Abstract
Background
Waning antibody levels post-vaccination and the emergence of variants of concern (VOCs) capable of evading protective immunity have raised the need for booster vaccinations. However, which combination of coronavirus disease 2019 (COVID-19) vaccines offers the strongest immune response against the Omicron variant is unknown.
Methods
This randomized, participant-blinded, controlled trial assessed the reactogenicity and immunogenicity of different COVID-19 vaccine booster combinations. A total of 100 BNT162b2-vaccinated individuals were enrolled and randomized 1:1 to either homologous (BNT162b2 + BNT162b2 + BNT162b2; “BBB”) or heterologous messenger RNA (mRNA) (BNT162b2 + BNT162b2 + mRNA-1273; “BBM”) booster vaccine. The primary end point was the level of neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) wild-type and VOCs at day 28.
Results
A total of 51 participants were allocated to BBB and 49 to BBM; 50 and 48, respectively, were analyzed for safety and immunogenicity outcomes. At day 28 post-boost, mean SARS-CoV-2 spike antibody titers were lower with BBB (22 382 IU/mL; 95% confidence interval [CI], 18 210 to 27 517) vs BBM (29 751 IU/mL; 95% CI, 25 281 to 35 011; P = .034) as was the median level of neutralizing antibodies: BBB 99.0% (interquartile range [IQR], 97.9% to 99.3%) vs BBM 99.3% (IQR, 98.8% to 99.5%; P = .021). On subgroup analysis, significant higher mean spike antibody titer, median surrogate neutralizing antibody level against all VOCs, and live Omicron neutralization titer were observed only in older adults receiving BBM. Both vaccines were well tolerated.
Conclusions
Heterologous mRNA-1273 booster vaccination compared with homologous BNT123b2 induced a stronger neutralizing response against the Omicron variant in older individuals.
Clinical Trials Registration
Keywords: COVID-19 vaccine booster, humoral immunity, Omicron, live virus neutralization
Spike antibody titers, surrogate virus neutralizing test levels, and live virus neutralization against severe acute respiratory syndrome coronavirus 2 wild-type, Omicron, and other variants of concern were significantly higher in older BNT162b2-vaccinated individuals who received messenger RNA-1273 compared with BNT162b2 as a booster.
Coronavirus disease 2019 (COVID-19) vaccination programs worldwide have focused on raising population immunity through the primary COVID-19 vaccination series. However, vaccine breakthrough infections have occurred with increasing frequency as a result of waning antibody levels and the emergence of variants of concern (VOCs), such as Omicron, that are capable of evading protective immunity [1, 2]. All COVID-19 vaccines currently approved by the World Health Organization (WHO) and included in their emergency use listing were developed with the wild-type severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strain that emerged in Wuhan in 2019 [3].
Within a few months after its discovery in November 2021, the Omicron variant supplanted Delta as the dominant strain detected worldwide [4]. Several immunogenicity studies of COVID-19 vaccines have demonstrated that a booster dose is needed to elicit an anti-Omicron neutralizing response [2, 4–6]. Vaccine booster combinations tested include homologous messenger RNA (mRNA) vaccines such as BNT162b2 [2, 4, 6] and mRNA-1273 [2], as well as nonreplicating viral vector vaccines AD26.COV2.3 [2] and AZD1222 [6]. However, it is not known whether homologous or heterologous mRNA booster vaccination regimens are better at inducing neutralizing antibodies against Omicron and whether different age groups respond differently to the various vaccine booster combinations.
In this interim analysis of a phase 4 randomized, participant-blinded clinical trial, we studied the immunogenicity of BNT162b2 vs mRNA-1273 booster vaccinations in individuals who had received the second dose of the BNT162b2 vaccine as a primary series at least 6 months prior to study enrollment. The study is still ongoing, and participants who received mRNA-1273 as their primary series will be included in later phases of the study. The primary end point was antibody levels against wild-type SARS-CoV-2 and VOCs as measured using a multiplex surrogate virus neutralization test (sVNT).
METHODS
PRIBIVAC is a participant-blinded, randomized, controlled trial to assess the immunogenicity and safety of heterologous booster COVID-19 vaccination compared with a homologous booster regimen. Participants were enrolled at the National Centre for Infectious Diseases, Singapore. The study protocol is provided in the Supplementary Materials.
Enrollment and Randomization
During the first phase of the study, from October 2021 through November 2021, we enrolled 100 individuals who had received BNT162b2 as their primary vaccine series at least 6 months earlier. Key exclusion criteria included a history of known SARS-CoV-1 or SARS-CoV-2 infection or an immunocompromising medical condition (eg, active leukemia or lymphoma, generalized malignancy, aplastic anemia, solid organ transplant, bone marrow transplant, current radiation therapy, congenital immunodeficiency, human immunodeficiency virus/AIDS with CD4 lymphocyte count <200 cells/mm3, and patients on immunosuppressant medications).
