Abstract
Background
Opaganib, an oral sphingosine kinase-2 inhibitor with antiviral and anti-inflammatory properties, was shown to inhibit severe acute respiratory syndrome coronavirus 2 replication in vitro. We thus considered that opaganib could be beneficial for moderate to severe coronavirus disease 2019 (COVID-19) pneumonia. The objective of the study was to evaluate the safety of opaganib and its effect on supplemental oxygen requirements and time to hospital discharge in COVID-19 pneumonia hospitalized patients requiring supplemental oxygen.
Methods
This Phase 2a, randomized, double-blind, placebo-controlled study was conducted between July and December 2020 in 8 sites in the United States. Forty-two enrolled patients received opaganib (n = 23) or placebo (n = 19) added to standard of care for up to 14 days and were followed up for 28 days after their last dose of opaganib/placebo.
Results
There were no safety concerns arising in this study. The incidence of ≥Grade 3 treatment-emergent adverse events was 17.4% and 33.3% in the opaganib and placebo groups, respectively. Three deaths occurred in each group. A numerical advantage for opaganib over placebo was observed in in this nonpowered study reflected by total supplemental oxygen requirement from baseline to Day 14, the requirement for supplemental oxygen for at least 24 hours by Day 14, and hospital discharge.
Conclusions
In this proof-of-concept study, hypoxic, hospitalized patients receiving oral opaganib had a similar safety profile to placebo-treated patients, with preliminary evidence of benefit for opaganib as measured by supplementary oxygen requirement and earlier hospital discharge. These findings support further evaluation of opaganib in this population.
Keywords: hospitalization, SARS-CoV-2, sphingosine-kinase-2, supplemental oxygen
Upon receiving opaganib, patients with COVID-19 pneumonia who were hospitalized and required supplemental oxygen demonstrated good safety with no new concerns as well as potential symptomatic clinical improvement compared with placebo, with less supplemental oxygen requirement and earlier hospital discharge.
Acute pneumonia due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection leading to coronavirus disease 2019 (COVID-19) can be life-threatening. Typical symptoms of SARS-CoV-2 infection include fever, fatigue, dry cough, and shortness of breath, but it can evolve to acute respiratory distress syndrome. Among patients with COVID-19, 80% present with symptoms of mild to moderate severity, with or without pneumonia, whereas 5%–14% develop serious or critical respiratory disease, requiring intensive care unit support [1–3]. The mortality rate of COVID-19 ranges from 0.1% to 19.7%, depending on country of origin, socioeconomic background, comorbidities such as diabetes and cardiovascular disease, and disease severity [4–8]. These comorbidities and advanced age (especially >65 years) increase the risk of mortality [9, 10].
Patients who are hospitalized with COVID-19 often require supplemental oxygen to maintain adequate oxygenation [3, 9]. Some pharmacological agents (corticosteroids and remdesivir) have been widely used as standard of care (SoC), with remdesivir first approved in the United States for emergency use in hospitalized COVID-19 patients in May 2020 [11].
Opaganib (RedHill Biopharma) is a first-in-class orally available, small molecule, selective sphingosine kinase-2 (SK-2) inhibitor with antiviral and anti-inflammatory properties [12]. Multiple ongoing clinical trials are evaluating opaganib as therapy for cancer. The antiviral effect of opaganib was demonstrated in various preclinical studies. Reid et al [13] showed that decreased expression or pharmacologic inhibition of SK-2 significantly inhibited Chikungunya virus replication. In a work by Xia et al [14], opaganib was shown to reduce fatality rates from influenza A infection in mice. Moreover, in a preliminary study using an in vitro model of human bronchial tissue, we recently demonstrated that opaganib has potent antiviral activity against SARS-CoV-2 [15]. We hypothesized that the antiviral and anti-inflammatory effects of opaganib would be beneficial for the treatment of SARS-CoV-2 infection, particularly for patients with moderate to severe COVID-19 pneumonia requiring supplemental oxygen. In a small cohort of patients with severe COVID-19 pneumonia, in which opaganib was offered through compassionate use, it appeared to improve clinical and laboratory parameters, and it was safe and well tolerated [16].
