Abstract
Background
Omicron variant viruses spread rapidly, even in individuals with high vaccination rates. This study aimed to determine the utility of the antibody against spike protein level as a predictor of the disease course of coronavirus disease 2019 (COVID-19) in vaccinated patients.
Methods
Between December 11, 2021, and February 10, 2022, we performed a prospective observational cohort study in South Korea, which included patients infected with Delta and Omicron variants. A multivariable logistic regression analysis to determine the association between antibody levels and outcomes was conducted. The relationship between antibody levels and cycle threshold (Ct) values was confirmed using a generalized linear model.
Results
From 106 vaccinated patients (39 Delta and 67 Omicron), the geometric mean titers of antibodies in patients with fever (≥37.5°C), hypoxia (≤94% of SpO2), pneumonia, C-reactive protein (CRP) elevation (>8 mg/L), or lymphopenia (<1100 cells/μL) were 1201.5 U/mL, 98.8 U/mL, 774.1 U/mL, 1335.1 U/mL, and 1032.2 U/mL, respectively. Increased antibody levels were associated with a decrease in the occurrence of fever (adjusted odds ratio [aOR], 0.23; 95% CI, 0.12–0.51), hypoxia (aOR, 0.23; 95% CI, 0.08–0.7), CRP elevation (aOR, 0.52; 95% CI, 0.29–0.0.94), and lymphopenia (aOR, 0.57; 95% CI, 0.33–0.98). Ct values showed a positive correlation between antibody levels (P = .02).
Conclusions
Antibody levels are predictive of the clinical course of COVID-19 in vaccinated patients with Delta and Omicron variant infections. Our data highlight the need for concentrated efforts to monitor patients with severe acute respiratory syndrome coronavirus 2 infection who are at risk of low antibody levels.
Keywords: antibody level, COVID-19 breakthrough infection, Delta variant, Omicron variant
Since the Omicron variant was first confirmed on November 24, 2021, it has spread rapidly worldwide [1]. As a result, global cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have increased at an unprecedented rate [2]. The Omicron variant can spread easily in individuals even if they have completed their vaccination course. Several mutations in the Omicron variant are expected to enable the virus to evade the immune system established by vaccination, resulting in increased infectivity [3, 4]. In addition, a decrease in immunity elicited by vaccines over time, namely waning immunity, may play an important role in the Omicron variant’s spread [5]. However, vaccines remain effective in protecting against severe diseases caused by the Omicron variant, although the effectiveness may have decreased in the Omicron variant rather than in previous variants [6]. Understanding the extent of vaccine effectiveness on clinical protection in patients with breakthrough infection caused by the Omicron variant is needed to take measures to minimize the damage from the current pandemic.
In South Korea, owing to quarantine and isolation guidelines, it took more time for the Omicron variant to become the dominant strain than in other countries. According to several reports, the Omicron variant virus presumably has weakened virulence, which is related to reduced severity, hospitalization, and mortality [7, 8]. However, the recent explosive increase in the number of Omicron variant infections has increased the total number of deaths, and Omicron’s spread has not slowed as of February 28, 2022. In this situation, to efficiently use limited medical resources, it is important to predict which patients will go through an unfavorable clinical course and put concentrated efforts into protecting them.
We performed a prospective cross-sectional study involving patients with Delta and Omicron variant infections who were admitted to an institution in South Korea. This study aimed to determine the vaccine’s effect on the clinical course of Delta and Omicron variant infections. Furthermore, we evaluated the usefulness of the antibody level against spike protein as a predictor of the disease course of COVID-19 in vaccinated patients.
METHODS
Study Design and Participants
We performed a prospective cross-sectional study involving SARS-CoV-2-confirmed adult patients (age >19 years) who were admitted to Yongin Severance Hospital. All SARS-CoV-2 cases were confirmed by polymerase chain reaction (PCR) tests at government-approved test centers, and a local public health center in South Korea was notified. Patients with specified symptoms or conditions were hospitalized for monitoring and treatment, and they were assigned to appropriate hospitals according to the severity of COVID-19 if they were willing to do so. Symptoms and conditions for which hospitalization was considered and classification of severity are described in Supplementary Tables 1 and 2. Yongin Severance Hospital has been in charge of hospitalization of SARS-CoV-2 cases with mild to moderate severity.
