Abstract
Background
The Simplified Acute Physiology Score (SAPS) 3 is a reliable score to predict mortality. This study aims to investigate the predictive values of SAPS 3 and other clinical parameters for death in critically ill coronavirus disease 2019 (COVID-19) patients.
Methods
This is a prospective study in a tertiary hospital for patients who required intensive care due to COVID-19 infection in northeast Brazil. Two distinct groups were constructed according to the epidemiological data: first wave and second wave. The severity of patients admitted was estimated using the SAPS 3 score.
Results
A total of 767 patients were included: 290 were enrolled in the first wave and 477 in the second wave. Patients in the first wave had more comorbidities, were put on mechanical ventilation and required dialysis and vasopressors more frequently (p<0.05). During the second wave, non-invasive ventilation was more often required (p<0.05). In both periods, older patients and higher SAPS 3 scores on admission were associated with death (p<0.05). Non-invasive ventilation use showed a negative association with death only in the second wave period. In the first wave, the SAPS 3 score was more useful (area under the curve [AUC] 0.897) in predicting death in critically ill COVID-19 patients than in the second wave (AUC 0.810).
Conclusion
The SAPS 3 showed very reliable predictive values for death during the waves of the COVID-19 pandemic, mostly together with kidney and pulmonary dysfunction.
Keywords: COVID-19, SARS-CoV-2, Simplified Acute Physiology Score, intensive care units, mortality
Introduction
The whole world has been facing the coronavirus disease 2019 (COVID-19) pandemic, which is currently the most important public health problem worldwide. The weakness of the health system in low- and middle-income countries has required intense optimization of resources, especially in the care of critically ill patients.1
The lack of an effective medication for COVID-19 treatment, the emergence of variants and non-compliance with mobility restrictions and quarantine measures remain critical issues to be overcome.2,3 These conditions have led to the occurrence of subsequent waves of COVID-19 spread and new outbreaks.3 Reducing transmission through established and proven disease control measures is central to the global strategy aimed at reducing the occurrence of mutations with negative public health implications.3
There are very few data comparing the first and second waves of COVID-19 and the predictor factors in critically ill patients. Between February 2020 and June 2021, the Brazilian Ministry of Health was notified of >18 million cases of COVID-19 and a massive number of deaths (around 512 000).4
During the COVID-19 pandemic, predictor models were able to explain the vulnerability of some patients and the demand for intensive care measures.5 There are mathematical models aimed at predicting the waves, but it is important to compare each wave in terms of severity and clinical features.6 The predictive tools may improve assessment of risk-group patients using factors with better predictive values for the target outcome and then optimize healthcare priorities for these patients.6
In the pandemic scenario, some studies have investigated the validity of well-established intensive care score systems. Among them, the Simplified Acute Physiology Score (SAPS) 3 was recently evaluated and showed a reliable performance to predict mortality in patients admitted to the intensive care unit (ICU) with COVID-19.7
This study aims to report the correlation of clinical and laboratory parameters of critically ill COVID-19 patients during the first and second waves in northeast Brazil. The mortality predictors were analysed, including SAPS 3 scores, in both waves.
Methods
Study design and patients’ characteristics
This is a prospective study. All recruited patients were admitted to Instituto Doutor José Frota Hospital (IJF), a tertiary care hospital in the state of Ceara, in the northeast of Brazil, from March 2020 to June 2021. This public hospital became the referral hospital for patients who required intensive care due to COVID-19 infection.
The inclusion criteria were defined according to the Brazilian Ministry of Health for cases of severe acute respiratory syndrome (SARS). Patients were adults ≥18 y of age, with dyspnoea/respiratory distress, non-invasive peripheral oximetry <95% without external oxygen support or blue coloration of the lips or face and laboratory confirmation of COVID-19 via reverse transcription polymerase chain reaction (RT-PCR) or positive immunoglobulin M test for COVID-19, even without being RT-PCR positive.8 Hospital admission due to external causes such as trauma, major burns, poisoning, firearm injuries or automobile accidents were excluded.
Brazil reported the first case of COVID-19 in Latin America in February 2020. During the first wave, the number of cases increased continuously until they reached their peak at the end of July 2020, with a gradual decrease after that. Analyses of incidence data indicated that the second wave of COVID-19 started in in Brazil in November 2020.9
Patients’ electronic medical records at the time of the diagnosis were evaluated and divided into two distinct groups according to the hospital admission date and allocated to the first or second wave. Administrative data were prospectively collected and analysed from a cloud database program (Epimed System). The analysed variables were age, gender, ethnicity/skin colour, diagnostic criteria at admission, respiratory support, length of hospital stay, outcome, use of vasoactive drugs and haemodialysis in the first 24 h, comorbidities and the variables included in the SAPS 3 score. The comorbidities analysed were reported on admission: non-dialysis chronic kidney disease (CKD), CKD on dialysis, chronic obstructive pulmonary disease (COPD), arterial hypertension and diabetes mellitus. Morbid obesity was defined as a body mass index >40 kg/m2. The oxygenation index was calculated using the ratio of arterial oxygen partial pressure (PaO2, in mmHg) to fractional inspired oxygen (FiO2).