Study participants were randomized 1:1 to receive 1 intramuscular dose of either BNT162b2 30 µg (0.3 mL) or mRNA-1273 50 µg (0.25 mL). Randomization was stratified by age (<60 years, ≥60 years) and time from second vaccine dose administered (6–9 months, >9 months). The study team from the Singapore Infectious Disease Clinical Research Network randomised study participants using a web-based randomization system hosted by the Singapore Clinical Research Institute. The randomization list was generated by the trial statistician with randomized permuted blocks.
Blood samples were collected pre-booster (day –28 to day 0) and at 7 days (±2 days) and 28 days (±7 days) post-booster for assessment of the immune response. Blood samples for immunogenicity assessment will also be collected at 6 months and 12 months. Participants were given a diary card to record solicited and unsolicited local and general symptoms experienced in the first 7 days after vaccination.
Primary End Point
The primary objective for this clinical trial is to determine whether a heterologous mRNA-1273 COVID-19 vaccine booster leads to noninferior humoral immunity against wild-type SARS-CoV-2 and/or VOCs at day 28 compared with homologous BTN162b2. This was assessed by an sVNT that detects total immunodominant neutralizing antibodies targeting the viral spike protein receptor-binding domain (RBD) in an isotype- and species-independent manner.
Interim Analyses and Stopping Guidelines
Interim analyses were performed for data safety monitoring board (DSMB) review after 10 participants from each of the intervention arms completed assessments at study day 28. The following criteria were established a priori for the DSMB to recommend discontinuation of participant enrollment to either study arm: an absolute difference of ≥25% in the proportion of participants with a serious adverse event (SAE); an absolute difference of ≥25% in the proportion of participants with grade 3 and 4 adverse events (AEs); and the geometric mean ratio of anti–SARS-CoV-2 antibody between either intervention group falling below 0.60.
Sample Size Calculation
Based on data from our ongoing COVID-19 vaccine immune-monitoring observational prospective study (SCOPE), the mean level of SARS-CoV-2 anti-spike immunoglobulins by the sVNT was 84% (standard deviation, 15%) at 28 days after the second dose [7]. We expect immunogenicity will be boosted back to the same level after the third booster dose in the control arm. Assuming an immunogenicity level of 84% in the control arm and a noninferiority margin of −10%, a sample size of 87 participants per arm is needed to conclude noninferiority of the intervention arm against the control arm with 80% power. The sample size is calculated at a 1-sided 2.5% significance level and accounts for an attrition rate of 15%.
Antibody Response Assays
Serum samples were tested with a newly developed multiplex-sVNT assay using the Luminex platform [8]. Briefly, AviTag-biotinylated RBD proteins from wild-type SARS-CoV-2 and 5 VOCs (Alpha, Beta, Gamma, Delta, Omicron) were coated on a MagPlex Avidin microsphere (Luminex) at 5 µg/1 million beads. RBD-coated microspheres (600 beads/antigen) were preincubated with serum at a final concentration of 1:20 or greater for 15 minutes at 37°C with 250 rpm agitation. After 15 minutes incubation, 50 µL of phycoerythrin-conjugated human angiotensin-converting enzyme 2 (GenScript 2 µg/mL) were added to the well and incubated for 15 minutes at 37°C with agitation, followed by 2 phosphate-buffered saline-1% bovine serum albumin washes. The final readings were acquired using the MAGPIX system.
Serological results were obtained using the Elecsys (Roche, Basel, Switzerland) anti–SARS-CoV-2 chemiluminescent immunoassays following the manufacturer’s instructions (anti-nucleocapsid [anti-N] and anti-spike protein RBD [anti-S]). Antibody titers in U/mL from the Elecsys anti-S assay are equivalent to the WHO standard binding antibody units per milliliter, with no conversion required [9].
Live Virus Inhibition Assay
The Omicron variant (B.1.1.529/BA.1) isolate M21021166 was originally isolated by Prof Gavin Screaton, University of Oxford, United Kingdom, and then obtained from Prof Wendy Barclay, Imperial College London, United Kingdom, through the Genotype to Phenotype National Virology Consortium. Sequencing confirmed it contained the variant defining mutations [10]. Viral stock of the SARS-CoV-2 Omicron isolate was generated in Vero/hSLAM cells with Dulbecco’s minimal essential medium (DMEM; Sigma) containing 4% fetal bovine serum (FBS; Sigma), 0.05 mg/mL gentamicin (Merck), and 0.4 mg/mL geneticin (G418; Thermo Fisher) and harvested 72 hours post-inoculation. Virus stocks were aliquoted and stored at −80°C as previously described [11].