This study enrolled participants from December 11, 2021, to February 10, 2022, which included 2 different waves of COVID-19, both the Delta and Omicron variants, in South Korea. Based on the national COVID-19 data, almost all SARS-CoV-2 cases were caused by the Delta variant until the start of study enrollment. However, the Omicron variant overtook the Delta variant in domestic SARS-CoV-2 cases from the third week of January 2022, and its detection rate exceeded 90% in the first week of February 2022 (Supplementary Figure 1). Patients who agreed to undergo PCR tests for the SARS-CoV-2 variant type and anti-SARS-CoV-2 antibody tests were eligible for enrollment in this study. Only participants with confirmed Delta or Omicron variant infections were included in the analysis.
Data Collection
We collected data on initial symptoms, reinfection identified via a questionnaire, diagnosis date, initial PCR cycle threshold (Ct) value, COVID-19 vaccination history, and household contacts from the COVID-19 investigation report provided by the epidemiological investigator. The patients’ baseline characteristics and clinical course were recorded during hospitalization. The Charlson comorbidity index (CCI) was used to categorize patients’ comorbidities [9]. Immunocompromised conditions were determined according to the UK Health Security Agency’s definition of severe immunosuppression [10]. Vaccination status was divided into 3 groups—unvaccinated or partially vaccinated, vaccinated, and booster-vaccinated. The unvaccinated or partially vaccinated group included patients who had never been vaccinated, those who received 1 dose within the past 3 weeks, and those who were partially vaccinated (received just 1 dose or received 2 doses within the past 2 weeks). The vaccinated group included patients who received a vaccination ≥2 weeks ago; a single dose of Janssen Ad26.COV2.S vaccine was considered vaccinated if it had been >2 weeks after administration. Of the vaccinated patients, individuals were classified into the booster-vaccinated group if they received a booster shot before 2 weeks of enrollment. The types of vaccines the participants received are presented in Supplementary Table 1.
Procedures
Serum sample preparation, anti-SARS-CoV-2 antibody assays, RNA extraction, and PCR for SARS-CoV-2 detection and variant typing are described in the Supplementary Data.
Outcomes
The study’s primary outcomes were to compare patients with Delta and Omicron variant infections and to determine the effect of antibodies on the clinical course of breakthrough infection caused by the Delta and Omicron variants. The secondary outcome was to determine viral dynamics according to antibody level.
Statistical Analysis
Only patients who were tested for antibody levels within 7 days of symptom onset or diagnosis, whichever was earlier, were included in the analyses using antibody titers. The 7-day period was designated to minimize the effect of current infection on antibody levels elicited by vaccination [11]. Furthermore, we performed sensitivity analyses using 3-day and 5-day thresholds. We investigated viral dynamics using Ct values obtained from the PCR test. Viral dynamic analysis was conducted only on data from patients who had undergone the PCR test—5–7 days after the initial diagnosis.
Continuous variables were analyzed using descriptive methods depending on their distribution and tested using the Shapiro-Wilk test. Variables with a normal distribution were described with means and standard deviations, and independent 2-sample t tests were performed. Non-normal variables were expressed as medians and interquartile ranges (IQRs). Categorical variables were described as frequencies and percentages. The chi-square or Fisher exact test was performed depending on the number of expected events. Multivariable logistic regression analysis was performed to determine the effect of antibody levels on the clinical course of breakthrough infections. We selected fever (≥37.5°C), hypoxia (≤94% oxygen saturation), pneumonia, C-reactive protein (CRP) elevation (>8 mg/L), and lymphopenia (<1100 cells/μL) as variables representing the clinical course. Relevant variables with a significance level of <.1 through univariate logistic regression analysis or with clinical significance were included in the multivariable model. The relationship between antibody levels and Ct values was confirmed using a generalized linear model. Statistical analyses were performed using SAS (version 9.4; SAS Institute) and R (version 4.1.1; R Foundation for Statistical Computing).
