from 2 large national laboratories. We performed segmented regression analysis for each state from a mixed-effects Poisson regression model with month as the main fixed predictor and state as a random intercept.
Results
During the pre–COVID-19 period (January 2019–March 2020), monthly HCV antibody and genotype tests decreased slightly whereas RNA tests and treatment initiations remained stable. Between March and April 2020, there were declines in the number of HCV antibody tests (37% reduction, P < .001), RNA tests (37.5% reduction, P < .001), genotype tests (24% reduction, P = .023), and HCV treatment initiations (31%, P < .001). Starting April 2020 through the end of 2020, there were significant increases in month-to-month HCV antibody (P < .001), RNA (P = .035), and genotype tests (P = .047), but only antibody testing rebounded to pre–COVID-19 levels. HCV treatment initiations remained low after April 2020 throughout the remainder of the year.
Conclusions
HCV testing and treatment dropped by >30% during April 2020 at the start of the COVID-19 pandemic, but although HCV testing increased again later in 2020, HCV treatment rates did not recover. Efforts should be made to link HCV-positive patients to treatment and revitalize HCV treatment engagement by healthcare providers.
Keywords: hepatitis C virus, coronavirus, testing, antibody, RNA
We analyzed monthly US state-level data on the hepatitis C virus (HCV) continuum, spanning January 2019 until December 2020, and found that HCV testing and treatment dropped by >30% in April 2020, but only HCV testing increased again later in 2020.
Acute hepatitis C virus (HCV) infections are increasing in the United States (US), with an estimated 3.5 million individuals being currently infected with HCV, and about half of those being unaware of their HCV diagnosis [1, 2]. The introduction of direct-acting antiviral therapy has since turned HCV infection into a curable disease [3, 4], and healthcare screening has become essential for early identification of disease before severe morbidity or mortality occurs. While previous HCV screening efforts mostly targeted Baby Boomers born between 1945 and 1965, in 2020 the Centers for Disease Control and Prevention has expanded its HCV screening recommendations and now recommends routine HCV screening for all adults aged ≥18 years for HCV infection [5]. An increasing number of healthcare settings have implemented these recommendations, for example, in their emergency departments (EDs) [6–9]. However, healthcare services were disrupted by the coronavirus disease 2019 (COVID-19) pandemic, reducing opportunities to conduct routine HCV antibody screening, clinical care, and treatment during the first half of 2020 [10, 11]. Dramatic decreases in patient volume were observed in many healthcare settings, driven by the fear of COVID-19 transmission and lower patient census. This resulted in reduced numbers of patients undergoing HCV screening, which decreased between 35% and 59% during April 2020 and rebounded to a 6%–20% reduction in July and August 2020 [10, 11]. Even more threatening, the ability to link HCV-positive individuals to care has stalled during the COVID-19 pandemic, when scheduling of in-person and walk-in visits was discouraged, creating significant delays in HCV linkage to care appointments [10], thereby reducing the linkage to care rate and HCV treatment prescriptions by 37%–43% between May and July 2020 [11].
While these reports indicated declines in HCV testing during the first half of 2020 in the US due to COVID-19, it remains unclear whether a longer-term impact on HCV testing and treatment initiations occurred. The objective of this study was to investigate the nationwide impact of the pandemic on HCV testing and treatment in the US through the end of 2020.
METHODS
Dataset
Monthly state-level data on the volumes of HCV antibody (Ab) testing, HCV RNA testing, HCV genotype testing, and HCV treatment initiations, stratified by age and gender, were obtained from the Symphony Health Dataverse, a nationally representative integrated dataset of medical, hospital, pharmacy, and laboratory data covering >317 million patients and >93% of prescriptions dispensed in the US (60%–70% capture rate from patient claims). The dataset includes laboratory test data from 2 large clinical laboratory test providers throughout the US. Deidentified aggregate data from HCV antibody immunoassay testing, HCV RNA testing and HCV genotype testing, and HCV treatments by state from 1 January 2019 through 31 December 2020 were included.
