Abstract
Objective
To describe adaptations necessary for effective use of direct-to-consumer (DTC) cameras in an inpatient setting, from the perspective of health care workers.
Methods
Our qualitative study included semi-structured interviews and focus groups with clinicians, information technology (IT) personnel, and health system leaders affiliated with the Mount Sinai Health System. All participants either worked in a coronavirus disease 2019 (COVID-19) unit with DTC cameras or participated in the camera implementation. Three researchers coded the transcripts independently and met weekly to discuss and resolve discrepancies. Abiding by inductive thematic analysis, coders revised the codebook until they reached saturation. All transcripts were coded in Dedoose using the final codebook.
Results
Frontline clinical staff, IT personnel, and health system leaders (N = 39) participated in individual interviews and focus groups in November 2020–April 2021. Our analysis identified 5 areas for effective DTC camera use: technology, patient monitoring, workflows, interpersonal relationships, and infrastructure. Participants described adaptations created to optimize camera use and opportunities for improvement necessary for sustained use. Non-COVID-19 patients tended to decline participation.
Discussion
Deploying DTC cameras on inpatient units required adaptations in many routine processes. Addressing consent, 2-way communication issues, patient privacy, and messaging about video monitoring could help facilitate a nimble rollout. Implementation and dissemination of inpatient video monitoring using DTC cameras requires input from patients and frontline staff.
Conclusions
Given the resources and time it takes to implement a usable camera solution, other health systems might benefit from creating task forces to investigate their use before the next crisis.
Keywords: COVID-19, electronic personal protective equipment (ePPE), telemedicine, patient safety, patient isolation, health care worker wellbeing
INTRODUCTION
The COVID-19 crisis emerged abruptly and has strained the health care systems around the globe. The rapidly increasing numbers of patients with COVID-19 needing hospitalization, staff shortages, and lack of sufficient personal protective equipment (PPE) generated many challenges, including the difficulty of providing enhanced monitoring of patients under isolation. Moreover, hospitals faced an urgent need to protect health care workers from highly infectious and deadly virus while providing optimal care to patients. Access to appropriate PPE and fear of infecting self and loved ones has been among the top sources of anxiety for health care workers and leaders during the pandemic.1 To address these concerns, many hospitals increased their use of telemedicine tools that do not require physical proximity for medical screening and evaluations, and tools now referred to as electronic PPE, or ePPE.2,3 One of the innovative responses that allowed adequate patient monitoring and helped to preserve traditional PPE (eg, N95 face masks) by minimizing non-critical contact with patients was using direct-to-consumer (DTC) cameras on COVID-19 floors.
DTC cameras have been familiar in non-health care settings for decades. For example, homeowners have used them in closed circuit television systems for security purposes; educators have used them in distant learning to reach remote learners; and parents have used them in baby monitoring systems to see and hear their infants from another room in the house. These systems have evolved, and now technology companies such as Google and Amazon sell their versions of products directly to customers, who can receive alerts of movement detection on their phone and speak with a delivery person through the doorbell camera installed outside their door. When the COVID-19 pandemic began, Turer et al2 proposed inpatient monitoring using commercially available software platforms that are familiar to staff to expedite the adoption and correct use in the context of the pandemic. Mount Sinai Health System (MSHS) in the greater New York City area and other hospital systems took this innovative approach to facilitate patient monitoring.4–6
This study examines the use of DTC cameras at a 3815-bed academic medical center where the daily census of patients with COVID-19 reached almost 2000 in early April 2020, including over 400 patients in intensive care units (ICUs). Many of these patients were suffering from respiratory distress and were connected to advanced respiratory devices that required enhanced monitoring. MSHS leadership needed cost-effective cameras with simple installation that could be used by staff easily with minimal training. Minimum requirements included sufficient video image resolution to discern patient discomfort and clear views of the digital readouts of vital monitors. Originally designed as home monitoring systems, Google Nest cameras met these criteria and were leased by MSHS free of charge as an ePPE solution for inpatient monitoring. The health systems received and installed 170 Google Nest cameras and implemented them within 2 weeks at 4 hospitals.
The scale and speed of implementation of this intervention was unprecedented due to the pressures of the pandemic. The few prior studies of inpatient video monitoring that exist have described the use of other forms including telecare phone calls, telemonitoring app,7 centralized video monitoring with in-room webcams,8 tele-intensive care units (tele-ICUs),9,10 and tele-critical care for family visitation during the COVID-19 pandemic.11 These studies show the promise of each of these technologies specifically. Our study offers insight specific to the use of DTC cameras, contributes to understanding whether the adaptations for other forms of ePPE generalize to DTC cameras, and thus informs future implementations of ePPE in hospitals. Adaptation, a key concept in implementation, is a process of thoughtful and deliberate alteration to the design or delivery of an intervention, with the goal of improving its fit and effectiveness in a given context.12,13 The objective of our study was to describe the adaptations needed to increase effective use of the cameras from the perspective of frontline clinical staff, information technology (IT) personnel, and hospital leaders.