Plaque reduction neutralization tests (PRNTs) were performed using African green monkey kidney C1008 (Vero E6) cells (Public Health England). Sera were heat-inactivated at 56°C for 1 hour and stored at −20°C until use. DMEM containing 2% FBS and 0.05 mg/mL gentamicin was used for serial 2-fold dilutions of patient plasma samples. SARS-CoV-2 at 800 plaque-forming units per milliliter was added to an equal volume of diluted plasma and incubated at 37°C for 1 hour. The virus–plasma dilution was inoculated onto Vero E6 cells in duplicate and incubated at 37°C for 1 hour. They were then overlaid with agarose as in standard plaque assays. Cells were incubated for 72 hours at 37°C and 5% carbon dioxide before being fixed with 10% formalin and stained with crystal violet solution (Sigma-Aldrich). The PRNT 90/80/50% was determined by the highest dilution with a 90/80/50% reduction in plaques compared with the control.
Statistical Methods
Demographic and baseline characteristics were summarized by vaccine and age group. For comparison of vaccine reactions, categorical data were compared using the Fisher exact test or χ2 test as appropriate. Anti-spike antibody titers were log10-transformed for all statistical analysis and compared using the Student t test. A multiple regression model of pre-vaccination antibody titers was constructed that included age (<60 years; ≥60 years), sex, and time since vaccination (in days) with the log10-transformed antibody titer as the dependent variable. Comparison of sVNT % inhibition level and the neutralization activity of plasma samples against Omicron was conducted using the Mann–Whitney U test. No adjustments were made for multiple testing. Statistical significance was defined as P < .05. Analyses were performed using R, and figures were generated using GraphPad Prism version 9.
Ethics Statement
Written informed consent was obtained from all study participants.
RESULTS
Participants
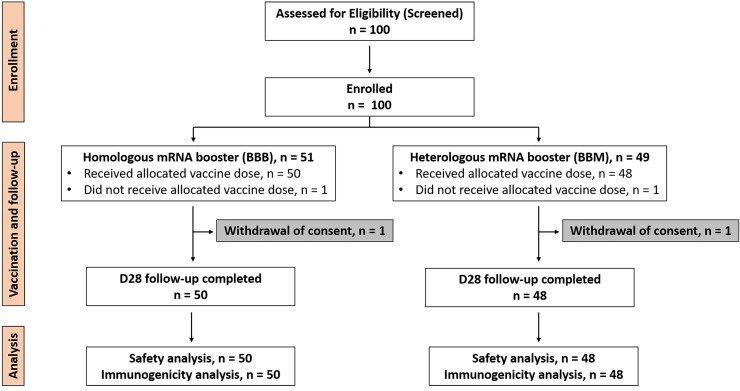
Among 100 participants who received 2 primary doses of BNT162b2, 51 were randomized to receive the homologous mRNA booster BNT162b2 (control group; BBB) and 49 to receive the heterologous mRNA booster mRNA-1273 (intervention group; BBM; Figure 1). One participant from each group withdrew from the study, resulting in an analysis sample size of 50 and 48 for BBB and BBM groups, respectively. Baseline demographic characteristics of the participants who received BBB or BBM in the younger (<60 years) and older (≥60 years) age groups are shown in Table 1.
Figure 1.
Consort flow diagram. Abbreviations: BBB, BNT162b2- BNT162b2- BNT162b2; BBM, BNT162b2-BNT162b2-mRNA-1273; D28, day 28; mRNA, messenger RNA.
Table 1.
Demographics of Study Participants
| Demographic | BNT162b2- BNT162b2- BNT162b2 (n = 50) | BNT162b2-BNT162b2-mRNA-1273 (n = 48) | ||
|---|---|---|---|---|
| Age group, years | <60 | ≥60 | <60 | ≥60 |
| N | 26 | 24 | 25 | 23 |
| Age, mean (range), years | 35 (21–58) | 68 (60–78) | 37 (23–59) | 67 (60–84) |
| Male sex, no. (%) | 9 (35) | 13 (54) | 12 (48) | 9 (39) |
| Chinese, no. (%) | 20 (77) | 23 (95) | 22 (88) | 23 (100) |
| Charlson comorbidity index, median (interquartile range) | 0 (0–0) | 0 (0–0.75) | 0 (0–0) | 0 (0–0) |
| Days since second dose, mean (range) | 254 (194–297) | 219 (190–280) | 252 (196–295) | 210 (189–257) |
| Current smoker | 1 | 0 | 0 | 1 |
No COVID-19 infections were recorded during the 28-day study period. All participants were negative for anti-N antibody at baseline, day 7, and day 28.
Safety
The number of participants with solicited local and systemic adverse reactions (ARs) was similar between the BBB and BBM groups (Supplementary Table 1, Supplementary Figure 1). The most common local AR was injection site pain, with 89% and 87% of participants who received BBB or BBM, respectively, experiencing pain at the injection site within 72 hours of a booster dose. The most common systemic AR was fatigue/tiredness (BBB 70% and BBM 67%), followed by muscle pain (BBB 61% and BBM 56%).
