Abstract
Most strains of the insecticidal bacterium Bacillus thuringiensis have a combination of different protoxins in their parasporal crystals. Some of the combinations clearly interact synergistically, like the toxins present in B. thuringiensis subsp. israelensis. In this paper we describe a novel joint activity of toxins from different strains of B. thuringiensis. In vitro bioassays in which we used pure, trypsin-activated Cry1Ac1 proteins from B. thuringiensis subsp. kurstaki, Cyt1A1 from B. thuringiensis subsp. israelensis, and Trichoplusia ni BTI-Tn5B1-4 cells revealed contrasting susceptibility characteristics. The 50% lethal concentrations (LC50s) were estimated to be 4,967 of Cry1Ac1 per ml of medium and 11.69 ng of Cyt1A1 per ml of medium. When mixtures of these toxins in different proportions were assayed, eight different LC50s were obtained. All of these LC50s were significantly higher than the expected LC50s of the mixtures. In addition, a series of bioassays were performed with late first-instar larvae of the cabbage looper and pure Cry1Ac1 and Cyt1A1 crystals, as well as two different combinations of the two toxins. The estimated mean LC50 of Cry1Ac1 was 2.46 ng/cm2 of diet, while Cyt1A1 crystals exhibited no toxicity, even at very high concentrations. The estimated mean LC50s of Cry1Ac1 crystals were 15.69 and 19.05 ng per cm2 of diet when these crystals were mixed with 100 and 1,000 ng of Cyt1A1 crystals per cm2 of diet, respectively. These results indicate that there is clear antagonism between the two toxins both in vitro and in vivo. Other joint-action analyses corroborated these results. Although this is the second report of antagonism between B. thuringiensis toxins, our evidence is the first evidence of antagonism between toxins from different subspecies of B. thuringiensis (B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. israelensis) detected both in vivo and in vitro. Some possible explanations for this relationship are discussed.
Bacillus thuringiensis is an entomopathogenic bacterium that is produced commercially and accounts for more than 90% of the world market for biological insecticides. During sporulation, the bacterial cells produce proteinaceous crystalline inclusions. These crystals are composed of protein protoxins that are modified in the insect gut to produce active toxins, the δ-endotoxins. These proteins are toxic to lepidopteran, dipteran, and coleopteran insects, and toxicity to nematodes, mites, and protozoans has also been reported (17). The mode of action of these proteins is based on solubilization and partial proteolysis in the insect intestine, in which the activated toxins interact with the membranes of columnar cells of the intestinal epithelium and damage the integrity of the gut lining; this is followed by paralysis of the host and death (10).
The amino acid or gene sequences of more than 100 different toxin proteins (Cry proteins) have been reported to date. Because of this sequence information, the relationships among the various Cry proteins that are active against lepidopteran, dipteran and coleopteran species have become apparent. In addition, a distinct group of toxins, the Cyt proteins, which are found only in mosquitocidal strains, has been detected. The Cyt and Cry proteins exhibit no sequence homology. Many strains of B. thuringiensis contain different combinations of toxins in their parasporal crystals; these toxins, such as the toxins present in strain HD-1 of B. thuringiensis subsp. kurstaki, may be closely related. Other strains, particularly strains exhibiting mosquitocidal activity, contain diverse Cry and Cyt proteins in their parasporal crystals (7).
Few workers have attempted to evaluate the combined action of toxins that naturally occur together or experimental mixtures of different toxins. Synergistic interactions between different δ-endotoxins have been described occasionally; these interactions include the synergism among B. thuringiensis subsp. kurstaki toxins and the synergism among B. thuringiensis subsp. israelensis toxins (16, 18). Other studies of interactions between δ-endotoxins have revealed additive effects, and there has been only one previous report concerning unambiguous antagonism between closely related toxins (13).
In the present paper we describe an antagonistic relationship between the following two quite different B. thuringiensis toxins: Cry1Ac1, a toxin with high activity toward members of the Lepidoptera, and Cyt1A1, which originated from a mosquitocidal strain but has a broad activity range due to its cytolytic capacity.
MATERIALS AND METHODS
Strains and plasmids.