MATERIALS AND METHODS
Setting
The early surge in the number of patients with COVID-19 in MSHS created the need to have continuous “eyes on the patient” from a distance. Within the first days of the pandemic, Mount Sinai Hospital added 60 ICU beds and converted 2 non-ICU units to accept patients with COVID-19. Unlike ICU floors, these floors had solid doors and walls, preventing needed external visualization of patient rooms. Being able to see patients was critical for timely detection of the need for intervention but the highly infectious and aerosolized nature of COVID-19 transmission required patients to be in isolation. Health care workers had to minimize in-person contact for their own and other patients’ safety. Recognizing the visual need and building barrier, MSHS leaders reached out to tech companies about cameras. A contact at Google proposed the use of their DTC solution (Google Nest security cameras for home), which could be customized for hospitals. Partnership between clinicians, Google, IT, and engineering departments aided a quick rollout, given the urgency of the situation. Google loaned these cameras to MSHS temporarily free of charge. A team of engineers from Google and a multidisciplinary team from Mount Sinai that consisted of frontline clinicians, IT, and leadership met daily to design the patient monitoring console. A working prototype was ready to be deployed after 2 weeks of design and development and then a few dozen of Google Nest cameras arrived and were quickly installed. This was the first of many deliveries as the program scaled across MSHS hospitals. MSHS received 170 cameras in total and had about 100 patients being monitored using these cameras at once during peak usage. Cameras were deployed on inpatient units at 4 hospitals and other care settings (eg, dialysis).
Study design
We employed a qualitative design using semi-structured in-depth interviews and focus group discussions, to assess the experiences and perceptions of MSHS staff about the use of Google Nest DTC cameras on COVID-19 floors.14,15 Our study was guided conceptually by the Non-adoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework which is pragmatic, evidence-based and theory informed.16 The framework was developed to help predict and evaluate the success of technology-supported health programs, including remote patient monitoring. The framework guides evaluation of technology adoption, non-adoption, and abandonment by focusing evaluation efforts on implementation constructs (domains) that have critical impact on program success. The NASSS framework includes 7 domains: condition, technology, value proposition, adopters, organization, wider system (ie, policy environment), and embedding and adaptation. We identified COVID-19 as the condition, DTC Google Nest cameras as technology. The value proposition was to improve patient safety, decrease staff anxiety about patients behind closed doors, and decrease staff exposure to COVID-19. Adopters (clinicians and IT personnel) and organization (organizational leaders) are key stakeholders involved in this early demonstration project within a single health system. We chose to conduct our study from their perspective because they were most knowledgeable about the adaptations needed for effective use of cameras.
Data collection
The interviews and focus groups took place between November 2020 and April 2021. During this period, New York City experienced a second wave of COVID-19 pandemic,17 with schools switching to remote instruction by the end of November; COVID-19 vaccines receiving emergency authorization in December 2020; and schools reopening for in-person classes in February–March 2021. Interviews, which were conducted by a single researcher, occurred via phone or video conferencing, while focus groups were facilitated by 2 team members on site (initials removed for blinding) using video conferencing equipment to connect with interviewers (initials removed for blinding). We used focus groups with clinical staff to explore opinions, attitudes, and beliefs about camera implementation in a time-efficient manner. We added individual interviews with nurse managers, registered nurses, patient care associates (PCAs), and nursing assistants (NAs) to clarify emerging themes, gather detailed descriptions of processes, and capture any divergent opinions that could be missed in a focus group setting. We interviewed some physicians in leadership positions, and report them as “health system leaders” rather than physicians here. We interviewed no physicians working on the COVID-19 units. This was an intentional decision based on whose workflows were affected by the DTC cameras the most: nurses and patient care associates. While aware of the cameras, physicians continued to round on patients in person and did not use the cameras in their work or interaction with patients. We used the same interview guide for interviews and focus groups. The study team completed frontline personnel interviews early in the project. In seeking to understand the technical and administrative context, we recruited additional interviewees in IT and leadership roles in the spring. The study was deemed exempt by the Mount Sinai Program for Protection of Human Subjects. All participants voluntarily consented to be interviewed and recorded for anonymized transcription, and de-identified reporting of their comments.
Participant recruitment
We recruited a variety of stakeholders, including frontline clinical staff, IT personnel, and MSHS leaders (N = 39) for individual interviews and focus groups. The clinical staff included PCAs, nurse managers, registered nurses, and NAs (N = 31 in 6 focus groups and 7 individual interviews). We also conducted individual interviews with project management personnel, executive leaders, IT staff members, and a technical engineer from Google (N = 8). The primary inclusion criterion was working on a COVID-19 floor with cameras installed or having direct experience with the project.