This Phase 2a proof-of-concept study was designed to provide a preliminary evaluation of the safety and efficacy of opaganib when added to SoC in hospitalized patients with COVID-19 pneumonia who required supplemental oxygen. Aside from safety and tolerability assessments, efficacy endpoints measured included change in oxygen requirements and clinical improvement parameters.
METHODS
Design
This was a Phase 2a, proof-of-concept, multicenter, randomized, double-blind, parallel arm, placebo-controlled study conducted in 8 sites in the United States. The study was not powered for efficacy. Eligible patients received opaganib or matching placebo twice daily added to SoC (per regional, institutional/physician practices). Eligible participants were adults (18–80 years inclusively), with SARS-CoV-2 infection determined by polymerase chain reaction analysis in a nasopharyngeal sample and pneumonia determined by chest x-ray. The full list of eligibility criteria is provided in the Supplementary Data, Section-1.
Intervention
Participants were randomized 1:1 to receive 2 × 250 mg of opaganib oral capsules or matching placebo every 12 hours for up to 14 days (Days 1–14). This dose had been determined in a Phase 1 clinical study in oncology patients as the maximum safe and tolerable dose [17]. Patients discharged before or at Day 10 completed only 10 days of treatment. After their last study drug dose, participants were followed for up to 28 days (safety follow-up). Treatment assignments remained blinded to the patients, investigators, hospital staff, and sponsor (RedHill Biopharma). The clinical trial (ClinicalTrials.gov Identifier NCT 04414618) was performed in accordance with the Declaration of Helsinki (1964), as revised most recently in Seoul (2008), US Food and Drug Administration (FDA) regulations, and the ICH Guideline for Good Clinical Practice, E6(R1) and local rules and regulations.
Outcomes
Given the exploratory nature of this proof-of-concept study, we sought to evaluate several clinically meaningful outcomes. We designed a primary outcome measure comparing total supplemental oxygen requirements from baseline to Day 14 (area under the curve) using daily oxygen flow measurements. Primary endpoint details are provided in the Supplementary Data, Section-2, together with a complete list of secondary, post hoc, safety, and exploratory endpoints. Presented herein are the safety outcomes, that is, incidence of treatment-emergent adverse events (TEAEs) and serious TEAEs (TESAEs) over the safety follow-up period, as well as the following clinically relevant secondary outcomes: (1) time to 50% reduction from baseline in supplemental oxygen requirement by Day 14, (2) percentage of patients no longer receiving supplemental oxygen for at least 24 hours by Day 14, (3) time to intubation and mechanical ventilation by Day 14, and (4) mortality by Day 30. The post hoc outcomes were as follows: (1) percentage of patients no longer receiving supplemental oxygen for at least 24 hours by Day 14 per SoC regimen, (2) time to intubation and mechanical ventilation by safety follow-up, (3) mortality by safety follow-up, (4) incidence of hospital discharge by Day 14 and by safety follow-up, and (5) time to ≥2 points improvement in the World Health Organization (WHO) Ordinal Scale [18] by Day 14.
Statistics
Because the sample size (∼40 patients) was not chosen for statistical consideration, efficacy interpretation is based on numerical comparisons of descriptive statistics. Statistical methodology details are provided in the Supplementary Data, Section-3. Baseline characteristics are presented for the intent-to-treat (ITT) population (all randomized patients). Safety evaluations were performed for the safety population (patients who took at least 1 dose of study drug). Efficacy endpoints were analyzed for the modified ITT (mITT) population (patients who took at least 1 dose of study drug).
Patient Consent Statement
Written consent was obtained from all patients who participated in this study. The design of the study has been approved by local ethical committees and by the national regulatory agencies in all the countries where the protocol was activated including the FDA, the Israeli Ministry of Health, and the Argentine Administration of Medicines, Food and Medical Technology.