The recombinant plasmids pHT3101-Cry1Ac1 and pWF45 were used to produce the Cry1Ac1 and Cyt1A1 proteins, respectively. These plasmids were kind gifts from Brian Federici (University of California, Riverside). Acrystaliferous strain cry−B of B. thuringiensis was used for transformation with the recombinant plasmids. Transformed bacteria were grown in Tris-G supplemented with 25 μg of erythromycin per ml (15).
Preparation of toxins.
Crystals were harvested from the transformed strains and purified by using the procedure described by López-Meza and Ibarra (15). Crystals were solubilized by using 50 mM Na2CO3 at 37°C for 2 h at pH 10.5 for Cry1Ac1 or at pH 9.5 for Cyt1A1 (1 μg of protein/ml); 25 mM dithiothreitol was also added to the preparations of Cry1Ac1. The concentrations of proteins in supernatants were determined by the method of Bradford (1) by using bovine serum albumin as the standard. The solubilized protoxins were activated by using a procedure described elsewhere (15).
Bioassays with BTI-Tn5B1-4 cells.
Cell line BTI-Tn5B1-4 of Trichoplusia ni was generously donated by Robert R. Granados (Boyce Thompson Institute) and was used to determine the biological activities of the toxins. This cell line was maintained in 25-cm2 culture bottles (Falcon) at 28°C by using TC-100 medium supplemented with 10% fetal bovine serum. To determine 50% lethal concentrations (LC50s), Cyt1A1 protein was tested at concentrations of 3.36 to 20 ng/ml, and concentrations between 468.75 and 15,000 ng/ml were used for Cry1Ac1 protein. Four different Cry1Ac1/Cyt1A1 ratios were also tested; these ratios were 99:1, 99.5:0.5, 99.75:0.25, and 99.875:0.125, and two replicates were tested for each ratio. The concentrations of the toxins in the test preparations ranged from 11.62 to 17.27 and from 1,723 to 9,905 ng/ml for Cyt1A1 and Cry1Ac1, respectively.
The in vitro bioassays were based on the assay of Chow and Gill (5), with minor modifications. Microtiter plates with 96 wells were inoculated with 50,000 cells/well. The cells were allowed to adhere for 1 h at 28°C, and each well was then inoculated with a different concentration of activated toxin(s). The cells were incubated in the presence of the toxin(s) for 4 h at 28°C, and then the preparations were centrifuged at 1,090 × g for 5 min. Living cells were quantified as described elsewhere (5).
Analysis.
The LC50s of toxins acting independently were estimated by a Probit analysis (8) based on at least three independent bioassays. When toxin mixtures were used, eight bioassays were performed for each toxin combination. The combined effect of the toxins was analyzed by the Tammes-Bakuniak graphical method by using isobolograms (2). The expected toxicity of a toxin mixture was calculated by using the following formula of Tabashnik (18):
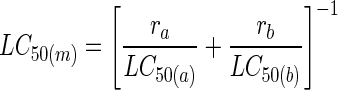 |
where LC50(m) is the expected LC50, which is the harmonic mean of the LC50s estimated for toxins a and b acting separately and ra and rb are the relative proportions of toxin a and toxin b in the mixture, respectively.
Bioassays performed with T. ni larvae.
T. ni larvae were maintained on a semisynthetic diet under insectary conditions by using previously described techniques (11). To determine the LC50 of a pure crystal preparation of Cry1Ac1, eight concentrations were tested; the maximum concentration was 40 ng of toxin/cm2 of diet, and a dilution factor of 0.6 was used to obtain the lower concentrations. To determine the equivalent value for pure preparations of Cyt1A1 toxin, concentrations of 0.01, 0.1, 1.0, and 10 μg/cm2 of diet were tested. In additional bioassays, larvae were offered a single dose consisting of 1 μg of Cyt1A1 activated toxin per larva.
The bioassays in which toxin combinations were used were performed by using the concentrations of Cry1Ac1 toxin described above and 1.0 or 0.1 μg of Cyt1A1 toxin per cm2 of diet. Mortality was recorded after incubation for 5 days under insectary conditions. The mortality data were subjected to a Probit analysis.