Patient Consent
This study was approved by the Institutional Review Board of Yonsei University Health System Clinical Trial Centre, and the study protocol adhered to the Declaration of Helsinki guidelines. Written informed consent was obtained from all participants (approval number: 9-2021-0156, approved on November 18, 2021).
RESULTS
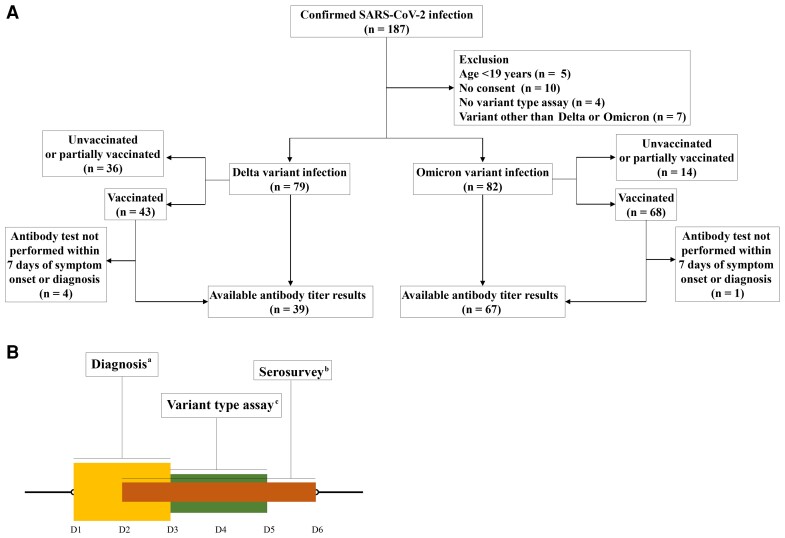
A total of 187 patients were admitted to Yongin Severance Hospital between December 11, 2021, and February 10, 2022. Of these, 172 patients underwent tests for SARS-CoV-2 variant type assay. The results of the assay revealed that 79 patients were infected with the Delta variant, 82 with the Omicron variant, and 7 with the undetermined type. There were no cases of re-infection. According to the inclusion criteria for analyses using antibody levels, 106 patients who underwent antibody testing within a defined period were selected from 111 vaccinated patients: 39 with Delta variant infections and 67 with Omicron variant infections (Figure 1A). Antibody levels were tested at a median (IQR) of 4 (2–6) days after symptom onset or diagnosis, and variant type assays were performed at 4 (3–5) days after the initial diagnosis (Figure 1B).
Figure 1.
Study flow diagram. A, Study flow of enrollment. B, Severe acute respiratory syndrome coronavirus 2 diagnosis, antibody to spike protein test, variant type assay. aPatients were diagnosed at a median (IQR) of 2 (1–3) days after symptom onset. bAntibody tests were performed at a median (IQR) of 4 (2–6) days after symptom onset or diagnosis, whichever was earlier. cVariant type assays were conducted at a median (IQR) of 4 (3–5) days after diagnosis. Abbreviation: IQR, interquartile range.
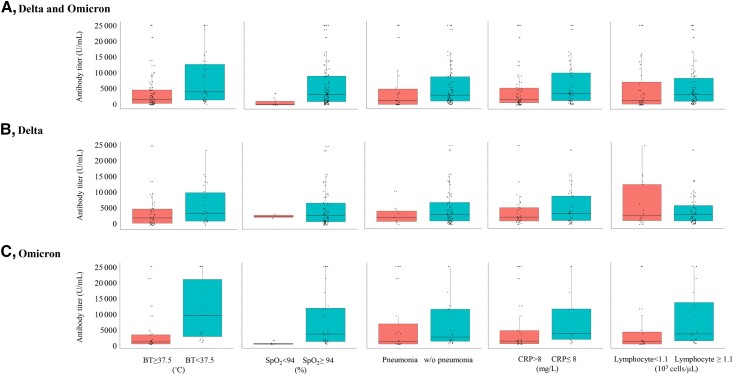
Figure 2.