Statistical Analysis
The number of HCV antibody tests, RNA tests, genotype tests, and treatment initiations were compared between the pre–COVID-19 period and the COVID-19 period as well as within each period. Months from January 2019 to March 2020 (months 1–15 on the graph) were designated the “pre–COVID-19 period” and April 2020 to December 2020 (months 16–24 on the graph) were designated the “COVID-19 period”. We applied methods from interrupted time series analysis and conducted segmented regression to estimate temporal changes within, as well as between, the pre–COVID-19 and COVID-19 periods [12]. First, mixed-effects Poisson regression models with month as the main fixed predictor were used to estimate the number of tests and treatment initiations, respectively, for each state, during each of the aforementioned 24 months. Tests of covariance parameters, based on the restricted likelihood, were conducted to assess if random intercepts or slopes were needed to be used in the models to account for possible baseline as well as temporal differences between the states. Consequently, for each state, a random intercept as well as a random slope with an autoregressive covariance structure (to account for within state correlations induced by the repeated measurements) was used to account for unmeasured differences between states at the beginning of the study as well as for the differences in the temporal trends in all analyses. The predicted values from the mixed-effects models were aggregated across states by summing the estimated number of tests and treatment initiations, respectively, for each of the 24 months. Then, the aggregated values were used as outcome variables in segmented regressions to predict trends in HCV antibody tests, HCV RNA tests, HCV genotype tests, and treatment initiations, within as well as between, the pre–COVID-19 and COVID-19 periods [12]. Three autoregressive terms (AR2, AR4, AR6) were included in the model on HCV antibody tests to account for positive autocorrelation, while no autoregressive terms were needed in the other segmented regressions models, as indicated by Durbin-Watson statistics [13]. Maximum likelihood estimation was used.
Segmented regression analyses for HCV antibody tests and treatment were also performed for various age groups (5–19, 20–34, 35–54, and ≥55 years, as well as 55–69 and ≥70 years separately) and gender (male, female). Subanalyses were performed for the states New York, California, Illinois, and Florida to evaluate whether there was an impact on timing of COVID-19 shutdowns on HCV testing rates. Another segmented regression analysis was performed for HCV treatment comparing states that fully adopted Medicaid expansion (California, Colorado, Connecticut, Delaware, District of Columbia, Hawaii, Pennsylvania, Alaska, Louisiana, Maryland, Massachusetts, Minnesota, Nevada, New Jersey, New York, Oregon, Rhode Island, Vermont, Washington, West Virginia) with those that did not adopt Medicaid expansion (Wyoming, South Dakota, Wisconsin, Kansas, Texas, Tennessee, Mississippi, Alabama, Georgia, South Carolina, North Carolina, Florida). The analyses were performed using SAS software, version 9.4 of the SAS System for Windows.
RESULTS
Among our dataset covering 2 large national laboratories and roughly 60%–70% of prescriptions claims in the US, 17 565 801 HCV antibody tests (7 284 917 in males and 10 280 583 in females; gender missing in 301), 242 624 HCV RNA tests, 454 097 HCV genotype tests, and 173 925 HCV treatment initiations (103 844 in males, 70 080 in females, missing gender in 1 in age group 0–4 years; 0.3% in ages 5–19 years, 15% in ages 20–34 years, 32% in ages 35–54 years, 46% in ages 55–69 years, and 7% in age ≥70 years) were observed during the observation period.
