Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused significant mortality, especially among older adults whose distinct immune system reflects immunosenescence. Multiple SARS-CoV-2 vaccines have received emergency use authorization and/or licensure from the US Food and Drug Administration and throughout the world. However, their deployment has heighted significant limitations, such by age-dependent immunogenicity, requirements for multiple vaccine doses, refrigeration infrastructure that is not universally available, as well as waning immunity. Thus, there was, and continues to be a need for continued innovation during the pandemic given the desire for dose-sparing, formulations stable at more readily achievable temperatures, need for robust immunogenicity in vulnerable populations, and development of safe and effective pediatric vaccines. In this context, optimal SARS-CoV-2 vaccines may ultimately rely on inclusion of adjuvants as they can potentially enhance protection of vulnerable populations and provide dose-sparing effects enabling single shot protection.
Keywords: precision vaccines, adjuvants, SARS-CoV-2, COVID-19, vulnerable populations
Traditional vaccine development has largely been empirical and has overlooked distinct immunity in vulnerable populations. Precision vaccinology promises to identify optimized vaccine strategies, such as adjuvantation systems, to better protect vulnerable populations against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and future pandemics.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) driven coronavirus disease 2019 (COVID-19) pandemic has caused significant mortality and morbidity, especially among the immunologically distinct older adults (ie, those >65 years of age), and those with chronic disease. Traditional vaccine development has largely been empirical and disregarded distinct immunity in vulnerable populations. Emergency use authorization (EUA) and licensure of mRNA and viral vector-based coronavirus vaccines is a major milestone in meeting the challenge of the SARS-CoV-2 vaccines pandemic [1, 2]. Although these mRNA vaccines appear safe and show 85–95% efficacy [3–6], limitations include (1) need for storage in costly freezer systems that do not consistently exist in rural and/or low- and middle- income regions of the world; (2) need for 2 dose series, which is both costly and logistically challenging; and (3) uncertainty regarding durability of protection, especially among the elderly who have demonstrated more rapid decline of antibody (Ab) titers [6]. Thus, although the newly authorized mRNA vaccines are an important breakthrough, substantial vaccinology remains to be done in optimizing coronavirus vaccines.
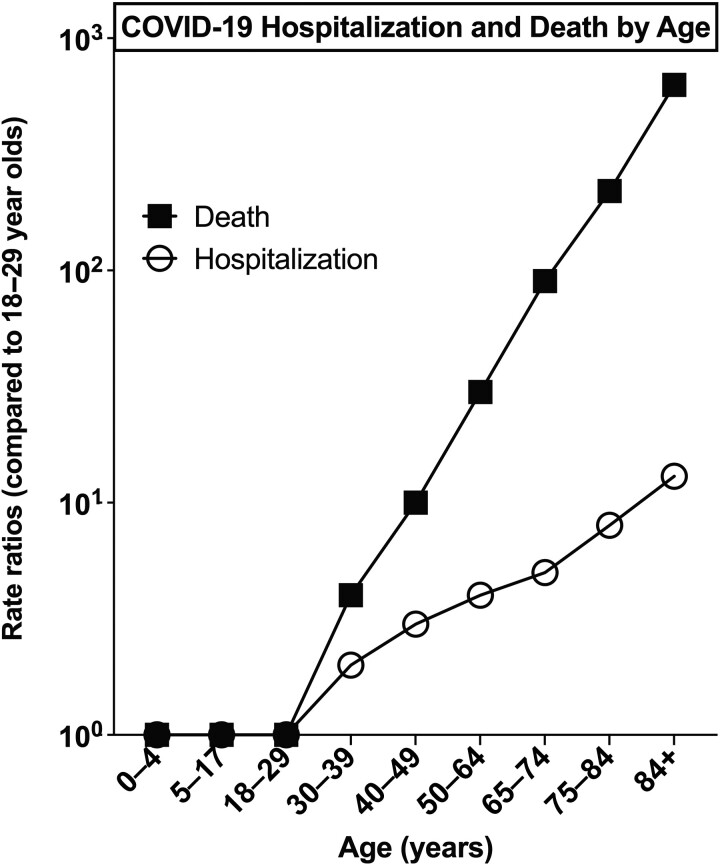
Vaccines have been traditionally developed with high reliance on poorly predictive animal models in pre-clinical testing and a presumption that they will demonstrate equivalent immunogenicity and protection in all individuals [7]. Indeed, adjuvants engage the innate immune system, which is hyper-variable between species [8]. Accordingly, until recently vaccine discovery and development has disregarded species and age-specificity and has not tailored vaccine activity to vulnerable populations such as the young, elderly, and/or immunocompromised [9]. Over the past few decades clinical and epidemiologic studies suggests fundamental differences in immune responses to vaccines based on demographic features such as age, sex, and geographic location [10]. Highlighting distinct immunity with age, elderly individuals and those with chronic illness such as asthma, diabetes, obesity and/or heart disease, are more vulnerable to developing severe COVID-19 [11], with increasing age identified early in the pandemic as the number 1 risk factor for severe COVID-19 in unvaccinated populations (Figure 1). In general, many vaccines are less immunogenic and effective in the older compared to younger adults [12–15], and early studies suggest diminished durability of mRNA vaccine-induced antibody (Ab) responses in those > 65 years of age [3, 16]. As such, the discovery and development of an adjuvanted SARS-CoV-2 vaccine optimized for vulnerable elders was therefore underrepresented in the early vaccine design considerations and as such and remains an urgent unmet need. This approach may be generalizable across multiple vaccine platforms.
