Abstract
Background
Evidence from the United States and United Kingdom suggests that ethnic minority populations are at an increased risk for developing severe coronavirus disease 2019 (COVID-19); however, data from other West-European countries are scarce.
Methods
We analyzed data from 1439 patients admitted between February 2020 and January 2021 to 4 main hospitals in Amsterdam and Almere, the Netherlands. Differences in the risk for hospitalization were assessed by comparing demographics to the general population. Using a population-based cohort as reference, we determined differences in the association between comorbidities and COVID-19 hospitalization. Outcomes after hospitalization were analyzed using Cox regression.
Results
The hospitalization risk was higher in all ethnic minority groups than in those of Dutch origin, with age-adjusted odds ratios ranging from 2.2 (95% confidence interval [CI], 1.7–2.6) in Moroccans to 4.5 (95% CI, 3.2–6.0) in Ghanaians. Hypertension and diabetes were similarly associated with COVID-19 hospitalization. For all other comorbidities, we found differential associations. Intensive care unit admission and mortality during 21-day follow-up after hospitalization was comparable between ethnicities.
Conclusions
The risk of COVID-19 hospitalization was higher in all ethnic minority groups compared to the Dutch, but the risk of adverse outcomes after hospitalization was similar. Our results suggest that these inequalities may in part be attributable to comorbidities that can be prevented by targeted public health prevention measures. More work is needed to gain insight into the role of other potential factors such as social determinants of health, which might have contributed to the ethnic inequalities in COVID-19 hospitalization.
Keywords: COVID-19, ethnic inequalities, migrants health, hospitalization, outcomes, The Netherlands
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]). Although the majority of COVID-19 patients are asymptomatic or present with mild respiratory symptoms, part of them develop a severe viral pneumonia leading to acute respiratory distress syndrome, hospital and intensive care unit (ICU) admission, and death [1]. Analyses from United States- and United Kingdom (UK)-based registries indicate that there is a relative overrepresentation of ethnic minorities among patients diagnosed with SARS-CoV-2 who require admission to hospital wards and ICUs [2–5]. The observed increased risk of severe COVID-19 disease in ethnic minorities might be related to underlying comorbid medical conditions and social determinants of health, which have been shown to vary across ethnic groups [6–8]. For instance, we have recently shown that cardiovascular risk factors, which have been shown to be differentially distributed across ethnic groups, are important predictors for adverse outcomes in patients hospitalized with COVID-19 [9]. Health inequalities in access to or quality of healthcare provide additional explanations for the increased risk of severe COVID-19 disease in ethnic minorities [10, 11].
At present, it is unknown whether the same observations translate to other Western European countries, where factors such as comorbidities or access to healthcare that may affect the severity of the COVID-19 disease could be differentially distributed between ethnic groups. In addition, it is unknown whether the impact of comorbidities on disease severity might differ between ethnic groups. We aimed to understand how the severity of COVID-19 disease differs between ethnic groups in a population living in Amsterdam and its neighboring city Almere, the Netherlands. We assessed ethnic inequalities in (1) the risk of COVID-19 hospitalization, (2) the relation between comorbidities and hospitalization, and (3) adverse clinical outcomes after hospitalization.
METHODS
Study Population
CovidPredict is a Dutch multicenter initiative to collect clinical data of patients hospitalized with COVID-19 [12]. For this analysis, we included data from Amsterdam UMC, Flevo Hospital, BovenIJ and OLVG hospitals, which together form all the general and university hospitals in Amsterdam and Almere. All admitted patients with either a confirmed positive SARS-CoV-2 polymerase chain reaction or high clinical suspicion for COVID-19, based on clinical presentation and computed tomography imaging of the chest (COVID-19 Reporting and Data System [CO-RADS] score 4 or 5), were included [13]. Patients transferred from another hospital or who were readmitted after a previous COVID-19 infection were excluded. Detailed information about the medical history, presentation at the hospital, and the clinical course after hospitalization were extracted from the electronic health record, as described elsewhere [14]. Comorbidities in the CovidPredict database were assessed using medical history as reported in the hospital discharge letter and the home medication list. Ethnicity was estimated based on country of birth. In the Netherlands, country of birth of the participant is a generally accepted and widely used indicator of ethnicity [15]. For patients for whom country of birth was unknown, surname and/or physician-reported information available in the electronic health record was used to indicate the ethnic background. Patients were classified as either from Dutch origin or migrants from Ghana, Suriname, Turkey, Morocco, or other non-European origin. These groups were chosen because they are among the largest ethnic groups residing in Amsterdam. Patients from Surinamese descent were further classified into South-Asian Surinamese and African Surinamese and other, using a previously validated list based on surname [16].