The severity of patients admitted to the ICU was estimated using the SAPS 3, which was calculated using the global standard equation.10 Data included in the analysis of SAPS 3 were collected within the first hour on admission. The score takes into account the arithmetic mean of the 20 variables distributed into three categories (see the supplemental table). In the first category, the conditions prior to the patient's admission to the ICU are considered, as well as the characteristics of this admission. The second category was the cause of hospitalization. The third category was physiological variables, such as age, Glasgow score, blood pressure, heart rate, oxygenation index, leucocyte count, pH and bilirubin and creatinine levels. For each analysed variable, a weight is assigned. As recommended, missing values were coded as ‘normal’ for each variable.10 We also collected the blood urea level, which is not included in the score. Patient discharge was defined as discharge from the hospital.
Statistical analysis
Categorical data were expressed as absolute count and relative frequencies as percentages. The χ2 test and Fisher’s exact test were used to evaluate associations between categorical data. Quantitative data were represented according to the data distribution. First, the Kolmogorov–Smirnov normality test was applied. Normal data were expressed as mean±standard deviation (SD) and non-normal data as median and interquartile range (IQR). To compare quantitative data between two independent groups, the Student's t-test or Mann–Whitney test was used according to normality.
Logistic regression analysis was performed to analyse predictive factors associated with death according to the wave period. The multivariate analysis used parameters with previously established statistical significance for death in each period (p<0.05). The most powerful predictive variables were selected in the multivariate regression models in each pandemic period using stepwise forward selection, using p≤0.20 to include the variable in the next step until the final model was achieved. The least absolute shrinkage and selection operator (LASSO) logistic regression was performed to avoid collinearity between variables and their overfitting. Moreover, multivariate LASSO regression models with selected variables were used to generate predictive values for each patient. The odds ratio (OR) with 95% confidence interval (CI) were calculated.
The prediction capacity of the different models with the respective predictive values were compared using the receiver operating characteristics (ROC) curve and the area under the ROC curve (AUC) with 95% CI. The data were evaluated using SPSS for Macintosh, version 23 (IBM, Armonk, NY, USA) and Orange for Macintosh, version 3.2 (Data Mining Toolbox in Python). For all analytical tests, a base value of p<0.05 was considered statistically significant.
Ethical approval
This study is part of the project ‘Clinical manifestations, complications, prognostic factors and treatment of hospitalized patients due to Coronavirus infection in Salvador and Fortaleza’, which was approved by the Brazilian National Research Ethics Committee (CAAE n. 30579020.4.1001.0008) and registered under approval number 4.026.888. Informed consent was obtained from all individual participants included in the study. As some patients admitted require immediate ICU care and were unable to sign, the consent was signed by a family member.
Results
Comparison of clinical and survival characteristics of patients admitted to the ICU due to COVID-19 between the first and second waves
In the studied period, 1023 critically ill patients were admitted to the ICU. Among them, 237 patients had another main diagnosis at ICU admission rather than COVID-19 and were excluded. Data were missing regarding the outcome of 19 patients and were also excluded. Finally, 767 patients admitted to the ICU due to COVID-19 infection were selected. Among them, 290 patients were enrolled in first wave period and 477 patients in second wave period (Figure 1).
Figure 1.
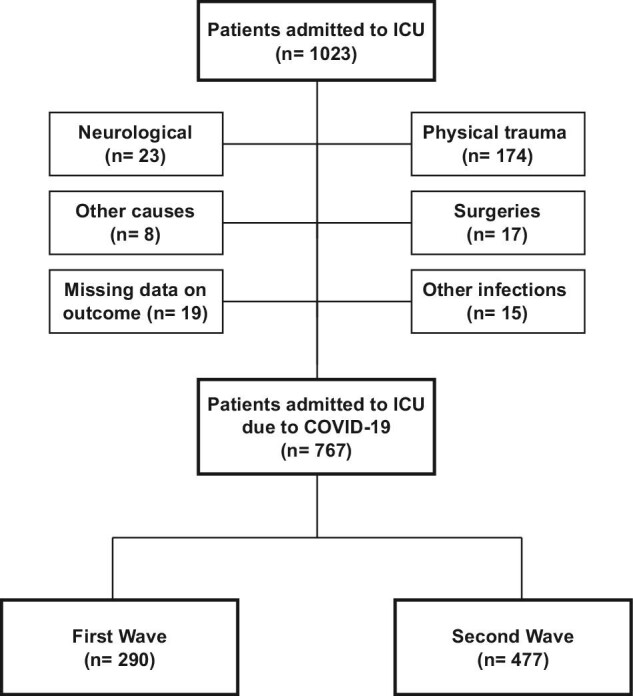
Flowchart of critically ill COVID-19 patients included in the study from each wave.