Comparison of antibody levels between vaccinated patients with or without specific signs during hospitalization. This analysis included 106 patients with Delta and Omicron variant infections whose serum samples were collected within 7 days of symptom onset or diagnosis. Antibody levels are described as box plots of medians with interquartile ranges. Abbreviations: BT, body temperature; CRP, C-reactive protein; SpO2, percutaneous oxygen saturation; w/o, without.
Of the 161 patients who had Delta or Omicron variant infection, 85 (53%) were women, and the mean age was 54.5 (±18.9) years. Patients with Omicron variant infection were younger than those with Delta variant infection (P = .017). The proportion of female patients, distribution of body mass index, frequency of immunocompromised status, and CCI ≥3 were similar among patients with Delta and Omicron variant infections. Although patients with Delta variant infection were more frequently asymptomatic at the time of diagnosis (P = .01), those with an Omicron variant infection had lower occurrence rates of pneumonia (P < .001) and hypoxia (P < .001) during hospitalization. Once patients experienced hypoxia, the duration of hypoxia was longer in patients with Delta infections than in those with Omicron variant infections (P = .002). Compared with patients with Omicron variant infections, those with Delta variant infections had higher levels of CRP (P < .001) and interleukin 6 (IL-6; P = .021) and were more likely to receive remdesivir (P = .029), dexamethasone (P = .002), and antimicrobial agents (P = .001) (Table 1).
Table 1.
Clinical Characteristics of Patients With SARS-CoV-2 Infection According to Variant Type
| Variable | Delta Variant | Omicron Variant | P Value |
|---|---|---|---|
| (n = 79) | (n = 82) | ||
| Demographic data | |||
| Age, y | 58.1 ± 15.7 | 51.0 ± 21.1 | .017 |
| Female, No. (%) | 40 (51.3) | 45 (54.9) | .590 |
| BMI, kg/m2 | 23.6 ± 3.1 | 23.7 ± 4.5 | .818 |
| Immunocompromised, No. (%) | 11 (13.9) | 12 (14.6) | .898 |
| CCI ≥3, No. (%) | 39 (49.4) | 28 (34.1) | .050 |
| Asymptomatic at diagnosis, No. (%) | 14 (17.7) | 4 (4.9) | .010 |
| Laboratory data (worst results during hospitalization) | |||
| CRP, mg/L | 47.6 ± 65.8 | 15.6 ± 23.1 | <.001 |
| Lymphocyte, 103 cells/μL | 1.1 ± 0.6 | 1.4 ± 0.6 | .001 |
| IL-6, pg/mL | 33.8 ± 73.7 | 9.8 ± 14.6 | .021 |
| D-dimer, mcgFEU/mL | 0.8 ± 1.4 | 0.6 ± 1.1 | .276 |
| Clinical courses during hospitalization | |||
| Fever (BT ≥37.5°C), No. (%) | 57 (72.2) | 50 (61.7) | .161 |
| Time to defervescence, d | 4.6 ± 2.9 | 3.6 ± 2.3 | .065 |
| Pneumonia,a No. (%) | 44 (55.7) | 12 (14.6) | <.001 |
| Hypoxia (SpO2 <94%), No. (%) | 20 (25.3) | 4 (4.9) | <.001 |
| Duration of oxygenation, d | 6.6 ± 2.8 | 4.0 ± 0.0 | .002 |
| Treatments | |||
| High flow, No. (%) | 3 (3.8) | 1 (1.2) | .361b |
| Regdanvimab, No. (%) | 8 (10.1) | 3 (3.7) | .104 |
| Remdesivir, No. (%) | 19 (24.1) | 9 (11.0) | .028 |
| Dexamethasone, No. (%) | 28 (35.4) | 12 (14.6) | .002 |
| Antimicrobial agents, No. (%) | 33 (41.8) | 15 (18.3) | .001 |
Data are expressed as mean ± SD or No. (%).
Abbreviations: BMI, body mass index; BT, body temperature; CCI, Charlson comorbidity index; CRP, C-reactive protein; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SpO2, percutaneous oxygen saturation.
Presence of pneumonia was determined using chest radiography.
P value was calculated using the Fisher exact test.
