Abstract
Background
Understanding the distribution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies from vaccination and/or prior infection is critical to the public health response to the pandemic. CalScope is a population-based serosurvey in 7 counties in California.
Methods
We invited 200 000 randomly sampled households to enroll up to 1 adult and 1 child between April 20, 2021 and June 16, 2021. We tested all specimens for antibodies against SARS-CoV-2 nucleocapsid and spike proteins, and each participant completed an online survey. We classified participants into categories: seronegative, antibodies from infection only, antibodies from infection and vaccination, and antibodies from vaccination only.
Results
A total of 11 161 households enrolled (5.6%), with 7483 adults and 1375 children completing antibody testing. As of June 2021, 33% (95% confidence interval [CI], 28%–37%) of adults and 57% (95% CI, 48%–66%) of children were seronegative; 18% (95% CI, 14%–22%) of adults and 26% (95% CI, 19%–32%) of children had antibodies from infection alone; 9% (95% CI, 6%–11%) of adults and 5% (95% CI, 1%–8%) of children had antibodies from infection and vaccination; and 41% (95% CI, 37%–45%) of adults and 13% (95% CI, 7%–18%) of children had antibodies from vaccination alone.
Conclusions
As of June 2021, one third of adults and most children in California were seronegative. Serostatus varied regionally and by demographic group.
Keywords: population-based, SARS-CoV-2 seroprevalence
By July 2021, the United States had recorded more than 34 million coronavirus disease 2019 (COVID-19) cases and 600 000 deaths, with over 3.7 million cases and 60 000 deaths in California [1]. Although all adults and children over 12 have been eligible for COVID-19 vaccination since May 2021 in California, vaccine uptake has been uneven; as of July 31, 2021, the percentage of persons fully vaccinated ranged from 24% to 79% across California counties.
The California Department of Public Health (CDPH) monitors COVID-19 burden and forecasts hospitalizations to determine when additional mitigation measures are required to avoid overwhelming the healthcare system [2]. Both prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and vaccination reduce the risk of symptomatic COVID-19 and hospitalization, although questions remain regarding the relative level and duration of risk reduction [3–7]. Accurately forecasting future COVID-19 surges requires estimating population immunity from prior infection or vaccination to determine how many people remain susceptible to infection. Estimating population immunity using routine surveillance data is challenging. Because COVID-19 may be asymptomatic and persons with mild illness may not seek testing, many infections are not recognized or reported. In recent studies, researchers estimated that 70% of SARS-CoV-2 infections in California were unaccounted for in the CDPH COVID-19 surveillance system by December 2020 [8].
Population-based serosurveys can estimate immunity from prior infection or vaccination without the limitations inherent in routine surveillance, and several seroprevalence studies have been completed or are currently underway throughout the United States [9–15]. However, ,the studies conducted thus far in California have been limited to convenience samples, restricted to narrow geographic regions, or only powered to produce statewide estimates, thereby limiting their utility for informing public health policy regionally in California [8, 16, 17]. Thus, CDPH launched a repeated cross-sectional population-based serosurvey (CalScope) to estimate the proportion of housed and noninstitutionalized Californians with evidence of immunity against SARS-CoV-2 from prior infection or vaccination. In this study, we present the results of the first wave of CalScope—conducted between May and June 2021.
METHODS
Study Design
CalScope is a repeated cross-sectional study using random address-based sampling of households in 7 counties in California. The study resamples households with replacement over 3 timepoints.
Sampling Strategy
We used a multistage sampling strategy to allow for region-specific seroprevalence estimates. The sampling approach was guided by principles of causal transportability [18] to ensure that the final study results could be appropriately and efficiently generalized to the general population (Supplementary Appendix). We sampled households in 7 counties: Alameda, El Dorado, Kern, Los Angeles, Monterey, San Diego, and Shasta.
We used an address-based sampling frame created by Marketing Systems Group to select a probability sample of households within each county. The frame uses the United States Postal Service Computerized Delivery Sequence File, which covers all residential delivery locations in the United States, with each address geocoded and linked to the 2015 American Community Survey (ACS) [19]. We oversampled households from census tracts with higher proportions of black households to ensure adequate representation. To enroll a total of 10 000 households, we sampled 200 000 households per wave distributed across the 7 counties proportional to each county’s population with a minimum of 15 000 households sampled per county.
Sampled households could enroll 1 adult and 1 child (6 months to 17 years old). To randomize which eligible household members participated, we instructed households to enroll the adult and child with the next upcoming birthday. Wave 1 enrollment was conducted from April 20, 2021 through June 15, 2021.
Survey Instruments
When registering for the study, participants completed a household enumeration form and could elect to order at-home antibody test kits. Participants who declined the antibody test could choose to only complete the survey instrument.
The adult survey asked about demographics of all household members, income, occupation, medical history, COVID-19 vaccination and testing history, and behaviors associated with COVID-19 risk—including mask use and social distancing. The child survey asked about the child participant’s demographics, medical history, COVID-19 vaccination and testing history, and behaviors associated with COVID-19 risk—including attendance in school and other social activities (Supplementary Appendix).
Antibody Testing
Participants were mailed at-home antibody test kits with instructions on how to collect a dried blood spot (DBS) specimen and were asked to return their sample to Enable Biosciences within 30 days. Specimens with inadequate volume or collected >30 days before receipt by the laboratory were rejected. All valid specimens received by the laboratory by August 1, 2021 were included in this analysis.
Specimens were tested for both antispike and anti-nucleocapsid antibodies using Enable’s ADAP SARS-CoV-2 total antibody assay. The assay procedures have been described previously (Supplementary Appendix) [20]. The assay cutoffs were established by testing 100 healthy controls and set at 99.7% percentile. The cutoffs for spike and nucleocapsid antibodies were 3.00 ΔCt and 1.50 ΔCt, respectively. In a validation study including 31 polymerase chain reaction [PCR]-positive COVID-19 cases and 80 healthy blood donors, the assays were shown to be 100% sensitive (95% confidence interval [CI], 89%–100%) and 100% specific (95% CI, 95%–100%) against the spike and nucleocapsid proteins [18].