The study team conducted 30-min interviews and focus groups via internet-based video conferencing. Participants were assigned identification (ID) codes to identify data collected from them. Focus group participants were reported as a single group without participants’ ID codes, because all focus groups were with clinical staff and the main focus was in documenting their experiences.
The interviewer ensured that each participant answered questions in all domains of the interview guide (Table 1) including participants’ roles in patient care, their experiences with the cameras, the impact on clinical workflows, patient safety, and staff morale, along with opportunities for improvement of camera use beyond the COVID-19 pandemic. Using an interview guide helped ensure consistency and reliability of collected data across participants.18,19 The interviewers used probes for clarification of concepts to ensure data credibility, or truth value to the participants and the context.20 Using probes can also elicit different explanations/details around certain constructs, thereby highlighting different facets of these phenomena. We reached saturation after we completed about two-thirds of the total number of interviews with clinical staff (4 out of 6 focus groups with clinical staff, N = 2 individual interviews with nursing staff, and N = 1 interview with a PCA). We followed accepted standards in qualitative research, which defines saturation as the point when new interviews yielded very little/no new information. We continued to interview a few more people beyond that point to make sure we have not missed anything. Then, we recruited IT personnel and executive leaders to add context and varying perspectives. Interviewers’ personal preconceptions or biases regarding DTC camera use were discussed within the research team and documented prior to interviews to reduce bias in participant selection and data analysis.
Table 1.
Interview domains and sample questions
| Domain | Sample question |
|---|---|
| Perception of being involved/being heard during the implementation/roll out | Tell me how this intervention was rolled out. |
| General feedback | What are your general thoughts about the intervention? |
| Changes to workflow | How did this intervention change your daily work, if at all? |
| Explaining to patients | Did you talk with your patients about the intervention? If so, what were those conversations? |
| Adverse events | Regarding your perceptions about potential to increase/decrease adverse events, how did you think the cameras affected patient safety? |
| Near misses | Can you think of any cases/examples when the intervention helped capture “a near miss”? |
| Staff morale | Do you think the intervention affected staff morale? How? (eg, less burden going in and out of the room) |
| Staff confidence and sense of security | Did the cameras help you feel more or less confident taking care of COVID-19 patients? Do you believe having the cameras gave you a sense of security that your patients would be ok? |
| Use of cameras with non-COVID patients | Did you still work on the unit when it was turned to non-COVID? Were the cameras still there? |
| Future implementation/scale-up | Do you see this kind of intervention being used outside of COVID context? |
| Other institutions | If other hospitals were to use Google Nest, what would you advise them? |
| Final thoughts | Is there anything we should have asked but didn’t? |
Data analysis
A professional transcription service transcribed recorded interviews. The study team verified transcripts with interviewers’ notes for consistency and accuracy and analyzed transcripts using the inductive technique, that is, using individual observations in the data to derive codes and themes.18 We used Dedoose qualitative analytic software21 to extract broad themes (aspects within the data that reflected single concepts) and assigned codes to them. Subsequently, we identified sub-codes under these main codes for more specific themes. Thematic analysis provided a flexible approach to identifying, analyzing, and reporting patterns.22 Three analysts (AM, KG, and EIIE) coded a subset of transcripts (N = 5) independently, one at a time, met to discuss discrepancies, and agreed on a set of codes and definitions (initial codebook). Then, the same coders used the initial codebook to code Transcript 2, met and discussed discrepancies, updated the codebook with the changes introduced at Transcript 2. This process was repeated with 5 interview transcripts, until the coders were applying the latest version of the codebook and the codebook was stable, that is, coders were no longer suggesting to add, change, split, or combine codes. At this point, one analyst (initials removed for blinding) applied the codebook to the complete data set.
We organized similar and related codes into broader themes through visual examination and meticulous consideration of their meanings. Research team discussions helped further refine codes. An integrated narrative was discussed among team members and colleagues to verify coherence of the themes and in-between themes, alongside the original research questions.
Analytical rigor was ensured through18:
Constant reference to participants’ ID codes to ensure data was appropriately associated with participants’ voices.
Consistency in data collection and monitoring of fidelity (use of interview guide, limited number of interviewers, mentorship and supervision of junior researchers).
Triangulation of methods (interviews and focus groups), participants (clinical staff, IT, leaders), and analysts (medical sociologist, 2 physicians).
Regular reference to source documentation, recordings and interviewers’ notes to ensure data accuracy.
Weekly team meetings during all stages of the analytic process, to ensure agreement and reliability of codes and results.
Appropriate documentation, recording and secure storage of data with subsequent analysis for independent auditing for research integrity.
Discussions with various cadres of health care workers at MSHS during development of themes identified in this study.
Parallel data collection and analysis.