To assess differences in COVID-19 hospitalization between ethnic group, we used population tables from Amsterdam and Almere generated by Statistics Netherlands to serve as denominator [17]. Finally, we used data from participants from the HELIUS (Healthy Life in an Urban Setting) study as control population [15]. The aims and design of the HELIUS study have been described elsewhere [15]. In short, HELIUS is a population-based cohort study, that aims to unravel the causes of inequalities in health between ethnic groups. It contains the data of ∼24 000 individuals, aged between 18 and 70 years at baseline (2011–2015; physical exam and questionnaire), who were sampled randomly from the Amsterdam municipality register, stratified by ethnic background [18]. For these analyses, participants of Dutch, Surinamese, Turkish, Moroccan, and Ghanaian ethnic origin were included. In HELIUS, participants of Surinamese ethnic origin were further classified into South-Asian Surinamese, African Surinamese, and other based on self-report. To compare cases to controls, in both the CovidPredict and the HELIUS, we defined for the HELIUS cohort history of hypertension as the use of blood pressure lowering medication. Chronic pulmonary disease was defined as the use of either inhaled corticosteroids or bronchodilating drugs. Obesity was defined as a body mass index larger than 30 kg/m2. Diabetes was defined based on medication use, fasting glucose levels above 7 mmol/L, or a self-reported history of diabetes. Chronic kidney disease was defined as either albuminuria (urinary albumin/creatinine ratio >30 mg/g) or an estimated glomerular filtration rate of less than or equal to 60 mL/min/1.73 m2 based on the CKD-EPI formula [19]. Current or previous malignancy was based on self-reported history obtained from the questionnaire.
Study Outcomes
First, we analyzed ethnic differences in the odds for COVID-19-related hospitalization using the general population of Amsterdam and Almere as the denominator. Second, we performed a case-control study to evaluate the relationship between comorbidities and the risk for hospitalization per ethnic group. Third, we investigated ethnic differences in the risk for ICU admission and mortality after hospital admission. For the latter, we performed a survival analysis starting from the hospitalization. We used (1) mortality as primary outcome and (2) the composite outcome of ICU admission and mortality as secondary outcome. Mortality was defined as either hospital mortality or discharge for palliative care within 21 days of hospitalization. Patients were considered event-free for the follow-up period of 21 days, if they were discharged to home or a nursing facility.
Statistical Analysis
We determined age-adjusted odds ratios (ORs) for each ethnicity for COVID-19 hospitalization by comparing the number of hospitalized COVID-19 patients to the general population of Amsterdam and Almere. The ORs were determined by calculating the ratio between the odds for hospitalization in each ethnic group and the odds for the Dutch group, which was taken as reference. Age adjustment was performed using the Cochran-Mantel-Haenszel method; by partitioning age into the following: <40; 40–50; 51–60; 61–70; and >70. We additionally performed a descriptive analysis of ethnic differences in medical history and presentation at the emergency department.
To assess ethnic differences in comorbid conditions between hospitalized COVID-19 patients and the risk of hospitalization, we matched COVID-19 patients from the COVID-predict database to HELIUS participants with complete data on comorbidities matched for ethnicity and a nearest neighbor match for age and sex with a 1:2 ratio between cases and controls. Using a logistic regression model with each comorbidity, ethnicity, and their interaction term, we assessed whether there were ethnic differences in the relation between hypertension, diabetes, obesity, chronic kidney disease, history of chronic pulmonary disease and malignancy, and hospital admission. We then calculated the OR for hospitalization for each comorbidity in the complete cohort and in groups stratified by ethnicity.
For the analysis of ethnic differences in outcomes during 21 days after hospitalization (third research question), we performed a survival analysis using Kaplan-Meier and a Cox-regression model with correction for age, sex, and time in the pandemic. The latter was included to account for treatment and presentation differences during the ongoing pandemic. We performed a sensitivity analysis using only patients admitted before (first wave) and after September 1, 2020 (second wave), respectively. All statistical analyses were conducted with R version 3.6.1 (Vienna, Austria).
Patient Consent Statement
For CovidPredict, we used data collected from the electronic health record. A waiver for the use of hospital record data was obtained from the Medical Ethical Committees of the participating centers and patients were given the opportunity to opt out. The HELIUS study protocols were approved by the Ethical Review Board of Amsterdam UMC, location AMC (NL32251.018.10). All participants provided written informed consent for participation in the HELIUS study.
RESULTS
Demographics and Patient Characteristics
We included 1439 patients, admitted between February 18, 2020 and January 30, 2021, with 826 patients (57%) admitted during the first wave (ie, before September 1, 2020). An overview of the inclusion stratified by hospital is given in Supplementary Table 1. Patients for whom data on ethnicity were missing (238 patients) or who were classified as “other” (261) were excluded. The overall median age was 64 (interquartile range [IQR], 54–57) years with Ghanaian and Moroccan patients being younger (median age 60 years) and patients from Dutch origin being older (median age 68 years). Overall, 42% of admitted COVID-19 patients were female with the highest proportion among African-Surinamese (50%) and the lowest proportion in Moroccans (38%).
Patients of Ghanaian descent were admitted more frequently during the first wave of the pandemic; with 79% of all Ghanaian patients admitted during the first wave. The time between the onset of symptoms and presentation in the hospital was similar across all ethnic groups; with a median of 7 (IQR, 5–11) days for the entire group. C-reactive protein was highest in the Turkish (105.7; IQR, 52.7–135.0) and the Moroccan group (98.1; IQR, 50.5–170.4) compared to the overall median of 83 (IQR, 41.5–142.9). Respiratory rate was the highest in the Ghanaian group with a median of 25.0 (standard deviation [SD] = 6.3), whereas the overall respiratory rate was 24.0 (SD = 7.0). An overview of the baseline characteristics is given in Table 1.
Table 1.