Local and systemic ARs between BBB and BBM in each age group were similar, except in the older age group where fever and weakness occurred more frequently in the BBM (35%) than the BBB group (5%).
There were 35 unsolicited AEs reported by 25 participants, 12 in the BBB group and 13 in the BBM group. No SAEs were reported in the 28 days after vaccination in either age group.
Immunogenicity Assessments
The levels of SARS-CoV-2 anti-S antibodies and neutralizing antibodies against the wild-type SARS-CoV-2 and VOCs were measured in serum samples collected before the booster dose (day –14 to day 0) and at days 7 and 28 after the booster dose. Before the booster dose and across all participants, the mean anti-S antibody titer in all participants was 555 IU/mL (95% confidence interval [CI], 484 to 635), and the median sVNT level was 48.0% (interquartile range [IQR], 36.5% to 59.3%) and similar between intervention groups. On multiple regression, baseline anti-S titers were significantly lower with older age (P = .0188) and among men (P = .0051), but not with time since primary vaccination series.
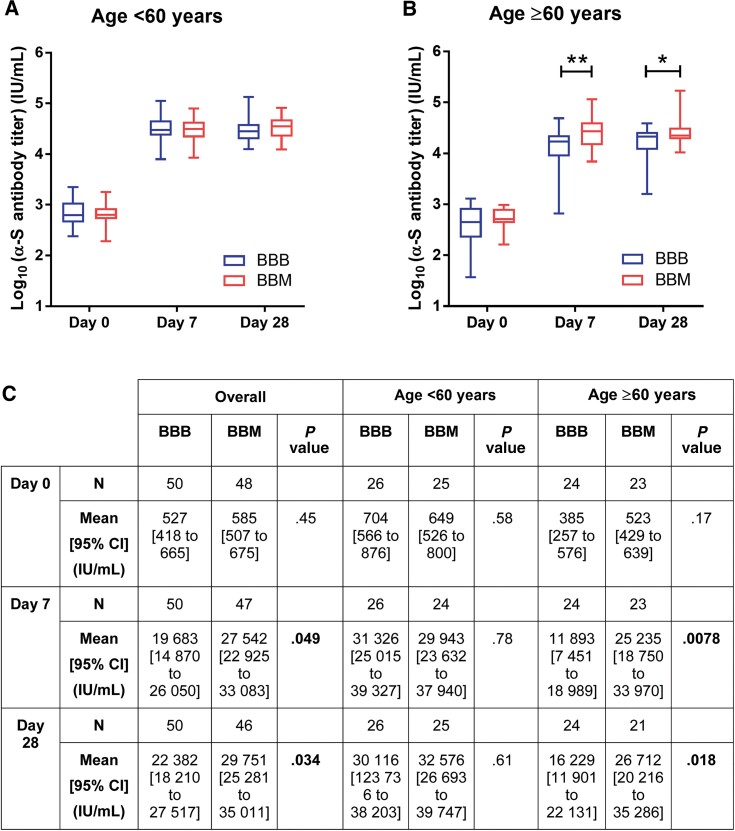
After the booster dose, the anti-S titer across both intervention groups increased by 35- to 49-fold at day 7 to a mean of 23 158 IU/mL (95% CI, 19 539 to 27 454), with only a modest further increase by day 28 (25 651 IU/mL; 95% CI, 22 444 to 29 322). Comparing study groups, antibody titers were higher at both day 7 (1.4-fold, P = .0496) and day 28 (1.3-fold, P = .0339) in the mRNA-1273 booster group compared with the BNT162b2 group (Figure 2). This finding was consistent when neutralization levels were compared against wild-type, Omicron, and most of the other variants (Figure 3).
Figure 2.
Level of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) anti-spike receptor-binding domain antibody in participants (A) aged <60 years, (B) participants aged ≥60 years, and (C) overall. Participants in the older age group (≥60 years old) who received a heterologous coronavirus disease 2019 vaccine booster (BBM) had significantly higher anti–SARS-CoV-2 immunoglobulin G antibodies than those who received a homologous messenger RNA booster (BBB) at days 7 and 28 post-vaccination. Data analyzed using the Student t test to compare the log10 anti-spike titer. Box represents 25th and 75th percentile, line is median, with whiskers denoting extremes. *P < .05, **P < .01. Abbreviations: BBB, BNT162b2- BNT162b2- BNT162b2; BBM, BNT162b2-BNT162b2-mRNA-1273; CI, confidence interval.