Sampling Weights
We anticipated that households that enrolled in the study and completed antibody tests would differ from those that did not respond. Thus, we constructed sampling weights to generalize our results from the study sample to the target population: the general population of noninstitutionalized, housed residents in each of the 7 sampled counties [21, 22].
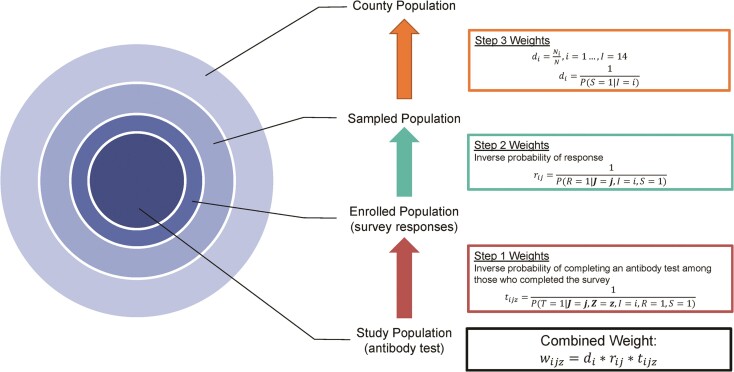
Within each county, there were 3 levels of selection between the final study sample and target population (Figure 1). The sampled population was all households that were mailed invitations to participate in CalScope; the registered population included all participants that registered for the study and completed a survey instrument; and the final study sample included all registered participants with a valid antibody test result and completed survey instrument. We estimated weights to generalize across each selection step: Step (1) from the final study sample to the registered population, Step (2) from the registered population to the sampled population, and Step (3) from the sampled population to the target population.
Figure 1.
Levels of selection between study sample and target population in each county and corresponding weighting steps. In the Step 3 Weights, S = 1 indicates that the household was sampled and I = i is the sampling stratum. In the Step 2 Weights, R = 1 indicates that at least 1 member of the household completed a survey instrument (enrolled). J is a vector of address-based characteristics including demographic characteristics from the American Community Survey, Healthy Places Index quartile, 2020 Presidential Election results by voter precinct, and COVID-19 vaccination coverage as of April 20, 2021 by zip code. In the Step 1 Weights, T = 1 indicates that an individual has a valid antibody test result, Z is a vector of individual-level measurements from the survey instrument. Step 3 and 2 weights were estimated at the household level. Step 1 weights were estimated at the individual level with weights estimated separately for adults and children. The combined weight is the product of all 3 weights.
We constructed selection diagrams to guide variable selection for estimating the weights in Steps 1 and 2 (Supplementary Figure 1) [18, 23]. Candidate variables for Step 1 included items from the survey instrument including the following: participant demographics, SARS-CoV-2 testing and vaccination history, mask use, ability to work remotely, household income, education, whether anyone in the household was considered an essential worker [24], and any known contacts with a COVID-19 case. Candidate variables also included neighborhood-level characteristics from the 2015 ACS (poverty, crowded living conditions, income, education, and race/ethnicity) [19], zip-code level COVID-19 vaccination coverage as of May 2021, and 2020 Presidential general election results by voting precinct [25]. Finally, we included the Healthy Places Index (HPI), a summary measure of neighborhood conditions that are associated with life-expectancy [26]. Residents in neighborhoods in HPI quartile 4 have longer life expectancies compared to those in HPI quartiles 1 to 3. Because we did not have survey responses from sampled households that never registered for the study, candidate variables for the Step 2 weights were limited to the neighborhood-level characteristics listed above.
We used a cross-validated ensemble machine learning algorithm, SuperLearner [27], to estimate inverse probability of selection weights for both Step 1 and Step 2. We included a mixture of parametric and machine learning algorithms in the SuperLearner. Weights for Step 1 were estimated separately for adults and children within each county. Step 2 weights were estimated at the household level within each county. Finally, we used the known sampling probabilities for each invited household to construct the Step 3 weights.
We multiplied all 3 weights and used iterative proportional fitting (raking) to calibrate the combined weights to ensure that the weighted distribution of age, sex, race/ethnicity, education, household income, and COVID-19 vaccination coverage matched the marginal distributions in the 2015 ACS and the state COVID-19 vaccine registry in each county [28].
Primary Outcomes
Participation in CalScope was anonymous, so we could not verify participants’ vaccination status. Instead, we used self-reported vaccination status, anti-nucleocapsid, and antispike antibody results to classify participants into 4 mutually exclusive serostatus categories: (1) Seronegative: negative nucleocapsid test and negative spike test regardless of self-reported vaccination status; (2) Prior Infection Only: positive nucleocapsid test AND negative spike test OR (positive nucleocapsid test OR positive spike test) AND self-reported not having received any doses of a COVID-19 vaccine; (3) Infected and Vaccinated: Positive nucleocapsid test AND positive spike tests AND self-reported at least 1 dose of any COVID-19 vaccine; and (4) Vaccinated Only: Negative nucleocapsid test AND positive spike test AND self-reported at least 1 dose of any COVID-19 vaccine.
Using the sampling weights, we estimated the proportion of the population in each serostatus category and with evidence of prior infection for the whole sample and stratified by county, age, race/ethnicity, and HPI quartile. We used a non-parametric bootstrap with 1000 replicates to obtain 95% confidence intervals.