Patient Characteristics of Hospitalized COVID-19 Patients; Stratified by Ethnic Origin
| Characteristics | Dutch | South-Asian Surinamese | African Surinamese | Ghanaian | Turkish | Moroccan | Other |
|---|---|---|---|---|---|---|---|
| n | 763 | 62 | 122 | 43 | 76 | 112 | 261 |
| Age, median [IQR] | 68.0 [58.0–77.0] | 61.0 [50.5–67.0] | 63.0 [55.0–70.0] | 60.0 [51.5–63.5] | 62.0 [52.0–72.0] | 60.0 [51.8–70.0] | 59.0 [47.0–68.0] |
| Women, n (%) | 333 (43.6) | 27 (43.5) | 61 (50.0) | 17 (39.5) | 32 (42.1) | 40 (35.7) | 100 (38.3) |
| Hypertension, n (%) | 301 (39.8) | 35 (56.5) | 73 (60.3) | 32 (76.2) | 47 (61.8) | 34 (30.4) | 93 (36.0) |
| Asthma or chronic pulmonary disease, n (%) | 194 (25.5) | 15 (24.2) | 32 (26.2) | 3 (7.0) | 24 (31.6) | 12 (10.7) | 48 (18.4) |
| Chronic kidney disease, n (%) | 78 (10.3) | 8 (12.9) | 16 (13.1) | 13 (30.2) | 6 (7.9) | 6 (5.4) | 26 (10.0) |
| Diabetes, n (%) | 177 (23.2) | 31 (50.0) | 44 (36.1) | 20 (46.5) | 39 (51.3) | 46 (41.1) | 77 (29.5) |
| Malignancy or chronic hematological disorder, n (%) | 104 (13.6) | 3 (4.8) | 13 (10.7) | 7 (16.3) | 8 (10.5) | 5 (45) | 23 (8.8) |
| Chronic cardiac disease, n (%) | 244 (32.1) | 18 (29.0) | 20 (16.4) | 9 (20.9) | 19 (25.0) | 18 (16.1) | 47 (18.1) |
| Obesity, n (%) | 181 (27.3) | 21 (38.2) | 60 (53.6) | 13 (35.1) | 32 (53.3) | 28 (33.7) | 65 (29.4) |
| Body mass index, mean (SD) | 27.9 (5.5) | 29.5 (6.5) | 30.3 (6.2) | 30.5 (5.9) | 31.8 (7.0) | 28.8 (5.1) | 28.1 (5.5) |
| Smoking, n (%) | 50 (8.4) | 2 (3.8) | 8 (9.1) | 3 (8.3) | 2 (3.3) | 1 (1.2) | 12 (6.0) |
| Respiratory rate, mean (SD) | 22.5 (6.3) | 25.0 (6.5) | 24.6 (7.2) | 25.7 (8.4) | 25.0 (6.3) | 24.8 (6.2) | 26.8 (8.0) |
| CRP (mg/L), median (IQR) | 75.0 [40.0–135.5] | 87.0 [46.2–122.8] | 95.7 [54.7–169.0] | 67.9 [34.7–180.1] | 105.7 [52.7–135.0] | 98.1 [50.5–170.4] | 86.0 [36.1–136.1] |
| WBC (109/L), median (IQR) | 6.5 [5.0–9.0] | 6.3 [5.6–8.5] | 6.6 [4.9–8.7] | 7.1 [5.8–9.1] | 6.1 [4.5–8.2] | 6.5 [5.3–8.6] | 6.4 [5.0–8.6] |
| ICU admission, n (%) | 121 (15.9) | 13 (21.0) | 21 (17.2) | 10 (23.3) | 16 (21.1) | 25 (22.3) | 58 (2.2) |
| Second wave of the pandemic, n (%) | 322 (42.2) | 34 (54.8) | 64 (52.5) | 9 (20.9) | 27 (35.5) | 46 (41.1) | 111 (42.5) |
| Time between hospitalization and March 2020 (days), median [IQR]) | 51.0 [28.0–246.0] | 214.0 [36.8–258.8] | 197.0 [41.2–242.8] | 34.0 [26.0–47.0] | 63.5 [29.0–234.0] | 66.5 [38.0–229.0] | 63.0 [33.0–239.0] |
| Time between onset of symptoms and admission (days), median (IQR) | 7.0 [5.0–10.0] | 8.0 [6.0–11.0] | 7.0 [5.0–11.2] | 7.0 [5.0–14.0] | 7.0 [5.0–10.0] | 8.0 [7.0–12.0] | 8.0 [6.0–11.5] |
Abbreviations: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; IQR, interquartile range; SD, standard deviation; WBC, white blood cells.
Risk of Hospitalization for Different Ethnicities
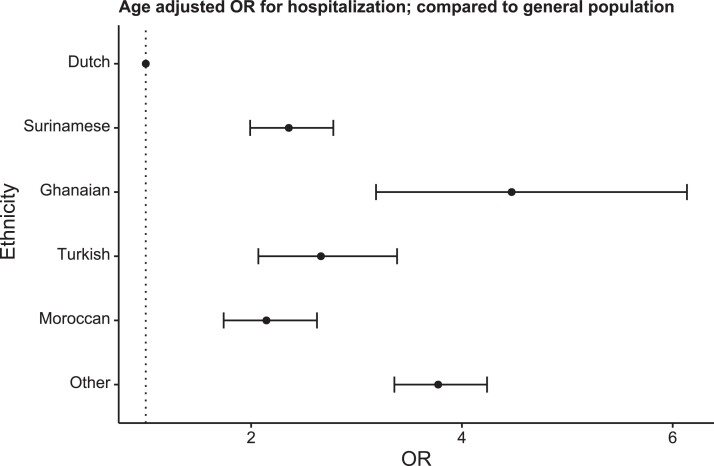
The risk for COVID-19 hospitalization among the population was significantly increased in all ethnic minority groups compared with Dutch (Figure 1). Ghanaians had the highest odds for COVID-19 hospitalization, with an age-adjusted OR of 4.5(95% CI, 3.2–6.0), whereas for all other ethnic minority groups the odds ranged between 2.1 and 3.7 (Supplementary Table 2).