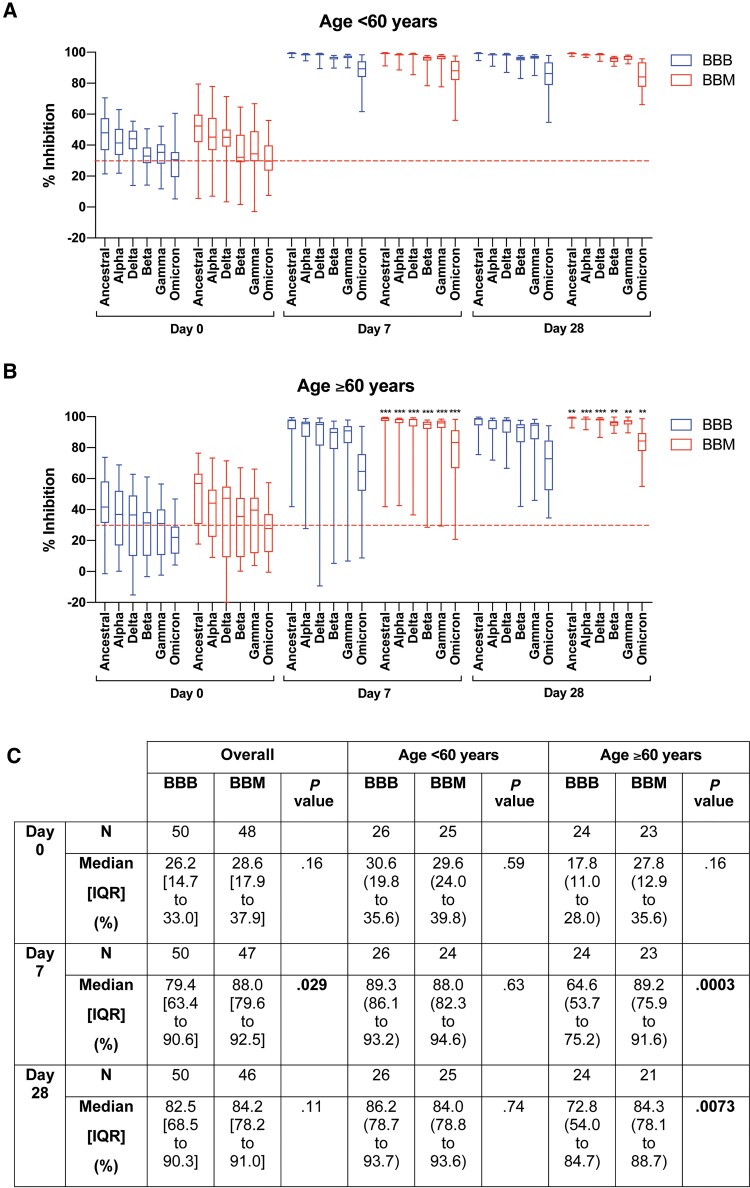
Figure 3.
Level of neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 and variants of concern in participants (A) aged <60 years, (B) participants aged ≥60 years, and (C) summary data for Omicron. Level of percent inhibition was determined using a multiplex surrogate virus neutralization test as previously described [8]. Data analyzed using the Mann–Whitney U test. Red dotted line indicates inhibition of 30% (nominal “seronegative” threshold). Data presented in box plot and the line in the box indicate median. **P < .01, ***P < .001. Abbreviations: BBB, BNT162b2- BNT162b2- BNT162b2; BBM, BNT162b2-BNT162b2-mRNA-1273; IQR, interquartile range.
At preplanned subgroup analysis, the anti-S antibody titers between BBB and BBM in the younger age group were not significantly different at days 7 and 28 post-booster, whereas receipt of BBM by older participants resulted in a significantly higher induction of anti-spike antibody levels than for those who received BBB. The mean anti-S titer was significantly higher with BBM than BBB by 2.1-fold (P = .0078) at day 7 and 1.6-fold (P = .0184) at day 28.
The same trend was observed in inhibition level measured by sVNT against the wild-type SARS-CoV-2 and VOCs. Older BBM participants had higher levels of neutralizing antibodies against SARS-CoV-2 and all known VOCs, including Omicron (Supplementary Tables 2–4). The median wild-type SARS-CoV-2 sVNT inhibition level was modestly different at day 28 (BBB 98.8%, IQR, 95.3% to 99.0% vs BBM 99.3%, IQR, 98.7% to 99.5%) likely due to saturation, although this achieved statistical significance (P = .003).
The largest absolute difference in inhibition level was observed against the Omicron variant in older participants (BBB 64.6%, IQR, 53.7% to 75.2% vs BBM 89.2%, IQR, 75.9% to 91.6%; P = .0003) at day 7 post-booster. At day 28 post-booster, the inhibition percent remained significantly higher against the Omicron variant in the BBM group (84.3%, IQR, 78.1% to 88.7%) than in the BBB group (72.8%, IQR, 54.0% to 84.7%; P = .0073).