HCV Antibody Testing
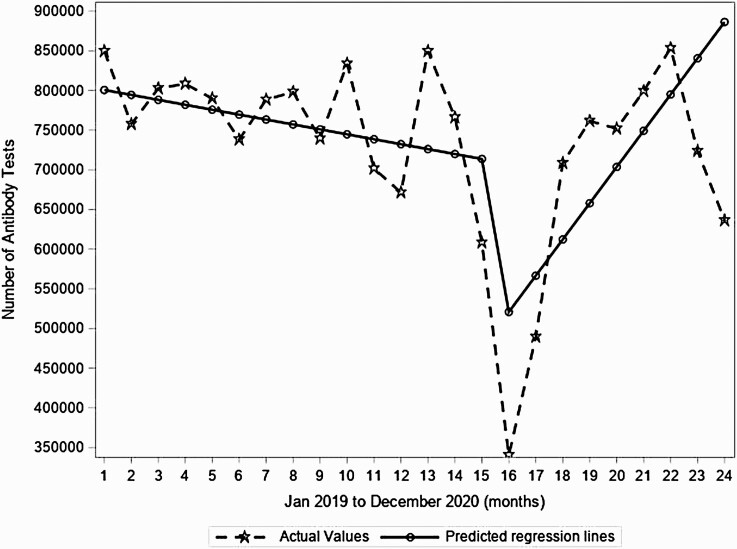
Right at the beginning of the observation period, the predicted number of tests in the US was 806 774. During the pre–COVID-19 period, there was a small (0.8%) yet statistically significant monthly decrease in the predicted number of HCV Ab tests. However, between March 2020 and April 2020, the predicted number of tests dropped abruptly and significantly by 238 473 tests. During the pre–COVID-19 period, the monthly number of HCV antibody tests in the US decreased by 0.8% monthly (decrease of 6207 tests each month, P = .008; Figure 1). Between March 2020 and April 2020, the predicted number of tests dropped abruptly and significantly by 37% (decrease of 238 473 tests, P < .001; similar trend in males and females and by state). During the COVID-19 period through the end of 2020 (compared to the month-to-month trend before the COVID-19 period), there was a significant increase in month-to-month testing of 51 880 tests (P < .001; Figure 1), rebounding to pre–COVID-19 levels by September 2020.
Figure 1.
Segmented regression analyses for monthly number of hepatitis C virus antibody tests in the United States from January 2019 to December 2020 from 2 large national laboratories.
Across states, similar HCV antibody test trends were observed (Supplementary Figure 1), which appeared independent of timing and extent of lockdown measures (Supplementary Figure 2 for New York, California, Illinois, and Florida). In subanalyses, there were also similar trends in HCV antibody tests across age groups (data not shown) and by gender (Supplementary Figures 3 and 4).
HCV RNA Testing
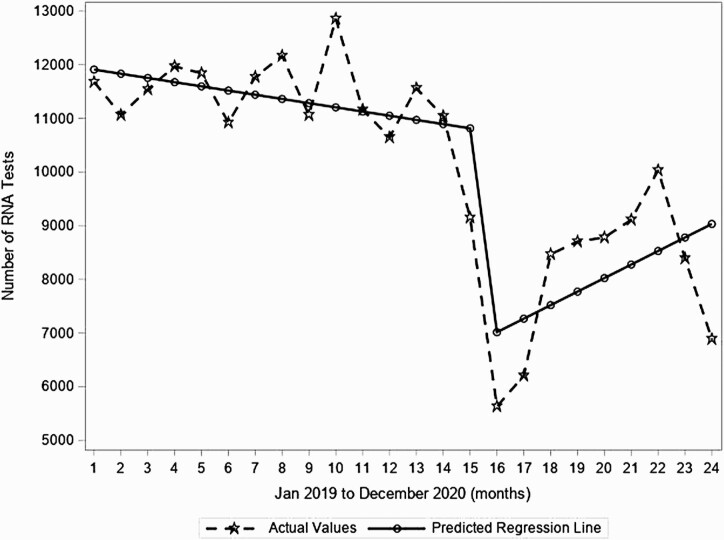
During the pre–COVID-19 period, there was a minor decrease in tests that occurred within each month (decrease of 0.7% or 78 tests per month from a baseline of 11 986 tests before the observation period; P = .217; Figure 2). Then, from March 2020 to April 2020, the predicted number of tests dropped significantly by 37.5% (ie, drop of 4051 tests, P < .001; Figure 2). This was followed by a significant increase in the month-to-month trend during the COVID-19 period compared to the month-to-month trend before the COVID-19 period (month-to-month increase of 3.6%, or 330 tests/month, P = .035; Figure 2). However, the RNA testing at the end of 2020 remained below pre–COVID-19 levels.