Figure 1.
COVID-19 hospitalization and death by age in unvaccinated populations. Early in the COVID-19 pandemic, increasing age was identified as the number 1 risk factor for developing hospitalization and death following SARS-CoV-2 infection. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Data: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.
Although clinical and translational studies have laid the basic rationale for targeted vaccine development, innovations in molecular biology, human in vitro modeling adjuvant discovery, adjuvant formulation science and systems biology have enabled new strategies to discover and develop targeted vaccines [17]. Current efforts are focused on leveraging novel approaches to vaccine discovery and development to optimize adjuvant systems to enhance vaccine immunogenicity while maintaining safety. Recently, alternative precision vaccine adjuvantation approaches based upon in vitro modeling that take species (human) and age-specificity into account early in the discovery process have demonstrated potential to accelerate and de-risk vaccine development. Supported by the US National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) Adjuvant Discovery and Development Programs, such approaches are now being applied to discover and develop SARS-CoV-2 vaccine candidates tailored for use in elderly individuals. Precision vaccinology promises to identify optimized vaccine strategies and adjuvantation systems to better protect immunological distinct and vulnerable populations against coronavirus (CoV) and future pandemics.
ADJUVANTS FOR INFECTIOUS DISEASES
There is a growing need to develop improved vaccine strategies for globally prevalent and emerging respiratory infections such as tuberculosis, pertussis, influenza and coronaviruses, including SARS-CoV-2 and variants of concerns (VOC) [18]. For practical, safety, and regulatory purposes, vaccine formulations should generally be kept as simple as possible— that is, additional components such as adjuvants should only be added if clearly necessary. Nevertheless, many antigens, as well as antigen-encoding mRNAs and viral vectors, delivered via vaccination require multiple doses to achieve sufficient immunogenicity and protection. Furthermore, vulnerable populations at the extremes of age and with distinct immunity often respond sub-optimally vaccinal antigens. The current data available suggest lower mRNA vaccine-induced antibody durability in adults over from 65 years of age and upwards [3, 16]. Based upon their innate immune activating effects, adjuvants can enhance the quantity and quality of adaptive immune responses and thereby vaccine immunogenicity [19–21]. Of note, immunologic effect of adjuvants can vary with demographic features such as the age of an individual [22, 23], such that adjuvants may not be “one size fits all” [24]. The use of optimized adjuvanted vaccine formulations targeted to a given population may overcome barriers in vaccine development and inclusion of adjuvantation systems can enable a vaccine to match or exceed natural infection with respect to immunogenicity [18, 25].