We estimated the ratio of the number of SARS-CoV-2 infections to confirmed cases in the CDPH’s COVID-19 case registry in the overall sample and stratified by county for both adults and children. To do this, we divided the proportion of the population with evidence of prior infection in CalScope by the proportion of the population that was a confirmed COVID-19 case as of 14 days before the median specimen collection date. A confirmed COVID-19 case was defined as a person with a positive PCR SARS-CoV-2 test; the cutoff date allowed for approximately 14 days between time of infection to seroconversion.
All analyses were conducted in R version 3.6.0 using the sl3 package for SuperLearner implementation, the anesrake package for iterative proportional fitting, and the survey package for analysis of the weighted data [27, 29, 30].
Patient Consent
The study protocol and materials were reviewed by the Committee for the Protection of Human Subjects for the State of California and by Stanford University’s Institutional Review Board and determined to be “Not Research/Exempt” under Public Health Practice/Surveillance. Therefore, our study does not include factors necessitating patient consent.
RESULTS
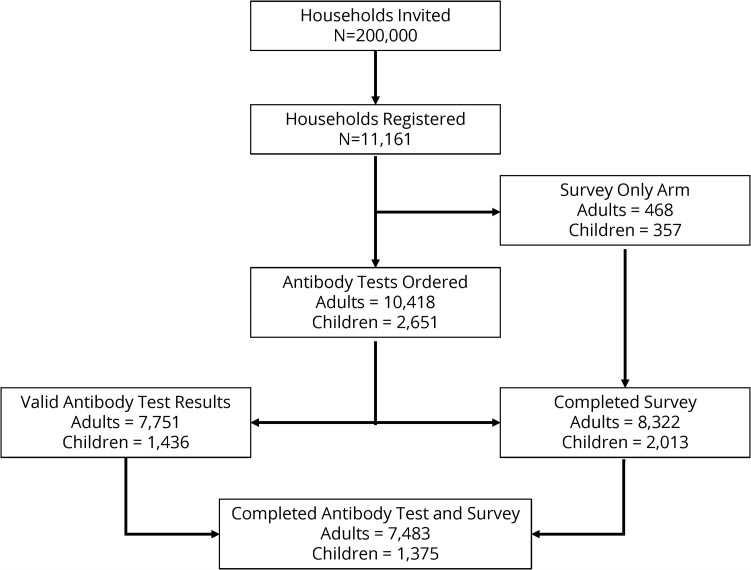
Of the 200 000 households invited, 11 161 registered for the study (5.6%) (Figure 2). A total of 8322 (74.6%) households completed an adult survey and 7751 households (69%) completed adult antibody testing. A total of 7483 (67%) adults completed the survey and returned a DBS specimen with valid antibody results. Of the 11 161 households that registered for the study, 3388 (30%) included at least 1 eligible child. A total of 2013 child surveys (65%) and 1436 (42.4%) child antibody tests were completed, and 1375 (40.6%) children completed both the survey and an antibody test. Households that chose to participate in CalScope had higher levels of education, higher household income, and were less likely to be Latinx compared to households that did not participate (Table 1 and Supplementary Table 1). Table 1 shows the demographics of the study sample before and after weighting. The median specimen collection date was May 22, 2021, with 60% of specimens collected in May 2021 and 90% of specimens collected in May or June 2021 (Supplementary Figure 2).
Figure 2.
Wave 1 consort diagram. The final study sample includes those who completed an antibody test and survey instrument.
Table 1.
Comparison of the Adult Sample Population Demographics and the Final Weighted Sample Demographics: The Weighted Sample Demographics Match the Distribution of Characteristics of Adults in the Target Population
| Sample | Weighted Sample | ||||
|---|---|---|---|---|---|
| Characteristic | N | Percent | N | Percent | |
| Overall | 7483 | 100 | 13 691 938 | 100 | |
| County | Alameda | 1012 | 13.5 | 1 673 845 | 12.2 |
| El Dorado | 803 | 10.7 | 180 384 | 1.3 | |
| Kern | 343 | 4.6 | 671 452 | 4.9 | |
| Los Angeles | 2225 | 29.7 | 7 810 740 | 57.0 | |
| Monterey | 724 | 9.7 | 332 740 | 2.4 | |
| San Diego | 1650 | 22.0 | 287 7651 | 21.0 | |
| Shasta | 726 | 9.7 | 145 126 | 1.1 | |
| Age | 18–25 | 285 | 3.8 | 1 083 541 | 7.9 |
| 26–40 | 1683 | 22.5 | 3 673 681 | 26.8 | |
| 41–65 | 3458 | 46.2 | 6 795 184 | 49.6 | |
| 65+ | 2057 | 27.5 | 2 139 532 | 15.6 | |
| Race/Ethnicity | Latino | 1133 | 15.1 | 6 564 547 | 47.9 |
| NH White | 4710 | 62.9 | 3 786 745 | 27.7 | |
| NH Asian | 811 | 10.8 | 1 625 260 | 11.9 | |
| NH Black | 319 | 4.3 | 752 348 | 5.5 | |
| Other | 510 | 6.9 | 963 038 | 7.0 | |
| Healthy Places Indexa | Quartile 1 | 994 | 13.3 | 4 629 711 | 33.8 |
| Quartile 2 | 2168 | 29.0 | 3 976 184 | 29.0 | |
| Quartile 3 | 1943 | 26.0 | 2 955 262 | 21.6 | |
| Quartile 4 | 2378 | 31.8 | 2 130 781 | 15.6 | |
| Education | Less than high school | 114 | 1.5 | 2 077 099 | 15.2 |
| High school/GED | 500 | 6.7 | 3 327 150 | 24.3 | |
| Some college | 1975 | 26.4 | 3 859 666 | 28.2 | |
| Bachelor’s degree | 2454 | 32.8 | 2 777 891 | 20.3 | |
| Master’s degree or higher | 2323 | 31.0 | 1 579 927 | 11.5 | |
| (Missing) | 117 | 1.6 | 70 204 | 0.5 | |
| Assigned Sex at Birth | Female | 4475 | 59.8 | 7 201 959 | 52.6 |
| Male | 3008 | 40.2 | 6 489 979 | 47.4 | |
| Household Income | <$25k | 885 | 11.8 | 3768643 | 31.9 |
| $25k–$75k | 2110 | 28.2 | 4 886 015 | 41.4 | |
| $75k–$100k | 1060 | 14.2 | 1 588 511 | 13.5 | |
| $100k–$150k | 1524 | 20.4 | 1 645 576 | 13.9 | |
| >$150k | 1904 | 25.4 | 1 803 192 | 15.3 | |
| Crowded Living Conditionsb | Yes | 528 | 7.1 | 1 382 886 | 10.1 |
| No | 6955 | 92.9 | 12 309 052 | 89.9 | |
Abbreviations: GED, General Educational Development; NH, non-Hispanic.