Figure 1.
Ethnic differences in the risk for coronavirus disease 2019 (COVID-19) hospitalization in the Amsterdam area population. Dots indicate Cochran-Mantel-Haenszel age-adjusted odds ratio (OR) for COVID-19-related hospitalization compared with the Dutch population; bars, 95% confidence interval.
Differences in Comorbidities Between Hospitalized Patients and Controls
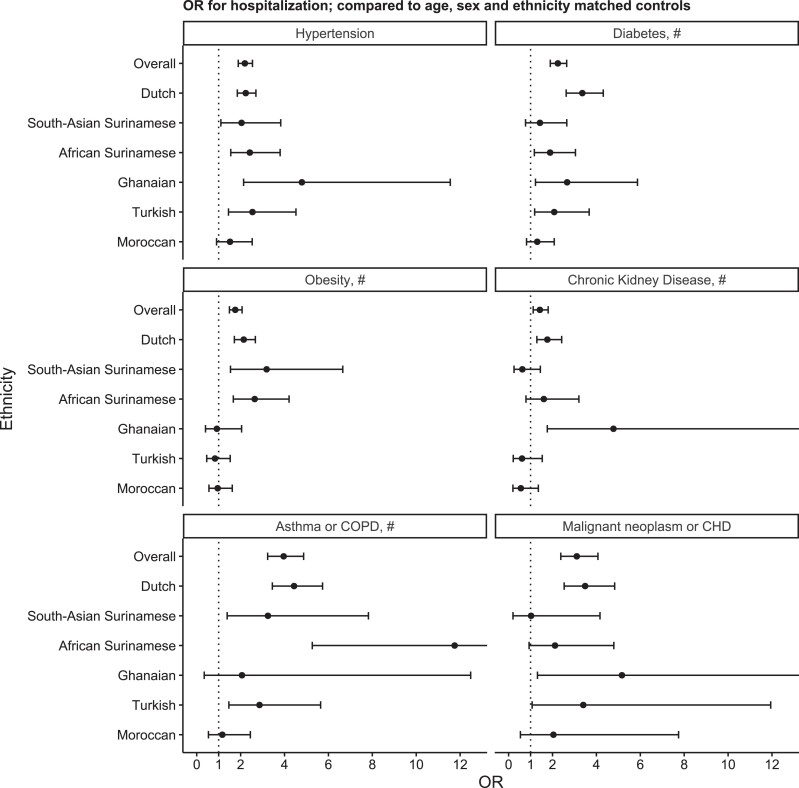
Overall, the prevalence of comorbidities was significantly higher in hospitalized patients compared to their matched controls. The association between each comorbidity and hospital admission differed significantly between ethnic groups (Figure 2). We observed the highest OR of 3.4 (95% CI, 2.6–4.3) between diabetes and COVID-19 hospitalization in the Dutch origin group. In South-Asian Surinamese, who had the highest prevalence of diabetes among controls (41.1%), we did not observe an increased OR for diabetes in hospitalized patients. Obesity, on the other hand, was significantly associated with hospitalization in the South-Asian Surinamese group, with an OR of 3.2 (95% CI, 1.5–6.6). For African Surinamese, ORs were similar to those in the Dutch origin group, except for asthma and chronic obstructive pulmonary disease (COPD). In the Ghanaian subgroup, we found that hypertension and chronic kidney disease were associated with hospitalization, with an OR of 4.8 (95% CI, 2.1–11.6) and 4.8 (95% CI, 1.8–14.0), respectively. In the Turkish group, we observed a high prevalence of obesity among both cases (53.3%) and controls (57.9%), leading to an OR of 0.8 (95% CI, 0.5–1.5). In Moroccan participants, we found the lowest OR for diabetes, chronic kidney disease, and asthma/COPD compared to the other groups (Supplementary Table 3).
Figure 2.
The relation between comorbidities and risk for coronavirus disease 2019 hospitalization overall and by ethnic group. Dots indicate odds ratio (OR) ratio; bars 95% confidence interval (CI), chronic hematologic disease (CHD). # indicates whether a significant interaction (P < 0.05) with ethnicity was observed. For chronic kidney disease, OR for Ghanaian was 8.1 (95% CI, 1.8–14.0). For asthma or chronic obstructive pulmonary disease (COPD), OR for African Surinamese was 11.8 (95% CI, 53–30.2). For malignant neoplasm or CHD, OR for Ghanaian was 5.2 (95% CI, 1.3–26.5).