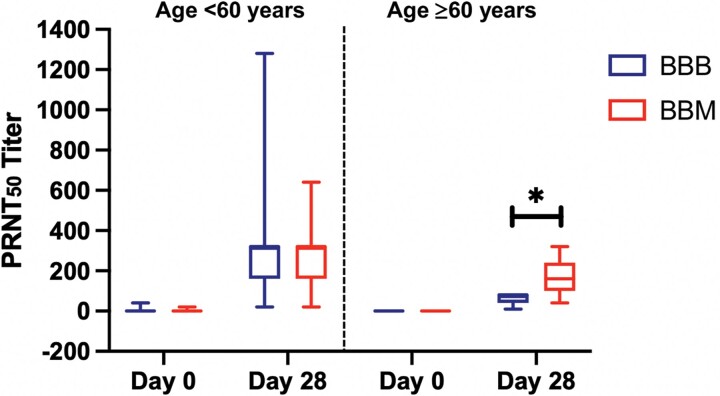
The neutralizing activity of plasma samples from a subgroup of 40 participants against the Omicron variant isolates was assessed using a live virus neutralization assay. The results corroborated the antibody and sVNT assay data, showing a significant increase in PRNT50 to Omicron at day 28 after booster vaccination (Figure 4A). In addition, older BBM participants had a higher PRNT50 against Omicron than BBB participants at day 28 post-booster (BBB 80, IQR, 40 to 80 vs BBM 160, IQR, 100 to 240; P = .022; Figure 4B). Similar results were observed with PRNT80 and PRNT90 (Supplementary Table 5).
Figure 4.
The neutralization activity of plasma samples against the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Plasma samples from participants who received a vaccine booster were collected prior to vaccination (day 0) and at day 28 after the booster vaccination and were screened for neutralizing activity against the Omicron variant of SARS-CoV-2. Plasma neutralizing activity comparison between participants who received the homologous (BBB) and heterologous (BBM) messenger RNA booster vaccine in the younger (aged <60 years, n = 28) and older (aged ≥60 years, n = 12) age groups. Box represents 25th and 75th percentile, line is median, with whiskers denoting extremes. Data analyzed using the Mann–Whitney U test. *P < .05. Abbreviations: BBB, BNT162b2- BNT162b2- BNT162b2; BBM, BNT162b2-BNT162b2-mRNA-1273; PRNT, plaque reduction neutralization test.
DISCUSSION
The Omicron variant of SARS-CoV-2 has opened a new chapter in the COVID-19 pandemic [12] due to its high transmissibility and large number of mutations in the RBD region of the spike protein [13], which may explain its partial or complete resistance to antibody neutralization in fully vaccinated or previously infected individuals. The increasing frequency of vaccine breakthrough infections and the variable supply for different vaccine products have raised the need and consideration for heterologous booster vaccinations. Recent studies have shown use of both homologous and heterologous boosting, irrespective of primary vaccine series, to increase neutralizing antibody titers [14, 15]. In Singapore, a recent study of data from the Delta variant outbreak found heterologous boosting to be associated with a lower incidence rate of SARS-CoV-2 infection compared with homologous boosting in adults aged ≥60 years [16]. However, the comparative effect of different booster vaccine regimens on the serum neutralizing activity against Omicron and other VOCs remains unknown.
This interim analysis describes the safety and immunogenicity of a homologous (BNT162b2, BBB) or heterologous (mRNA-1272, BBM) mRNA booster dose in fully vaccinated adults against clinically important VOCs such as Omicron. The ARs after single booster injections with BNT162b2 or mRNA-1273 were comparable between BBB and BBM groups and similar to those observed after the BNT162b2 primary series and commonly include pain at the site of injection, lethargy, and muscle pain.
Six months after the primary vaccine series, mean neutralizing antibody titers against the wild-type SARS-CoV-2 declined to 40%–60% in all groups. Additional reduction of neutralizing activity against VOCs compared with the wild-type SARS-CoV-2 is a common trend in all participants that is not influenced by age. Declining neutralization against the wild-type SARS-CoV-2 and low neutralizing activity against Omicron after complete BNT162b2 vaccination call for an effective booster vaccine regimen to increase immune responses and protection. In this interim analysis, we demonstrate that a booster dose can effectively enhance serum neutralizing activity against the wild-type SARS-CoV-2 and all known VOCs (Alpha, Beta, Gamma, Delta, and Omicron) as early as day 7 post-booster. More importantly, we evaluated and compared the choice of booster dose for different age groups. For the vulnerable older age group, in particular, a heterologous booster COVID-19 vaccine regimen induces a higher anti-spike antibody titer and a stronger neutralizing antibody response against the highly infectious Omicron variant (approximately 20% higher neutralization) than a homologous booster regimen.
This analysis is limited to healthy individuals receiving the BNT162b2 primary vaccine series; a recent study has shown that immunogenicity may be affected by the order of vaccine products, though apparently less so than the combination [17, 18]. Currently, it is not clear to what extent the higher antibody levels observed in older BBM participants are due to superiority of mRNA-1273 vs BNT126b2 or an effect of heterologous boosting. The PRIBIVAC study is ongoing; later phases of the study will include individuals who received mRNA-1273 as their primary vaccine series to address this question. In addition, it is not known whether these higher antibody peaks after vaccination will persist for the long term. Study participants will continue to be followed up at 6 months and 12 months after their booster vaccination to measure the rate of waning.
