ABSTRACT
Background
Several cases of idiopathic nephrotic syndrome (INS) relapse following the administration of coronavirus disease 2019 (COVID-19) vaccines have recently been reported, raising questions about the potential relationship between the immune response to COVID-19 vaccination and INS pathogenesis.
Methods
We performed a retrospective multicentre survey describing the clinical and biological characteristics of patients presenting a relapse of INS after COVID-19 vaccination, with an assessment of outcome under treatment.
Results
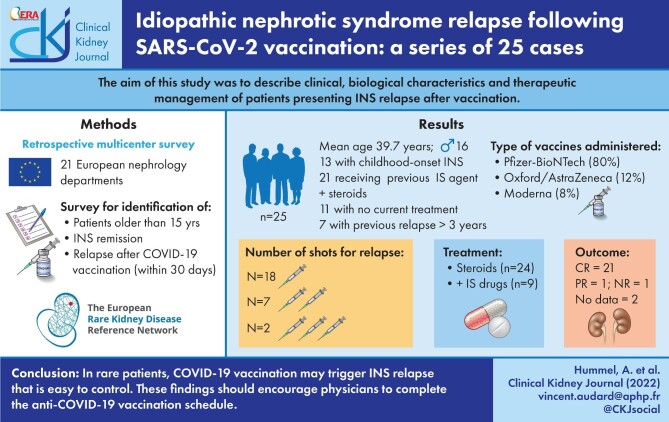
We identified 25 patients (16 men and 9 women) presenting a relapse within 1 month of a COVID-19 vaccine injection. The glomerular disease was of childhood onset in half of the patients and most patients (21/25) had received at least one immunosuppressive drug in addition to steroids for frequently relapsing or steroid-dependent nephrotic syndrome (NS). All patients were in a stable condition at the time of injection and 11 had no specific treatment. In five patients, the last relapse was reported >5 years before vaccine injection. The Pfizer-BioNTech (BNT162b2) vaccine was used in 80% of the patients. In 18 cases, INS relapse occurred after the first injection, a mean of 17.5 days after vaccination. A second injection was nevertheless administered in 14 of these patients. Five relapses occurred after administration of the second dose and two relapses after the administration of the third dose. All but one of the patients received steroids as first-line treatment, with an additional immunosuppressive agent in nine cases. During follow-up, complete remission was achieved in 21 patients, within 1 month in 17 cases. Only one patient had not achieved at least partial remission after 3 months of follow-up.
Conclusions
This case series suggests that, in rare patients, COVID-19 vaccination may trigger INS relapse that is generally easy to control. These findings should encourage physicians to persuade their patients to complete the COVID-19 vaccination schedule.
Keywords: COVID-19, idiopathic nephrotic syndrome, minimal change disease, relapse, vaccination
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The molecular mechanisms underlying the pathophysiological processes of idiopathic nephrotic syndrome (INS) remain poorly understood, but this glomerular disease, which includes two principal pathological entities, minimal change disease (MCD) and primary focal and segmental glomerulosclerosis (FSGS), is currently considered to be an immune-mediated disease [1–4]. Indeed, relapses of INS may be triggered by immunological stimuli, such as viral infections (particularly those of the upper airways), immunization or allergens, supporting the hypothesis of a primary immune system disorder leading to changes in glomerular capillary permeability and disorganization of the podocyte cytoskeleton [1–4]. Vaccination has long been considered a trigger of INS relapse, even though very few prospective data are available. A recent study in children with steroid-dependent nephrotic syndrome (SDNS) found no close relationship between vaccine administration and an increase in the risk of relapse [5]. INS patients are well known to be at high risk of episodes of infection, due to the use of immunosuppressive agents and alterations to both humoral and cell-mediated immunity during active phases of the disease. Thus, in INS patients, the benefit:risk ratio is clearly in favour of prophylactic vaccination provided that the contraindication of live vaccines is respected in cases of ongoing immunosuppression [6]. In the context of the massive coronavirus disease 2019 (COVID-19) vaccination campaign worldwide, some cases of new-onset MCD [7–14] or of MCD relapse [14–20] have been reported, suggesting a potential relationship between the immune response to COVID-19 vaccination and MCD pathogenesis [21]. In contrast to the published cases of MCD, new-onset or relapses of primary FSGS following COVID-19 vaccination seem to be an exceptional finding [22]. The demographic, clinical and biological characteristics of patients with INS relapse following COVID-19 vaccination remain to be determined. In this European multicentre survey, we describe the main features of 25 patients with a prior history of INS, all in remission (with or without specific immunosuppressive drugs) at the time of vaccination, with reported relapses of MCD or FSGS within 1 month of the first, second or third administration of the COVID-19 vaccine.