Ethnic Differences in Outcomes After Hospitalization
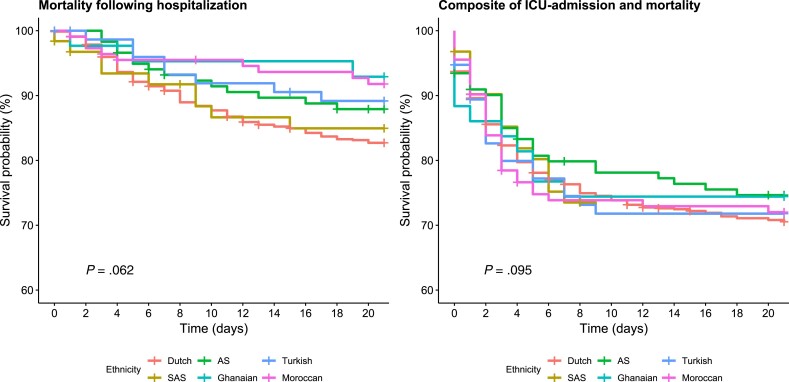
We included 1178 patients in the survival analysis after exclusion of patients with other or missing ethnicity. During 21-day follow-up, 169 (14.3%) patients died. Fifty-eight (4.9%) of the patients were transferred to another hospital and outcome data were missing in 16 patients (1.4%). A total of 206 (17.5%) patients were admitted to the ICU. Figure 3 shows the Kaplan-Meier curves stratified by ethnicity, without adjustment for confounders. Mortality was highest in patients from Dutch origin (17.3%), South-Asian Surinamese (15.1%), and African Surinamese (12.1%) at 21 days after hospitalization (Table 2). After adjustment for sex, age, and time in the pandemic, there were no significant differences between the ethnic groups (P = 0.66). The incidence rate for the composite outcome of ICU admission and mortality ranged from 25.4% to 29.5%. In both unadjusted (P = 0.95) and adjusted (P = 0.99) analyses there were no statistically significant differences between the ethnic groups. These results were similar during the first and second wave (Supplementary Table 4).
Figure 3.
Kaplan-Meier curves for primary and secondary outcome after hospitalization without adjustment for confounders. P values show log-rank test for ethnic difference in mortality and the composite outcome of intensive care unit (ICU) admission and mortality. AS, African Surinamese; SAS, South-Asian Surinamese.
Table 2.
Ethnic Differences in Outcomes After Hospitalizationa
| Mortality | ||||
|---|---|---|---|---|
| Ethnic Origin | Event Rate | 95% CI | HR | 95% CI |
| Dutch | 17.3 | 14.5–20.0 | 1.00 | |
| South-Asian Surinamese | 15.1 | 5.5–23.7 | 1.55 | 0.78–3.10 |
| African Surinamese | 12.1 | 5.9–17.8 | 1.00 | 0.57–1.76 |
| Ghanaian | 7.1 | 0.0–14.5 | 0.77 | 0.24–2.45 |
| Turkish | 10.8 | 3.5–17.6 | 0.86 | 0.42–1.76 |
| Moroccan | 8.2 | 2.9–13.2 | 0.69 | 0.35–1.38 |
| Composite of ICU-Admission Mortality | ||||
| Dutch | 29.5 | 26.1–32.7 | 1.00 | |
| South-Asian Surinamese | 28.2 | 15.9–38.7 | 1.13 | 0.69–1.87 |
| African Surinamese | 25.4 | 17.1–32.8 | 0.97 | 0.66–1.42 |
| Ghanaian | 25.6 | 11.3–37.5 | 1.06 | 0.57–1.95 |
| Turkish | 28.2 | 17.2–37.7 | 1.09 | 0.69–1.71 |
| Moroccan | 28.0 | 19.1–35.9 | 1.09 | 0.75–1.60 |
Abbreviations; CI, confidence interval; ICU, intensive care unit; HR, hazard ratio.
Event rate denotes estimated event rate from Kaplan-Meier model. HR shows age and sex adjusted hazard ratio derived from Cox-regression model.
DISCUSSION
Our analysis shows that the risk of COVID-19 hospitalization per population is higher in all ethnic minority groups than in the Dutch host population, but the risk of ICU admission and hospital mortality after COVID-19 hospitalization was not different. The case-control analyses show differential associations between comorbid conditions and COVID-19 hospitalization between ethnic groups.
The relative high risk of COVID-19 hospitalization among ethnic minority groups in our study is consistent with the findings from the UK and the United States, and it corroborates with the higher rates of SARS-CoV-2 infections diagnosed among these populations [20, 21]. The relatively high rates of COVID-19 hospitalization among ethnic minority groups infected with COVID-19 have been attributed to numerous factors including a higher infection rate in these groups. A study among a subsample of respondents of the HELIUS study has confirmed this hypothesis for the population in Amsterdam as well [21].