Figure 2.
Segmented regression analyses for monthly number of hepatitis C virus RNA tests in the United States from January 2019 to December 2020 from 2 large national laboratories.
HCV Genotype Testing
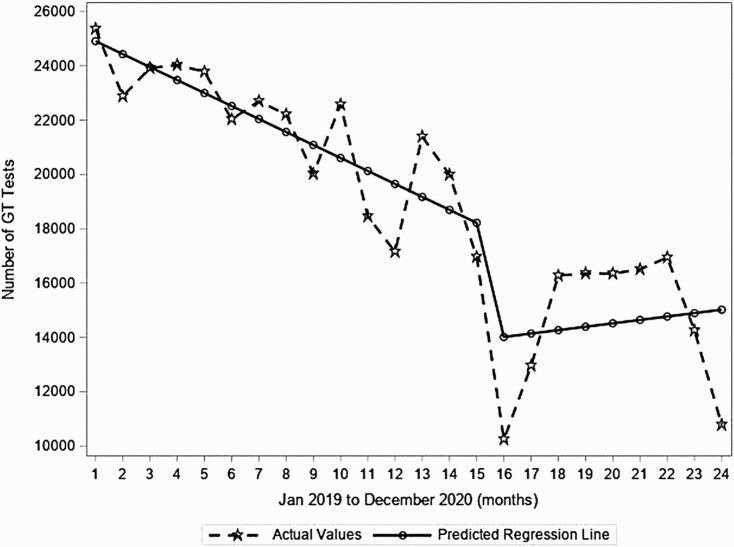
During the pre–COVID-19 period, there was a significant decrease in genotype tests with a decrease of 1.9% or 478 tests each month from a predicted number of 25 386 tests before the observation period (P < .001; Figure 3). Then, from March to April 2020, the predicted number of tests dropped significantly by 24% (decrease of 4328 tests/month, P = .023). This was followed by a significant increase in the month-to month trend during the COVID-19 period (0.9% increase, +603 tests per month; P = .047, Figure 3). However, the RNA testing at the end of 2020 remained below pre–COVID-19 levels.
Figure 3.
Segmented regression analyses for monthly number of hepatitis C virus genotype (GT) tests in the United States from January 2019 to December 2020 from 2 large national laboratories.
HCV Treatment Initiations
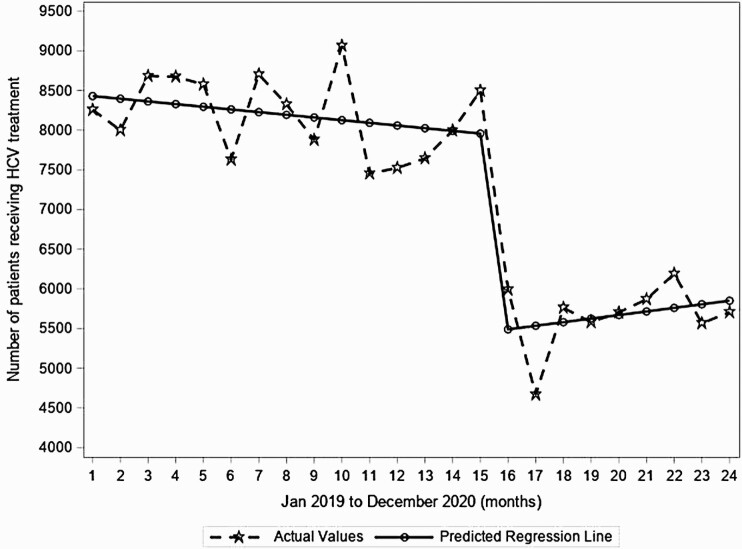
During the pre–COVID-19 period, HCV treatment initiations (predicted n = 8463 before the observation period) were stable, but dropped significantly from March 2020 to April 2020 by 31% (decrease of 2512 treatment initiations/month, P < .001; Figure 4) and remained low throughout the remainder of the year during the COVID-19 period.