RESULTS
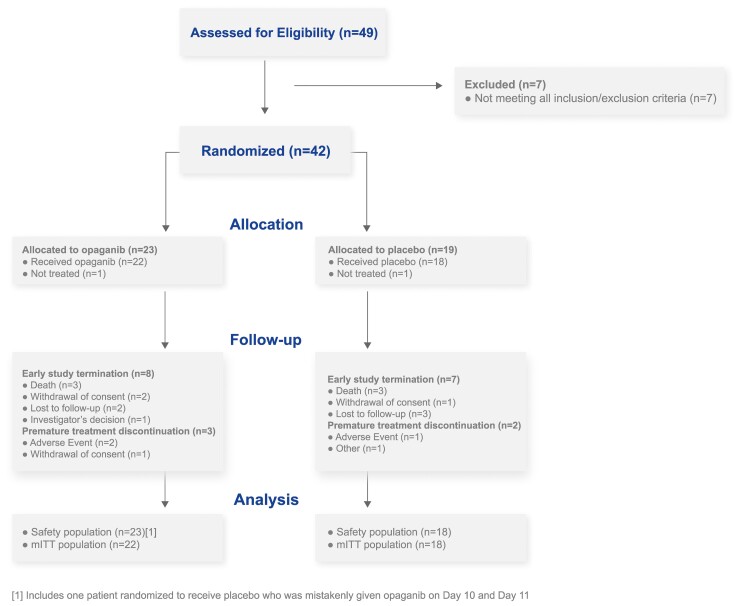
A diagram of the study flow is presented in Figure 1. The ITT population comprised 42 eligible patients, enrolled in July–November 2020. Of these, 23 patients were randomized to receive opaganib and 19 patients were randomized to receive placebo (randomization details are provided in the Supplementary Data, Section-4. One patient in each group did not receive study drug and was excluded from the safety and mITT populations. One patient randomized to placebo was mistakenly given opaganib on Days 10 and 11.
Figure 1.
Study flow diagram. mITT, modified intent-to-treat population.
Demographic and Other Baseline Characteristics
The main demographic and baseline characteristics of patients in the ITT population are presented in Table 1. Most patients were male (64.3%) with an overall median age of 58.0 years. Randomization resulted in a difference between the opaganib and placebo arms in median age (years; 52.0 and 61.0, respectively) and median baseline oxygen requirement (L/minute; 6.0 and 10.5, respectively). The median time (days) from onset of symptoms to randomization was 10.0 for the opaganib group and 9.0 for placebo.
Table 1.
Main Patient Demographic and Baseline Characteristics in the Opaganib and Placebo Groups and Overall, and Kaplan-Meier Estimate of the Cumulative Incidence of No Longer Receiving Supplemental Oxygen for at Least 24 Hours by Day 14 in the Opaganib and Placebo Groups per Standard-of-Care Regimen of Interest
| Parameter (ITT Population) | Opaganib (N = 23) | Placebo (N = 19) | Overall (N = 42) |
|---|---|---|---|
| Age (years), median (range) | 52.0 (29–80) | 61.0 (35–80) | 58.0 (29–80) |
| Age (years), n (%) | |||
| <70 | 20 (87.0) | 15 (78.9) | 35 (83.3) |
| ≥70 | 3 (13.0) | 4 (21.1) | 7 (16.7) |
| Gender, n (%) | |||
| Male | 16 (69.6) | 11 (57.9) | 27 (64.3) |
| Female | 7 (30.4) | 8 (42.1) | 15 (35.7) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 12 (52.2) | 8 (42.1) | 20 (47.6) |
| Race, n (%) | |||
| White | 18 (78.3) | 15 (78.9) | 33 (78.6) |
| American Indian or Alaska Native | 1 (4.3) | 1 (5.3) | 2 (4.8) |
| Asian | - | 1 (5.3) | 1 (2.4) |
| Black or African American | 3 (13.0) | 2 (10.5) | 5 (11.9) |
| Other | 1 (4.3) | - | 1 (2.4) |
| Smoking Status, n (%) | |||
| Never | 20 (87.0) | 14 (73.7) | 34 (81.0) |
| Former | 2 (8.7) | 4 (21.1) | 6 (14.3) |
| Current | 1 (4.3) | 1 (5.3) | 2 (4.8) |
| HbA1c (%) at Screening, n (%) | |||
| <6.5 | 13 (56.5) | 10 (52.6) | 23 (54.8) |
| ≥6.5 | 10 (43.5) | 9 (47.4) | 19 (45.2) |
| Weight at baseline (Kg), median (range)a | 104.33 (74.8–163.3) | 79.00 (55.3–140.0) | 96.76 (55.3–163.3) |
| Oxygen Requirement at Baseline (L/minute) | |||
| n | 22 | 18 | 40 |
| Median (min, max) | 6.0 (0, 40) | 10.5 (1, 55) | 6.0 (0, 55) |
| Time from symptoms onset to hospitalization (days), median (range)b | 7.0 (−4 to 39) | 6.5 (0–11) | 7.0 (−4 to 39) |