Classes of adjuvants that are used in currently licensed vaccines include aluminum salts, oil-in-water emulsions (eg, MF59), pattern recognition receptor (PRR) agonists (eg, TLR4 agonist MPLA), saponins (eg, QS21) and combination adjuvantation systems such AS03 (squalene/polysorbate/a-tocopherol) or AS04 (Alum + MPL) [25]. Over the past 2 decades, greater understanding of innate immunity, including identification of PRRs has informed discovery and development of additional adjuvants [21, 25]. Synthetic single-stranded oligodeoxynucleotides (ODNs) containing unmethylated cytosine phosphate guanine (CpG) motifs (CpG ODNs) found in bacterial DNA, have demonstrated adjuvanticity [26]. CpG-ODN enhances Ab responses and enhances Th1 cell responses [27]. In humans, CpG motifs are recognized by TLR9 expressed on natural killer (NK) cells, B cells, and plasmacytoid DCs but not myeloid DCs and monocytes [28]. As compared to 3 doses of conventional alum adjuvanted hepatitis B vaccine, 2 doses of the recently licensed TLR9A CpG-adjuvanted hepatitis B vaccine, Heplisav-B induces superior immunogenicity in older adults and the elderly (40–70 years of age) [29, 30].
PRECISION ADJUVANTATION APPROACHES TO TAILOR VACCINES FOR VULNERABLE POPULATIONS
The growing realization that vaccine immunogenicity and protection can vary by demographics has prompted innovation in adjuvant discovery and development [17]. In this context, a major national priority, as articulated by the US NIH NIAID, is the discovery and development of adjuvants that are tailored to optimally enhance immune responses in distinct and vulnerable populations such as the young and elderly [31]. A key approach in defining optimal adjuvants for a given population with distinct immunity is human in vitro modeling. Human in vitro systems include whole blood assays, monocyte-derived dendritic cell arrays and 3-dimensional microphysiologic systems such as the tissue construct [22, 32–36]. These employ primary human leukocytes and autologous plasma, a rich source of age-specific immunomodulatory factors [37, 38]. Multiple such in vitro studies have tested adjuvants and licensed vaccines suggesting that these systems mirror and/or predict in vivo responses [32, 33, 36].
Examples of age-dependent adjuvant discovery using human in vitro modeling include TLR7/8 agonists [19], which demonstrate robust activation of human and nonhuman primate antigen-presenting cells in vitro and have subsequently proven effective in greatly enhancing immunogenicity of pneumococcal conjugate vaccine in neonatal nonhuman primates in vivo [32]. Combinations of adjuvants, such as TLR and CLR agonists, have demonstrated age-specificity such that adjuvant combinations that may be synergistic with respect to activation of Th1-poalrizing responses in 1 age group may be additive or even antagonistic in other age groups [22, 39]. Multiple such PRR adjuvants are now in human clinical trials [40].
PRE-PANDEMIC TO EARLY PANDEMIC: LIMITED KNOWLEDGE OF ADJUVANTS FOR CORONAVIRUS VACCINES
Before the COVID-19 pandemic, there were few publications regarding adjuvantation of coronavirus vaccines. Some adjuvants had been assessed as an approach to optimize candidate Middle East respiratory syndrome (MERS)-CoV, SARS-CoV, and SARS-CoV-2 vaccines [41]. Compared to the unadjuvanted protein vaccine, administration to mice of a MERS-CoV S 377-588-Fc protein-based vaccine containing the oil-in-water adjuvant MF-59 demonstrated greater induction of neutralizing antibodies (NAbs) and enhanced protection against MERS-CoV challenge [42]. Adjuvantation of a recombinant MERS-CoV S nanoparticle vaccine with a saponin-based Matrix-M1 adjuvant enhanced production of NAbs, conferred significant antigen-sparing, and protected mice from MERS-CoV challenge [43].
As the pandemic unfolded, results accumulated indicating the potential value of adjuvants to enhance immunogenicity of SARS-CoV-2 vaccines. Alum enhanced total immunoglobulin G (IgG) responses to purified inactivated SARS-CoV-2 virus vaccine candidate SARS-CoV-2-specific NAbs in mice, rats, and nonhuman primates [44]. Aluminum-based adjuvants also enabled high titers of NAbs to the S protein of SARS-CoV-2 [45] but were most effective when used in combination with other PRR agonist adjuvants, especially in designing vaccines for vulnerable populations with distinct immunity such as the young and elderly [32, 46]. As compared to single adjuvants, a combination of CpG-1018 with Alum enhanced Th1-polarized responses to SARS-CoV-2 S antigen correlating with the most effective murine immunogenicity profile in vivo in young animals [47]. Sigma adjuvant system (SAS) (oil-in-water emulsion, TLR4 agonist (monophosphoryl lipid A) (MPLA) and trehalose dicorynomycolate) induced IgG2a and IgG1 subclass S-binding Abs, indicating a balanced Th1/Th2 response in young mice [48], whereas AddaVax (a generic version of the oil-in-water adjuvant MF59) was useful in enhancing immunogenicity of different spike variants in the mouse model [49]. As of 2021, the most advanced adjuvanted SARS-CoV-2 vaccine was the NVX-CoV2373 formulation, which includes a recombinant SARS-CoV-2 nanoparticle vaccine composed of trimeric full-length SARS-CoV-2 spike glycoproteins and Matrix-M1 adjuvant. As of the time of writing this article (spring 2022), this formation had completed phase 3 clinical trials and has received AUE in more than 1 country. NVX-CoV2373 appeared safe, and induced humoral immune responses that exceeded those measured in the sera of a cohort of those who have recovered from COVID-19 CD4+ T-cell responses that were T helper (Th)1-biased [50]. These examples suggested that adjuvants may enhance effectiveness of SARS-CoV-2 vaccines. However, the growing pipeline of candidate adjuvants had yet to be systematically compared in the context of SARS-CoV-2 vaccines.