Figure 4.
Segmented regression analyses for monthly number of hepatitis C virus (HCV) treatment initiations in the United States from January 2019 to December 2020 from 2 large national laboratories.
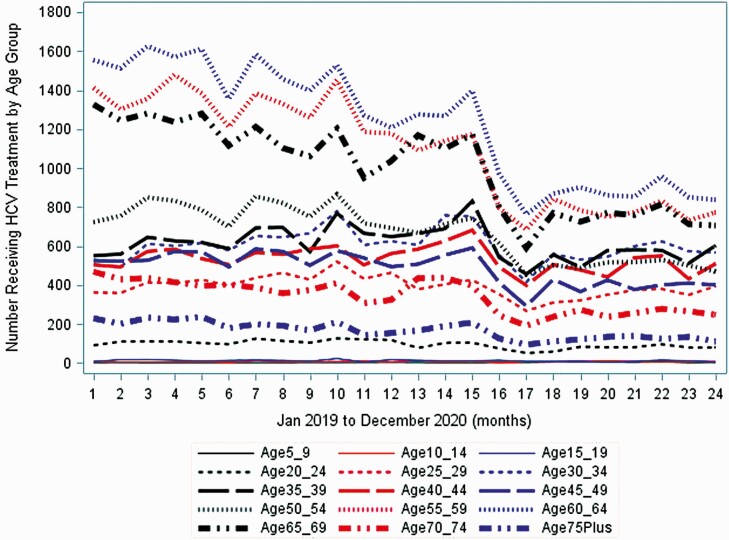
States that did not adopt Medicaid expansion did have a significant month-to-month decrease in HCV treatment initiations during the pre–COVID-19 period (P = .014), whereas the states that adopted Medicaid did not (P = .3) (Supplementary Figures 5 and 6). However, there was no difference in the trend after April 2020 between states that adopted Medicaid and those that did not (both P < .001; Supplementary Figures 5 and 6). In subanalyses, there were also similar trends in HCV treatment declines across age groups (Figure 5) and by state (Supplementary Figure 7, New York, California, Illinois, and Florida).
Figure 5.
Segmented regression analyses for monthly number of hepatitis C virus (HCV) treatment initiations in the United States from January 2019 to December 2020, by age group.
DISCUSSION
We analyzed monthly US state-level volumes of HCV antibody, RNA and genotype testing, and HCV treatment initiation, stratified by age and gender, spanning from January 2019 until December 2020, and found that monthly HCV Ab and RNA testing as well as treatment volumes dropped by >30% during the first months of the COVID-19 pandemic. While HCV Ab testing rebounded to pre–COVID-19 levels by the end of 2020 and HCV RNA and genotype testing increased, although not reaching pre–COVID-19 levels, HCV treatment rates remained at a constant low level through the end of 2020. This sustained disruption of HCV treatment initiation could potentially result in increased transmission and HCV-associated morbidity and mortality.
The observed decrease in HCV Ab testing numbers during the early phases of the COVID-19 pandemic is mostly in line with a decrease in ED and hospital census during that time period [10]. In fact, during the early pandemic period between late March and April 2020, the total number of US ED visits was 42% lower than during the same period a year earlier [14]. In a report from the Boston Medical Center, hospital-wide HCV testing decreased by 49.6% in the 3.5 months following 16 March 2020, particularly driven by a 72% decrease in ambulatory testing [15]. The decline in HCV testing during the early phase of COVID-19 observed in our analysis of 37% was mostly in line with these previous observations but lower than the 59% decrease observed during April 2020 in a previous analysis of about 12 million HCV Ab tests in the US [11]. Compared to previous studies, the longer observation period in this analysis allowed us to investigate whether HCV Ab testing would rebound to pre–COVID-19 levels later in 2020, and indeed we found that the drop in HCV Ab test was only temporary. The same was true for HCV RNA testing, which dropped in line with HCV antibody testing in our study (37.5% drop), and HCV genotype testing where a 24% drop was observed between March and April 2020.