RESULTS
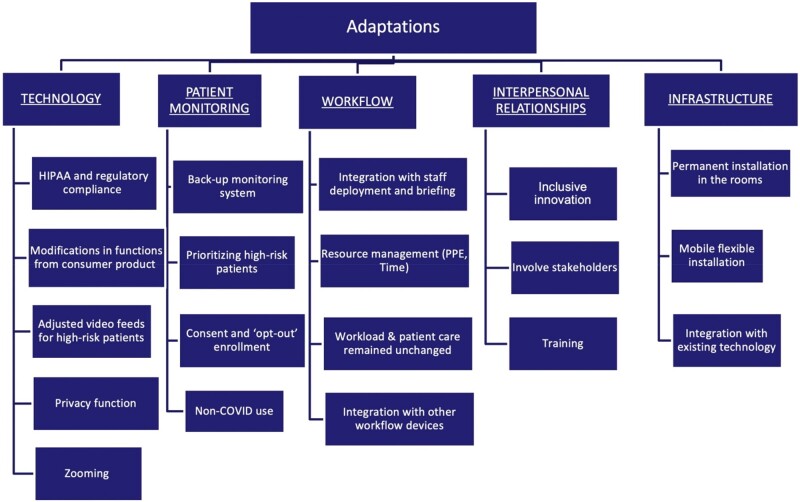
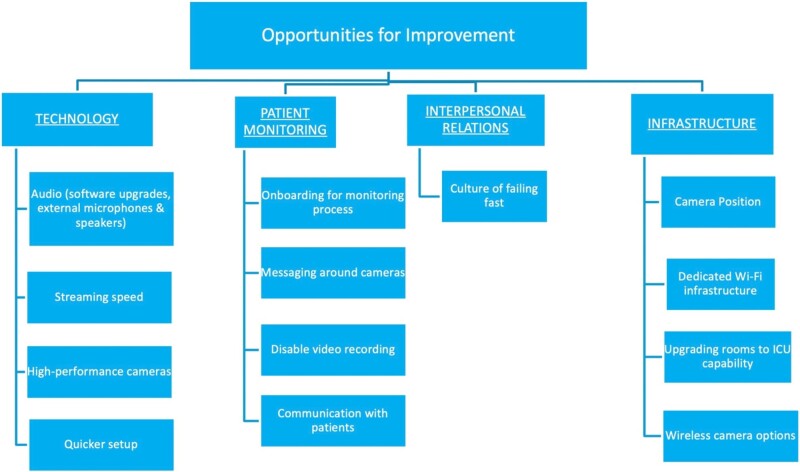
Interviewees’ comments revealed 5 areas for adaptation required for effective DTC camera use for inpatient monitoring: technology, patient monitoring, workflows, interpersonal relationships, and infrastructure. Within each theme, respondents discussed solutions to challenges that surfaced during the implementation, or adaptations (Figure 1 and Table 2). Respondents also highlighted challenges that remained at the time of the interview (8–13 months after camera implementation), which we term opportunities for improvement (Figure 2 and Table 3). Their comments indicated that these opportunities would need to be addressed for sustained DTC use. Participants noted that patients had declined to use DTC after the units reversed to their pre-pandemic designations and began to care for non-COVID-19 patients. We also explored differences in perceptions of camera implementation and use among clinicians on 2 units that participated in this study. In the end, we did not identify any striking differences in the perceptions of clinical staff from the 2 units.
Figure 1.
Documented adaptations.
Table 2.
Documented adaptations
| Themes and definitions | Illustrative quotes |
|---|---|
| 1.Technological Adaptations: software-related adaptations that ensured secure, effective, compliant use of DTC cameras |
|
| 2. Patient Monitoring: collecting information about the patient in real time, with or without the use of technology, to identify any changes in status that may require intervention. |
|
| 3.Workflow: a sequence or pattern of activities to complete a task. |
|
| 4.Interpersonal Relations: communication between hospital leaders, managers, and staff; training and learning; organizational culture |
|
| 5.Infrastructure: hardware, power supply, buildings and facilities, maintenance |
|
Figure 2.
Opportunities for improvement.
Table 3.
Opportunities for improvement
| Themes and definitions | Illustrative quotes |
|---|---|
| 1.Technology: improvements related to software, additional devices, and network characteristics. |
|
| 2.Patient Monitoring: improvements needed in collecting information about the patient in real time, with or without the use of technology, to identify any changes in status that may require intervention. |
|
| 3.Interpersonal Relations: improvements needed in relations between and among hospital leaders, managers, and staff |
|
| 4.Infrastructure: hardware, power supply, facilities and buildings, maintenance |
|
Technological adaptations
The Google Nest cameras needed technical adaptations for compliance with federal regulations concerning protections of patient data and privacy (Table 2, 1a). IT personnel modified functions to improve security of transmitted data by creating 2 levels of users (administrators and monitors) with varying permissions to video feeds. Then, Mount Sinai IT personnel created an enterprise solution in collaboration with Google that limited video feeds to designated viewers on MS campus (Table 2, 1b). Settings were modified so that no data from remote monitoring was sent back to Google.