MATERIALS AND METHODS
Study population
Participants were recruited by the network of French rare disease centres dedicated to the management of INS. We conducted this retrospective study by sending a questionnaire to all French nephrology departments, asking them to identify patients of ≥15 years of age with a prior history of INS (regardless of age at onset) who presented a relapse within 30 days of a first, second or third dose of COVID-19 vaccine. Additional cases from European centres belonging to the European Rare Kidney Disease Reference Network were also included in the survey. At each centre of the network, patients were identified from electronic medical records. This study was performed in accordance with the ethical standards of the Helsinki Declaration. The inclusion criteria for patients with adult-onset INS were biopsy-proven MCD or FSGS with therapeutic sensitivity to steroids and/or immunosuppressive agents. For childhood-onset INS, patients could be included without a systematic renal pathological study if they had a prior history of steroid-sensitive NS with or without relapses. In addition, all patients included in this study had to have been in remission for at least 2 months before COVID-19 vaccination. Two of the patients in this series were reported in previous publications, but detailed information was supplied by their physicians via the survey [15, 16].
Data collection
Demographic, clinical, biological and histological data obtained at the initial diagnosis of INS and at the time of relapse after COVID-19 vaccine administration were assessed for each patient. NS was defined as a urine protein:creatinine ratio (uPCR) >3 g/g and a serum albumin concentration <30 g/L. MCD was diagnosed on the basis of an absence of visible alterations on light microscopy examination and an absence of immunoglobulin and/or complement deposits in immunofluorescence studies [3]. FSGS diagnosis was based on the presence of segmentally collapsed glomerular capillaries with areas of glomerular scarring associated with the focal and segmental granular deposition of immunoglobulin M and/or complement 3 within the areas of segmental glomerular sclerosis [4]. Complete remission of NS was defined as the normalization of uPCR to a level <0.3 g/g and a serum albumin concentration >30 g/L. INS relapse in patients previously in complete remission was defined as an increase in uPCR to ˃3 g/g and/or a serum albumin concentration <30 g/L. Acute kidney injury (AKI) was defined according to the Kidney Disease: Improving Global Outcome (KDIGO) criteria [23]. Specific treatments for INS (steroid therapy and/or immunosuppressive drugs) were noted for all patients at the time of initial diagnosis and at the time of relapse. Follow-up involved assessments of proteinuria and serum albumin concentration 1 and/or 3 months after relapse.
RESULTS
Demographic, clinical and biological characteristics of patients with INS before post-COVID-19 vaccination relapse
We retrospectively identified 25 patients (16 men and 9 women) with a mean age of 25.6 years (range 1–75) at the first episode of INS who presented a relapse after the COVID-19 vaccination. Their demographic, clinical and biological data from INS diagnosis until the last relapse are summarized in Table 1. The glomerular disease had a childhood onset in 13 patients (52%). All but three patients (patients 9, 18 and 20, with childhood-onset steroid-sensitive NS) had undergone renal biopsy. All 10 children with biopsy-proven INS presented typical features of MCD. In patients with adult-onset INS, a renal biopsy revealed typical pathological lesions consistent with a diagnosis of MCD in 10 cases and FSGS lesions in two cases (tip variant in both cases). The therapeutic approaches used to control underlying glomerular disease differed between individuals, with one to six lines of treatment prescribed during the course of the disease. In 21 patients, at least one immunosuppressive drug in addition to steroids was administered for the frequently relapsing nephrotic syndrome (FRNS) or SDNS. Four patients received steroids only and displayed a complete remission of NS; two of these patients had two relapses each, which were also treated with steroids alone. A total of 15 patients (10 with childhood-onset and 5 with adult-onset INS) had received at least three lines of immunosuppressive treatment to control their INS due to FRNS or SDNS.
Table 1.
Demographic, clinical and biological data at the time of INS diagnosis and during follow-up before the last relapse
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | M | F | M | F | F | F | M | M | M | M | F | M | F | M | M | M | M | M | M | M | F | F | M |
| Past history of INS | |||||||||||||||||||||||||
| Age at first episode of INS (years) | 3 | 66 | 74 | 45 | 5 | 27 | 35 | 36 | 4 | 1 | 34 | 1 | 32 | 75 | 47 | 3 | 2 | 4 | 5 | 2 | 5 | 68 | 3 | 6 | 58 |
| Relapses, n | >10 | 1 | 0 | 1 | >10 | 2 | 2 | 2 | NA | 5 | >10 | 0 | NA | NA | 2 | NA | NA | NA | >10 | >10 | NA | 3 | >10 | >10 | >10 |
| Lines of treatment, n | 5 | 3 | 1 | 2 | 5 | 3 | 1 | 2 | 3 | 5 | 2 | 1 | 3 | 2 | 1 | 2 | 3 | 3 | 5 | 6 | 2 | 3 | 4 | 4 | 4 |
| Previous specific treatment for INS | Cs Lev CYC CNI MMF |
Cs PE MMF |
Cs | Cs CNI |
Cs CYC CNI MMF R |
Cs MMF R |
Cs | Cs CNI |
Cs Lev MMF |
Cs Lev MMF CYC R |
Cs MMF |
Cs | Cs CNI R |
Cs CNI |
Cs | Cs MMF |
Cs CNI R |
Cs CNI MMF |
Cs CYC Lev MMF R |
Cs CYC Lev CNI MMF R |
Cs AZA |
Cs CNI R |
Cs MMF CNI R |
Cs MMF CNI R |
Cs MMF CNI R |
| Renal biopsy findings | MCD | FSGS | MCD | MCD | MCD | MCD | MCD | MCD | NP | MCD | MCD | MCD | FSGS | MCD | MCD | MCD | MCD | NP | MCD | NP | MCD | MCD | MCD | MCD | MCD |
| Last biological evaluation before the occurrence of the relapse | |||||||||||||||||||||||||
| Serum alb level (g/L) | NA | 40 | 37 | 43 | 32.3 | 47 | 40 | 43 | NA | 47 | 42 | 42 | 39 | 40 | 43 | 45 | 48 | NA | NA | 44 | NA | 33 | 45.7 | 42 | 38 |
| Proteinuria (g/g) | 0 | 0.3 | 0 | 0 | 0 | 0 | 0.11 | 0 | 0 | 0 | 1.7 | 0 | 0.1 | 0 | 0.06 | 0 | 0.11 | 0.21 | NA | 0.12 | NA | 1.2 | 0.09 | 0.09 | 0 |
Cs, corticosteroids; CYC, cyclophosphamide; R, rituximab; Lev, levamisol; PE, plasma exchange; AZA, azathioprine; MCD, minimal change disease; NA, not available; NP, not performed; alb, albumin.