In contrast to HCV testing, HCV treatment initiations, after dropping 31% during the early phase of COVID-19, did not rebound in 2020. These results add to recent observations that HCV treatment prescriptions dropped by 37% to 43% between May and July 2020, when compared to the same months the year before [11], indicating that the reduction of HCV treatment initiations has in fact continued beyond mid-2020. While a delay is expected between HCV diagnosis and subsequent treatment initiation [16], the temporary drop in HCV Ab tests and HCV diagnoses does not explain sufficiently the low rates of HCV treatment initiations in 2020. In fact, it has been shown that HCV linkage to care has stalled during the early phase of COVID-19. In a universal HCV screening program in 2 University of California, San Diego EDs, median time to linkage to care was 18 weeks between March and June 2020 and therefore double the normally observed median of 9 weeks between December 2018 and February 2020 [10]. While universal HCV screening has been a success, an increase of HCV diagnoses outside the birth cohort has also led to an increase of barriers to care among the diagnosed population, including higher frequency of active substance use and housing instability [10], both of which have also increased during the COVID-19 pandemic [17, 18]. These barriers present major challenges to the linkage to care process and are likely to persist even after the lockdown phase when appointments at primary care providers and HCV treatment providers become more readily available. Potential longer-term solutions could involve remote telehealth visits for HCV treatment initiation.
Interestingly, the findings and timing of HCV tests and treatment initiations were consistent across states and did not parallel the differences in COVID-19 surges and restrictions observed during the pandemic between the states (eg, no lockdowns in Florida vs lockdown in New York). Whether this reflects patient hesitancy or healthcare availability or whether there are other reasons for this finding needs to be explored further. In a previous study it was shown that presence or absence of Medicaid restrictions had a significant impact on changes in HCV screening and diagnosis rates, with HCV screening rates increased in states without restrictions, whereas rates declined in those with restrictions [19]. Conversely, HCV RNA positivity rates per 100 000 persons declined significantly when comparing states without vs with restrictions. Our study found that during the pre–COVID-19 period, HCV treatment initiation numbers declined significantly only in states that did not adopt the Medicaid expansion, However there was no difference in the trend after April 2020 between states that adopted Medicaid and those that did not.
Importantly, for HIV and hepatitis B a similar trajectory with a significant decrease in testing and linkage to care during the first months of the COVID-19 pandemic in 2020, with only testing mostly rebounding later in 2020, had also been described [10, 20–25]. Particularly for HIV, relinkage to care of known HIV positives out of care was impacted by COVID-19, also in part due to conflicting priorities of county healthcare departments that came with the COVID-19 pandemic [10].
There were several strengths of this study, including the largest US dataset on HCV testing and treatment initiation analyzed to date and the broad and representative geographic coverage of the data sets. Also we were able to draw conclusions beyond the early phase of COVID-19 as data until the end of 2020 were analyzed. In addition, we were able to analyze differences between the states that adopted and those that did not adopt Medicaid during the pre–COVID-19 period. While only states that did not adopt Medicaid expansion did have a significant month-to-month decrease during pre–COVID-19, there was no difference in the trend after April 2020, indicating that the pandemic disrupted care irrespective of other variables. There are also several limitations. First, we were unable to assess differences by race/ethnicity with our dataset. Both HCV and COVID-19 disproportionately affect communities of color [26–29]. HCV infection rates are highest among non-Hispanic Blacks, and yet their HCV treatment initiation rates are lower [27, 30]. In our analysis, despite the fact that trends were consistent across age groups, states, and gender, it is possible that disparities by race/ethnicity in screening and treatment initiation could have been exacerbated by the COVID-19 pandemic and merits further analysis. Last, although a strength of our analysis is the use of a large dataset that covers 2 large national laboratories and a majority of all prescription claims in the US, the fact that it does not capture all HCV testing and treatment in the US limits generalizability. Notably, the Symphony Health dataset excludes data from the following groups: Veterans, prisons, Department of Defense, and Indian Health Services, and so our results are not generalizable to them.