Regarding the epidemiological data, the patients admitted in the first wave period were older than the second wave patients (59.8±15.2 vs 57.1±14.9; p=0.015). Patients admitted to the ICU in the first wave showed a more severe profile according to SAPS 3 scores compared with the second wave (p<0.001) (Table 1). However, no statistical difference was observed regarding the death rates between the first and second waves (54% vs 48%, respectively; p=0.112).
Table 1.
Critically ill COVID-19 patients’ characteristics, support interventions, SAPS 3 score and outcome in the first and second waves
| Characteristics | First wave(n=290) | Second wave(n=477) | p-Value |
|---|---|---|---|
| Age (years), mean±SD | 59.8 ± 15.2 | 57.1 ± 14.9 | 0.015 |
| Age groups (years), n (%) | 0.206 | ||
| 18–40 | 35 (12.1) | 74 (15.5) | |
| 41–60 | 112 (38.6) | 196 (41.1) | |
| >60 | 143 (49.3) | 207 (43.4) | |
| Gender, n (%) | 0.309 | ||
| Male | 175 (60.3) | 270 (56.6) | |
| Female | 115 (39.7) | 207 (43.4) | |
| Comorbidities, n (%) | |||
| Non-dialysis CKD | 31 (13.1) | 11 (2.8) | <0.001 |
| CKD on dialysis | 4 (1.7) | 7 (1.8) | 0.873 |
| Severe COPD | 8 (3.4) | 9 (2.3) | 0.472 |
| Arterial hypertension | 153 (64.8) | 232 (59.2) | 0.198 |
| Diabetes | 91 (38.6) | 119 (31.8) | 0.088 |
| Morbid obesity | 56 (23.7) | 132 (33.7) | 0.016 |
| Support interventions in the ICU, n (%) | |||
| Mechanical ventilation | 233 (80.6) | 332 (67.2) | <0.001 |
| Non-invasive ventilation | 2 (0.7) | 126 (25.5) | <0.001 |
| Vasopressors | 197 (68.2) | 197 (39.9) | <0.001 |
| RRT | 102 (35.3) | 83 (16.8) | <0.001 |
| Vital signs and kidney and pulmonary aspects on ICU admission | |||
| Lowest systolic blood pressure (mmHg), mean±SD | 89.2±8.5 | 100.6±23.7 | <0.001 |
| Lowest diastolic blood pressure (mmHg), mean±SD | 52.8±9.4 | 56.7±16.6 | <0.001 |
| Lowest mean arterial pressure (mmHg), mean±SD | 64.8±8.6 | 71.3±18.4 | <0.001 |
| Highest respiratory rate (breaths/min), mean±SD | 33.5±2.7 | 29.1±4.3 | <0.001 |
| Highest creatinine level (mg/dL), median (IQR) | 1.6 (0.9–2.5) | 0.9 (0.7–2.1) | <0.001 |
| Lowest oxygenation index, median (IQR) | 39 (25–73) | 59 (34–93) | <0.001 |
| Urea (mg/dL), median (IQR) | 80 (45–130) | 64 (39–112) | 0.092 |
| SAPS 3 score, median (IQR) | 70 (55–78) | 59 (49–70) | <0.001 |
| Intensive care unit stay (days), median (IQR) | 11 (6–18) | 11 (7–18) | 0.941 |
| Outcome, n (%) | 0.112 | ||
| Survivors | 133 (45.9) | 247 (51.8) | |
| Non-survivors | 157 (54.1) | 230 (48.2) |
In general, patients admitted in the first COVID-19 wave showed more comorbidities, except morbid obesity. Regarding intensive care support interventions, mechanical ventilation (81 vs 67%; p<0.001), vasopressor (68 vs 40%; p<0.001) and renal replacement therapy (RRT; 35 vs 17%, p<0.001) were more often observed in the first wave. Non-invasive ventilation was more often performed in the second wave (35 vs 0.7%; p<0.001) (Table 1).
Clinical variables on ICU admission were more severe in the first wave period. In first wave, the following showed the lowest decrease: systolic and diastolic blood pressure, mean arterial pressure and oxygenation index; and the following showed the greatest increase: respiratory rate and serum creatinine (Table 1).
Association of clinical and outcome characteristics with death in patients admitted to the ICU due to COVID-19 in the first and second waves
In both the first and second wave periods, older patients and those with a higher SAPS 3 score on admission were associated with death (p<0.05). However, non-dialysis CKD was associated with death only in the first wave (Table 2). Regarding support interventions, higher rates of mechanical ventilation, vasopressors and RRT were associated with death. Non-invasive ventilation showed a negative association with death in the second wave period (p<0.001) (Table 2). There was no statistical association between death and vital signs, morbid obesity and diabetes in either of the periods.