There were 111 vaccinated patients with Delta variant (n = 43) and Omicron variant (n = 68) infections. The comparison of Delta and Omicron variant infections in these patients was similar to that in 161 patients whose vaccination status was not classified (Supplementary Table 3). Unvaccinated or partially vaccinated patients (n = 50) more commonly experienced fever, pneumonia, and hypoxia during hospitalization than vaccinated patients did. Vaccinated patients had a shorter time to defervescence than unvaccinated or partially vaccinated patients. Laboratory results did not differ between the vaccinated and unvaccinated or partially vaccinated patients, except for lymphocyte counts. Vaccinated patients were less likely to be treated with drugs for COVID-19 than were unvaccinated or partially vaccinated patients (Supplementary Table 4).
When we classified vaccinated patients with Delta and Omicron variant infection into booster-vaccinated (n = 47) and booster-unvaccinated (n = 64) groups, as expected from a recent national vaccination program recommending a booster shot, the time since the last vaccination to confirm SARS-CoV-2 infection was shorter in the booster-vaccinated group than in the booster-unvaccinated group. Symptoms, signs, laboratory results, and treatment drugs, except for dexamethasone, did not differ between groups (Supplementary Table 5). Comparisons of vaccinated and unvaccinated or partially vaccinated patients and of booster-unvaccinated and booster-vaccinated patients in each variant type are shown in Supplementary Tables 6–9.
Data from 106 patients whose serum samples were collected within 7 days of symptom onset or diagnosis were used for analyses using antibody titers (Figure 1B). The geometric mean antibody titers in patients who experienced fever, hypoxia, pneumonia, CRP elevation, and lymphopenia during hospitalization were 1201.5 U/mL, 98.8 U/mL, 774.1 U/mL, 1335.1 U/mL, and 1032.2 U/mL, respectively, which were lower than those in patients who did not (Supplementary Table 10, Supplementary Figure 2). Similar results were observed when the selected patients were divided into Delta and Omicron variant infection groups. Sensitivity analyses using 3-day and 5-day thresholds for time since symptom onset or diagnosis to antibody test did not differ from the main analyses (Supplementary Figures 2 and 3).
The multivariable model showed that an increase in antibody levels in vaccinated patients with Delta or Omicron variant infection was independently associated with a decrease in the occurrence of fever (adjusted odds ratio [aOR], 0.231; 95% CI, 0.105–0.511), hypoxia (aOR, 0.229; 95% CI, 0.075–0.703), CRP elevation (aOR, 0.524; 95% CI, 0.293–0.0.938), and lymphopenia (aOR, 0.568; 95% CI, 0.33–0.0.976) during hospitalization (Table 2). Although the occurrence of pneumonia was associated with antibody levels in the unadjusted analysis, statistical significance was not observed in the adjusted analysis (aOR, 0.526; 95% CI, 0.249–1.112).
Table 2.
Association of Antibody Titers and Variables With Clinical Courses During Hospitalization in Vaccinated Patients With Breakthrough Infections Caused by the Delta and Omicron Variants
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Fevera (BT ≥37.5°C) | 0.245 (0.116–0.517) | <.001 | 0.231 (0.105–0.511) | <.001 |
| Hypoxiab (SpO2 ≥94%) | 0.191 (0.074–0.490) | <.001 | 0.229 (0.075–0.703) | .010 |
| Pneumoniac,d | 0.427 (0.246–0.743) | .003 | 0.526 (0.249–1.112) | .093 |
| CRP elevatione (CRP >8 mg/L) | 0.503 (0.292–0.868) | .014 | 0.524 (0.293–0.938) | .030 |
| Lymphopeniaf (lymphocyte <1100 cells/μL) | 0.506 (0.304–0.841) | .009 | 0.568 (0.330–0.976) | .041 |
Multivariable logistic regression analysis was performed to determine the effect of antibody levels on the clinical course of breakthrough infections. Fever, hypoxia, pneumonia, CRP elevation, and lymphopenia were selected as variables representing the clinical course. Confounding factors were included in each multivariable model, as described below. Antibody titers were log10-transformed for analyses.