Clinical characteristics and treatment just before post-COVID-19 vaccination relapse
The mean age of our patients at the time of relapse after vaccination was 39.7 years (range 15–83) (Table 2). The time from the last relapse (before vaccination) and the relapse after vaccination was known for 21 patients (mean 56 months, range 2–468 months). In all, 10 of the patients had a relapse in the last 12 months. For four patients, the last relapse was diagnosed between 1 and 3 years before the relapse after vaccination and seven patients had experienced no relapse in the previous 3 years. For one 40-year-old patient (patient 12), the only episode of the disease had occurred 39 years previously, at the age of 1 year. Biological data (uPCR and/or albumin level) during the year preceding vaccination were available for 20 patients (Table 1). uPCR was <0.5 g/g in all but two patients: patient 13, for whom uPCR was 1.7 g/g but albumin levels were in the normal range (42 g/L) before vaccination, decreasing significantly to 16 g/L during relapse, and patient 22, whose uPCR was 1.2 g/g, increasing to 3.3 g/g, but the albumin level decreased to 23 g/L after vaccination. At the time of the relapse, 11 patients were not receiving any specific treatment for INS. In contrast, steroid therapy was ongoing, with or without immunosuppressive agents, in 14 patients, to maintain complete remission of INS (5 patients received steroids alone, 4 received mycophenolate mofetil, 2 received rituximab and 3 received steroids plus another immunosuppressive treatment).
Table 2.
Demographic, clinical and biological data at the time of relapse following vaccination against COVID-19
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at time of INS relapse (years) | 38 | 67 | 74 | 46 | 23 | 30 | 36 | 41 | 16 | 19 | 48 | 40 | 46 | 83 | 53 | 25 | 19 | 15 | 31 | 21 | 42 | 72 | 18 | 16 | 72 |
| Treatment at the time of relapse | MMF | Cs | Cs | Cs | Cs R |
– | Cs | – | – | – | MMF | – | R | Cs CNI |
– | MMF | R | – | – | – | – | Cs R |
MMF | – | Cs |
| Time since last relapse (months) | 60 | 5 | 3 | 6 | 5 | 20 | 2 | 25 | 72 | 27 | NA | 468 | 7 | NA | 5 | NA | NA | 54 | 90 | 15 | 240 | 10 | 49 | 11 | 9 |
| Type of vaccine | AZ Pf |
Pf Pf |
Pf Pf |
Pf |
Pf Pf |
Pf Pf |
Pf Pf |
Pf * |
Pf Pf |
Pf Pf |
Mo Mo |
Pf NA |
Pf Pf |
AZ AZ |
Pf Pf |
Pf Pf |
Pf Pf |
Pf Pf |
Pf Pf |
Pf Pf |
AZ | Pf Pf Pf |
Pf Pf |
Mo Mo |
Pf Pf Pf |
| Injection number followed by relapse (days) | 1 (14) | 1 (18) | 1 (21) | 1 (11) | 1 (21) | 2 (6) | 1 (10) 2 (5) |
1 (30) | 1 (15) | 1 (21) | 1 (7) | 1 (7) | 2 (20) | 2 (20) | 1 (26) | 1 (21) | 2 (25) | 1 (28) | 1 (21) | 1 (20) | 1 (11) | 3 (7) | 1 (14) 2 (9) |
2 (1) | 3 (2) |
| Laboratory parameters at the time of relapse | |||||||||||||||||||||||||
| Serum albumin concentration (g/L) | 26 | 19 | 36 | 24.5 | 27 | 37 | 37 | 37 | 28 | 42 | 16 | 27 | 22 | 20 | 32 | 43 | 32 | NA | 12 | 19 | 13 | 23 | ND | ND | 22 |
| Proteinuria (g/g) | 5.2 | 9.2 | 1.4 | 7 | 3.9 | 1.7 | 3 | 1.6 | 6.6 | 6 | 8.5 | 8 | 9 | 3.5 | 3.6 | 7.2 | 2.8 | 4.6 | 5 | 7.2 | 7.3 | 3.3 | 10.3 | 4.9 | 10.1 |
| Serum creatinine concentration (mg/L) | 10 | 37 | 11 | 6.3 | 8.2 | 6.1 | 8.6 | 8.2 | NA | 6 | 19 | 13 | 21 | 17 | 9 | 8 | 9.3 | 9.4 | 9 | 7.2 | 12.7 | 13.7 | ND | ND | 23.8 |
| Specific treatment at time of relapse | Cs MMF |
Cs MMF |
Cs CNI |
Cs CNI |
Cs Obi |
Cs R |
Cs R |
Cs CNI |
Cs | Cs | Cs MMF |
Cs | CYC | Cs | Cs | Cs MMF |
Cs | Cs | Cs | Cs | Cs | Cs MMF |