Table 2.
Demographic parameters, SAPS 3 score and outcome of critically ill COVID-19 patients in the first and second waves according to outcome
| First wave (n=290) | Second wave (n=477) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Survivors (n=133) | Non-survivors (n=157) | p-Value | Survivors (n=247) | Non-survivors (n=230) | p-Value |
| Age (years), mean±SD | 55.2±14.1 | 63.7±15 | <0.001 | 53.5±14.5 | 60.8±14.4 | <0.001 |
| Age groups (years), n (%) | 0.001 | <0.001 | ||||
| 18–40 | 23 (17.3) | 12 (7.6) | 52 (21.1) | 22 (9.6) | ||
| 41–60 | 60 (45.1) | 52 (33.1) | 115 (46.6) | 81 (35.2) | ||
| >60 | 50 (37.6) | 93 (59.2) | 80 (32.4) | 127 (55.2) | ||
| Gender, n (%) | 0.762 | 0.738 | ||||
| Male | 79 (59.4) | 96 (61.1) | 138 (55.9) | 132 (57.4) | ||
| Female | 54 (40.6) | 61 (38.9) | 109 (44.1) | 98 (42.6) | ||
| Comorbidities, n (%) | ||||||
| Non-dialysis CKD | 8 (7.7) | 23 (17.4) | 0.028 | 3 (1.7) | 8 (4.1) | 0.155 |
| CKD on dialysis | 3 (2.9) | 1 (0.8) | 0.323 | 3 (1.7) | 4 (2.1) | 1.000 |
| Severe COPD | 6 (5.8) | 2 (1.5) | 0.143 | 3 (1.7) | 6 (3.1) | 0.505 |
| Arterial hypertension | 65 (62.5) | 88 (66.7) | 0.506 | 104 (57.5) | 119 (61.7) | 0.461 |
| Diabetes | 42 (40.4) | 49 (37.1) | 0.609 | 50 (27.6) | 69 (35.8) | 0.092 |
| Morbid obesity | 29 (27.9) | 27 (20.5) | 0.183 | 66 (36.5) | 57 (29.5) | 0.154 |
| Support interventions, n (%) | ||||||
| Mechanical ventilation | 78 (59.1) | 155 (98.7) | <0.001 | 122 (49.4) | 200 (87.3) | <0.001 |
| Non-invasive ventilation | 2 (1.5) | 0 (0) | 0.208 | 82 (33.2) | 36 (15.7) | <0.001 |
| Vasopressors | 52 (39.4) | 145 (92.4) | <0.001 | 54 (21.9) | 141 (61.6) | <0.001 |
| RRT | 13 (9.8) | 89 (56.7) | <0.001 | 20 (8.1) | 63 (27.5) | <0.001 |
| Vital signs and kidney and pulmonary aspects on ICU admission | ||||||
| Lowest systolic blood pressure (mmHg), mean±SD | 92.7±7 | 86.2±8.6 | <0.001 | 104.5±24.5 | 97.8±23 | 0.003 |
| Lowest diastolic blood pressure (mmHg), mean±SD | 57.4± .4 | 48.9±9.1 | <0.001 | 60.4±17.2 | 53.9±15.7 | <0.001 |
| Highest respiratory rate (breaths/min), mean±SD | 33.1±2.9 | 33.8±2.6 | 0.026 | 28.5±4.7 | 29.5±4.1 | <0.001 |
| Highest creatinine level (mg/dL), median (IQR) | 1 (0.8–1.8) | 2 (1.4–3) | <0.001 | 0.8 (0.6–1.1) | 1.4 (0.75–3) | <0.001 |
| Lowest oxygenation index, median (IQR) | 27.7 (21.9–50.7) | 59.2 (30.9–95.2) | <0.001 | 45.3 (30.1–79.0) | 72.1 (41.0–104.2) | <0.001 |
| Urea (mg/dL), median (IQR) | 46.5 (37– 85) | 106 (68–146) | <0.001 | 46 (34–73.5) | 90 (60–144) | <0.001 |
| Intensive care unit length of stay (days), median (IQR) | 11 (11–17) | 11 (11–18) | 0.873 | 12 (12–17) | 11 (1– 18) | 0.535 |
| SAPS 3 score, median (IQR) | 54 (54–65) | 76 (76–84) | <0.001 | 52 (52–60) | 68.5 (68.5–75) | <0.001 |
Predictive risk factors for death in each period: first and second waves
Logistic stepwise regression was used with SAPS 3 and other admission parameters, with or without support interventions, aimed at evaluating models with the most powerful variables to predict death. In the first wave of the pandemic, SAPS 3, lowest oxygenation index and RRT remained statistically associated with death after adjusting the multivariate models. Without support intervention use in the multivariate analysis, in addition to SAPS 3, the lowest oxygenation index and urea level remained statistically significant for death (Table 3).