PRECISION VACCINOLOGY TO MEET A PANDEMIC CHALLENGE
In the context of the current pandemic, the rationale for identifying optimal adjuvantation systems for a precision elderly SARS-CoV-2 vaccine is four-fold, as adjuvants may (1) broaden an immune response to enhance protection, (2) enable robust vaccine immunogenicity in a vulnerable population with distinct and relatively weak immunity, (3) have dose-sparing effects which may be crucial from a pharmaco-economic standpoint to scaling a SARS-CoV-2 vaccine as hundreds of millions to billions of vaccine doses may be required, and (4) increase durability of an immune response, potentially enabling single dose long-term protection. As such, among the priorities in the field is the generation of a toolbox of adjuvants that (a) have a well-defined mechanisms of action, (b) are applicable to old and/or new vaccines against diverse pathogens, (c) can be used in combination to produce uniquely beneficial safe and effective vaccines, and (d) include agents that can, alone or in combination, induce the desired protective immune profiles in vulnerable populations, such as infants, elders, and/or those with chronic illness [31].
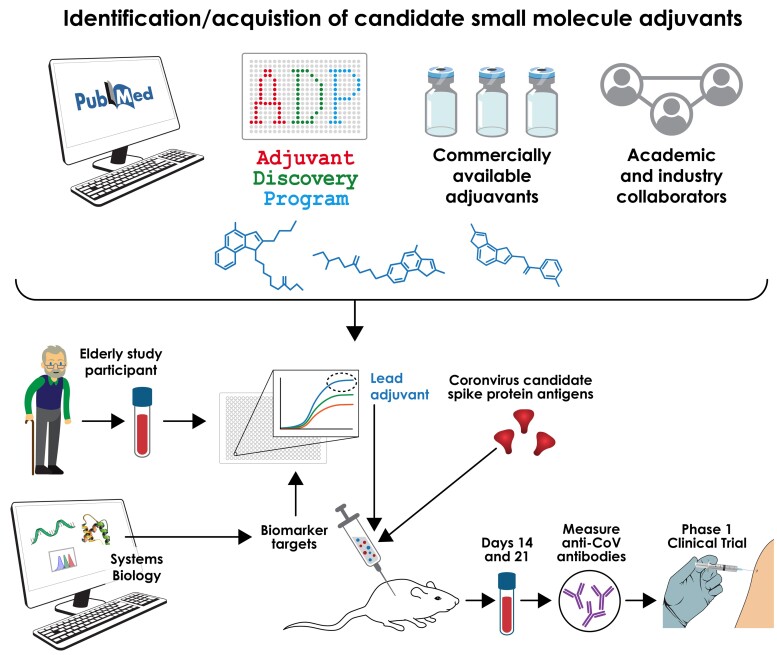
In January 2020 and supported by NIH/NIAID’s Adjuvant Discovery and Development Program, our PVP team initiated an effort to discover and develop adjuvant systems to enhance optimal SARS-CoV-2 vaccine immunogenicity in those most vulnerable to severe COVID—- that is, older adults (those > 65 years of age). With respect to identifying optimal adjuvants to help address the current coronavirus pandemic, innovative approaches we choose to undertake, included a broad adjuvant discovery pipeline and, use of age-specific animal models as well as human in vitro modeling of vulnerable populations innate and adaptive immune response [51]. These approaches are designed to provide more precise and accurate go/no go decision points and accelerate and de-risk vaccine development tailored for such vulnerable populations (Figure 2). To maximize the likelihood that a candidate SARS-CoV-2 vaccine tailored to be optimally effective for the most vulnerable, adjuvants were screened for in vitro activity toward human elderly leukocytes in order to identify leads for formulation together with an optimized CoV antigen (Table 1).