A study of this size is not likely to be able to determine vaccine effectiveness against infection, and the clinical impact of this antibody difference in older adults needs to be determined [16]. Further studies are underway to characterize cell-mediated immunity in this cohort that may indicate effectiveness against severe infection.
This study was initiated initially with only 2 arms (the control arm [BBB] and intervention arm [BBM]) as the availability of other vaccine formulations are subjected to rigorous regulatory scrutiny before they can be used in Singapore. We present interim results from this study obtained before reaching our initial planned sample size due to new inclusion of Covaxin as a booster dose to the study platforms adaptive protocol. It is unlikely that the study findings will change with a larger sample size given the large difference in the Omicron-specific neutralizing levels among older adults. Singapore has rapidly expanded its COVID-19 booster vaccination campaign; currently, 65% of adults aged ≥60 years have received a booster dose.
Variant-specific vaccines may be necessary for optimal protection against SARS-CoV-2 variants such as Omicron [19]. Clinical trials are currently ongoing. However, even if successful, these vaccines are not expected to be available until late in 2022. Thus, there is an urgent need for an effective standard booster vaccination regimen, particularly in vulnerable populations, to reduce the risk of severe disease. The present data provide evidence that a heterologous booster vaccination in older individuals induces more robust neutralization against the immune-evasive Omicron variant. This information is of paramount importance to inform future COVID-19 booster programs (third dose in other countries or fourth dose in Singapore) for older individuals to better protect them against SARS-CoV-2 infection and severe disease. Future follow-up analyses can provide further insights into the durability of the neutralizing antibody response of the different vaccine booster combinations, as well as the neutralizing ability against new VOCs.
CONCLUSIONS
Although the Omicron variant exerts considerable humoral immune escape in BNT162b2 fully vaccinated individuals, a booster dose with BNT162b2 or mRNA-1273 is capable of increasing the serum neutralizing activity against Omicron by more than 50% by day 7 post-booster. In older individuals who received BNT162b2 as their primary vaccine series, a heterologous booster regimen with mRNA-1273 induced a higher anti-spike antibody titer and a stronger neutralizing response against the Omicron variant than a homologous booster regimen.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Xuan Ying Poh, National Centre for Infectious Diseases, Singapore, Singapore.
Chee Wah Tan, Emerging Infectious Diseases Programme, Duke-National University of Singapore Medical School, Singapore, Singapore.
I Russel Lee, National Centre for Infectious Diseases, Singapore, Singapore.
Jean-Marc Chavatte, National Centre for Infectious Diseases, Singapore, Singapore; National Public Health Laboratory, Singapore, Singapore.
Siew-Wai Fong, A*STAR Infectious Diseases Lab, Agency for Science Technology and Research, Singapore, Singapore.
Tessa Prince, Department of Infection Biology, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom.
Catherine Hartley, Department of Infection Biology, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom.
Aileen Y Y Yeoh, Emerging Infectious Diseases Programme, Duke-National University of Singapore Medical School, Singapore, Singapore.
Suma Rao, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore.
Po Ying Chia, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
Sean W X Ong, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore.
Tau Hong Lee, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore.
Sapna P Sadarangani, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
Ray J H Lin, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore.
Clarissa Lim, National Centre for Infectious Diseases, Singapore, Singapore.
Jefanie Teo, National Centre for Infectious Diseases, Singapore, Singapore.
Daniel R X Lim, National Centre for Infectious Diseases, Singapore, Singapore; National Public Health Laboratory, Singapore, Singapore.
Wanni Chia, Emerging Infectious Diseases Programme, Duke-National University of Singapore Medical School, Singapore, Singapore.
Julian A Hiscox, A*STAR Infectious Diseases Lab, Agency for Science Technology and Research, Singapore, Singapore; Department of Infection Biology, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom.
Lisa F P Ng, A*STAR Infectious Diseases Lab, Agency for Science Technology and Research, Singapore, Singapore; Department of Infection Biology, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom; Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Ee Chee Ren, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; Singapore Immunology Network, Agency for Science Technology and Research, Singapore, Singapore.
Raymond T P Lin, National Centre for Infectious Diseases, Singapore, Singapore; National Public Health Laboratory, Singapore, Singapore.
Laurent Renia, A*STAR Infectious Diseases Lab, Agency for Science Technology and Research, Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore; School of Biological Sciences, Nanyang Technological University, Singapore, Singapore.
David Chien Lye, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore; Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Lin-Fa Wang, Emerging Infectious Diseases Programme, Duke-National University of Singapore Medical School, Singapore, Singapore.