Table 3.
Logistic regression to evaluate an independent association between SAPS 3 score and other clinical parameters analysed in the first 24 h of ICU admission
| Death | |||
|---|---|---|---|
| Variables | OR | 95% CI | p-Value |
| First wave | |||
| Model 1 (SAPS 3, admission data and support interventions) | |||
| SAPS 3 score | 1.153 | 1.095 to 1.213 | <0.001 |
| Lowest oxygenation index | 1.016 | 1.001 to 1.03 | 0.038 |
| RRT | 4.632 | 1.733 to 12.384 | 0.002 |
| Model 2 (SAPS 3 and admission data, without support interventions) | |||
| SAPS 3 score | 1.156 | 1.098 to 1.216 | <0.001 |
| Urea level | 1.01 | 1.002 to 1.018 | 0.013 |
| Lowest oxygenation index | 1.018 | 1.003 to 1.033 | 0.016 |
| Second wave | |||
| Model 1 (SAPS 3, admission data and support interventions) | |||
| SAPS 3 score | 1.111 | 1.063 to 1.162 | <0.001 |
| Urea level | 1.005 | 0.997 to 1.012 | 0.200 |
| Lowest oxygenation index | 1.008 | 0.999 to 1.017 | 0.085 |
| Mechanical ventilation | 2.356 | 1.062 to 5.226 | 0.035 |
| Model 2 (SAPS 3 and admission data, without support interventions) | |||
| SAPS 3 score | 1.133 | 1087 to 1.182 | <0.001 |
| Lowest oxygenation index | 1.008 | 1.000 to 1.017 | 0.064 |
| Urea level | 1.006 | 0.998 to 1.013 | 0.135 |
The stepwise forward method was applied to models that had variables with a significance of p≤0.2.
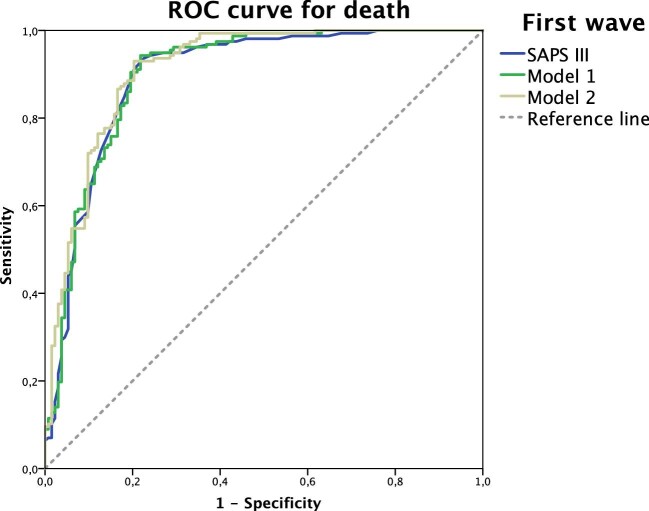
The SAPS 3 score and predictive values were generated by LASSO regression. The different models and their performance to predict death were evaluated using the ROC curve. In the first wave, the SAPS 3 was more useful (AUC 0.897) in predicting death in critically ill COVID-19 patients than in the second wave period (AUC 0.810).
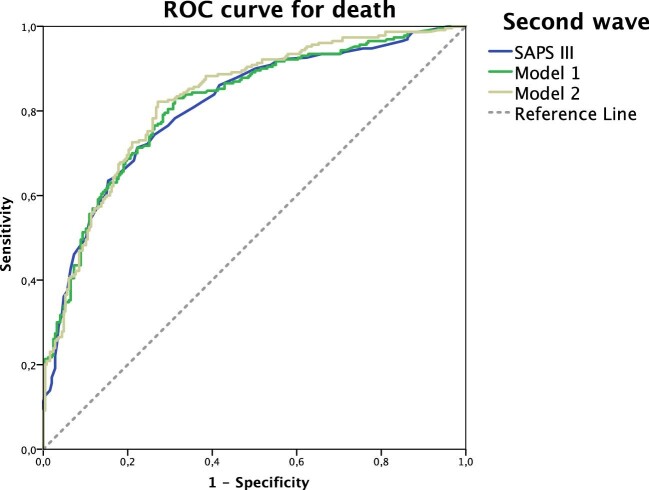
The use of SAPS 3 with urea level and the lowest oxygenation index in the first 24 h of the ICU stay improved the AUC to 0.901 in the first wave. When evaluating the model with support intervention (RRT), the AUC improved to 0.912 (Table 4, Figure 2). Similarly, in the second wave period, the use of SAPS 3 with urea and the lowest oxygenation index in the first 24 h of the ICU stay improved the AUC to 0.816 and subsequently to 0.827, using support intervention as the powerful variable, which was mechanical ventilation (Table 4, Figure 3).