RESULTS
Effects of toxins on BTI-Tn5B1-4 cells.
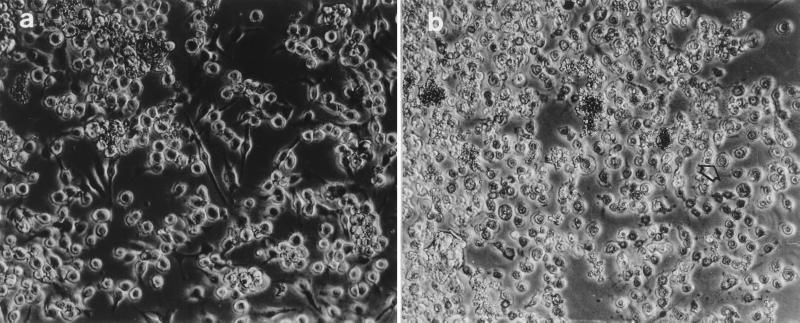
Both Cyt1A1 and Cry1Ac1 toxins caused cytolytic damage when they were tested on monolayers of BTI-Tn5B1-4 cells (Fig. 1). The cell responses to the toxins included cell rounding, followed by swelling and the formation of membrane vesicles and finally lysis. The Cyt1A1 toxin was far more active than Cry1Ac1, producing complete lysis more quickly and at lower concentrations. The typical spindle shape of the BTI-Tn5B1-4 cells (Fig. 1a) disappeared after 1 h of exposure to Cyt1A1 (Fig. 1b). Moreover, following the 4-h incubation period of the assay, the cells disintegrated completely, leaving only cell debris (Fig. 1b, arrow).
FIG. 1.
BTI-Tn5B1-4 cell line. (a) Normal cells. (b) Cells 1 h after treatment with Cyt1A1. The arrow indicates a cell exhibiting typical symptoms caused by this toxin.
Toxicity in vitro.
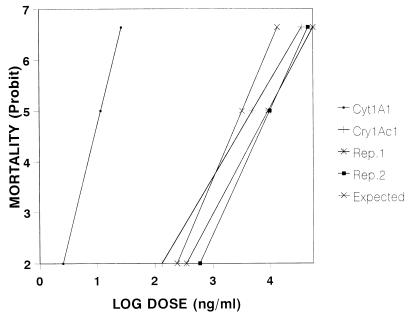
When the toxins were assayed individually with cells, the LC50s were estimated to be 11.64 ng/ml for CytA1 and 4,957 ng/ml for Cry1Ac1 (Table 1), which indicated that Cyt1A1 was more than 400 times more active than Cry1Ac1 with the cell line used. When mixed toxins were assayed in four different proportions, the estimated LC50s varied from 1,740 to 9,308 ng of total protein per ml (sum of the two toxins) (Table 1). The LC50s increased as the proportion of the Cry1Ac1 toxin increased. The narrow confidence limits of the calculated LC50s indicate that our estimates are very reliable. The high level of toxicity of Cyt1A1 compared to Cry1Ac1 was also evident from the marked separation of the corresponding regression lines and the location of the Cyt1A1 line far to the left on the graph (Fig. 2).
TABLE 1.
Estimated LC50s of Cry1Ac1 and Cyt1A1 toxins acting individually and in mixtures against the BTI-Tn5B1-4 cell line and comparison with the expected LC50s of mixtures
| Cry1Ac1/Cyt1A1 ratio | Estimated LC50 (ng/ml) | Higher fiducial limit (ng/ml) | Lower fiducial limit (ng/ml) | Expected LC50 (ng/ml)a |
|---|---|---|---|---|
| 100:0 | 4,957.00 | 5,786 | 4,286 | |
| 0:100 | 11.64 | 13.35 | 10.3 | |
| 99:1 | 1,740.00 | 1,935 | 1,608 | 956 |
| 99:1 | 1,744.00 | 3,506 | 1,378 | 956 |
| 99.5:0.5 | 2,726.00 | 4,678 | 2,028 | 1,598 |
| 99.5:0.5 | 2,733.00 | 4,010 | 2,126 | 1,598 |
| 99.75:0.25 | 5,934.00 | 8,635 | 4,878 | 2,415 |
| 99.75:0.25 | 6,212.00 | 9,320 | 5,052 | 2,415 |
| 99.875:0.125 | 9,905.00 | 11,612 | 8,729 | 3,248 |
| 99.875:0.125 | 9,308.00 | 16,028 | 6,845 | 3,248 |
As determined by the formula of Tabashnik (18).