In addition to the higher incidence of infections, an increased risk of severe COVID-19 disease in ethnic minority groups might explain their higher risk of hospital admission, potentially driven by high burden of pre-existing comorbidities [22–24], poor access to health education in prevention measures, and delay in seeking healthcare potentially due to several factors such as language barriers, misinformation, and lack of trust in public officials [25, 26]. Evidence indicates that the risk of COVID-19 hospitalization is higher in individuals with pre-existing comorbidities than those without comorbidities [27, 28]. In our study, we found that the prevalence of comorbid conditions was significantly higher in COVID-19 hospitalized patients compared to matched controls from the general population. Studies in the Netherlands have consistently found higher rates of comorbid conditions such as diabetes, hypertension, and chronic kidney disease among ethnic minority groups compared to the population of Dutch origin [29–31]. Data from the HELIUS study, for example, show that the rate of diabetes is 3 to 12 times higher, and the rate of chronic kidney disease is 1.5 to 2.6 times higher in different ethnic minority groups compared to the Dutch host population [30, 31]. These high rates of comorbidity among ethnic minority groups might have contributed to their relatively high rates of COVID-19 hospitalizations. However, the impact of the pre-existing comorbid conditions on COVID-19 hospitalization seems to vary between the ethnic groups. We observed important ethnic disparities in the associations between cormorbid conditions and COVID-19 hospitalization in the population in our study. For instance, obesity was a strong predictor for COVID-19 hospitalization among the Dutch, African Surinamese, and South-Asian Surinamese origin population, but not among Moroccans, Turkish, and Ghanaian ethnic groups, whereas chronic kidney disease was a strong predictor of hospitalization in Dutch and Ghanaians only. These differential associations may be related to the differences in the distribution of chronic diseases among these population. For example, obesity was highly prevalent in Moroccans, Turkish, and Ghanaians in both patients with COVID-19 and patients without COVID-19 compared with other groups. Our current finding corroborates with earlier study findings in the UK where the association of obesity with an elevated risk of in-hospital COVID-19 outcomes was strongest in African Caribbean compared with European and South Asian ethnicities [32]. These important ethnic disparities in these associations seem to suggest that pre-existing comorbidities may have a different impact on COVID-19-related complications differently among ethnic groups. Lifestyles and social determinants of health such as socioeconomic status, access to preventive services and healthcare, healthy food, and environment that shape pre-existing chronic conditions vary among ethnic minority groups, which may underline the differential associations between the ethnic groups [33, 34].
It is well established that ethnic minority groups have been overrepresented in COVID-19-related ICU admissions in the UK and United States. In the UK’s Intensive Care National Audit and Research Centre report, approximately one third of critically ill COVID-19 patients during the early phase of the pandemic were from ethnic minority background despite them representing approximately 14% of the UK population [35]. Similarly, in the Netherlands, the overrepresentation of ethnic minority groups in hospitals including the ICU has also been well reported in a previous study and in the Dutch media [36]. Despite the well publicized overrepresentation of ethnic minority groups in ICU, the evidence in the literature regarding this remains inconsistent [20]. In our study, the risk of ICU admission and mortality after hospitalization was similar between the ethnic groups. Our finding is consistent with studies that found no ethnic differences in the risk of ICU admission [2, 37], but it contrasts those that found higher risk of ICU admission among ethnic minority groups [38, 39]. Similarly, our finding of the lack of increased risk of deaths among ethnic minority groups after hospital admission with COVID-19 is also consistent with earlier studies [2, 37], although not all [40]. The lack of increased risk of death after COVID-19 hospitalization seems to suggest that the higher mortality observed in ethnic minority groups in the Netherlands is not as a result of a poorer prognosis after hospitalization, but rather a higher chance of being hospitalized possibly due to a higher incidence of infections and a higher risk of serious illness from COVID-19 infection [20, 41]. Our current findings suggest that the increased risk of severe COVID-19 disease may in part be attributable to comorbid conditions that can be prevented by targeted health promotion measures and healthcare interventions that aim at mitigating ethnic inequalities in these conditions.
Strengths and Limitations
Current data on ethnic minority groups are mainly based on major ethnic groups (1) in the United States such as African Americans and Hispanic Americans and (2) in the UK such as African Caribbean and South Asians [5]. Data on major ethnic groups from the Mediterranean, including individuals of Turkish and Moroccan descent, are limited. Our study therefore provides new insight into multiethnic populations in Europe. In addition, our study combined HELIUS cohort data with hospital data to shed light on pre-existing comorbidities and their associations with COVID-19 hospitalization among various ethnic groups. Our data were limited to hospitals in greater Amsterdam catchment areas, and therefore they might not be representative of multiethnic communities living in the Netherlands or elsewhere in Europe. Nevertheless, our current studies encompass the largest ethnic minority groups in Europe, and they are mainly concentrated in cities, and therefore our current findings are likely to be representative of these populations. Furthermore, data on ethnicity of the hospitalized patients were based on country of birth, surname, and physician-reported information available in the electronic health record, which could have led to misclassification. However, surveillance data on COVID hospitalization from the regional public health service [42] during the first wave showed similar results compared with the data derived from the hospital databases in our study. Moreover, some of the measures such as previous malignancy status were based on self-reported data. Finally, although combining HELIUS with hospital data was a key strength of our study, the sample sizes of the subgroups were still relatively small, making it difficult to assess the interaction between ethnicity and comorbidities. Despite these limitations, our study provides new comprehensive data on inequalities in COVID-19 hospitalization and prognosis among major ethnic minority groups in Europe in whom data are currently lacking.
CONCLUSIONS
In conclusion, our study findings indicate that the risk of COVID-19 hospitalization is higher in ethnic minority groups than in the population of Dutch origin. Our findings suggest that part of the ethnic inequalities in hospitalization may be attributable to comorbid conditions that can be prevented by targeted public health prevention measures and healthcare interventions that are aimed at mitigating the high burden of these conditions in ethnic minority groups. However, we did not find any indications to suggest an impaired quality of care for ethnic minority groups once hospitalized, because the risk of ICU admission and mortality in hospitalized patients was similar between the ethnic groups.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank the CovidPredict consortium (www.covidpredict.org) for their efforts in providing the patient data.