| Time from symptoms onset to randomization (days), median (range)b | 10.0 (4–41) | 9.0 (2–18) | 9.0 (2–41) |
| Time from diagnosis to hospitalization (days), median (range) | 0.0 (−5 to 20) | 0.0 (−1 to 14) | 0.0 (−5 to 20) |
| Time from diagnosis to randomization (days), median (range) | 4.0 (1–33) | 4.0 (2–18) | 4.0 (1–33) |
| Kaplan-Meier Estimate of the Cumulative Incidence of No Longer Receiving Supplemental Oxygen for at Least 24 Hours by Day 14: Standard-of-Care Regimen Groupc,d | |||
| Overall (N) | 22 | 18 | |
| Cumulative incidence at Day 14 | 50.00 | 22.22 | |
| Treated with combination of remdesivir and high-dose corticosteroids (N) | 9 | 9 | |
| Cumulative incidence at Day 14 | 44.44 | 22.22 | |
| Treated only with high-dose corticosteroids (N) | 11 | 9 | |
| Cumulative incidence at Day 14 | 54.55 | 22.22 | |
Abbreviations: HbA1c, hemoglobin A1c; ITT, intent-to-treat population.
Two patients in each group had missing information on weight at baseline, resulting in n = 21 patients in the opaganib group and n = 17 patients in the placebo group.
One patient in the placebo group had missing information on symptoms onset, resulting in n = 18 patients in the placebo group.
The number of patients treated with specific standard-of-care regimen is presented.
Death was censored at 14 days.
Remdesivir and/or high-dose corticosteroids (dexamethasone, prednisone, and methylprednisolone) comprised known effective SoC for COVID-19 and were coadministered with opaganib in 22 (95.7%) patients, and with placebo in all 18 (100%) patients. This included remdesivir in 10 (43.5%) patients on opaganib and 9 (50.0%) on placebo, and high-dose corticosteroids in 21 (91.3%) and all 18 (100%) patients, respectively.
Treatment Compliance
Compliance was assessed in a subset of the safety population comprising 21 patients on opaganib and 18 patients on placebo (further details are provided in the Supplementary Data, Section-5). For 2 patients on opaganib, compliance was not defined: for the patient randomized to placebo who took opaganib on one day, the expected number of opaganib doses was zero; the second patient was lost to follow-up, and thus compliance could not be assessed. The mean (±standard deviation) compliance was 90.05% (±21.523) in the opaganib and 98.41% (±3.917) in the placebo groups, respectively.
Treatment-Emergent Adverse Events
The incidence of TEAEs was similar between groups (52.2%, in the opaganib group versus 50.0% in the placebo group) (Table 2). In general, each unique TEAE term was reported singly by 1 patient (either in the opaganib or placebo group). The only TEAEs reported by 2 patients (8.7% in the opaganib group), and with a higher incidence in the opaganib group than in placebo, were hypokalemia (reported in 1 patient on placebo) and diarrhea (not reported in patients on placebo).
Table 2.