Figure 2.
A comprehensive multidisciplinary approach to defining precision adjuvants for a coronavirus vaccine active in older adults. Traditional vaccine development can lead to sub-optimal vaccine efficacy outcomes, normally attributed to an overreliance on nonhuman species-models and underappreciated value of incorporating age-specificity readout into the preclinical vaccine development pipeline. An alternative precision vaccine approach aims to accelerate development of optimal vaccine approaches, increasing the probably the final formulations will better protect the most vulnerable by (a) defining a very broad adjuvant pipeline, (b) rapidly screening adjuvants for activity toward human elderly PBMCs, and (c) combining lead adjuvants with multiple SARS-CoV-2 antigens. Lead adjuvanted vaccines are then advanced to age-specific animal studies and beyond. Abbreviations: PBMC, peripheral blood mononuclear cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Lessons Learned From Undertaking a Precision Approach to Discovery and Development of Adjuvanted Vaccines During a Pandemic Effecting a Vulnerable Group
| Aspect of Vaccine Discovery and Development | Limitation of Classical Vaccine Development Approaches | Advantages of a Precision Approach |
|---|---|---|
| Identification of lead formulation | Large number of antigens, adjuvants, and multiple distinct vulnerable populations, and differences across methodologies limit meaningful comparison across studies, precluding actionable conclusions. | Systematic/multidisciplinary approach benchmarks candidate adjuvants vs prior art. Prioritizes and speeds down-selection of most effective formulations, over easiest to deploy. |
| Species considerations | Heavy reliance on animal models that may not be predictive and may include bioethical concerns. | Considers needs of vulnerable populations including by age, race, sex, and medical conditions. |
| Distinct human populations | Traditional approach initially disregards species- and age-specificity resulting in high failure rate and inability to predict efficacy in vulnerable populations such as young and elderly. | Leverage human population (eg, age) in vitro immunization models to de-risk and accelerate selection of antigen, adjuvant, tailored to a given vulnerable population (eg, elderly). |
Specifically, we de-risked 3 adjuvanted formulations that were shown to effectively protect mice from mouse-adapted SARS-CoV-2. First, we undertook a study aimed to evaluate an optimal adjuvant formulation to enhance immunogenicity and efficacy of RBD-based subunit vaccines in older adults, which is otherwise reduced as an effect of aging. To this end, we performed a comprehensive comparison of PRR agonists, including 2′3′-cGAMP, a ligand of the stimulator of interferon (IFN) genes (STING), Poly (I:C) (TLR3 ligand), PHAD (synthetic MPLA, TLR4 ligand), and CpG-ODN 2395 (TLR9 ligand). Each PRR agonist was formulated ±aluminum hydroxide (AH). We also included AS01B (liposome-based MPLA/saponin QS-21 adjuvant) as a clinical-grade benchmark. We demonstrated that an AH:CpG adjuvant formulation induces potent anti-RBD responses in both young and aged mice and overcomes both the poor immunogenicity of the antigen and impaired immune responses in the aged [52]. We discovered unique immunological properties of the AH:CpG adjuvant formulation that demonstrated in vitro synergistic enhancement of human leukocyte/peripheral blood mononuclear cell (PBMC) activation and MoDC-dependent memory T cell activation. These observations indicate that formulating RBD with AH:CpG is a promising approach to develop a practical, thermostable, scalable, effective, and affordable vaccine that may be effective across multiple age groups and could potentially incorporate multiple RBD proteins to achieve cross-strain protection [52]. Indeed, this approach represents the discovery and conceptual basis for the Corbevax vaccine (Biological E Ltd [BioE]) recently authorized in India [52, 53]. Furthermore, the US International Development Finance Corporation (DFC) plans to fund the expansion of BioE's manufacturing capabilities, to support production in large scale (approximately 1 billion doses by end of 2022) [54].