Table 4.
Performance of SAPS 3 and selected models with powerful variables that predicted death in critically ill patients due to COVID-19 in the first and second waves
| ROC for death | |||
|---|---|---|---|
| Variables | AUC | 95% CI | p-Value |
| First wave | |||
| SAPS 3 | 0.897 | 0.858 to 0.936 | <0.001 |
| SAPS 3, urea level, lowest oxygenation index | 0.901 | 0.863 to 0.939 | <0.001 |
| SAPS 3, urea level, lowest oxygenation index, RRT | 0.912 | 0.877 to 0.946 | <0.001 |
| Second wave | |||
| SAPS 3 | 0.810 | 0.771 to 0.848 | <0.001 |
| SAPS 3, urea, lowest oxygenation index | 0.816 | 0.777 to 0.854 | <0.001 |
| SAPS 3, urea, lowest oxygenation index, mechanical ventilation | 0.827 | 0.790 to 0.864 | <0.001 |
Figure 2.
Comparisons between ROC curves for death in the first wave using only SAPS 3 (blue line), model 1 (green line) and model 2 (yellow line). Model 1: SAPS 3 with urea level and lowest oxygenation index. Model 2: SAPS 3, urea level, lowest oxygenation index and RRT.
Figure 3.
Comparisons between ROC curves for death in the second wave using only SAPS 3 (blue line), model 1 (green line) and model 2 (yellow line). Model 1: SAPS 3 with urea level and lowest oxygenation index. Model 2: SAPS 3, urea level, lowest oxygenation index and mechanical ventilation.
Discussion
In this study, performed in one of the most affected cities in Brazil, critically ill COVID-19 patients in the first wave arrived with a more severe profile for ICU care than those who arrived during the second wave period. Despite that, the mortality remained similar between the periods. Moreover, the SAPS 3 score and kidney and pulmonary disturbances were important predictors of death in both waves, even in adjusted models with other associated factors. The differences between the first and second waves of COVID-19 disease are fundamental for a better understanding of the outcomes throughout the pandemic, identifying risk factors and prognostic indicators to decrease morbidity and mortality.
According to this study, some baseline epidemiological features were very similar during the first and second waves of COVID-19 in northeast Brazil, such as ICU length of stay and the predominance of the male gender, but the second wave showed a younger age profile. This scenario is quite variable around the world. In some countries the patients in the second wave were younger than in the first wave,11,12 while other countries did not disclose important differences.13
Regarding comorbidities, the first wave had a higher prevalence of hypertension and diabetes in ICU-admitted patients, as well as non-dialysis CKD compared with the second wave. Several studies have shown that the presence of comorbidities is related to more severe forms of COVID-19 and the hospital length of stay.14,15 Although some studies have reported a higher occurrence of comorbidities in the first wave,16 recent studies have not reported differences in the prevalence of comorbidities between the two waves.12,13 It is important to note that vaccination in Brazil started in January 2021, with the elderly and patients with comorbidities as a priority, which is probably one of the factors that would explain the change in the age group observed in the second wave and, consequently, a lower prevalence of comorbidities.
In the current study, non-dialysis CKD was associated with death only in patients in the first wave period, in more severe patients according to the SAPS 3 score. Despite extensive reports of the COVID-19 impact on the kidneys in the acute setting and its association with in-hospital mortality in patients with any kidney involvement, much less information has been published on the impact of COVID-19 in patients with underlying CKD.17 Importantly, some studies have described a wide range of CKD prevalence in COVID-19 on hospital admission, varying from 0.7 to 47.6%.18–20 Additionally, the literature has reported that CKD is an important risk factor associated with the severity of COVID-19.19 We considered that detailed knowledge about the disease by both health professionals and the population during the second wave contributed to medical care earlier in non-dialysis CKD patients, avoiding COVID-19 disease progression and worsening of kidney function, and thus improving the prognosis.