Abbreviations: BT, body temperature; CCI, Charlson comorbidity index; CRP, C-reactive protein; OR, odds ratio; SpO2, percutaneous oxygen saturation.
Multivariable analysis adjusted for age and sex.
Multivariable analysis adjusted for age, sex, immunocompromised status, and variant type.
Multivariable analysis adjusted for age, sex, immunocompromised status, CCI, and variant type.g
Presence of pneumonia was determined using chest radiography.
Multivariable analysis adjusted for sexg and variant type.
Multivariable analysis adjusted for sex, CCI, and variant type.
In addition to antibody titer, the Omicron variant was associated with a decrease in the occurrence of pneumonia (OR, 0.150; 95% CI, 0.050–0.448); female sex was associated with a decrease in the occurrence of CRP elevation (OR, 0.431; 95% CI, 0.194–0.955).
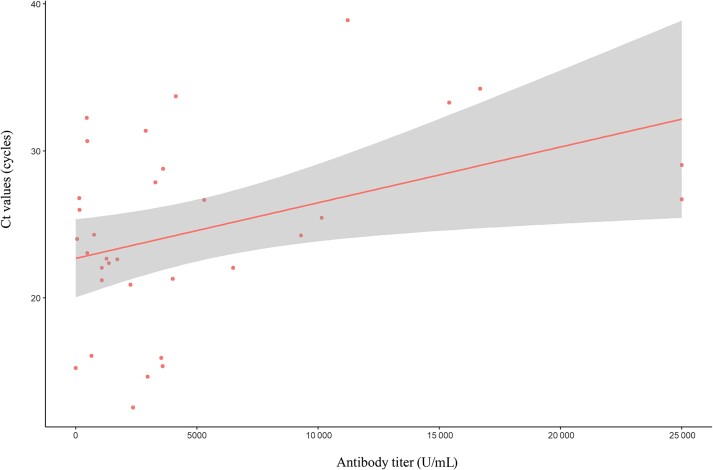
Therefore, we investigated the association between antibody levels and viral dynamics. Among 106 patients included in the analyses using antibody levels, 33 patients who had Ct values from the PCR tests 5–7 days after the initial diagnosis were selected. Patients with higher antibody levels had higher Ct values at 5–7 days after the initial diagnosis, indicating lower viral loads than those with lower antibody levels (P = .022) (Figure 3). We also evaluated the association between antibody levels and Ct values at the initial diagnosis. Although several different commercial PCR kits were used at initial diagnosis because the patients were tested in different institutions at the time of diagnosis, we found no difference in Ct values at the time of diagnosis according to antibody level (data not shown).
Figure 3.
Association of antibody titers and Ct values. Data from 33 patients, with Ct values measured 5–7 days after diagnosis, showed a positive correlation between antibody levels and Ct values (slope, 0.0004; P = .022). Abbreviations: Ct, cycle threshold; PCR, polymerase chain reaction.
DISCUSSION
We identified that the vaccine was still effective against SARS-CoV-2 infection, including the Delta and Omicron variants. Furthermore, our data showed that antibody titers were independently associated with the occurrence of specific signs, as indicators of an unfavorable clinical course, in patients with SARS-CoV-2 breakthrough infection, regardless of virus type or booster vaccination status. Our study provides important information for taking measures to minimize the damage caused by the current pandemic.
Vaccines against SARS-CoV-2 are effective in the prevention of adverse outcomes in patients with infections, such as hospitalization and death [12, 13]. Neutralizing antibody responses are highly predictive of protection in vaccinated individuals [14]. However, identifying neutralizing antibodies is not feasible for practical use in clinical settings because it is a labor-intensive and time-consuming task. Instead, monitoring antibody levels to spike proteins using commercial kits is more convenient and logistically feasible. Our study provides evidence that antibody level is an important predictor of the clinical course in patients with breakthrough infection by showing a close relationship between low antibody levels and an unfavorable disease course such as fever, hypoxia, pneumonia, CRP elevation, and lymphopenia. Our data fill the gap of missing data on the impact of variable antibody responses to vaccination on the disease course of COVID-19.