Second, based on Dectin-2-binding mannans isolated from Candida albicans admixed with alumOH (mannan-alumOH) and formulated with pre-fusion stabilized SARS-CoV-2 spike trimer [55]. Mannan-alumOH greatly enhanced SARS-CoV-2 spike-specific neutralizing Abs, and protected mice from viral infection. Mannan-alumOH also elicited a robust anti-spike type 1 immunity included spike-specific IgG2c and IFNγ-producing T cells, via the adjuvant strategy enabled by modulation of the physical properties of the microbial ligands. Soluble mannans were shown to be “immunosilent” in the periphery but elicited a potent pro-inflammatory response in the draining lymph node (dLN). By modulating the physical form of mannans, we developed a formulation that targets both the periphery and the dLN. When combined with viral glycoprotein antigens, this mannan formulation broadens epitope recognition, elicits potent antigen-specific neutralizing antibodies, and confers protection against viral infections of the lung [55].
The potential of a third adjuvated formulation was noted with respect to RBD-nanoparticles (RBD-NPs) [56] composed of multimeric RBD displayed on a protein scaffold of 60 subunits of the self-assembling bacterial protein lumazine synthase [57]. By screening multiple adjuvant formulations with RBD-NP we found that a squalene-based oil-in-water (O/W) emulsion with carbohydrate fatty acid monosulphate derivative (O/W:CMS) [58, 59], enhanced anti-RBD serum Ab titers and SARS-CoV-2 cross-neutralizing titers in both young adult and aged mice. OIW:CMS-adjuvanted RBD-NP vaccine elicited the highest concentrations of anti-RBD IgG Abs amplifying both anti-RBD IgG1 and IgG2a titers, and potent inhibition of RBD binding to hACE2 and SARS-CoV-2 neutralization. Immunization of aged mice resulted in lower anti-RBD Ab titers compared to young mice [56].
As the rollout of various and competing adjuvanted SARS-CoV vaccine formulations continues, including lipid nanoparticle–mRNA (LNP–mRNA) based vaccines, which have inherent, if understudied adjuvanticity properties [60], it will be become increasing important to benchmark each licensed and approved product against each other. Ongoing questions to naturally arise will include: which vaccine class(es) induces the desired long lived durability [52, 61], which support Ab affinity maturation [62], T-cell mediated immunity, including B -cell germinal-center maturation [52], epitope broadening for variant protection [55], immunologically distinct vulnerable populations and what can be learned from comparing and contrasting cellular and molecular pathways that underly effective adjuvanticity [63]. Overall, head-to-head adjuvant comparison demonstrates that precision adjuvantation can overcome poor immunogenicity of RBD/spike proteins and immunosenescence, supporting our approach for discovery of a scalable, affordable, and safe SARS-CoV-2 vaccine suitable for older adults. Ultimately, the potential benefits arising from such research and innovation can only be fully utilized if they are backed by promotion of vaccine confidence [64]. Such an effort will be augmented by thoughtful and transparent engagement of the lay public regarding how the latest vaccine science is aimed at generating safe and effective precision vaccines [65].
Contributor Information
David J Dowling, Precision Vaccines Program; Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Ofer Levy, Precision Vaccines Program; Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Broad Institute of Massachusetts Institute of Technology (MIT) and Harvard, Cambridge, Massachusetts, USA.
Notes
Author contributions . D. J. D. and O. L. conceived, wrote, and edited the article.
Acknowledgments . The authors thank Dr Gary Fleisher Physician-In-Chief and Dr Kevin Churchwell CEO of Boston Children’s Hospital for their support of the Precision Vaccines Program. Kristin Johnson created the artwork in Figure 2. D. J. D. thanks Siobhan McHugh, Geneva Boyer, Lucy Conetta, and the staff of Lucy’s Daycare, the staff of the Young Men's Christian Association (YMCA) of Greater Boston, Bridging Independent Living Together (BILT), Inc, and the Boston Public Schools for childcare and educational support during the COVID-19 pandemic.
Financial support . The PVP is supported in part by US National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID) awards including Molecular Mechanisms of Combination Adjuvants (grant number 1U01AI124284-01), Adjuvant Discovery (grant numbers HHSN272201400052C and 75N93019C00044) and Development (grant number HHSN272201800047C) Program Contracts, Development of Vaccines for the Treatment of Opioid Use Disorder (grant number 75N93020C00038), and Human Immunology Project Consortium (HIPC) U19AI118608-01A1 to O. L. D. J. D.’s laboratory is supported by NIH grant number 1R21AI137932-01A1, Adjuvant Discovery Program (grant number 75N93019C00044) and Development of Vaccines for the Treatment of Opioid Use Disorder (grant number 75N93020C00038). The Precision Vaccines Program is supported in part by the BCH Department of Pediatrics and the Chief Scientific Office.