In conclusion, we found that monthly HCV antibody testing and treatment initiation volumes dropped by >30% during the early phase of the COVID-19 pandemic. While HCV antibody testing rebounded to pre–COVID-19 levels, HCV treatment rates remained at a constant low level through the end of 2020, representing missed hepatitis C treatment opportunities. Efforts should be made to link HCV Ab–positive patients to treatment and revitalize HCV treatment engagement by healthcare providers.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The views expressed are those of the authors and not necessarily those of the National Institutes of Health (NIH).
Financial support. This work was supported by Gilead Sciences. N. K. M. is supported by the National Institute of Allergy and Infectious Diseases (NIAID)/National Institute on Drug Abuse (NIDA) (grant number R01 AI147490) and the University of California, San Diego Center for AIDS Research, an NIH-funded program (grant number P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, National Cancer Institute, National Institute of Mental Health, NIDA, National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, National Institute on Aging, National Institute of General Medical Sciences, and National Institute of Diabetes and Digestive and Kidney Diseases.
Potential conflicts of interest. N. K. M. has received unrestricted research grants from Gilead and Merck. M. H. has received grants from Gilead, MSD, Pfizer, Astellas, Euroimmun, F2G, and the NIH. N. R. has received grants from AbbVie, Gilead, Abbott, Salix, and Intercept and consulting fees from Gilead, Salix, Intercept, and Antios; has participated on a data and safety monitoring board or advisory board for Arbutus; and reports a leadership or fiduciary role in the American Association for the Study of Liver Diseases (Clinical Liver Disease editor), American Board of Internal Medicine (Gastroenterology subspeciality board), and the Accreditation Council for Graduate Medical Education Review Committee. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: The Liver Meeting 2021, Virtual, 12–15 November 2021. Presentation 913.
Contributor Information
Martin Hoenigl, University of California San Diego, San Diego, California, USA; Medical University of Graz, Graz, Austria.
Daniela Abramovitz, University of California San Diego, San Diego, California, USA.
Ricardo E Flores Ortega, University of California San Diego, San Diego, California, USA.
Natasha K Martin, University of California San Diego, San Diego, California, USA; University of Bristol, Bristol, United Kingdom.
Nancy Reau, Rush University Medical Center, Chicago, Illinois, USA.
References
- 1. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T.. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015; 62:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryerson AB, Schillie S, Barker LK, Kupronis BA, Wester C.. Vital signs: newly reported acute and chronic hepatitis C cases—United States, 2009-2018. MMWR Morb Mortal Wkly Rep 2020; 69:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaillon A, Mehta SR, Hoenigl M, et al. . Cost-effectiveness and budgetary impact of HCV treatment with direct-acting antivirals in India including the risk of reinfection. PLoS One 2019; 14:e0217964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin NK, Thornton A, Hickman M, et al. . Can hepatitis C virus (HCV) direct-acting antiviral treatment as prevention reverse the HCV epidemic among men who have sex with men in the United Kingdom? Epidemiological and modeling insights. Clin Infect Dis 2016; 62:1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB.. CDC recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recomm Rep 2020; 69:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowan EA, Dinani A, Brandspiegel S, et al. . Nontargeted hepatitis C screening in an urban emergency department in New York City. J Emerg Med 2021; 60:299–309. [DOI] [PubMed] [Google Scholar]