Booster vaccination increases humoral immunity to prevent hospitalization or severe disease progression in patients with Omicron variant infection as well as Delta variant infection [15, 16]. We also observed higher antibody levels among vaccine-completed individuals with booster vaccination, compared with primary vaccination (Supplementary Tables 5, 7, and 9). However, booster vaccination was not associated with a favorable disease course in patients with breakthrough infections caused by the Delta and Omicron variants in this study. We postulate that this finding is attributable to the fact that the indicator of adverse outcome used in this study could be sufficiently prevented by the level of antibodies mounted by primary vaccination within 4–5 months. This study could not evaluate the occurrence of severe outcomes (eg, mechanical ventilation or mortality) as indicators of adverse outcome because of the specific baseline conditions of the included patients. Therefore, the effect of antibodies according to different titers on these outcomes should be evaluated in future studies involving other populations.
Previous studies have reported that the Omicron variant has weakened virulence, which in turn results in reduced severity even if infection occurs [17, 18]. We also found similar findings in Omicron variant infections compared with Delta variant infections (Table 1). However, in breakthrough infections caused by Delta and Omicron variants, the variant type was not associated with the occurrence of the specific signs observed in this study, except for pneumonia (Table 2). We assume that our findings are owing to the reduced effectiveness of vaccines against Omicron variants. Indeed, the Omicron variant has multiple mutations that enable evasion of the vaccine-induced immune system [19, 20]. A decrease in immunity against symptomatic Omicron variant infection may offset the protective effect of the impaired virulence of the Omicron variant. Our postulation needs to be corroborated by further studies that have an extended period of observation to determine the effect of variant type on the clinical course.
We found differences in viral kinetics among vaccinated patients with a variety of antibody levels. The viral load decline rate was positively correlated with antibody levels. In other words, more rapid viral clearance was observed in patients with higher antibody levels than in those with lower antibody levels. These findings are in line with those of previous studies, which suggest that the host immune system determines viral clearance [21, 22]. Although we did not evaluate the association of clinical courses and viral kinetics in this study, owing to the limited number of participants who underwent PCR testing 5–7 days after the initial diagnosis, it is possible that rapid viral clearance may have contributed to the reduction in the occurrence of specific signs observed in this study among patients with breakthrough infection. This hypothesis should be tested in future studies.
Our study had several limitations. First, we could not exclude the effects of previous or current infections on antibody levels. However, none of the participants had a previous infection, although this information was self-reported. Furthermore, our findings were robust in a sensitivity analysis involving patients who tested antibody levels within 3 or 5 days of symptom onset or diagnosis. Second, as our study hospital has specific criteria for admission, which allowed only patients with mild to moderate severity to be admitted, we could not investigate the effects of antibody levels on outcomes such as moving to the severe phase (n = 2) or death (n = 0). Therefore, further studies involving a larger number of patients with a wide range of disease severities are warranted. Third, the diagnosis of pneumonia in this study was based on chest radiographic findings. Chest radiography is limited in its ability to detect subtle pneumonia. Furthermore, we did not routinely perform follow-up chest radiography if the initial test did not report abnormal findings and if the patient’s condition did not change. Therefore, the results associated with pneumonia should be cautiously interpreted. Fourth, we did not consider the type of vaccine (eg, adenoviral vector–based, mRNA-based) when analyzing antibody levels. Most previous studies have demonstrated the effectiveness of vaccines based on homologous vaccination data. However, the real-world population receives heterologous vaccines from different platforms for several reasons, such as the disruption of vaccine supply and vaccine-associated adverse events. Moreover, booster immunization programs have increased the possibility of receiving different types of vaccines. In this regard, we did not classify the participants according to the type of vaccine but only vaccination status to reflect real-world population data. Lastly, we explored the relationship of antibody levels with individual variables associated with poor outcomes instead of collective variables that predict adverse outcomes. As there is no consensus on the validity of the scoring system in predicting outcomes, we decided not to use it.