In the present study, the SAPS 3 score calculated on ICU admission was an important predictor of death in COVID-19 patients in the first and second waves. Moreover, according to SAPS 3, patients admitted in the first wave of COVID-19 had more severe disease. In fact, they required more intensive care support in the first 24 h of ICU admission, such as a higher prevalence of mechanical ventilation use, use of vasopressor drugs and RRT. The SAPS 3 is a well-validated scoring system widely used around the world for the prediction of hospital mortality.21,22 However, a recent study investigated the performance of the SAPS 3 score in 30.571 patients admitted to private ICUs in Brazil. The authors investigated if adjustments in the SAPS 3 score modifies its performance and suggested that SAPS 3 should be used with caution for mortality prognosis in critically ill patients with COVID-19.23
Based on the SAPS 3 score in the current study, statistical models were generated and parameters of kidney and pulmonary disturbances were more powerful predictors for death in both waves. Kidney disease indicators, such as elevated urea observed in the present study, were early associated with in-hospital death in other important prospective studies, even after adjusting for age, sex, disease severity and comorbidities.24 In addition, it is well recognized that COVID-19 mortality is associated with pulmonary disturbances. We reported patients with an elevated SAPS 3 score added to a worse oxygenation index and invasive mechanical ventilation requirement were independent factors for death, mostly in the second wave period. We hypothesize that during the second wave, well-defined criteria for indicating non-invasive support improved hypoxemic acute respiratory failure, reducing hospital length of stay and the need for invasive mechanical ventilation and decreasing the number of deaths. The increasing use of non-invasive mechanical ventilation might have a decisive impact on mortality.25,26 In fact, the use of non-invasive ventilation such as Elmo (assisted breathing helmet) and high-flow therapy through a high-flow nasal cannula was one of many changes in the management of critically ill patients during the COVID-19 pandemic.
Greater knowledge of COVID-19 pathophysiology and disease evolution led to lower mortality rates in the second wave. Additionally, greater access to diagnostic tests, the emergence of protocols, better structuring of units to receive critically ill patients and the use of pharmacological treatments known to improve the survival of these patients, such as dexamethasone and anticoagulants, were relevant factors in the second wave.27–29
Limitations
There were some limitations in our study. It was a retrospective, single-centre study and there were missing data for some patients, especially the occurrence of comorbidities. It was not possible to specify the length of hospital stay of patients before arrival at the ICU and how many patients received vaccines in the second wave. We did not evaluate the SAPS 3 score weight in comparison with other different scores studied in the COVID-19 population.30
Conclusions
The SAPS 3 score has been shown to be a very reliable predictive value for death during the first and second waves of the COVID-19 pandemic, mostly together with kidney and pulmonary dysfunction. In a sample of critically ill Brazilian patients admitted during the first wave, serum urea level, lowest oxygenation index and RRT use were associated with higher mortality rates. On the other hand, during the second wave, mechanical ventilation use was an important mortality predictor. Further studies are required to understand the association between kidney and respiratory dysfunction.
Supplementary Material
Acknowledgements
We would like to give special thanks to the Brazilian agencies that funded this project: Coordination for the Improvement of Higher Education Personnel, Ceará Foundation for the Support of Scientific and Technological Development and the National Council for Scientific and Technological Development. We are also grateful to the Toxicological Assistance Center and Laboratory of Instituto Doutor Jose Frota Hospital.
Contributor Information
Ana Paula Pires Lázaro, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil; Post-Graduate Program in Public Health, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Polianna Lemos Moura Moreira Albuquerque, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil; Instituto Doutor Jose Frota Hospital, R. Barão do Rio Branco, 1816, Fortaleza, CE, 60025-061, Brazil.
Gdayllon Cavalcante Meneses, Post-Graduate Program in Medical Sciences, Federal University of Ceara, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Marza de Sousa Zaranza, Instituto Doutor Jose Frota Hospital, R. Barão do Rio Branco, 1816, Fortaleza, CE, 60025-061, Brazil; Post-Graduate Program in Medical Sciences, Federal University of Ceara, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Ana Beatriz Batista, Instituto Doutor Jose Frota Hospital, R. Barão do Rio Branco, 1816, Fortaleza, CE, 60025-061, Brazil.
Natalia Linhares Ponte Aragão, Instituto Doutor Jose Frota Hospital, R. Barão do Rio Branco, 1816, Fortaleza, CE, 60025-061, Brazil.
Andrea Mazza Beliero, Instituto Doutor Jose Frota Hospital, R. Barão do Rio Branco, 1816, Fortaleza, CE, 60025-061, Brazil.
Álvaro Rolim Guimarães, Federal University of Ceara, Av. da Universidade, 2853, Fortaleza, CE, 60020-181, Brazil.
Nilcyeli Linhares Aragão, Post-Graduate Program in Medical Sciences, Federal University of Ceara, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Alessandra Marjorye Maia Leitão, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Marcelo Costa Freire de Carvalho, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Maria Isabel de Alencar Cavalcante, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Fabio Augusto Xerez Mota, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Elizabeth De Francesco Daher, Post-Graduate Program in Medical Sciences, Federal University of Ceara, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Alice Maria Costa Martins, Post-Graduate Program in Pharmaceutical Sciences, Federal University of Ceara, Rua Capitão Francisco Pedro, 1210 , Fortaleza, CE, 60.430-370, Brazil.
Geraldo Bezerra da Silva Junior, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil; Post-Graduate Program in Public Health, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil; Post-Graduate Program in Medical Sciences, University of Fortaleza, Av. Washington Soares, 1321, Fortaleza, CE, 60811-905, Brazil.