TEAEs by MedDRA Preferred Term in the Opaganib and Placebo Groups (Safety Population)
| Opaganib (N = 23) | Placebo (N = 18) | |
|---|---|---|
| Safety Population | n (%)a | n (%)a |
| Any TEAE | 12 (52.2) | 9 (50.0) |
| Any TEAE Grade 3 and above | 4 (17.4) | 6 (33.3) |
| Any Serious TEAE | 3 (13.0) | 5 (27.8) |
| Any treatment-related TEAE | 2 (8.7) | - |
| Any treatment-related Grade 3 and above TEAE | 1 (4.3) | - |
| Any treatment-related serious TEAE | - | - |
| TEAEs Grade 3 and Above by MedDRA Preferred Termb | ||
| MedDRA Preferred Term | n (%) | n (%) |
| Anemia | 1 (4.3) | 1 (5.6) |
| COVID-19 pneumonia | 1 (4.3) | 1 (5.6) |
| Sepsis | 1 (4.3) | - |
| Septic shock | - | 1 (5.6) |
| Fibrin D dimer increased | 1 (4.3) | - |
| Troponin I increased | - | 1 (5.6) |
| White blood cell count increased | - | 1 (5.6) |
| Glucose tolerance impaired | - | 1 (5.6) |
| Hypocalcemia | - | 1 (5.6) |
| Acute kidney injury | 1 (4.3) | 2 (11.1) |
| Respiratory failure | 2 (8.7) | 2 (11.1) |
| Pneumonia aspiration | - | 1 (5.6) |
| Rash | 1 (4.3) | - |
| Shock | - | 1 (5.6) |
Abbreviations: COVID-19, coronavirus disease 2019; MedDRA, Medical Dictionary for Regulatory Activities; TEAEs, treatment-emergent adverse events.
The n refers to number of patients with the respective TEAEs, not number of TEAEs.
A total of 8 Grade 3 and above TEAEs were recorded in 4 patients in the opaganib arm, and 13 Grade 3 and above TEAEs were recorded in 6 patients on placebo, with some patients experiencing multiple TEAEs.
The TESAEs were reported in 13.0% of patients on opaganib and 27.8% patients on placebo. The TESAEs by Medical Dictionary for Regulatory Activities System Organ Class included the following: infections and infestations (8.7% in the opaganib and 11.1% in the placebo group); respiratory, thoracic, and mediastinal disorders (8.7% and 16.7%, respectively); and renal and urinary disorders (4.3% and 11.1%, respectively). None of the TESAEs were deemed as treatment related by the investigator.
The TEAEs of Grade 3 and above were reported in 4 patients (17.4%) on opaganib and 6 patients (33.3%) on placebo, with no TEAEs of Grade 3 and above reported at a higher frequency in the opaganib group compared with placebo (Table 2). Treatment-related TEAEs were recorded in 2 patients in the opaganib group, including 1 patient with Grade 1 diarrhea and 1 patient with Grade 3 rash. Both events were nonserious, and in both cases, the drug was withdrawn, and the patients recovered.
There were no reported TEAEs resulting in dose reductions. The TEAEs leading to treatment withdrawal were reported in 4 patients (17.4%) in the opaganib group (including a case of Grade 1 heart palpitations [not related to study treatment], a case of Grade 1 diarrhea [possibly related to study treatment], a case of Grade 4 sepsis and Grade 4 acute kidney injury [both unlikely related to study treatment], and a case of Grade 3 rash [possibly related to study treatment]) and 1 patient (5.6%) in the placebo group, who suffered from multiple TEAEs resulting in drug withdrawal.
Three death cases were reported in each group, which occurred due to (1) COVID-19 pneumonia, sepsis, and respiratory failure in the opaganib arm and (2) acute kidney injury and respiratory failure (2 cases) in placebo. None of the deaths were assessed by the investigator as related to the treatment.
In addition to the safety analysis, several efficacy analyses (presented below) were performed with the aim of providing initial data on potential beneficial effects of opaganib in the study population. Because the study was not powered for efficacy, the findings from these analyses provide a preliminary evaluation of opaganib’s potential benefits, and they should be regarded as such.
Total Supplemental Oxygen Requirement Using the Maximal Daily Oxygen Flow Over 14 Days
A relative benefit derived for each group was calculated (as described in the Supplementary Data, Section-2). The relative benefit calculated was 61.6% for opaganib (n = 21) and 46.7% for placebo (n = 18) (Supplementary Figure 1). This was a post hoc analysis to the predefined primary endpoint; as more information on the course of COVID-19 pneumonia became available, this endpoint, namely, quantifying total supplemental oxygen required over time (across various devices), proved to be of less clinical value than initially anticipated.
Requirement of Supplemental Oxygen by Day 14
The cumulative incidence for no longer requiring supplemental oxygen for at least 24 hours by Day 14 was estimated using the Kaplan-Meier analysis. The analysis showed a higher estimated cumulative incidence in patients on opaganib (50%) compared with those on placebo (22.2%) (Supplementary Figure 2, Table 1).