IT personnel added a function to switch between livestream video and a series of snapshots taken every 5 s on 1 unit to relieve network limitations in streaming multiple videos simultaneously. On that unit, critical patients were prioritized for full streaming, while others had a series of snapshots at 5-s intervals (Table 2, 1c). Other features included zoom-in and “privacy” enabling camera shut-off at patients’ request (eg, while changing), until a nurse approves further monitoring, a light on the camera indicated if monitoring was taking place. To preserve confidentiality, IT completely deactivated the recording feature (Table 2, 1d and e).
Patient monitoring
Our respondents described the cameras as “extra eyes” on the entire floor, for example, when there was a critical event with one patient, enabling the team to focus on the crisis, with one staff deployed to monitor the floor through the feeds (Table 2, 2a). Participants reported that without the cameras, they would have been worried about their other patient deteriorating while helping the patient in critical condition. Staff applied labels (paper post-it notes) to the screen to prioritize high-risk patients based on their oxygen modality and frequency of interventions during the previous shift. Supplementary Appendix A includes a handoff sheet that helped streamline the process of contacting the patient by phone or their assigned nurse to do a physical check-in (Table 2, 2b). The staffing model included patient monitoring, that is, one staff member was always watching the cameras, even if the unit was short staffed.
The units used opt-out consent, with all patients having cameras unless they declined (Table 2, 2c). One clinician mentioned that patients had to meet certain criteria to have the camera turned off. Some patients asked to have cameras turned off or pulled them off the wall. Patients were less receptive when they were not pre-informed (eg, admitted in a confused state), or when the unit went from COVID to non-COVID (Table 2, 2d).
Workflows
The cameras modified staff deployment and briefing. Nurse managers reported that they had to ensure they had a staff member (usually a Patient Care Associate, PCA, or a Nursing Assistant, NA) watching the screen showing the feed of all cameras (N = 38), even if they were short staffed (Table 2, 3a). The NA alerted assigned PCA or nurse when walk-ins were needed, through a speaker system or ‘Vocera’, a wearable voice over internet protocol communication device (eg, accidental bell push or patient removing an oxygen device), thus having PCAs and nurses prepared to handle the situation before they entered the room (Table 2, 3b). The cameras did not change the workload or nature of patient care, though they did help triage non-urgent requests (Table 2, 3c). Cameras also provided extended floor coverage when staff had assignments to patients in other parts of the unit (Table 2, 3d).
The cameras affected staff processes in terms of length of stay in patient rooms, frequency of checking on patients, and reduced the number of times they needed to don and doff PPE. With the goal of reducing staff exposure to the virus in isolation rooms and in the context of staff shortages, cameras allowed clinical staff to check on patients remotely some of the time (Table 2, 3e and f).
Interpersonal relationships
The implementation team and frontline clinicians designed a handoff sheet (Supplementary Appendix A) to create an integrated team strategy and facilitate training of personnel (Table 2, 4a and c). It included patient information, assigned PCA’s and nurse’s names, patient oxygen modalities, fall risk, and phone number of the room. NAs on duty could use the phone to call the patient and ask them, for example, to put their oxygen device back on. Direct input of frontline staff was key to implementing the intervention because the need for cameras emerged organically from the units and was not imposed from above. This secured staff buy-in (Table 2, 4b).
Infrastructure
Engineering teams temporarily secured cameras on ceilings, which was useful in moving them around, especially for patients who could move around the room (Table 2, 5a and c). Flexibility in camera integration with existing technology was also useful. For example, 1 unit experimented with having patient video streams on iPads outside patient rooms, allowing nurses to be by the “bedside” monitoring their patients in the hallway (Table 2, 5b).
Technology-related opportunities for improvement
The most frequently stated concern by participants was ability to communicate with patients via camera microphone and speaker (Table 3, 1a). They stressed that the cameras were distant from the patient, located on the opposite wall to capture their full body and the noise of medical equipment and extractor fans in isolation rooms interfered with the audio quality. Bedside teams called the phone in the patient rooms and had patients use call bells. External microphones and speakers would be desirable enhancements in future applications (Table 3, 1a).
Delays in data transmission also affected video and audio quality. Reducing lag from cloud transmission and routing feeds directly to the browser interface, might improve video and audio quality (Table 3, 1b). Respondents recommended considering newer camera models with higher capabilities (Table 3, 1c), better picture quality and motion sensors for coverage of patients who could walk and improved quality to zoom-in on monitors (eg, pulse oximeters) without entering the rooms. Quick and convenient deployment of cameras between rooms was critical during the pandemic, and participants mentioned the ease of deploying and using new cameras as essential (Table 3, 1e).