Barnaby E Young, National Centre for Infectious Diseases, Singapore, Singapore; Department of Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore; Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore.
Notes
Author contributions. B. E. Y. had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Concept and design: L. F. P. N., L. R., E. C. R., R. T. P. L., D. C. L., L.-F. W., B. E. Y. Acquisition, analysis of data interpretation: X. Y. P., W. Chia, R. Lee, J.-M. C., S. W. F., T. P., C. H., A. Yeoh, S. R., P. Y. Chia, S. W. X. O., T. H. Lee, R. T. P. L., S. P. S., D. Lim, J. T., C. W. T., R. T. P. L., D. C. L., L.-F. W., B. E. Y. Drafting of the manuscript: X. Y. P., W. Chia, R. Lee, B. E. Y. Critical revision of the manuscript for important intellectual content: X. Y. P., W. Chia, R. Lee, J.-M. C., S. W. F., A.Yeoh, S. R., P. Y. Chia, S. W. X. O., T. H. Lee, R. T. P. L., S. P. S., Lim, J. T., D. Lim, C. W. T., L. F. P. N., L. R., E. C. R., R. T. P. L., D. C. L., L.-F. W., B. E. Y. Statistical analysis: X. Y. P., W. Chia, T. P., S. W. F., B. E. Y. Obtained funding: J. A. H., L. R., D. C. L., L.-F. W., B. E. Y. Administrative, technical, or material support: J. A. H., L. F. P. N., L. R., D. C. L., L.-F. W., B. E. Y. Supervision: J. A. H., L. F. P. N., L. R., D. C. L., L.-F. W., B. E. Y.
Acknowledgments. We thank Jinyan Zhang and Wee Chee Yap for technical assistance and the Data and Safety Monitoring Board (Jenny Low, David Allen, Alex R. Cook) for their review and guidance. We also thank the Singapore Clinical Research Institute for their project management and monitoring of this study.
Data sharing. All data sharing requests should be addressed to the corresponding authors.
Disclaimer. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Financial support. The study is supported in part by grants from the Singapore National Medical Research Council (STPRG-FY19-001, COVID19RF-003, COVID19RF-011, COVID19RF-018, COVID19RF-060, and OFLCG19May-0034). This live virus inhibition work was partially funded by the US Food and Drug Administration Medical Countermeasures Initiative (contract 75F40120C00085). This work was also supported by the Medical Research Council (MR/W005611/1 to J. A. H.) Genotype to Phenotype National Virology Consortium, a national virology consortium to address phenotypic consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genomic variation.
References
- 1. World Health Organization . Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. 2021; Available at: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 18 January 2022.
- 2. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022; 185:457–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . 10 vaccines granted emergency use listing (EUL) by WHO. 2022; Available at: https://covid19.trackvaccines.org/agency/who/. Accessed 21 February 2022.
- 4. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022; 602:671–5. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt F, Muecksch F, Weisblum Y, et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med 2022; 386:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022; 185:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laurent R, Yun Shan G, Angeline R, et al. Durable T cell responses contrast with faster antibody waning in BNT162b2-vaccinated elderly at 6 month. Nature Portfolio [Preprint]. 9 December 2021 [cited 11 May 2022]. Available at: 10.21203/rs.3.rs-1103804/v1 [DOI] [Google Scholar]
- 8. Tan C-W, Chia W-N, Young BE, et al. Pan-sarbecovirus neutralizing antibodies in BNT162b2-immunized SARS-CoV-1 survivors. N Engl J Med 2021; 385:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkmann T, Perkmann-Nagele N, Koller T, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spectr 2021; 9:e0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 Omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022; 399:234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prince T, Dong X, Penrice-Randal R, et al. Analysis of SARS-CoV-2 in Nasopharyngeal Samples from Patients with COVID-19 Illustrates Population Variation and Diverse Phenotypes, Placing the Growth Properties of Variants of Concern in Context with Other Lineages. mSphere [in press]. Available at: 10.1128/msphere.00913-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021; 398:2126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma W, Yang J, Fu H, et al. Genomic perspectives on the emerging SARS-CoV-2 Omicron variant. Genom Proteom Bioinf [in press]. Available at: 10.1016/j.gpb.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous covid-19 booster vaccinations. N Engl J Med 2022; 386:1046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clemens SA C, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet 2022; 399:521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan SHX, Pung R, Wang L-F, et al. Association of homologous and heterologous vaccine boosters with COVID-19 incidence and severity in Singapore. JAMA 2022; 327:1181–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021; 398:856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker EPK, Desai S, Marti M, et al. Emerging evidence on heterologous COVID-19 vaccine schedules—To mix or not to mix? Lancet Infect Dis 2022; 22:438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dolgin E. Omicron is supercharging the COVID vaccine booster debate. Nature [in press]. Available at: 10.1038/d41586-021-03592-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.