The subgroup analysis based on SoC regimen showed consistent findings in patients on high-dose corticosteroids and patients on remdesivir and corticosteroids (Table 1). Specifically, the estimated cumulative incidence of no longer receiving supplemental oxygen by Day 14 in patients receiving opaganib on top of remdesivir in combination with high-dose corticosteroids, and in those receiving opaganib on top of high-dose corticosteroids only, was consistent with that observed in the overall opaganib group and higher than in the respective subgroups receiving placebo on top of the SoC (44.4% versus 22.2% and 54.6% versus 22.2%, respectively).
The median Kaplan-Meier estimated time to 50% reduction from baseline in supplemental oxygen requirement based on oxygen flow (L/minute) Day 1–Day 14 was 5 days in the opaganib versus 8 days in the placebo group. The Kaplan-Meier estimated cumulative incidence of this event by Day 14 was 81% in the opaganib group compared to 66.7% in the placebo group.
Intubation and Mechanical Ventilation, and Mortality During the Course of the Study and at the End of Follow-up
The Kaplan-Meier estimated cumulative incidence of intubation and mechanical ventilation at Day 14 was 9.6% in the opaganib group and 11.1% in placebo. There were 2 intubation and mechanical ventilation events in each group, and there were no subsequent intubations and mechanical ventilations through the end of safety follow-up.
Three patients on opaganib and 2 patients on placebo died by Day 30. The Kaplan-Meier estimated cumulative incidence of death by Day 30 was 15.0% in the opaganib and 11.9% in the placebo group. An additional death occurred in the placebo group by the end of safety follow-up. The Kaplan-Meier estimated cumulative incidences of death by safety follow-up were 15.0% and 19.2% in the opaganib and placebo groups, respectively.
Hospital Discharge
The Kaplan-Meier estimated cumulative incidence of hospital discharge was 68.2% in the opaganib and 50.0% in the placebo groups by Day 7 and 86.4% and 55.6%, respectively, by Day 14 (Supplementary Figure 3). A related post hoc analysis, whereby the 2-point improvement in the WHO Ordinal Scale was derived for patients, is presented in Supplementary Table 1.
DISCUSSION
This proof-of-concept study explored the safety and effectiveness of oral opaganib in hospitalized patients with COVID-19 pneumonia requiring supplemental oxygen. Opaganib’s safety profile in this study was not materially different than that of placebo. Results suggest that clinical improvement, as measured by reduced need for supplemental oxygen, improved WHO level in the scale for clinical improvement and in time to discharge (Supplementary Table 2).
Several lines of preliminary evidence suggested that opaganib may be particularly relevant to COVID-19 therapy. Before the onset of the COVID-19 pandemic, opaganib had demonstrated antiviral and anti-inflammatory effects in several preclinical models, including the following: amelioration of Pseudomonas aeruginosa-induced lung injury and reduction of fatality rates from influenza infection [19, 20]; inhibition of host inflammatory responses in several disease models [21, 22]; and exertion of various effects on the immune system [23]. Opaganib has since been shown to have potent antiviral activity against SARS-CoV-2 in a preclinical study using an in vitro model of human bronchial tissue [15] and was associated with favorable outcomes when offered through compassionate use to patients with moderate to severe COVID-19 pneumonia requiring supplemental oxygen via high-flow nasal cannula [16]. Because opaganib targets a host cell factor rather than the virus directly, development of resistance is less likely. This is of particular interest in SARS-CoV-2 infection, given its rapidly emerging mutations and viral strains.
The antiviral agent remdesivir has been approved for treatment in certain stages of COVID-19; however, it is administered intravenously and lacks anti-inflammatory properties [5], whereas opaganib is an oral agent with both antiviral and anti-inflammatory properties. Moreover, opaganib is stable at room temperature for 5 years (RedHill Biopharma Ltd., unpublished data, 2022). These factors support opaganib as a much-needed, practical, and convenient potential treatment of COVID-19 pneumonia.