Supplement sponsorship. This supplement is sponsored by the Precision Vaccines Program of Boston Children's Hospital.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity 2020; 52:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krammer F. SARS-CoV-2 vaccines in development. Nature 2020; 586:516–27. [DOI] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med 2020; 383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol 2014; 35:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werling D, Jann OC, Offord V, et al. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol 2009; 30:124–30. [DOI] [PubMed] [Google Scholar]
- 9. Poland GA, Ovsyannikova IG, Kennedy RB. Personalized vaccinology: a review. Vaccine 2018; 36:5350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flanagan KL, van Crevel R, Curtis N, Shann F, Levy O. Heterologous (“nonspecific”) and sex-differential effects of vaccines: epidemiology, clinical trials, and emerging immunologic mechanisms. Clin Infect Dis 2013; 57:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015; 109:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dugan HL, Henry C, Wilson PC. Aging and influenza vaccine-induced immunity. Cell Immunol 2020; 348:103998. [DOI] [PubMed] [Google Scholar]
- 14. Heo JY, Song JY, Noh JY, et al. Effects of influenza immunization on pneumonia in the elderly. Hum Vaccin Immunother 2018; 14:744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinberger B. Vaccines for the elderly: current use and future challenges. Immun Ageing 2018; 15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nanishi E, Levy O, Ozonoff A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: a rapid review. Hum Vaccin Immunother 2022; 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soni D, Van Haren SD, Idoko OT, et al. Towards precision vaccines: lessons from the second international precision vaccines conference. Front Immunol 2020; 11:590373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barman S, Soni D, Brook B, Nanishi E, Dowling DJ. Precision vaccine development: cues from natural immunity. Front Immunol 2022; 12:662218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dowling DJ. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immuno Horizons 2018; 2:185–97. [DOI] [PubMed] [Google Scholar]
- 20. Nanishi E, Dowling DJ, Levy O. Toward precision adjuvants: optimizing science and safety. Curr Opin Pediatr 2020; 32:125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597–608. [DOI] [PubMed] [Google Scholar]
- 22. van Haren SD, Dowling DJ, Foppen W, et al. Age-specific adjuvant synergy: dual TLR7/8 and mincle activation of human newborn dendritic cells enables Th1 polarization. J Immunol 2016; 197:4413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weinberger B. Adjuvant strategies to improve vaccination of the elderly population. Curr Opinion Pharmacol 2018; 41:34–41. [DOI] [PubMed] [Google Scholar]
- 24. Kollmann TR, Levy O, Montgomery RR, et al. Innate immune function by toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dowling DJ, Levy O. Pediatric vaccine adjuvants: components of the modern vaccinologist’s toolbox. Pediatr Infect Dis J 2015; 34:1395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines 2003; 2:305–15. [DOI] [PubMed] [Google Scholar]
- 27. Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 2000; 18:1755–62. [DOI] [PubMed] [Google Scholar]
- 28. Roda JM, Parihar R, Carson WE III. CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J Immunol 2005; 175:1619–27. [DOI] [PubMed] [Google Scholar]
- 29. Halperin SA, Ward B, Cooper C, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine 2012; 30:2556–63. [DOI] [PubMed] [Google Scholar]
- 30. Heyward WL, Kyle M, Blumenau J, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine 2013; 31:5300–5. [DOI] [PubMed] [Google Scholar]
- 31. National Institute of Health . NIAID Strategic Plan for Research on Vaccine Adjuvants. 2018.