In conclusion, this study showed the significance of antibody levels as a predictor of the clinical course of COVID-19 in patients with breakthrough infections caused by Delta and Omicron variants. Moreover, in our study, enhanced viral clearance was observed in vaccinated patients with higher antibody levels than in those with lower antibody levels. We highlight the need for concentrated efforts to monitor patients with SARS-CoV-2 infection who have conditions related to poor antibody response to vaccination (eg, immunocompromised status) or waning immunity after vaccination (eg, adenoviral vector–based vaccine) as they are more likely to undergo an unfavorable disease course.
Supplementary Material
Acknowledgments
We would like to thank all of the nursing and laboratory staff as well as the physicians who supported this project. Finally, we give credit to all of the patients who took part in this study.
Financial support. This research received no specific grant from and funding agency, commercial or not-for-profit sectors.
Potential conflicts of interest. The authors declare that they have no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data availability. The data set supporting the conclusions of this article is included within Supplemental data (Appendix 2. Raw data used in the analysis).
Author contributions. Min Hyung Kim, Yooju Nam and Nak-Hoon, Son was responsible for data analysis and contributed to the drafting and writing of the manuscript. Yong Chan Kim was the chief investigator and was responsible for the conception, design of the study and contributed to drafting and writing of the manuscript. Namwoo Heo, Bongyoung Kim, Eawha Kang, Areum Shin and Andrew Jihoon Yang were involved in data acquisition and interpretation. Yoon Soo Park and Heejung Kim contributed to data curation. Taeyoung Kyong supervised the entire process and provided feedback. All authors approved the final version of the manuscript. Min Hyung Kim, Yooju Nam, and Nak-Hoon Son contributed equally to this manuscript. Yong Chan Kim and Taeyoung Kyong contributed equally as corresponding authors.
Contributor Information
Min Hyung Kim, Division of Infectious Disease, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Yooju Nam, Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Nak Hoon Son, Department of Statistics, Keimyung University, Daegu-si, Republic of Korea.
Namwoo Heo, Division of Infectious Disease, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Bongyoung Kim, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Republic of Korea.
Eawha Kang, Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Areum Shin, Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Andrew Jihoon Yang, Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Yoon Soo Park, Division of Infectious Disease, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Heejung Kim, Department of Laboratory Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Taeyoung Kyong, Department of Hospital Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Yong Chan Kim, Division of Infectious Disease, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin-si, Republic of Korea.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. World Health Organization . Classification of Omicron (B.1.1.529) SARS-CoV-2 variant of concern. Available at: https://www.who.int/news/item/26-11-2021-classification-of-Omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 11 February 2022.
- 2. World Health Organization WHO coronavirus (COVID-19) dashboard . Available at: https://covid19.who.int/. Accessed 11 February 2021.
- 3. Hu J, Peng P, Cao X, et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol 2022; 19:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu J, Peng P, Cao X, et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol 2022; 19:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2022; 386:494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis 2022; 116:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA 2022; 327:583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57:1288–94. [DOI] [PubMed] [Google Scholar]
- 10. UK Health Security Agency . Stepdown of infection control precautions and discharging COVID-19 patients and asymptomatic SARS-CoV-2 infected patients. Available at: https://www.gov.uk/government/publications/covid-19-guidance-for-stepdown-of-infection-control-precautions-within-hospitals-and-discharging-covid-19-patients-from-hospital-to-home-settings/guidance-for-stepdown-of-infection-control-precautions-and-discharging-covid-19-patients. Accessed 1 December 2021.
- 11. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]
- 12. Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;. 373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med 2021; 385(7):585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 15. Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta infection. medRxiv 2021.12. 30.21268565 [Preprint]. 28 January 2022. Available at: 10.1101/2021.12.30.21268565. Accessed 11 February 2022. [DOI] [Google Scholar]
- 16. Burki TK. Omicron variant and booster COVID-19 vaccines. Lancet Respir Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global Omicron variant COVID-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe 2021; 29:44–57.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021; 19:409–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caillard S, Benotmane I, Gautier Vargas G, Perrin P, Fafi-Kremer S. SARS-CoV-2 viral dynamics in immunocompromised patients. Am J Transplant 2021; 21:1667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS-CoV-2 Delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.