Healthy Places Index is a summary measure of neighborhood conditions associated with life-expectancy. Quartile 4 represents neighborhoods with longer life-expectancy compared to quartiles 1 to 3.
Crowded living conditions is defined as more than 1 person per room living in a residence.
Spike and Nucleocapsid Seroprevalence
Overall, 6625 of 7483 (89%) adults and 581 of 1375 (42%) children had detectable spike antibodies; 846 of 7483 (11%) adults and 224 of 1375 (16%) children had detectable nucleocapsid antibodies. The weighted spike seroprevalence was 67% (95% CI, 63%–71%) for adults and 41% (95% CI, 35%–47%) for children; the weighted nucleocapsid seroprevalence was 22% (95% CI, 18%–26%) among adults and 25% (95% CI, 19%–31%) in children (Table 2).
Table 2.
Nucleocapsid and Spike Antibody Test Results
| Nucleocapsid | Spike | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unweighted | Weighted | Unweighted | Weighted | ||||||||
| n | N | % | Seroprevalence | 95% CI | n | N | % | Seroprevalence | 95% CI | ||
| Overall | Adult | 846 | 7483 | 11% | 22% | 18%–26% | 6625 | 7483 | 89% | 67% | 63%–71% |
| Child | 224 | 1375 | 16% | 25% | 19%–31% | 581 | 1375 | 42% | 41% | 35%–47% | |
| Alameda | Adult | 51 | 1012 | 5% | 10% | 4%–16% | 959 | 1012 | 95% | 69% | 61%–77% |
| Child | 14 | 165 | 8% | 13% | 0%–29% | 74 | 165 | 45% | 27% | 7%–47% | |
| El Dorado | Adult | 70 | 803 | 9% | 10% | 4%–16% | 694 | 803 | 86% | 58% | 48%–68% |
| Child | 24 | 162 | 15% | 17% | 5%–29% | 73 | 162 | 45% | 30% | 16%–44% | |
| Kern | Adult | 60 | 343 | 17% | 25% | 13%–37% | 285 | 343 | 83% | 61% | 45%–77% |
| Child | 22 | 84 | 26% | 16% | 4%–28% | 41 | 84 | 49% | 36% | 14%–58% | |
| Los Angeles | Adult | 323 | 2225 | 15% | 26% | 20%–32% | 2004 | 2225 | 90% | 68% | 62%–74% |
| Child | 84 | 429 | 20% | 34% | 22%–46% | 193 | 429 | 45% | 49% | 37%–61% | |
| Monterey | Adult | 70 | 724 | 10% | 24% | 14%–34% | 659 | 724 | 91% | 73% | 63%–83% |
| Child | 23 | 115 | 20% | 30% | 10%–50% | 56 | 115 | 49% | 45% | 23%–67% | |
| San Diego | Adult | 168 | 1650 | 10% | 19% | 13%–25% | 1442 | 1650 | 87% | 65% | 59%–71% |
| Child | 37 | 311 | 12% | 19% | 7%–31% | 98 | 311 | 32% | 40% | 26%–54% | |
| Shasta | Adult | 104 | 726 | 14% | 19% | 13%–25% | 582 | 726 | 80% | 63% | 55%–71% |
| Child | 20 | 109 | 18% | 23% | 11%–35% | 46 | 109 | 42% | 36% | 22%–50% | |
Abbreviations: CI, confidence interval.
Serostatus
Among adults, we estimated that 33% (95% CI, 28%–37%) were seronegative; 18% (95% CI, 14%–22%) had antibodies from previous infection but not vaccination; 9% (95% CI, 6%–11%) had antibodies from prior infection and vaccination; and 41% (95% CI, 37%–45%) had antibodies from vaccination alone (Table 3). Among children, 57% (95% CI, 48%–67%) were seronegative; 26% (95% CI, 19%–32%) had antibodies from prior infection but not vaccination; 5% (95% CI, 1%–8%) had antibodies from prior infection and vaccination; and 13% (95% CI, 7%–18%) had antibodies from vaccination alone (Table 4). Serostatus varied for adults and children across the 7 counties. For example, seronegativity in adults varied from 27% (95% CI, 15%–38%) in Monterey County to 42% (95% CI, 29%–54%) in El Dorado County. For children, seronegativity ranged from 51% (95% CI, 34%–67%) in Los Angeles County to 68% (95% CI, 41%–96%) in El Dorado County.
Table 3.