- 32. Dowling DJ, van Haren SD, Scheid A, et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2017; 2:e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dowling DJ, Scott EA, Scheid A, et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J Allergy Clin Immunol 2017; 140:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh DY, Dowling DJ, Ahmed S, et al. Adjuvant-induced human monocyte secretome profiles reveal adjuvant- and age-specific protein signatures. Mol Cell Proteomics 2016; 15:1877–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dowling DJ, Sanders H, Cheng WK, et al. A meningococcal outer membrane vesicle vaccine incorporating genetically attenuated endotoxin dissociates inflammation from immunogenicity. Front Immunol 2016; 7:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sanchez-Schmitz G, Stevens CR, Bettencourt IA, et al. Microphysiologic human tissue constructs reproduce autologous age-specific BCG and HBV primary immunization in vitro. Front Immunol 2018; 9:2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Philbin VJ, Dowling DJ, Gallington LC, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 2012; 130:195–204 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pettengill MA, van Haren SD, Levy O. Soluble mediators regulating immunity in early life. Front Immunol 2014; 5:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Haren SD, Ganapathi L, Bergelson I, et al. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine 2016; 83:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schijns V, Fernández-Tejada A, Barjaktarović Z, et al. Modulation of immune responses using adjuvants to facilitate therapeutic vaccination. Immunol Rev 2020; 296:169–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupta T, Gupta SK. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int Immunopharmacol 2020; 86:106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang N, Channappanavar R, Ma C, et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol 2016; 13:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coleman CM, Venkataraman T, Liu YV, et al. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine 2017; 35:1586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020; 369:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hotez PJ, Corry DB, Strych U, Bottazzi ME. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat Rev Immunol 2020; 20:399–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coccia M, Collignon C, Hervé C, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNgamma response promoting vaccine immunogenicity. NPJ Vaccines 2017; 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuo TY, Lin M-Y, Coffman RL, et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci Rep 2020; 10:20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020; 586:567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amanat F, Strohmeier S, Rathnasinghe R, et al. Introduction of two prolines and removal of the polybasic cleavage site lead to higher efficacy of a recombinant spike-based SARS-CoV-2 vaccine in the mouse model. mBio 2021; 12:e02648–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keech C, Albert G, Cho I, et al. Phase 1-2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020; 383:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Soni D, Bobbala S, Li S, Scott EA, Dowling DJ. The sixth revolution in pediatric vaccinology: immunoengineering and delivery systems. Pediatr Res 2021; 89:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nanishi E, Borriello F, O’Meara TR, et al. An aluminum hydroxide:CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor binding domain vaccine in aged mice. Sci Transl Med 2022; 14:eabj5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. CTRI . http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=48329, 2020. Accessed 17 February 2022.
- 54. DFC . https://www.dfc.gov/media/press-releases/dfc-announces-support-manufacturing-vaccines-during-quad-summit, 2021. Accessed 17 February 2022.
- 55. Borriello F, Poli V, Shrock E, et al. An adjuvant strategy enabled by modulation of the physical properties of microbial ligands expands antigen immunogenicity. Cell 2022; 185:614–29.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Borriello F, Nanishi E, Seo H-S, et al. An adjuvanted SARS-CoV-2 RBD nanoparticle elicits neutralizing antibodies and fully protective immunity in aged mice. BioRxiv [Preprint]. 2021. doi: 10.1101/2021.09.09.45966. [DOI]
- 57. Zhang X, Meining W, Fischer M, Bacher A, Ladenstein R. X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 A resolution: determinants of thermostability revealed from structural comparisons. J Mol Biol 2001; 306:1099–114. [DOI] [PubMed] [Google Scholar]
- 58. Hilgers LAT, Platenburg PPLI, Bajramovic J, et al. Carbohydrate fatty acid monosulphate esters are safe and effective adjuvants for humoral responses. Vaccine 2017; 35, 3249–55. [DOI] [PubMed] [Google Scholar]
- 59. Blom AG, Hilgers LA. Sucrose fatty acid sulphate esters as novel vaccine adjuvants: effect of the chemical composition. Vaccine 2004; 23:743–54. [DOI] [PubMed] [Google Scholar]
- 60. Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021; 54:2877–92.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dowling D, Nanishi E, McGrath M, et al. mRNA booster vaccination protects extremely aged mice against the SARS-CoV-2 Omicron variant. Research Square 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Akkaya M, Akkaya B, Kim AS, et al. Toll-like receptor 9 antagonizes antibody affinity maturation. Nat Immunol 2018; 19:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nanishi E, Angelidou A, Rotman C, et al. Precision vaccine adjuvants for older adults: a scoping review. Clin Infect Dis 2022; ciac302. doi: 10.1093/cid/ciac302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet 2020; 396:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Piot P, Larson HJ, O’Brien KL, et al. Immunization: vital progress, unfinished agenda. Nature 2019; 575:119–29. [DOI] [PubMed] [Google Scholar]