Cs MMF |
Cs | Cs |
| Outcome M1 | CR | PR | PR | CR | CR | CR | CR | CR | CR | CR | CR | CR | NR | NA | CR | CR | CR | CR | CR | NR | PR | CR | CR | ||
| Outcome M3 | CR | CR | NR | PR | CR | CR | |||||||||||||||||||
Pf, Pfizer–BioNTech vaccine; AZ, Oxford/AstraZeneca vaccine; Mo, Moderna vaccine; Cs, corticosteroids; CYC, cyclophosphamide; R, rituximab; Obi, obinutuzumab; CR, complete remission; PR, partial remission; NR, no remission; NA, not available.
*Patient 8 developed confirmed COVID-19 after the first dose.
Vaccination and clinical and biological characteristics at relapse
All patients received their first injection of the COVID-19 vaccine between January and September 2021 (Table 2). The vaccine used was the Pfizer–BioNTech vaccine (BNT162b2) in 20 cases, the Oxford–AstraZeneca vaccine (AZD1222) in 3 cases and the Moderna vaccine [messenger RNA (mRNA)-1273] in 2 cases. In 18 cases, the relapse of NS occurred after the first injection, a mean of 17.5 days after vaccination (range: 7–28 days). No clinical and/or laboratory findings of relapse were reported in the first week after vaccination, seven relapses occurred in the second week, eight in the third week and three in the fourth week. Despite the occurrence of a relapse, all but four patients received a second dose of vaccine, of the same type as the first, except for one young patient (patient 1) who was vaccinated with the BNT162b2 vaccine 3 months after an initial AZD1222 vaccine injection (the AZD1222 vaccine was no longer authorized for people <55 years of age in France at the time of his second injection). One of the four patients who received only one dose had a positive polymerase chain reaction test for SARS-CoV-2 at the time at which he should have received the second injection (patient 8). Seven relapses were noted after the second injection of the vaccine. These relapses occurred a mean of 12 days (range 1–25 days) after vaccination. Finally, two patients had relapses 2 and 7 days after their third injection of the BNT162b2 vaccine, despite having experienced no relapse after their second injection 6 months previously. Two patients (patients 7 and 23) experienced a relapse after each of their two injections of the vaccine. INS relapse was confirmed by biological evidence of a NS in 14 patients and was strongly suspected due to a significant increase in proteinuria relative to the last assessment, but without a decrease in albumin level to ˂30 g/L in nine patients. Two young adults (patients 23 and 24) did not undergo albumin determinations, but their proteinuria increased from 0.09 to 10.3 g/g and 4.9 g/g. Proteinuria in the entire cohort ranged from 1.4 to 10.3 g/g (mean 5.6 g/g). Two patients had severe NS with albumin levels ˂15 g/L. Four patients (patients 3, 6, 8 and 17) had no nephrotic-range proteinuria at the time of relapse, but the significant increase in proteinuria level led the clinicians to consider this increase as the first manifestation of post-vaccination relapse requiring specific therapy that was started within days. Five patients experienced AKI at the time of post-vaccine relapse: three had AKI KDIGO stage 1 (patients 11, 14 and 25), one had AKI KDIGO stage 2 (patient 13) and one had AKI KDIGO stage 3 (patient 2), but none of these patients underwent either renal biopsy or renal replacement therapy at this time. Venous thrombosis occurred in two patients (patients 1 and 2) at the time of relapse.