Adult Serostatus by Region, Age, Race/Ethnicity, and HPI Quartile
| Evidence of Prior Infection | Serostatus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seronegative | Prior Infection Only | Prior Infection and Vaccination | Vaccination Only | ||||||||
| Characteristic | Weighted Percent | 95% CI | Weighted Percent | 95% CI | Weighted Percent | 95% CI | Weighted Percent | 95% CI | Weighted Percent | 95% CI | |
| Overall | 27% | 23%–31% | 33% | 28%–37% | 18% | 14%–22% | 9% | 6%–11% | 41% | 37%–45% | |
| County | Alameda | 19% | 11%–27% | 31% | 20%–41% | 15% | 6%–23% | 4% | 1%–7% | 51% | 38%–63% |
| El Dorado | 14% | 8%–20% | 42% | 29%–54% | 10% | 4%–16% | 5% | 2%–7% | 44% | 34%–54% | |
| Kern | 27% | 15%–39% | 38% | 18%–57% | 14% | 5%–22% | 13% | 3%–24% | 35% | 20%–51% | |
| Los Angeles | 30% | 24%–36% | 32% | 25%–39% | 20% | 13%–26% | 11% | 7%–15% | 38% | 32%–44% | |
| Monterey | 26% | 14%–38% | 27% | 15%–38% | 15% | 6%–24% | 12% | 4%–20% | 47% | 31%–64% | |
| San Diego | 23% | 17%–29% | 35% | 27%–43% | 18% | 11%–24% | 5% | 3%–7% | 42% | 36%–48% | |
| Shasta | 22% | 16%–28% | 36% | 26%–46% | 17% | 10%–23% | 5% | 3%–7% | 42% | 36%–49% | |
| Age | 18–25 | 40% | 18%–62% | 33% | 13%–52% | 33% | 11%–54% | 8% | 2%–13% | 27% | 13%–42% |
| 26–40 | 31% | 21%–41% | 34% | 25%–44% | 22% | 13%–31% | 9% | 6%–13% | 34% | 27%–42% | |
| 41–65 | 26% | 20%–33% | 33% | 27%–39% | 17% | 12%–22% | 9% | 5%–14% | 41% | 34%–47% | |
| 65+ | 13% | 7%–19% | 28% | 17%–40% | 7% | 3%–11% | 6% | 2%–10% | 59% | 48%–69% | |
| Race/Ethnicity | Latinx | 36% | 27%–45% | 24% | 17%–31% | 23% | 15%–30% | 13% | 8%–18% | 40% | 33%–48% |
| Non-Latinx (all races) | 18% | 15%–22% | 41% | 34%–47% | 14% | 10%–17% | 4% | 3%–6% | 41% | 37%–45% | |
| Non-Latinx White | 12% | 12%–20% | 44% | 37%–52% | 12% | 7%–16% | 4% | 3%–6% | 40% | 35%–45% | |
| Non-Latinx Asian | 21% | 12%–30% | 26% | 15%–37% | 16% | 7%–25% | 3% | 3%–7% | 53% | 44%–61% | |
| Non-Latinx Black | 16% | 7%–25% | 53% | 27%–79% | 12% | 4%–21% | 4% | 1%–6% | 31% | 18%–45% | |
| HPI | Quartile 1 | 30% | 21%–40% | 29% | 19%–38% | 16% | 8%–24% | 15% | 9%–21% | 41% | 32%–50% |
| Quartile 2 | 28% | 20%–37% | 33% | 25%–41% | 21% | 14%–29% | 7% | 4%–10% | 39% | 32%–45% | |
| Quartile 3 | 23% | 16%–30% | 42% | 32%–52% | 17% | 11%–24% | 6% | 3%–8% | 35% | 29%–42% | |
| Quartile 4 | 20% | 12%–28% | 27% | 20%–35% | 17% | 9%–25% | 3% | 2%–5% | 52% | 46%–59% | |
Abbreviations: CI, confidence interval; HPI, Healthy Places Index.
Table 4.
Child Serostatus by Region, Age, Race/Ethnicity, and HPI Quartile
| Evidence of Prior Infection | Serostatus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seronegative | Prior Infection Only | Prior Infection and Vaccination | Vaccination Only | ||||||||
| Characteristic | Weighted Percent | 95% CI | Weighted Percent | 95% CI | Weighted Percent | 95% CI | Weighted Percent | 95% CI | Weighted Percent | 95% CI | |
| Overall | 30% | 24%–36% | 57% | 48%–66% | 26% | 19%–32% | 5% | 1%–8% | 13% | 7%–18% | |
| County | Alameda | 15% | 1%–29% | 68% | 35%–100% | 15% | 0%–30% | 0% | 0%–0% | 17% | 0%–40% |
| El Dorado | 19% | 7%–31% | 69% | 40%–97% | 19% | 5%–33% | 0% | 0%–1% | 12% | 6%–19% | |
| Kern | 18% | 6%–30% | 63% | 35%–92% | 17% | 7%–26% | 2% | 0%–4% | 18% | 0%–43% | |
| Los Angeles | 37% | 25%–49% | 51% | 34%–67% | 28% | 15%–41% | 9% | 0%–18% | 12% | 4%–21% | |
| Monterey | 39% | 19%–59% | 56% | 27%–85% | 34% | 12%–56% | 5% | 0%–13% | 6% | 0%–16% | |
| San Diego | 31% | 17%–45% | 57% | 38%–76% | 29% | 11%–47% | 1% | 0%–4% | 12% | 1%–23% | |
| Shasta | 27% | 13%–41% | 64% | 40%–87% | 24% | 11%–38% | 2% | 0%–5% | 9% | 4%–15% | |
| Age | 6 months–5 years | 29% | 7%–51% | 71% | 39%–100% | 29% | 7%–51% | 0% | 0%–0% | 0% | 0%–0% |
| 5 years–11 years | 36% | 21%–52% | 64% | 46%–81% | 36% | 21%–52% | 0% | 0%–0% | 0% | 0%–0% | |
| 12 years–17 years | 27% | 17%–37% | 52% | 39%–65% | 20% | 12%–28% | 8% | 2%–14% | 21% | 12%–29% | |
| Race/Ethnicity | Latinx | 35% | 23%–46% | 51% | 38%–65% | 28% | 17%–38% | 7% | 1%–13% | 14% | 6%–22% |
| Non-Latinx (all races) | 24% | 15%–33% | 65% | 52%–79% | 23% | 14%–32% | 1% | 0%–3% | 10% | 5%–16% | |
| Non-Latinx White | 26% | 15%–38% | 66% | 50%–82% | 26% | 14%–37% | 1% | 0%–1% | 8% | 6%–10% | |
| Non-Latinx Asian | 21% | 3%–39% | 52% | 27%–77% | 20% | 2%–38% | 1% | 0%–2% | 27% | 2%–52% | |
| Non-Latinx Black | 39% | 10%–68% | 59% | 2%–94% | 35% | 7%–64% | 3% | 0%–10% | 2% | 1%–4% | |
| HPI | Quartile 1 | 38% | 23%–54% | 47% | 29%–64% | 29% | 16%–42% | 9% | 1%–17% | 15% | 5%–26% |
| Quartile 2 | 29% | 15%–43% | 58% | 40%–75% | 25% | 13%–38% | 4% | 0%–10% | 13% | 3%–24% | |
| Quartile 3 | 23% | 10%–37% | 70% | 46%–94% | 23% | 9%–37% | 0% | 0%–1% | 7% | 4%–10% | |
| Quartile 4 | 21% | 9%–34% | 68% | 46%–89% | 21% | 8%–33% | 1% | 0%–1% | 11% | 7%–15% | |
Abbreviations: CI, confidence interval; HPI, Healthy Places Index.