Author contributions. D. C., K. S., M. P., B.-J. H. v. d. B., and C. A. conceptualized and designed the study. D. C., L. C., K. B., M. B., N. B., R. A. D., P.E., H. G., and M. t. W. contributed substantially to the acquisition of data. D. C. and L. C. performed the data analysis. D. C., K. S., V. H., B.-J. H. v. d. B., and C. A. drafted the manuscript. K.B., M. B., N. B., R. A. D., P.E., H. G., M. t. W., and M. P. critically revised the manuscript. All authors provided final approval of the version to be submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support. This work was funded by ZonMw (10430022010002 and 10430012010003). The HELIUS study is conducted by Amsterdam UMC, location Academic Medical Center and the Public Health Service of Amsterdam. Both organizations provided core support for HELIUS. The HELIUS study is also funded by the Dutch Heart Foundation (2010 T084), ZonMw (200500003), the European Union (FP-7) (278901), and the European Fund for the Integration of non-EU immigrants (EIF) (2013EIF013).
Potential conflicts of interests. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Didier Collard, Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; Internal Medicine, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands.
Karien Stronks, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Vanessa Harris, Department of Infectious Diseases, Amsterdam Institute for Infection and Immunity, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Global Health, Amsterdam Institute of Global Health and Development, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Liza Coyer, Department of Infectious Diseases, Amsterdam Institute for Infection and Immunity, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Infectious Diseases, Public Health Service of Amsterdam, Amsterdam, the Netherlands.
Kees Brinkman, Internal Medicine, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands.
Martijn Beudel, Department of Neurology, Amsterdam Neuroscience Institute, Amsterdam University Medical Center, Amsterdam, the Netherlands.
Nejma Bokhizzou, Internal Medicine, BovenIJ Hospital, Amsterdam, the Netherlands.
Renee A Douma, Internal Medicine, Flevo Hospital, Almere, the Netherlands.
Paul Elbers, Department of Intensive Care, Amsterdam Medical Data Science, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Henrike Galenkamp, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Marije ten Wolde, Internal Medicine, Flevo Hospital, Almere, the Netherlands.
Maria Prins, Department of Infectious Diseases, Amsterdam Institute for Infection and Immunity, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Infectious Diseases, Public Health Service of Amsterdam, Amsterdam, the Netherlands.
Bert Jan H van den Born, Department of Vascular Medicine, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Charles Agyemang, Department of Public and Occupational Health, Amsterdam Public Health Research Institute, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
References
- 1. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogedegbe G, Ravenell J, Adhikari S, et al. . Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open 2020; 3:e2026881. 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nafilyan V, Islam N, Mathur R, et al. . Ethnic differences in COVID-19 mortality during the first two waves of the coronavirus pandemic: a nationwide cohort study of 29 million adults in England. Eur J Epidemiol 2021; 36:605–17. 10.1007/s10654-021-00765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mathur R, Rentsch CT, Morton CE, et al. . Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet 2021; 397:1711–24. 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agyemang C, Richters A, Jolani S, et al. . Ethnic minority status as social determinant for COVID-19 infection, hospitalisation, severity, ICU admission and deaths in the early phase of the pandemic: a meta-analysis. BMJ Glob Heal 2021; 6:1–14. 10.1136/bmjgh-2021-007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drefahl S, Wallace M, Mussino E, et al. . A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun 2020;11:5097. 10.1038/s41467-020-18926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhala N, Curry G, Martineau AR, et al. . Sharpening the global focus on ethnicity and race in the time of COVID-19. Lancet 2020; 395:1673–6. 10.1016/S0140-6736(20)31102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang S, Pierson E, Koh PW, et al. . Mobility network models of COVID-19 explain inequities and inform reopening. Nature 2021; 589:82–7. 10.1038/s41586-020-2923-3. [DOI] [PubMed] [Google Scholar]
- 9. Collard D, Nurmohamed NS, Kaiser Y, et al. . Cardiovascular risk factors and COVID-19 outcomes in hospitalised patients: a prospective cohort study. BMJ Open 2021; 11:1–7. 10.1136/bmjopen-2020-045482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burstrom B, Tao W. Social determinants of health and inequalities in COVID-19. Eur J Public Health 2020; 30:617–8. 10.1093/eurpub/ckaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open 2021; 4:1–12. 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. COVID-PREDICT Werkgroep . [Klinisch beloop van covid-19 in Nederland - Een overzicht van 2607 ziekenhuispatiënten uit de eerste golf]. Ned Tijdschr Geneeskd 2021; 165:D5085.33651497 [Google Scholar]
- 13. Prokop M, Everdingen WV, Rees Vellinga TV, et al. . CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19—definition and evaluation. Radiology 2020; 296:E97–104. 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peters EJG, Collard D, Assen SV, et al. . Outcomes of persons with COVID-19 in hospitals with and without standard treatment with (hydroxy)chloroquine. Clin Microbiol Infect 2020; 27:264–8. 10.1016/j.cmi.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stronks K, Kulu-Glasgow I, Agyemang C. The utility of ‘country of birth’ for the classification of ethnic groups in health research: the Dutch experience. Ethn Health 2009; 14:255–69. 10.1080/13557850802509206. [DOI] [PubMed] [Google Scholar]
- 16. Oudhof K, Harmsen C. De maatschappelijke situatie van Surinaamse bevolkingsgroepen in Nederland. Bevolkingstrends 2011: 46–60, Statistics Netherlands. Avalaible at: https://www.cbs.nl/nl-nl/achtergrond/2011/51/de-maatschappelijke-situatie-van-surinaamse-bevolkingsgroepen-in-nederland.