Treatment and outcome
All but one of the patients received steroids as a first-line treatment for INS relapse. A total of 9/11 patients without specific treatment at the time of post-vaccine relapse received steroids alone for treatment of the relapse, whereas the other two received steroids together with a calcineurin inhibitor (CNI; patient 8) or rituximab (patient 6). The steroid dose was increased for the patients on steroid treatment alone at the time of relapse and all but one of these five patients received an additional immunosuppressive drug: mycophenolate mofetil (MMF) for patient 2, CNI for patients 3 and 4 and rituximab for patient 7. For the four patients on MMF only, steroids were added to the treatment regimen at the time of relapse. One patient on rituximab was treated with steroids, one patient on CNI and low-dose steroids was treated by increasing the steroid dose and two patients on rituximab and steroids were switched to obinutuzumab for patient 5 and MMF for patient 22 along with a higher dose of steroids. The last patient (patient 13) was treated with oral cyclophosphamide alone. Of note, among 18 patients with available data, an assessment of humoral immune response to SARS-CoV-2 found that 11 patients exhibited a positive antibody titre (>7.1 BAU/mL) within 2 months after the first vaccine administration. During follow-up, 21/25 patients achieved complete remission, within 1 month in 17 cases. One patient (patient 14) was in partial remission at the last follow-up and one patient had no remission (patient 13). This particular patient displayed INS due to FSGS and had been in remission on rituximab for 7 months. His relapse started 20 days after the second dose of the vaccine and was treated with cyclophosphamide; this treatment remained unsuccessful at the last follow-up. The two patients (patients 22 and 25) who relapsed after their third dose had just started steroid therapy at the time of writing.
DISCUSSION
COVID-19 vaccines were developed in <1 year in response to the pandemic threat. The three main vaccines used in Europe are based on either new mRNA technology [24] for the BNT162b2 vaccine and the mRNA-1273 vaccine or an adenovirus vector for the AZD1222 vaccine. These vaccines have formed the backbone of a massive vaccination campaign worldwide, heralding a new era of preventive medicine. Nevertheless, a number of concerns have been raised about the potential toxicity and reactivity of these vaccines, and pharmacovigilance reports for these new vaccines have been monitored particularly closely, due to their novel mode of action. By 18 August 2021, 1.8 billion people had been completely vaccinated against COVID-19 and only 295 cases of glomerulonephritis (new onset or relapsing), including 46 cases of MCD, had been reported to VigiBase [25]. COVID-19 vaccines are designed to activate immune responses by inducing the production of several pro-inflammatory cytokines, but they could also potentially trigger autoimmune conditions in predisposed individuals. In agreement with this hypothesis, Caza et al. [26] reported a series of 29 patients with a broad spectrum of immune-mediated glomerular diseases (including 7 MCD, 10 immunoglobulin A nephropathy, 6 crescentic glomerulonephritis and 3 membranous nephropathy) [26]. We cannot rule out the possibility of these relapses or new onsets of MCD being related to vaccine administration, but these events seem to be a very rare finding [21].
We report here the first series of 25 INS relapses occurring within 1 month of COVID-19 vaccine injection. We also excluded six other known cases from this series because the biological data were insufficient to confirm INS relapse. The 20 French patients were referred from different regions of France after several calls for cases sent to all French nephrologists by the French Society of Nephrology Dialysis and Transplantation and the French rare disease centre for INS management. This study was retrospective and suffers from the limitations inherent in this type of approach and missing cases are, of course, likely. Unfortunately, in the context of the massive worldwide vaccination campaign against COVID-19, it is difficult to determine accurately the incidence of relapse after vaccination, because vaccination status is not sufficiently well recorded for all INS patients. Nevertheless, at one centre participating in our study (Necker Hospital, Adult Nephrology Department), 106 adult patients were contacted individually to record vaccination status and the occurrence of relapse: 80 of these patients had had at least one injection and 2 (2.5%) had experienced a relapse (one after the first dose and the other after the third dose).
We cannot rule out the possibility that the INS relapses observed in the context of SARS-CoV-2 vaccination reflect a fortuitous association rather than a related disorder, but the pathophysiological processes potentially involved merit consideration. It has been suggested that INS is the clinical manifestation of a primary disorder of T-lymphocyte function leading to cytokine release, resulting in alterations to the glomerular filtration barrier [1]. The signature of the immune response to COVID-19 vaccination seems to involve several crucial effectors of adaptive immunity (CD4+/CD8+ T lymphocytes, natural killer T cells, memory B lymphocytes and T follicular cells) [24]. Sahin et al. [27] showed that, following stimulation, the supernatants of peripheral blood mononuclear cells from vaccinated participants contained pro-inflammatory cytokines such as tumour necrosis factor, interleukin (IL)-1β and IL-12p70, which could potentially be involved in INS pathogenesis [2]. A close association between Human Leucocyte Antigen system variants and steroid-sensitive MCD has also been suggested [1]. Interestingly, Caso et al. [28] showed that genetic predispositions could influence the response to COVID-19 vaccines and their adverse effects [28]. Molecular mimicry, involving a cross-reaction with a host protein sequence, may also trigger autoimmune responses following COVID-19 vaccination [29]. As observed in seven previously published case reports (after exclusion of two cases previously reported and added to our case series [15, 16]) summarized in Table 3, the close temporal relationship (17.5 days after a first injection, 12 days after a second injection and 4.5 days after a third injection) between vaccination against COVID-19 and INS relapse suggests a possible pathophysiological link between these two conditions, although it is not yet possible to draw definitive conclusions.
Table 3.