Patient monitoring opportunities for improvement
Increasing reliability of communication between patients and staff was among the most frequently suggested improvements (Table 3, 2a). However, external speakers may compromise patient privacy, especially in double occupancy rooms. Patients’ and caregivers’ ideas can be incorporated in the consent process and parameters developed for assessing consent capacity (Table 3, 2b). Standardized consent workflows may improve consent rates, thereby improving patient safety. Positive messaging about cameras (eg, telling patients that “this is a tool that we offer for your safety”), and letting them opt out may also improve consent rates and decrease disconnection rates. Recording may be disabled by vendors prior to rollout due to privacy and liability risks.
Interpersonal relationships opportunities for improvement
One respondent emphasized that a culture of failing fast should be encouraged outside of crisis or pandemic context (Table 3, 3a). This hospital leader described their process of innovating in a crisis: “Once you have the problem, figure out who are the stakeholders, bring them together, and then just brainstorm. What are the gaps? What do we have? What can we try? What’s our ideal state? What experiment can we do? How quickly can we do it? How quickly can we come back to debrief on it? Did it work? Did it not work? Do we have to modify?”
Infrastructure opportunities for improvement
Camera positioning is crucial for adequate coverage of the rooms and enhanced video quality. Some rooms had 1 camera per 2 patients as the number of patients rapidly increased, resulting in poor camera positioning, creating blind spots that compromised patient safety, and warranted regular walk-ins (Table 3, 4a). Participants in one focus group emphasized that ideally, each room should have more than 1 camera, strategically positioned at equal distances around the bed and the door, for full coverage of the rooms, hallways, and bathrooms. More power outlets in the rooms will reduce the distance between cameras and enhance clearer visualization of the floors (Table 3, 4b).
Relevant internet upgrades suitable for high-quality video streams will aid visualization of readings from diagnostic equipment over the cameras (Table 3, 4c). Upgrading all hospital rooms to have the potential for ICU-level visualization and hard-wired monitoring capability will help in a potential future surge (Table 3, 4d). Minimally, these should include transparent doors and/or walls for direct visualization of patients. Wireless cameras may reduce the risk of tripping and falling (Table 3, 4e).
DISCUSSION
This is the first study to our knowledge that describes the use of DTC cameras as ePPE in a hospital setting, with the goals of improving patient and health care worker safety. Our study focused on the implementation process of deploying DTC cameras in the inpatient setting. The DTC cameras were deployed rapidly during the initial surge in New York. Several leaders we interviewed spoke about the need to adapt these technologies to the unique contexts of their institutions, which varied even within the same health system. Table 4 summarizes general recommendations for implementing DTC cameras in an inpatient setting.
Table 4.
Recommendations for DTC camera implementation in an inpatient setting
| Technology recommendations: |
| Use a combination of high-quality external microphones and speakers to enhance patient—clinician communication. Consider using motion detection cameras. |
| Patient monitoring recommendations: |
| Transparent communication about camera monitoring between patients and clinicians may help improve patient acceptance and consent rates, as well as decrease disconnection rates. |
| Workflow recommendations: |
| Camera interventions require a modified staffing model with 1–2 personnel always watching the cameras. |
| Integrating cameras with existing communication systems (Vocera, phones in patient rooms, etc.) requires input from frontline staff. |
| A flowsheet to track communication and high-risk patients must be adapted to the local context and patient needs. |
| Interpersonal relations: |
| Accept failure quickly if DTC cameras are not accepted by clinicians or patients. Engage frontline staff in designing workflows around patient monitoring. Expect this to take time. Start before the next crisis/surge. |
| Infrastructure recommendations: |
| Ideally, each room should have more than one camera in order to increase patient visibility from multiple angles, especially in rooms with two or more patients. |
| Upgrading all hospital rooms to have potential for ICU visualization may ease future transitions to ICU-level care. Equipping rooms with transparent doors and walls can make adaptation to ICU rooms both easier and safer for patients. When possible, choose wireless cameras to reduce tripping hazards. |
| General recommendations: |
| Technologies ought to be adapted to local hospital context and clinical needs. |
| Discussions around privacy and safety are ongoing and will require further clarifications. |
In contrast with studies of tele-ICUs, which reported some pushback from staff, we found that frontline clinicians at MSHS initiated and implemented many adaptations to make camera use more effective.9 This finding is consistent with early reports about using telemedicine at ePPE during the COVID-19 pandemic.2,3 This may be explained by several factors. First, video monitoring was conducted on the same floor, by NAs who were part of the unit staff (rather than external staff in several tele-ICU studies), and at the request of the unit. Secondly, the camera intervention was implemented during a global pandemic, and promised to reduce the exposure to the virus and thus reduce their risk of falling ill with the novel pathogen. The fear of contagion and the risk of infecting self or loved ones was likely a strong impetus to embracing the project. With the scarcity of the PPE, anything that preserved PPE was well appreciated by the frontline staff. Third, cameras helped staff to feel more confident in their ability to perform their duties while someone else was watching their other patients. Prior to the implementation of DTC cameras, nursing staff reported feeling anxious about their ability to deliver quality care behind closed doors with no visibility. Research shows that feeling helpless was common among health care workers during the pandemic, and is in fact one of the indicators of professional burnout.23
Designing a standardized workflow for identifying patients who can benefit from video monitoring, timeframes when they need to be monitored (eg, 24/7 or only at night), developing patient-centric protocols to identify patients who should be offered to opt in rather than using the global opt-out strategy, and investing in health care specific plug-and-play cameras can protect patient lives, improve patient and family satisfaction, protect health care personnel from infection and burnout and reduce health care costs. While video monitoring has been used in health care in the past to enforce compliance to protocols such as hand hygiene24 our study indicates that both patients and staff will likely accept being monitored if it is meant to protect them from harm. However, vendors need to develop products specifically tailored for health care and adhering to regulations regarding patient privacy.