Opaganib was shown to be well tolerated in this study, with no new safety signals emerging. The only TEAE of Grade 3 and above reported at a higher frequency in the opaganib group than placebo was a Grade 3 rash in a single patient, which resolved upon stopping study drug. Rash is a known uncommon toxicity of opaganib. Three patients on opaganib and 5 patients on placebo experienced TESAEs. The TEAEs leading to treatment withdrawal were reported in 17.4% of patients on opaganib and 5.6% of patients on placebo. By the safety follow-up timepoint, there were 3 deaths recorded in each group, and none were deemed treatment related. The overall incidence of TEAEs and TESAEs were similar in both treatment groups.
The current study was not powered for efficacy, and, as such, its efficacy findings provide a preliminary evaluation of opaganib’s beneficial effects in the study population. A numerical superiority was found for opaganib over placebo across several clinical outcomes. The prespecified primary efficacy analysis of change in total supplemental oxygen requirements proved difficult to analyze and required a post hoc analysis utilizing percentage change. This post hoc analysis demonstrated an apparent difference, with less total supplemental oxygen requirement for patients on opaganib compared with those on placebo. As the pandemic progressed, improvement in supplemental oxygen requirement had become a commonly used criterion for hospital discharge in COVID-19 patients [24]. Hence, the predefined secondary analysis of no longer requiring supplemental oxygen for at least 24 hours was the most clinically relevant endpoint. A number of other prespecified and post hoc analyses measuring clinical benefit supported the improvement in oxygen requirements. Of note, however, there was an imbalance in median age between the 2 arms of the study, with a younger population in the opaganib group (52.0 years) compared with placebo (61.0 years). In addition, the mean baseline oxygen requirement (L/minute) was lower in the opaganib group (6.0) than in placebo (10.5). Because younger individuals are expected to have generally better disease outcomes than their older counterparts, we cannot rule out that these imbalances may have impacted the preliminary efficacy results of the study, contributing to opaganib’s observed benefit in the studied population. However, such potential impact remains hypothetical, because most studies looking at the effect of age on disease severity report the age of >65 years as a risk factor.
Limitations
The main limitation of this study is its small sample size, which allows for descriptive analyses rather than meaningful statistical interpretation. A further limitation is the imbalance in age and supplemental oxygen requirement at baseline, which is a result of the small sample size despite randomization. The clinical interpretation of the primary endpoint was limited, as described under Discussion, leading to the addition of post hoc analyses to help with the overall interpretation of the data. An additional limitation of the study is that certain predefined secondary endpoints that relied on inpatient data collection could not be assessed due to changing standard of practice leading to earlier discharge of patients than anticipated at the time of protocol development. Furthermore, discharge criteria were somewhat subjective, depending on hospital capacity and regulations. It is notable that a greater proportion of patients on opaganib versus placebo were discharged before Day 14.
CONCLUSIONS
Taken together, the results of this Phase 2a study suggest that opaganib is safe and well tolerated and suggest a potential benefit of opaganib across various clinically meaningful outcome measures in patients with COVID-19 pneumonia. Based on these findings, further evaluation of opaganib is currently ongoing in larger randomized studies as a potential treatment for moderate to severe COVID-19 pneumonia in hospitalized patients requiring supplemental oxygen (a global Phase 2/3 [ClinicalTrials.gov Identifier NCT04467840] already completed enrollment [15]).
Supplementary Material
Acknowledgments
We acknowledge the patients who participated in the study, as well as the dedicated healthcare professionals who cared for them at risk of their own lives. We thank Dr. Dana Savulescu (Bioforum Group, Ltd.) for assistance in manuscript editing.
Financial support. This work was funded by RedHill Biopharma, Ltd.
Potential conflicts of interest. A. B. and G. R. are employees of RedHill Biopharma Ltd. P. A., R. F., V. K. B.-Y., and M. L. L. are full-time consultants for RedHill Biopharma Ltd. G. E. and K. L. W. are consultants to RedHill Biopharma Ltd. A. W. S.’ spouse purchased RedHill Biopharma Ltd. stocks. A. M. R. and H. S. M. have received research support from other companies. M. S. G. has worked as an oncology consultant to RedHill Biopharma Ltd. and has participated in research studies conducted by other companies.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Kevin L Winthrop, Oregon Health & Science University, Portland, Oregon, USA.
Alan W Skolnick, HD Res., Houston, Texas, USA.
Adnan M Rafiq, Mem. Hermann Southeast Hospital, Houston, Texas, USA.