Serostatus also varied across age groups, with the lowest seronegativity among people >65 years (28%; 95% CI, 17%–40%) and highest proportion seronegative in children <5 years old (71%; 95% CI, 40%–100%). Seropositivity due to vaccination alone was highest in people >65 years (59%; 95% CI, 48%–69%), whereas people between ages 18 and 25 years were more likely to have antibodies from prior infection alone (33%; 95% CI, 11%–54%) compared to other age groups. When comparing across race and ethnicity, the lowest percentage seronegative was in Latinx adults (24%; 95% CI, 17%–46%); non-Latinx Asian adults were most likely to have antibodies due to vaccination alone (53%; 95% CI, 44%–61%) (Table 3).
Finally, adults living in HPI quartiles 1 or 4 were less likely to be seronegative than adults living in HPI quartiles 2 or 3 (Table 3). In contrast, children living in HPI quartile 1 were less likely to be seronegative compared to those in the higher HPI quartiles (Table 4).
Evidence of Prior Infection and Infection-to-Case Ratio
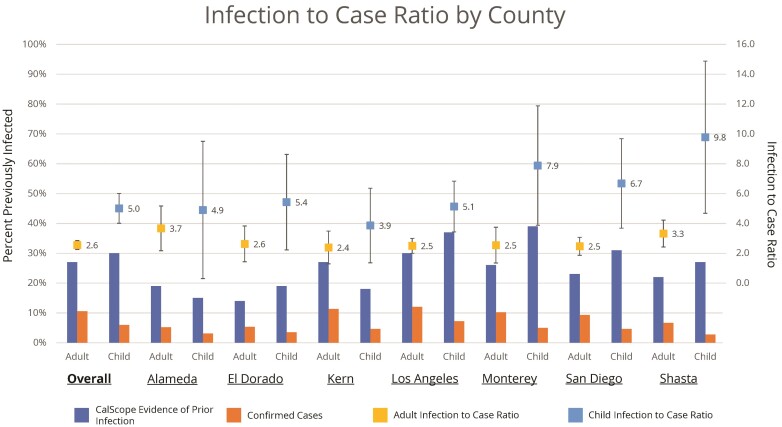
Overall, 27% (95% CI, 23%–31%) of adults and 30% (95% CI, 24%–36%) of children had evidence of prior infection. In contrast, 11% of adults and 6% of children were confirmed COVID-19 cases as of May 8, 2021 in the COVID-19 case registry in the 7 CalScope counties. Among people who were previously infected, 33% (95% CI, 19%–48%) had also been previously vaccinated. The estimated infection-to-case ratio was 2.6 (95% CI, 2.2–2.9) for adults and 5.0 (95% CI, 4.0–5.0) for children (Figure 3).
Figure 3.
Infection to case ratio by county. The infection to case ratio is the ratio of the percentage of the population with evidence of prior infection based on antibody test results to the percentage of the population with a polymerase chain reaction-confirmed infection in California Department of Public Health’s coronavirus disease 2019 surveillance database with an episode date on or before May 8, 2020.
Evidence of prior infection among adults varied across the 7 counties from 14% (95% CI, 8%–20%) in El Dorado County to 30% (95% CI, 24%–36%) in Los Angeles County. Likewise, the percentage of children with antibody evidence of prior infection varied from 15% (95% CI, 1%–29%) in Alameda County to 39% (95% CI, 19%–59%) in Monterey County. Infection-to-case ratios were consistently higher for children than adults in all counties.
Across all age groups, 18- to 25-year-olds had the highest percentage with antibodies from prior infection (40%; 95% CI, 18%–62%) (Table 3). Among children, those between 5 and 11 years old were most likely to have evidence of prior infection (36%; 95% CI, 21%–51%) (Table 4). Adults >65 years were least likely to have evidence of prior infection (13%; 95% CI, 7%–19%).
Latinx adults and children were more likely to have antibodies from prior infection (adults: 36%, 95% CI = 27%–45%; children: 35%, 95% CI = 23%–46%) compared with non-Latinx adults or children, and non-Latinx Asian adults and children were least likely to have antibody evidence of prior infection (adults: 21%, 95% CI = 12%–30%; children: 21%, 95% CI = 3%–39%).
Finally, seroprevalence of antibodies from prior infection was highest among adults and children living in the lowest HPI quartile and was lowest in adults and children living in neighborhoods in the highest HPI quartile.