- 17. CBS Statline, Statistics Netherlands. Available at: https://opendata.cbs.nl/. Accessed 2021.
- 18. Snijder MB, Galenkamp H, Prins M, et al. . Cohort profile: the healthy life in an urban setting (HELIUS) study in Amsterdam, the Netherlands. BMJ Open 2017; 7:1–11. 10.1136/bmjopen-2017-017873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Workgroup . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3:4. [Google Scholar]
- 20. Sze S, Pan D, Nevill CR, et al. . Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine 2020; 29-30:100630. 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coyer L, Boyd A, Schinkel J, et al. . SARS-CoV-2 antibody prevalence and correlates of six ethnic groups living inAmsterdam, the Netherlands: a population-based cross-sectional study, June–October 2020 BMJ Open 2022;12:e052752. 10.1136/bmjopen-2021-052752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 2020; 8:e35. [PMC free article] [PubMed] [Google Scholar]
- 23. Yancy CW. COVID-19 and African Americans. JAMA 2020; 323:1891–2. 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 24. Wang B, Li R, Lu Z, et al. . Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020; 12:6049–57. 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egede LE, Walker RJ. Structural racism, social risk factors, and Covid-19—a dangerous convergence for Black Americans. N Engl J Med 2020; 383:e77. 10.1056/NEJMp2023616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bil JP, Zuure FR, Alvarez-del Arco D, et al. . Disparities in access to and use of HIV-related health services in the Netherlands by migrant status and sexual orientation: a cross-sectional study among people recently diagnosed with HIV infection. BMC Infect Dis 2019; 19:906. 10.1186/s12879-019-4477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gottlieb M, Sansom S, Frankenberger C, et al. . Clinical course and factors associated with hospitalization and critical illness among COVID-19 patients in Chicago, Illinois. Acad Emerg Med 2020; 27:963–73. 10.1111/acem.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrilli CM, Jones SA, Yang J, et al. . Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agyemang C, Snijder MB, Adjei DN, et al. . Ethnic disparities in CKD in the Netherlands: the healthy life in an urban setting (HELIUS) study. Am J Kidney Dis 2016; 67:391–9. 10.1053/j.ajkd.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 30. Snijder MB, Agyemang C, Peters RJ, et al. . Case finding and medical treatment of type 2 diabetes among different ethnic minority groups: the HELIUS study. J Diabetes Res 2017; 2017:1–8. 10.1155/2017/9896849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Agyemang C, Kieft S, Snijder MB, et al. . Hypertension control in a large multi-ethnic cohort in Amsterdam, The Netherlands: the HELIUS study. Int J Cardiol 2015; 183:180–9. 10.1016/j.ijcard.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 32. Yates T, Zaccardi F, Islam N, et al. . Obesity, ethnicity, and risk of critical care, mechanical ventilation, and mortality in patients admitted to hospital with COVID-19: analysis of the ISARIC CCP-UK cohort. Obesity 2021; 29:1223–30. 10.1002/oby.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yau A, Adams J, White M, et al. . Differences in diet quality and socioeconomic patterning of diet quality across ethnic groups: cross-sectional data from the HELIUS Dietary Patterns study. Eur J Clin Nutr 2020; 74:387–96. 10.1038/s41430-019-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agyemang C, Van Den BB. Non-communicable diseases in migrants: an expert review. J Travel Med 2019; 26:1–9. 10.1093/jtm/tay107. [DOI] [PubMed] [Google Scholar]
- 35. Intensive Care National Audit and Research Centre report on COVID-19 in critical care, April 30, 2020. Available at: https://www.icnarc.org/our-audit/audits/cmp/reports. Accessed 2021.
- 36. NOS . Corona-problemen bij migranten hadden eerder erkend moeten worden. Available at: https://nos.nl/artikel/2381750-corona-problemen-bij-migranten-hadden-eerder-erkend-moeten-worden]. Accessed 2021.
- 37. Kim L, Garg S, O’Halloran A, et al. . Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis 2021; 72:e206–14. 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Price-Haywood EG, Burton J, Fort D, et al. . Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020; 382:2534–43. 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Golestaneh L, Neugarten J, Fisher M, et al. . The association of race and COVID-19 mortality. EClinicalMedicine 2020; 25:100455. 10.1016/j.eclinm.2020.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harrison SL, Fazio-Eynullayeva E, Lane DA, et al. . Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med 2020; 17:e1003321. 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Visser M, Kunst A, Stoeldraijer L, Harmsen C. Sociaal-demografische verschillen in COVID-19-sterfte tijdens de eerste golf van de corona-epidemie. Statistics Netherlands, Stat Trends, 2021. Available at: https://www.cbs.nl/nl-nl/longread/statistische-trends/2021/sociaal-demografische-verschillen-in-covid-19-sterfte-tijdens-de-eerste-golf-van-de-corona-epidemie. Accessed in 2021.
- 42. Coyer L, Wynberg E, Buster M, et al. . Hospitalisation rates differed by city district and ethnicity during the first wave of COVID-19 in Amsterdam the Netherlands. BMC Public Health 2021; 21:1–24. 10.1186/s12889-021-11782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.