Scott H Beegle, Albany Med. Coll., Albany, New York, USA.
Julian Suszanski, Henry Ford Hospital, Detroit, Michigan, USA.
Guenther Koehne, Miami Cancer Inst., Miami, Florida, USA.
Ofra Barnett-Griness, Bioforum Ltd., Ness Ziona, Israel.
Aida Bibliowicz, RedHill Biopharma Ltd., Tel-Aviv, Israel.
Reza Fathi, RedHill Biopharma Ltd., Tel-Aviv, Israel.
Patricia Anderson, RedHill Biopharma Ltd., Tel-Aviv, Israel.
Gilead Raday, RedHill Biopharma Ltd., Tel-Aviv, Israel.
Gina Eagle, G.E.T Pharma Consulting LLC, Lambertville, New Jersey, USA.
Vered Katz Ben-Yair, RedHill Biopharma Ltd., Tel-Aviv, Israel.
Harold S Minkowitz, HD Res., Houston, Texas, USA.
Mark L Levitt, Levitt Oncology Associates Ltd., Hashmonaim, Israel.
Michael S Gordon, Honor Health Res. Inst., Scottsdale, Arizona, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Adil MT, Rahman R, Whitelaw D, et al. SARS-CoV-2 and the pandemic of COVID-19. Postgrad Med J 2021; 97:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) . Available at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed.
- 3. Daher A, Balfanz P, Aetou M, et al. Clinical course of COVID-19 patients needing supplemental oxygen outside the intensive care unit. Sci Rep 2021; 11:2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. RECOVERY Collaborative Group . Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2020; 396:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Solidarity Trial Consortium . Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med 2021; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Available at: https://coronavirus.jhu.edu/data/mortality. Accessed.
- 7. Mishra V, Seyedzenouzi G, Almohtadi A, et al. Health inequalities during COVID-19 and their effects on morbidity and mortality. J Healthc Leadersh 2021; 13:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naylor-Wardle J, Rowland B, Kunadian V. Socioeconomic status and cardiovascular health in the COVID-19 pandemic. Heart 2021; 107:358–65. [DOI] [PubMed] [Google Scholar]
- 9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021; 384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Available at: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/table-of-contents/. Accessed 15 February 2022.
- 12. French KJ, Zhuang Y, Maines LW, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther 2010; 333:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reid SP, Tritsch SR, Kota K, et al. Sphingosine kinase 2 is a chikungunya virus host factor co-localized with the viral replication complex. Emerg Microbes Infect 2015; 4:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia C, Seo Y-J, Studstill CJ, et al. Transient inhibition of sphingosine kinases confers protection to influenza A virus infected mice. Antiviral Res 2018; 158:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Available at: https://www.prnewswire.com/news-releases/redhill-biopharma-completes-enrollment-of-oral-opaganib-phase-23-covid-19-study. Accessed 15 June 2021.
- 16. Kurd R, Ben-Chetrit E, Karameh H, Bar-Meir M. Compassionate use of opaganib for patients with severe COVID-19. J Emerg Dis Virol 2020; 5. [Google Scholar]
- 17. Britten CD, Garrett-Mayer E, Chin SH, et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2017; 23:4642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO R&D Blueprint . COVID-19 Therapeutic Trial Synopsis. 2020.
- 19. Xia C, Seo Y-J, Studstill CJ, et al. Transient inhibition of sphingosine kinases confers protection to influenza A virus infected mice. Antiviral Res 2018; 158:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ebenezer DL, Berdyshev EV, Bronova IA, et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax 2019; 74:579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maines LW, French KJ, Wolpert EB, et al. Pharmacologic manipulation of sphingosine kinase in retinal endothelial cells: implications for angiogenic ocular diseases. Investig Opthalmology Vis Sci 2006; 47:5022. –31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maines LW, Fitzpatrick LR, French KJ, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci 2008; 53:997–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Q, Rehman H, Shi Y, et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS One 2012; 7:e41834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greysen SR, Auerbach AD, Mitchell MD, et al. Discharge practices for COVID-19 patients: rapid review of published guidance and synthesis of documents and practices at 22 US academic medical centers. J Gen Intern Med 2021; 36:1715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.