Published case reports for relapses of INS following COVID-19 vaccinationa
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Reference | 17 | 18 | 19 | 19 | 14 | 14 | 20 |
| Age (years) | 22 | 60 | 30 | 40 | 33 | 34 | 33 |
| Sex | M | M | M | F | F | F | F |
| Vaccine | Pf | Pf | AZ | AZ | Mo | Pf | ND |
| Relapse after first dose (interval between vaccination and relapse in days) | 3 | 8 | 2 | 2 | No | No | No |
| Relapse after second dose (interval between vaccination and relapse in days) | No | NA | NA | NA | 21 | 28 | 14 |
| Treatment | Cs + CNI | CS + CNI | Cs | Cs | NA | NA | Cs |
| Course of relapse (time in days) | CR (17) | CR (14) | CR (10) | CR (14) | NA | NA | NA |
The main objective of our study was the identification of risk factors or patient profiles at particular risk of INS relapse following vaccination against COVID-19. Our study suggests that INS may relapse in patients with childhood- or adult-onset disease, but that relapses occur preferentially in patients with ‘difficult-to-treat’ INS, as 84% of cases were already on treatment with immunosuppressive agents to control underlying glomerular disease. A total of 10 of our patients had suffered a relapse in the preceding 12 months, suggesting that their INS remained particularly active before vaccination. Nevertheless, 11 patients were on no specific treatment and the occurrence of a relapse ˃20 years after the previous episode in two patients highlights the potential role of vaccination as a trigger for INS relapse. In our series, 80% of the patients had received the BNT162b2 vaccine, but INS relapse has been described with most of the vaccines used in Europe and North America. Adding our 25 patients to the other 7 published cases of post-vaccination relapse and new-onset NS linked to MCD [7–20, 25], the BNT162b2 vaccine had been administered in 30 patients (68%), the mRNA-1273 vaccine in 5 patients (11%), the AZD1222 vaccine in 7 patients (16%), the Janssen vaccine in 1 patient (2%) and information about the type of vaccine was unavailable in 1 patient. These figures may simply reflect the availability of each of the vaccines. For example, in France, the BNT162b2 vaccine accounts for almost 80% of COVID-19 vaccine injections. However, one pharmacovigilance report reported an odds ratio of 2.13 for MCD development after vaccination with the BNT162b2 vaccine relative to other vaccines [25]. The clinical and biological presentation was not unusual for this condition. In four patients without nephrotic-range proteinuria at the time of post-vaccination relapse, after careful evaluation of their medical records, spontaneous remission of NS was not suspected. AKI occurred in five patients (three with AKI KDIGO 1) but resolved rapidly with treatment in all but one patient who did not achieve remission and two patients had uncomplicated thrombophlebitis. As expected, the post-vaccination relapses of all patients were treated with steroids, the cornerstone of INS treatment. Based on specific previous renal disease history, treatment with additional immunosuppressive agents was initiated in nine cases. Excluding the two patients who presented a relapse after the third injection and who had only just started treatment, 21/23 patients achieved rapid remission on treatment and an additional patient achieved partial remission. These observations are consistent with the various case reports published to date (Table 3) and suggest that INS relapse following COVID-19 vaccination is highly sensitive to steroid therapy. Because renal biopsy was not systematically performed at the time of COVID-19 vaccination relapse, alternate glomerular pathologies cannot be definitively ruled out. Nevertheless, biopsy-proven INS at the time of the first episode, previous history of a difficult-to-treat form of INS (frequently relapsing or steroid-dependent NS) and successful use of steroids to control post-vaccination relapse highly suggest that the patients presented in this study displayed a relapse of their primary glomerular disease rather than the occurrence of another glomerular disease.
Strikingly, despite the relapse after the first administration of the vaccine in 18 patients, the patients’ clinicians were not reluctant to perform a second injection in 14 patients (with a vaccine from the same manufacturer in 13 patients). In 12 of these 14 patients, no flare-up was observed after the second dose. The other two patients had a minor relapse that resolved rapidly when the steroid dose was increased slightly. Only three of the seven published cases received a second dose, and none presented proteinuria requiring a change of treatment. These findings should encourage physicians to persuade their patients to complete the COVID-19 vaccination schedule. Given the severity of COVID-19 in immunocompromised patients [30], even if the production of vaccine-specific antibodies is impaired in patients on immunosuppressive agents [31, 32], we believe that prophylactic strategies against infectious pathogens, including approaches for reducing the risk of COVID-19, remain crucial for INS patients. Nevertheless, our data suggest that INS patients receiving COVID-19 vaccines should be carefully monitored, with reinforced screening for proteinuria after vaccination, to ensure that potential relapses are detected early.
In conclusion, our case series suggests that, in rare patients with no identified specific risk factors, COVID-19 vaccines may trigger INS relapse, which is easy to control in most cases, but the precise molecular mechanisms underlying proteinuria in this context remain to be determined.
Contributor Information
Aurélie Hummel, Assistance Publique des Hôpitaux de Paris (AP-HP), Hôpital Universitaire Necker-Enfants Malades, Service de Néphrologie et Transplantation, Centre de Référence Maladie Rare “Syndrome Néphrotique Idiopathique” (SNI), Paris, France.