DISCUSSION
During Wave 1 of CalScope, 33% of adults and 56% of children did not have antibodies against SARS-CoV-2 as of June 2021, with 27% of adults and 30% of children having evidence of prior SARS-CoV-2 infection. Overall, the infection-to-case ratio was 2.6 for adults and 5.0 for children suggesting that through June 2021, similar numbers of infections had occurred in adults and children, but infections in children were less likely to be diagnosed. Because children are less likely to have symptomatic SARS-CoV-2 infections, they are also less likely to be tested for SARS-CoV-2.
Serostatus differed across region, race/ethnicity, age, and HPI quartile reflecting disparate patterns of infection and vaccination. For example, California has been prioritizing equity in its COVID-19 response by using the HPI to target vaccination campaigns, testing, and other COVID-19 mitigation measures towards more disadvantaged neighborhoods, which have borne a larger burden of the COVID-19 pandemic thus far [31]. Our results suggest that these targeted vaccination campaigns have been effective—seropositivity due to vaccination is similar for adults in the lowest and highest HPI quartiles (56% in both).
Although the proportion of children and adults who had been previously infected was similar, most children remained seronegative as of June 2021 because they were not yet eligible for vaccination. Even among those eligible (age 12–17), vaccination coverage has been low, and many were still seronegative. With in-person schooling resuming in much of the state, it will be important to encourage vaccination of all age eligible children.
Other studies using remnant commercial laboratory specimens have found that seroprevalence from prior infection may be higher among children than adults [32, 33]. These studies may overestimate seroprevalence from prior infection in children because children receiving bloodwork may be more likely to be sick compared to the general population. However, our estimates are uncertain and do not preclude the possibility that seroprevalence from prior infection is higher in children than adults.
Antibody waning below the limit of detection is dependent on the assay used [34]. The ADAP assays used in CalScope are highly sensitive and specific, but the assays have only been validated up to 4 months postinfection. Thus, we do not know the extent of antibody waning below the limit of detection after >4 months. This means that our estimates of seroprevalence due to prior infection might underestimate true cumulative incidence—particularly in populations who were infected in the Spring of 2020.
Although antibody levels are associated with reduced risk of infection, there is currently no established threshold above which someone is considered completely protected from infection [35, 15, 36–39]. Individuals whose antibodies wane below detectable levels after vaccination or infection may be less susceptible to subsequent infection or COVID-19 disease than immunologically naive individuals because of cell-mediated immunity [40]. In addition, although the correlation between antibody levels and risk of severe COVID-19 disease has not been established, prior studies have found that protection likely differs between infection-induced, vaccine-induced, and hybrid immunity. This relationship is likely evolving as immunity wanes and as new variants emerge; nevertheless, the serostatus estimates from CalScope are being used to calibrate and refine the California Department of Public Health’s COVID-19 modeling.
We anticipated that those who enrolled in our study might not be representative of our target populations, so we used causal transportability to design our study and survey instrument to generalize our results to our target populations. Our weighted results can be considered unbiased estimates of SARS-CoV-2 serostatus in our target population only if we assume that we were able to measure and adjust for all the differences between the study sample and target population that were associated with SARS-CoV-2 serostatus. Given the low response rate in CalScope, it is likely that our weights may have excluded some relevant characteristics that differed between the sample and target populations, and our estimates may still suffer from residual nonresponse bias. In addition, we relied on self-reported vaccination to classify serostatus, which may be subject to social desirability or recall biases. However, our results are in line with those from other studies and known patterns of vaccination and infection, so residual biases are unlikely to meaningfully affect our results.
CONCLUSIONS
Overall, we found that similar proportions of adults and children had been infected as of April- June 2021, but serostatus varied substantially across region, age group, and by race/ethnicity. Although seroprevalence studies such as CalScope are unable to measure all aspects of the immune response, spike antibodies are a correlate of protection for SARS-CoV-2 infection and symptomatic disease [41–43]. As vaccination and transmission continues, the population that remains most vulnerable to COVID-19 infection and disease will evolve. It is critical that public health agencies monitor who does not have SARS-CoV-2 antibodies to accurately forecast future COVID-19 surges. CalScope will begin collecting data for Wave 2 in Fall 2021 and Wave 3 will occur in the first half of 2022.
Supplementary Material
Acknowledgments
We thank Daniela Valenzuela, Evelyn Cubias, Mauricio Ollervides, Edgar Martinez, Kara Gionfriddo, Lourdes Mariana Ponte-Cordova, and Stefanie Medlin for their work in addressing participant questions and concerns. We thank Thomas Quarre and Siddarth Satish for support in designing and maintaining the CalScope website.
Disclaimer. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views or opinions of the California Department of Public Health or the California Health and Human Services Agency.
Financial support. This work was supported by the State of California and the Centers for Disease Control Epidemiology and Laboratory Capacity Grant.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Megha L Mehrotra, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Esther Lim, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Katherine Lamba, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Amanda Kamali, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Kristina W Lai, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Erika Meza, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Irvin Szeto, Infectious Diseases, Stanford University, School of Medicine, Palo Alto, California, USA.
Peter Robinson, Enable Biosciences, South San Francisco, California, USA.
Cheng-ting Tsai, Enable Biosciences, South San Francisco, California, USA.
David Gebhart, Enable Biosciences, South San Francisco, California, USA.
Noemi Fonseca, Enable Biosciences, South San Francisco, California, USA.
Andrew B Martin, Infectious Diseases, Stanford University, School of Medicine, Palo Alto, California, USA.
Catherine Ley, Infectious Diseases, Stanford University, School of Medicine, Palo Alto, California, USA.
Steve Scherf, Gauss Surgical, Menlo Park, California, USA.
James Watt, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
David Seftel, Enable Biosciences, South San Francisco, California, USA.
Julie Parsonnet, Infectious Diseases, Stanford University, School of Medicine, Palo Alto, California, USA.