- 7. Hoenigl M, Mathur K, Blumenthal J, et al. . Universal HIV and birth cohort HCV screening in San Diego emergency departments. Sci Rep 2019; 9:14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathur K, Blumenthal J, Horton LE, et al. . HIV screening in emergency departments: linkage works but what about retention? Acad Emerg Med 2021; 28:913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford JS, Chechi T, Toosi K, et al. . Universal screening for hepatitis C virus in the ED using a best practice advisory. West J Emerg Med 2021; 22:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lara-Paez G, Zuazo M, Blumenthal J, Coyne CJ, Hoenigl M.. HIV and HCV screening in the emergency department and linkage to care during COVID-19: challenges and solutions. J Acquir Immune Defic Syndr 2021; 88:e14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaufman HW, Bull-Otterson L, Meyer WA III, et al. . Decreases in hepatitis C testing and treatment during the COVID-19 pandemic. Am J Prev Med 2021; 61:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D.. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 13. Durbin J, Watson GS.. Testing for serial correlation in least squares regression. II. Biometrika 1951; 38(1-2): 159–78. [PubMed] [Google Scholar]
- 14. Hartnett KP, Kite-Powell A, DeVies J, et al. . Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sperring H, Ruiz-Mercado G, Schechter-Perkins EM.. Impact of the 2020 COVID-19 pandemic on ambulatory hepatitis C testing. J Prim Care Community Health 2020; 11:2150132720969554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rice DP, Ordoveza MA, Palmer AM, Wu GY, Chirch LM.. Timing of treatment initiation of direct-acting antivirals for HIV/HCV coinfected and HCV monoinfected patients. AIDS Care 2018; 30:1507–11. [DOI] [PubMed] [Google Scholar]
- 17. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med 2020; 173:61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubey MJ, Ghosh R, Chatterjee S, Biswas P, Chatterjee S, Dubey S.. COVID-19 and addiction. Diabetes Metab Syndr 2020; 14:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reau NM-SG, Joshi AD, Dylla DE, et al. . Change in HCV epidemiology from 2018 to 2019: a comparison between states with no Medicaid restrictions and states with several medicaid restrictions. In: The Liver Meeting, Virtual, 13–16 November 2020. [Google Scholar]
- 20. Stanford KA, McNulty MC, Schmitt JR, et al. . Incorporating HIV screening with COVID-19 testing in an urban emergency department during the pandemic. JAMA Intern Med 2021; 181:1001–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stanford KA, Friedman EE, Schmitt J, et al. . Routine screening for HIV in an urban emergency department during the COVID-19 pandemic. AIDS Behav 2020; 24:2757–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hensley KS, Jordans CCE, van Kampen JJA, et al. . Significant impact of coronavirus disease 2019 (COVID-19) on human immunodeficiency virus (HIV) care in hospitals affecting the first pillar of the HIV care continuum. Clin Infect Dis 2021; 74:521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rick F, Odoke W, van den Hombergh J, Benzaken AS, Avelino-Silva VI.. Impact of coronavirus disease (COVID-19) on HIV testing and care provision across four continents. HIV Med 2022; 23:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pley CM, McNaughton AL, Matthews PC, Lourenço J.. The global impact of the COVID-19 pandemic on the prevention, diagnosis and treatment of hepatitis B virus (HBV) infection. BMJ Glob Health 2021; 6:e004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mandel E, Peci A, Cronin K, et al. . The impact of the first, second and third waves of covid-19 on hepatitis B and C testing in Ontario, Canada. J Viral Hepat 2022; 29:205–8. [DOI] [PubMed] [Google Scholar]
- 26. Balakrishnan M, Kanwal F.. The HCV treatment cascade: race is a factor to consider. J Gen Intern Med 2019; 34:1949–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hall EW, Rosenberg ES, Sullivan PS.. Estimates of state-level chronic hepatitis C virus infection, stratified by race and sex, United States, 2010. BMC Infect Dis 2018; 18:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arena PJ, Malta M, Rimoin AW, Strathdee SA.. Race, COVID-19 and deaths of despair. EClinicalMedicine 2020; 25:100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore KJ, Gauri A, Koru-Sengul T.. Prevalence and sociodemographic disparities of hepatitis C in Baby Boomers and the US adult population. J Infect Public Health 2019; 12:32–6. [DOI] [PubMed] [Google Scholar]
- 30. Nili M, Luo L, Feng X, Chang J, Tan X.. Disparities in hepatitis C virus infection screening among Baby Boomers in the United States. Am J Infect Control 2018; 46:1341–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.