With a number of adaptations to local context, DTC cameras are a promising tool and can be used in a variety of health care settings, such as inpatient units caring for patients with brain injury, delirium, dementia, or on certain medications. Further research is needed to evaluate how and to what extent inpatient video monitoring could improve patient safety and health care workforce psychological well-being.
Our study has some limitations. This was a single case study that may not generalize to other hospitals and health systems. MSHS is a large health system located in New York City, with access to advanced monitoring technology and Google Nest cameras during the pandemic. Other hospitals may not have these resources at their disposal. Another limitation involves generalizability of our findings outside of the COVID-19 pandemic context. It is unclear whether and what kind of the adaptations would be needed in other, less urgent and threatening conditions. Given the uncertainty and intensity of the COVID-19 pandemic, patients and staff may have been more likely to welcome these new forms of monitoring. However, in a non-public emergency environment, patients and staff may value their privacy and be reluctant about being subjected to constant monitoring.
In sum, our findings indicate that the effective use of ePPE and DTC cameras is contingent on adaptations, based on the observations of frontline staff and leaders. As our study shows, developing the workflows takes time, and discussions around patient privacy and everyone’s safety are still ongoing. Using camera solutions during “normal” times may help improve patient safety and reduce staff anxiety on units caring for patients with limited physical and/or cognitive capacity, such as dementia, or on certain medications. We urge health care leaders to begin this conversation with their frontline clinicians today rather than wait for the next crisis.
FUNDING
This work was supported by the Office of Clinical Innovation, Mount Sinai Health System.
AUTHOR CONTRIBUTIONS
All the authors meet criteria for authorship as stated in the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. Study concept and design: KG, RF, and MM. Acquisition of data: KG, AM, PH, TS, LX, and TS. Analysis and interpretation of data: KG, AM, EIIE, and IN. Drafting of the manuscript: KG, AM, EIIE, SP, MM, DR, PH, RF, and CS. Critical revision of the manuscript for important intellectual content: KG, AM, EIIE, SP, PH, TS, IN, CS, LX, DR, MM, and RF.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie Tchen for her assistance with interviewing clinical personnel and Kathleen Sutcliffe for her thoughtful comments on the draft of this paper in the context of the Health Care Management Division of the American Academy of Management Research Incubator.
CONFLICT OF INTEREST STATEMENT
The authors have the following financial or personal conflicts to disclose: KG received salary support for conducting this study from the Office of Clinical Innovation, Mount Sinai Health System. All other authors have no conflicts to disclose.
Contributor Information
Ksenia Gorbenko, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Institute for Health Care Delivery Science, Mount Sinai Health System, New York, New York, USA.
Afrah Mohammed, Department of Clinical Innovation, Mount Sinai Health System, New York, New York, USA.
Edward I I Ezenwafor, Institute for Health Care Delivery Science, Mount Sinai Health System, New York, New York, USA.
Sydney Phlegar, Institute for Health Care Delivery Science, Mount Sinai Health System, New York, New York, USA.
Patrick Healy, Department of Clinical Innovation, Mount Sinai Health System, New York, New York, USA.
Tamara Solly, Department of Nursing, The Mount Sinai Hospital, Mount Sinai Health System, New York, New York, USA.
Ingrid Nembhard, Department of Health Care Management, Wharton School of Business, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Lucy Xenophon, Chief Transformation Officer, The Mount Sinai Morningside Hospital, Mount Sinai Health System, New York, New York, USA.
Cardinale Smith, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Robert Freeman, Department of Clinical Innovation, Mount Sinai Health System, New York, New York, USA.
David Reich, Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA; President, Mount Sinai Hospital and Mount Sinai Queens, Mount Sinai Health System, New York, New York, USA.
Madhu Mazumdar, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Institute for Health Care Delivery Science, Mount Sinai Health System, New York, New York, USA.