Seema Jain, California Department of Public Health, Epidemiology, Surveillance and Modeling Section, COVID-19 Response, Richmond, California, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention . United States COVID-19 Cases and Deaths by State over Time. Available at: https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36. Accessed 4 October 2021.
- 2. California COVID Assessment Tool . Available at: https://calcat.covid19.ca.gov/cacovidmodels/. Accessed 4 October 2021.
- 3. Baden LR, El Sahly HM, Essink B, et al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gazit S, Shlezinger R, Perez G, et al. . The incidence of SARS-CoV-2 reinfection in persons with naturally acquired immunity with and without subsequent receipt of a single dose of BNT162b2 vaccine. Ann Intern Med 2022: DOI: 10.7326/M21-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. León TM. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis—California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep 2022; 71:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhuang C, Liu X, Chen Q, et al. . Protection duration of COVID-19 vaccines: waning effectiveness and future perspective. Front Microbiol 2022; 13:828806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamba K, Bradley H, Shioda K, et al. . SARS-CoV-2 cumulative incidence and period seroprevalence: results from a statewide population-based serosurvey in California. Open Forum Infect Dis 2021; 8:ofab379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robertson MM, Kulkarni SG, Rane M, et al. . Cohort profile: a national, community-based prospective cohort study of SARS-CoV-2 pandemic outcomes in the USA—the CHASING COVID Cohort study. BMJ Open 2021; 11:e048778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegler AJ, Sullivan PS, Sanchez T, et al. . Protocol for a national probability survey using home specimen collection methods to assess prevalence and incidence of SARS-CoV-2 infection and antibody response. Ann Epidemiol 2020; 49:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan PS, Siegler AJ, Shioda K, et al. . Severe acute respiratory syndrome coronavirus 2 cumulative incidence, United States, August 2020–December 2020. Clin Infect Dis 2022; 74:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bajema KL, Wiegand RE, Cuffe K, et al. . Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med 2020; 181:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havers FP, Reed C, Lim T, et al. . Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med 2020: DOI: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- 14. Ng DL, Goldgof GM, Shy BR, et al. . SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun 2020; 11:4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anand S, Montez-Rath M, Han J, et al. . Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet 2020; 396:1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bendavid E, Mulaney B, Sood N, et al. . COVID-19 antibody seroprevalence in Santa Clara County, California. Int J Epidemiol 2021; 50:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sood N, Simon P, Ebner P, et al. . Seroprevalence of SARS-CoV-2–specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA 2020; 323:2425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearl J, Bareinboim E. Transportability of Causal and Statistical Relations: A Formal Approach, 2011 IEEE 11th International Conference on Data Mining Workshops, Vancouver, BC, Canada, December 11, 2011. [Google Scholar]
- 19. U.S. Census Bureau; American Community Survey, 2015 American Community Survey 1-Year Estimates . Available at: data.census.gov. Accessed 6 October 2021.
- 20. Karp DG, Danh K, Espinoza NF, Seftel D, Robinson PV, Tsai C. A serological assay to detect SARS-CoV-2 antibodies in at-home collected finger-prick dried blood spots. Sci Rep 2020; 10:20188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pearl J, Bareinboim E. External validity: from do-calculus to transportability across populations. Statistical Science 2014; 29:579–95. [Google Scholar]
- 22. Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: the ACTG 320 trial. Am J Epidemiol 2010; 172:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen ML. Compound treatments, transportability, and the structural causal model: the power and simplicity of causal graphs. Epidemiology 2011; 22:378–81. [DOI] [PubMed] [Google Scholar]
- 24. Essential Critical Infrastructure Workers . State of California. Available at: https://covid19.ca.gov/essential-workforce/. Accessed 6 October 2021.
- 25. Statewide Database . 2020 General Election Precinct Data. Available at: www.statewidedatabase.org/d10/g20.html. Accessed 5 August 2021.
- 26. Public Health Alliance of Southern California . The California Healthy Places Index (HPI). Available at: www.healthyplaces.org. Accessed 5 August 2021.
- 27. van der Laan MJ, Rose S. Targeted Learning. New York, NY: Springer New York, 2011. [Google Scholar]
- 28. Bacharach M. Estimating nonnegative matrices from marginal data. Int Econ Rev 1965; 6:294–310. [Google Scholar]
- 29. Coyle J, Hejazi N, Malenica I, et al. Pipelines for Machine Learning and Super Learning . Available at: https://tlverse.org/sl3/index.html. Accessed 5 October 2021.
- 30. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2018https://www.R-project.org/. [Google Scholar]
- 31. California Department of Public Health. Blueprint for a Safer Economy . Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/COVID19CountyMonitoringOverview.aspx. Accessed 5 October 2021.
- 32. Couture A, Lyons BC, Mehrotra ML, et al. . Severe acute respiratory syndrome coronavirus 2 seroprevalence and reported coronavirus disease 2019 cases in US children, August 2020–May 2021. Open Forum Infect Dis 2022; 9:ofac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention . COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker. Accessed 26 March 2021.
- 34. Peluso MJ, Takahashi S, Hakim J, et al. . SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci Adv 2021; 7:eabh3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilbert PB, Montefiori DC, McDermott AB, et al. . Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Beltran WF, St. Denis KJ, Hoelzemer A, et al. . mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022; 185: 457–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. . Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khoury DS, Cromer D, Reynaldi A, et al. . Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 39. Cromer D, Steain M, Reynaldi A, et al. . Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022; 3:e52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cassaniti I, Percivalle E, Bergami F, et al. . SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clinical Microbiol Infect 2021; 27:1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Corbett KS, Nason MC, Flach B, et al. . Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science 2021; 373:eabj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harvey RA, Rassen JA, Kabelac CA, et al. . Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Internal Medicine 2021; 181: 672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Addetia A, Crawford KHD, Dingens A, et al. . Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol 2020; 58:e02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.