Authors’ contributions
APPL, PLMMA and GCM conceived the study and conducted the selection, interpretation of data and project administration. MSZ, ABB, NLPA, AMB, ARG, NLA, AMML, MCFC, MIAC and FAXM contributed to the study design and selection of the studies. AMCM, GBSJ and EFD revised the manuscript. GCM performed the design and interpretation of data and formal analysis. EFD and PLMMA were responsible for funding acquisition. All authors read and approved the final version of the manuscript.
Funding
CAPES supported the research through the following grants: 88881.505364/2020-01, call 09/2020, Program Epidemics (to EFD) and 88882.306447/2018-01 (to PLMMA).
Competing interests
None declared.
Ethical approval
This study is part of the project ‘Clinical manifestations, complications, prognostic factors and treatment of hospitalized patients due to coronavirus infection in Salvador and Fortaleza’, which was approved by the Brazilian National Research Ethics Committee (CAAE n. 30579020.4.1001.0008) and registered under the approval number 4.026.888. Informed consent was obtained from all individuals participating in the study.
Data availability
The authors confirm that the data supporting the findings of this study are available in the article.
References
- 1. Tan E, Song J, Deane AMet al. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest. 2021;159(2):524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stipic D, Bradac M, Lipic Tet al. Effects of quarantine disobedience and mobility restrictions on COVID-19 pandemic waves in dynamical networks. Chaos Solitons Fractals. 2021;150:111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Tracking SARS-CoV-2 variants. Geneva: World Health Organization; 2021. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [accessed 10 May 2022]. [Google Scholar]
- 4. Worldometer . Worldometer COVID-19 data. Available from: https://www.worldometers.info/coronavirus/about/ [accessed 10 May 2022]. [Google Scholar]
- 5. Mani VR, Kalabin A, Valdivieso SCet al. New York Inner City Hospital COVID-19 experience and current data: retrospective analysis at the epicenter of the American coronavirus outbreak. J Med Internet Res. 2020;22(9):e20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scharbarg E, Moog CH, Mauduit Net al. From the hospital scale to nationwide: observability and identification of models for the COVID-19 epidemic waves. Annu Rev Control. 2020;50:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metnitz PGH, Moreno RP, Fellinger Tet al. Evaluation and calibration of SAPS 3 in patients with COVID-19 admitted to intensive care units. Intensive Care Med. 2021;47(8):910–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministério da Saúde . Diretrizes para diagnóstico e tratamento da COVID-19. Secretaria de Ciência, Tecnologia, Inovação e Insumos Estratégicos em Saúde. 2020. Available from: https://www.gov.br/saude/pt-br [accessed 10 May 2022]. [Google Scholar]
- 9. Silva SJR, Pena L.. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13:100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreno RP, Metnitz PG, Almeida Eet al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Domingo P, Pomar V, Mur Iet al. Not all COVID-19 pandemic waves are alike. Clin Microbiol Infect. 2021;27(7):1040.e7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iftimie S, López-Azcona AF, Vallverdú Iet al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16(3):e0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brehm TT, Heyer A, Roedl Ket al. Patient characteristics and clinical course of covid-19 patients treated at a German tertiary center during the first and second waves in the year 2020. J Clin Med. 2021;10(11):2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrilli CM, Jones SA, Yang Jet al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson S, Hirsch JS, Narasimhan Met al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saito S, Asai Y, Matsunaga Net al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82(4):84–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kant S, Menez SP, Hanouneh Met al. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. 2020;21:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan WJ, Ni ZY, Hu Yet al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arentz M, Yim E, Klaff Let al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adapa S, Chenna A, Balla Met al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 2020;12(6):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Metnitz PG, Moreno RP, Almeida Eet al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31(10):1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Merwe E, Kapp J, Pazi Set al. The SAPS 3 score as a predictor of hospital mortality in a South African tertiary intensive care unit: a prospective cohort study. PLoS One. 2020;15(5):e0233317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurtz P, Bastos LSL, Salluh JIFet al. SAPS-3 performance for hospital mortality prediction in 30,571 patients with COVID-19 admitted to ICUs in Brazil. Intensive Care Med. 2021;47(9):1047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Y, Luo R, Wang Ket al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang BJ, Koh Y, Lim CMet al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–32. [DOI] [PubMed] [Google Scholar]
- 26. Ricard JD, Roca O, Lemiale Vet al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020;46(12):2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hattori T, Saito A, Chiba Het al. Characteristics of COVID-19 patients admitted into two hospitals in Sapporo, Japan: analyses and insights from two outbreak waves. Respir Investig. 2021;59(2):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karagiannidis C, Windisch W, McAuley DFet al. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med. 2021;9(5):e47–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators . Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385(9):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vicka V, Januskeviciute E, Miskinyte Set al. Comparison of mortality risk evaluation tools efficacy in critically ill COVID-19 patients. BMC Infect Dis. 2021;21:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available in the article.