Julie Oniszczuk, AP-HP, Hôpitaux Universitaires Henri Mondor, Service de Néphrologie et Transplantation, Centre de Référence Maladie Rare SNI, Créteil, France; Université Paris Est Créteil, Institut National de la Santé et de la Recherche Médicale (INSERM) U955, Institut Mondor de Recherche Biomédicale, Equipe 21, Créteil, France.
Delphine Kervella, Institut de Transplantation Urologie Néphrologie, Centre Hospitalo Universitaire (CHU) Nantes, Nantes, France; Centre de Recherche en Transplantation et Immunologie, Unité Mixte de Recherche 1064, INSERM, Université de Nantes, Nantes, France.
Marina Charbit, AP-HP, Hôpital Universitaire Necker-Enfants Malades, Service de Néphrologie Pédiatrique, Centre de Référence MARHEA, Centre de Référence SNI, Institut Imagine, Université de Paris, Paris, France.
Dominique Guerrot, Department of Nephrology, Hemodialysis and Transplantation, Rouen University Hospital, Rouen, France; Normandy University, UNIROUEN, INSERM U1096, Rouen, France.
Angelo Testa, Expansion Centre Hémodialyse de l'Ouest, Rezé, France.
Carole Philipponnet, Service Nephrologie Dialyse et Transplantation Rénale CHU de Clermont-Ferrand, Clermont-Ferrand, France.
Cécile Chauvet, Service de Néphrologie, Centre Hospitalier Saint Joseph Saint Luc, Lyon, France.
Thomas Guincestre, Service de Néphrologie, Centre Hospitalier de Roubaix, Roubaix, France.
Karine Brochard, Service de Néphrologie-Rhumatologie-Médecine Interne pédiatrique, Centre de Référence des Maladies Rénales Rares du Sud-Ouest, Hôpital des Enfants, Toulouse, France.
Ariane Benezech, Service de Néphrologie-Rhumatologie-Médecine Interne pédiatrique, Centre de Référence des Maladies Rénales Rares du Sud-Ouest, Hôpital des Enfants, Toulouse, France.
Lucile Figueres, Institut de Transplantation Urologie Néphrologie, Centre Hospitalo Universitaire (CHU) Nantes, Nantes, France; Centre de Recherche en Transplantation et Immunologie, Unité Mixte de Recherche 1064, INSERM, Université de Nantes, Nantes, France.
Xavier Belenfant, Groupe Hospitalier Grand Paris Nord Est, Hôpital André Grégoire, Service de Néphrologie-Dialyse, Montreuil, France.
Andrea Guarnieri, Nephrology Dialysis and Transplant Unit, Azienda Ospedaliero-Universitaria Senese, Siena, Italy.
Nathalie Demoulin, Nephrology Division, Cliniques Universitaires Saint-Luc, Brussels, Belgium; Institut de Recherche Expérimentale et Clinique, UC Louvain, Brussels, Belgium.
Elisa Benetti, Pediatric Nephrology, Dialysis and Transplant Unit, Department of Women's and Children's Health, Padua University Hospital, Padua, Italy.
Marius Miglinas, Nephrology Center, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
Kathleen Dessaix, Université de Montpellier, Service de Nephrologie, CHU Montpellier, Hôpital Lapeyronie, Montpellier, France.
Johann Morelle, Nephrology Division, Cliniques Universitaires Saint-Luc, Brussels, Belgium; Institut de Recherche Expérimentale et Clinique, UC Louvain, Brussels, Belgium.
Andrea Angeletti, Division of Nephrology, Dialysis, Transplantation, IRCCS Giannini Gaslini Children's Hospital, Genova, Italy.
Anne-Laure Sellier-Leclerc, Centre de Référence des Maladies Rénales Rares Néphrogones, Service de Néphrologie Rhumatologie et Dermatologie Pédiatriques, Hôpital Femme Mère Enfant, Faculté de Médecine Lyon Est, Bron, France.
Bruno Ranchin, Centre de Référence des Maladies Rénales Rares Néphrogones, Service de Néphrologie Rhumatologie et Dermatologie Pédiatriques, Hôpital Femme Mère Enfant, Faculté de Médecine Lyon Est, Bron, France.
Guillaume Goussard, Service de Néphrologie et Transplantation CHU Poitiers, Poitiers, France.
Laurent Hudier, Service de Néphrologie, Centre Hospitalier Broussais, Saint Malo, France.
Justine Bacchetta, Centre de Référence des Maladies Rénales Rares Néphrogones, Service de Néphrologie Rhumatologie et Dermatologie Pédiatriques, Hôpital Femme Mère Enfant, Faculté de Médecine Lyon Est, Bron, France.
Aude Servais, Assistance Publique des Hôpitaux de Paris (AP-HP), Hôpital Universitaire Necker-Enfants Malades, Service de Néphrologie et Transplantation, Centre de Référence Maladie Rare “Syndrome Néphrotique Idiopathique” (SNI), Paris, France.
Vincent Audard, AP-HP, Hôpitaux Universitaires Henri Mondor, Service de Néphrologie et Transplantation, Centre de Référence Maladie Rare SNI, Créteil, France; Université Paris Est Créteil, Institut National de la Santé et de la Recherche Médicale (INSERM) U955, Institut Mondor de Recherche Biomédicale, Equipe 21, Créteil, France.