Data Availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. Anonymized data will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Shanafelt T, Ripp J, Trockel M.. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA 2020; 323 (21): 2133–4. [DOI] [PubMed] [Google Scholar]
- 2. Turer RW, Jones I, Rosenbloom ST, Slovis C, Ward MJ.. Electronic personal protective equipment: A strategy to protect emergency department providers in the age of COVID-19. J Am Med Inform Assoc 2020; 27 (6): 967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai M-J, Tsai W-T, Pan H-S, et al. Deployment of information technology to facilitate patient care in the isolation ward during COVID-19 pandemic. J Am Med Inform Assoc 2020; 27 (11): 1819–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mount Sinai deploys Google Nest cameras for COVID-19 patient monitoring and communication | TechCrunch [Internet]. https://techcrunch.com/2020/05/11/mount-sinai-deploys-google-nest-cameras-for-covid-19-patient-monitoring-and-communication/ Accessed March 7, 2022.
- 5. Naik BN, Gupta R, Singh A, Soni SL, Puri GD.. Real-time smart patient monitoring and assessment amid COVID-19 pandemic—an alternative approach to remote monitoring. J Med Syst 2020; 44 (7): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouabida K, Malas K, Talbot A, et al. Remote patient monitoring program for COVID-19 patients following hospital discharge: a cross-sectional study. Front Digit Health 2021; 3: 721044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cournan M, Fusco-gessick B, Wright L.. Improving patient safety through video monitoring. 2018; 43 (2): 111–5. [DOI] [PubMed] [Google Scholar]
- 8. Sand-Jecklin K, Johnson J, Tylka S.. Protecting patient safety: can video monitoring prevent falls in high-risk patient populations? J Nurs Care Qual 2016; 31 (2): 131–8. [DOI] [PubMed] [Google Scholar]
- 9. Lilly CM, Thomas EJ.. Tele-ICU: Experience to date. J Intensive Care Med 2010; 25 (1): 16–22. [DOI] [PubMed] [Google Scholar]
- 10. Chu-Weininger L, Wueste L, Lucke JF, Weavind L, Mazabob J, Thomas EJ.. The impact of a tele-ICU on provider attitudes about teamwork and safety climate. Qual Saf Health Care 2010; 19 (6): e39. [DOI] [PubMed] [Google Scholar]
- 11. Sasangohar F, Dhala A, Zheng F, Ahmadi N, Kash B, Masud F.. Use of telecritical care for family visitation to ICU during the COVID-19 pandemic: an interview study and sentiment analysis. BMJ Qual Saf 2020; 30 (9): 715–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiltsey Stirman S, Gamarra JM, Bartlett BA, Calloway A, Gutner CA.. Empirical examinations of modifications and adaptations to evidence-based psychotherapies: methodologies, impact, and future directions. Clin Psychol Sci Pract 2017; 24 (4): 396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiltsey Stirman S, Gutner CA, Crits-Christoph P, Edmunds J, Evans AC, Beidas RS.. Relationships between clinician-level attributes and fidelity-consistent and fidelity-inconsistent modifications to an evidence-based psychotherapy. Implement Sci 2015; 10 (1): 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley EH, Curry LA, Devers KJ.. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007; 42 (4): 1758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patton MQ. Qualitative Research. In: Everitt BS, Howell DC, eds. Encyclopedia of Statistics in Behavioral Science. John Wiley & Sons; 2005. https://doi.org/10.1002/0470013192.bsa514
- 16. Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017; 19 (11): e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.New York’s Spring of Optimism: Finally, the Second Coronavirus Wave is Ebbing. The New York Times. https://www.nytimes.com/2021/04/28/nyregion/covid-cases-new-york-city-second-wave.html Accessed March 8, 2022.
- 18. Colorafi K, Evans B.. Qualitative descriptive methods in health science research. HERD 2016; 9 (4): 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutton J, Austin Z.. Qualitative research: data collection, analysis, and management. Can J Hosp Pharm 2015; 68 (3): 226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mills AJ, Durepos G, Wiebe E.. Credibility. In: Encyclopedia of Case Study Research. Thousand Oaks, CA, USA: SAGE Publications, Inc.; 2010. http://methods.sagepub.com/reference/encyc-of-case-study-research/n91.xml Accessed March 8, 2022. [Google Scholar]
- 21.Dedoose Version 9.0.17, web application for managing, analyzing, and presenting qualitative and mixed method research data. Los Angeles, CA: SocioCultural Research Consultants, LLC; 2021. www.dedoose.com.
- 22. Braun V, Clarke V.. Using thematic analysis in psychology. Qual Res Psychol 2006; 3 (2): 77–101. [Google Scholar]
- 23. Gorbenko K, Franzosa E, Masse S, et al. I felt useless”: a qualitative examination of COVID-19’s impact on home-based primary care providers in New York. Home Health Care Serv Q 2021; 40 (2): 121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sánchez-Carrillo LA, Rodríguez-López JM, Galarza-Delgado DÁ, et al. Enhancement of hand hygiene compliance among health care workers from a hemodialysis unit using video-monitoring feedback. Am J Infect Control 2016; 44 (8): 868–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. Anonymized data will be shared on reasonable request to the corresponding author.