FUNDING
The authors received no specific financial support for this study.
AUTHORS’ CONTRIBUTIONS
A.H. and V.A. designed the research study. A.H., J.O., A.S. and V.A. analysed the data. J.A., D.K., M.C., D.G., A.T., C.P., C.C., T.G., K.B., A.B., L.F., X.B., A.G., N.D., E.B., M.M., K.D., J.M., A.A., A.L.-S.L., B.R., G.G., L.H. and J.B. collected the data, provided clinical and biological information and critically reviewed the article. A.H. and V.A. wrote the article. Each author contributed important intellectual content during article drafting. All authors read and approved the final version of the article.
CONFLICT OF INTEREST STATEMENT
V.A. received consulting fees from Addmedica, Travere, Alnylam and AstraZeneca outside of the submitted work. None of the other authors has any conflicts of interest to declare.
REFERENCES
- 1. Colucci M, Corpetti G, Emma F et al. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol 2018; 33: 573–584 [DOI] [PubMed] [Google Scholar]
- 2. Sahali D, Sendeyo K, Mangier M et al. Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin Immunopathol 2014; 36: 421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vivarelli M, Massella L, Ruggiero B et al. Minimal change disease. Clin J Am Soc Nephrol 2017; 12: 332–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2017; 12: 502–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angeletti A, Bruschi M, Bianchin S et al. Vaccines and disease relapses in children with nephrotic syndrome. Clin J Am Soc Nephrol 2021; 16: 937–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyer O, Baudouin V, Bérard E et al. Vaccine recommendations for children with idiopathic nephrotic syndrome. Nephrol Ther 2020; 16: 177–183 [DOI] [PubMed] [Google Scholar]
- 7. Lebedev L, Sapojnikov M, Wechsler A et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021; 78: 142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maas RJ, Gianotten S, van der Meijden WAG. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021; 78: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Agati VD, Kudose S, Bomback AS et al. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int 2021; 100: 461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holzworth A, Couchot P, Cruz-Knight W et al. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 2021; 100: 463–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weijers J, Alvarez C, Hermans MMH. Post-vaccinal minimal change disease. Kidney Int 2021; 100: 459–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anupama YJ, Patel RGN, Vankalakunti M. Nephrotic syndrome following ChAdOx1 nCoV-19 vaccine against SARScoV-2. Kidney Int Rep 2021; 6: 2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leclerc S, Royal V, Lamarche C et al. Minimal change disease with severe acute kidney injury following the Oxford-AstraZeneca COVID-19 vaccine: a case report. Am J Kidney Dis 2021; 78: 607–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salem F, Rein JL, Yu SM et al. Report of three cases of minimal change disease following the second dose of mRNA SARS-CoV-2 COVID-19 vaccine. Kidney Int Rep 2021; 6: 2523–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mancianti N, Guarnieri A, Tripodi S et al. Minimal change disease following vaccination for SARS-CoV-2. J Nephrol 2021; 34: 1039–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kervella D, Jacquemont L, Chapelet-Debout A et al. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int 2021; 100: 457–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwotzer N, Kissling S, Fakhouri F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine”. Kidney Int 2021; 100: 458–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Komaba H, Wada T, Fukagawa M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021; 78: 469–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morlidge C, El-Kateb S, Jeevaratnam P et al. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int 2021; 100: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Özkan G, Bayrakçı N, Karabağ S et al. Relapse of minimal change disease after inactivated SARS-CoV- 2. vaccination: case report. Int Urol Nephrol 2022; 54: 971–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Izzedine H, Bonilla M, Jhaveri KD. Nephrotic syndrome and vasculitis following SARS-CoV-2 vaccine: true association or circumstantial? Nephrol Dial Transplant 2021; 36: 1565–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klomjit N, Alexander MP, Fervenza FC et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep 2021; 6: 2969–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 12: 1–138 [Google Scholar]
- 24. Linares-Fernández S, Lacroix C, Exposito JY et al. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med 2020; 26: 311–323 [DOI] [PubMed] [Google Scholar]
- 25. Kronbichler A, Jung SY, Kim MS et al. Distinct glomerular disease association after vaccination with BNT162b2 and mRNA-1273: a Vigibase analysis. Kidney Int 2021; 101: 415–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caza TN, Cassol CA, Hannoudi A et al. Glomerular disease in temporal association with SARS-CoV-2 vaccination: a series of 29 cases. Kidney360 2021; 2: 1770–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sahin U, Muik A, Derhovanessian E et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature 2020; 586: 594–599 [DOI] [PubMed] [Google Scholar]
- 28. Caso F, Costa L, Ruscitti P et al. Could SARS-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev 2020; 19: 102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020; 217: 108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caillard S, Anglicheau D, Matignon M et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int 2020; 98: 1549–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colucci M, Piano Mortari E, Zotta F et al. Evaluation of immune and vaccine competence in steroid-sensitive nephrotic syndrome pediatric patients. Front Immunol 2021; 12: 602826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyarsky BJ, Werbel WA, Avery RK et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021; 325: 1784–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]