FIG. 2.
Concentration-mortality relationship for independent pure suspensions of Cry1Ac1 and Cyt1A1 toxins and for toxin mixtures, as determined with BTI-Tn5B1-4 cells. Rep. 1 and Rep. 2, observed regression lines obtained from bioassays 1 and 2, respectively, in which the Cry1Ac1/Cyt1A1 ratio was 99.875:0.125.
Joint-action analysis. (i) Regression lines.
When an expected regression line was constructed by using the expected data for the different concentrations tested and a Cry1Ac1/Cyt1A1 ratio of 99.875:0.125, this line was significantly separated (especially at the higher concentrations) from the regression lines obtained by using data from the bioassays performed with the same toxin ratio (Fig. 2).
(ii) Tabashnik’s formula.
We compared the estimated LC50s obtained in the bioassays in which all of the different ratios and replicates of toxin mixtures were used with the LC50s calculated by the formula of Tabashnik (18). The results indicated that the expected LC50s were always significantly lower (based on their fiducial limits; P = 0.05) than the estimated LC50s (Table 1) and that the combinations of the two toxins exhibited less toxicity than each toxin acting independently.
(iii) Tammes-Bakuniak graphical analysis.
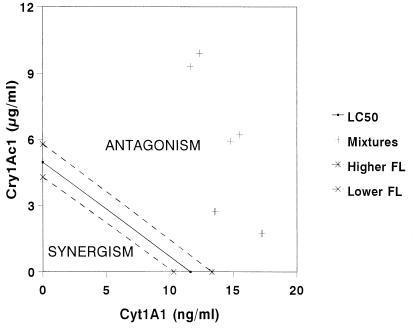
All eight points for the observed LC50s in the mixed-toxin assays fell above the isobole generated from assays in which each toxin was used alone. Moreover, the points were above the 95% confidence interval for the single-toxin isobole (Fig. 3). The locations of these points on the graph indicate that there was antagonism between the two toxins.
FIG. 3.
Isobolograms produced by the Tammes-Bakuniak graphical method for the Cry1Ac1 and Cyt1A1 toxins acting in combination. FL, fiducial limit.
In vivo toxicity tests.
The in vivo toxicity tests performed with T. ni larvae indicated that Cry1Ac1 had a high level of toxic activity (LC50, 2.41 ng/cm2 of diet), whereas Cyt1A1 had no toxic effect even at the highest concentration tested (10 μg/cm2 of diet) or even when the toxin was activated (Table 2). When combinations of these toxins were tested, however, there was a significant reduction in the activity of Cry1Ac1. At Cyt1A1 toxin concentrations of 0.1 and 1.0 μg/cm2, the LC50s of Cry1Ac1 (15.64 and 19.05 ng/cm2) increased 6.5-fold and 8-fold, respectively.
TABLE 2.
Comparison of LC50s estimated from bioassays performed with first-instar larvae of T. ni, in which Cry1Ac1 was used alone and in combination with two concentrations of Cyt1A1
| Toxin(s)a | LC50 (ng/cm2) | SD | Coefficient of variation (%) | Statistical significanceb |
|---|---|---|---|---|
| Cry1Ac1 | 2.412 | 0.26 | 10.7 | A |
| Combination I | 19.051 | 3.3 | 17.3 | B |
| Combination II | 15.643 | 3.5 | 22.2 | B |
For combination I Cry1Ac1 was mixed with 1.0 μg of Cyt1A1 per cm2; for combination II Cry1Ac1 was mixed with 0.1 μg of Cyt1A1 per cm2.
Different letters indicate that values were statistically different (P = 0.99, as determined by the Tukey test).
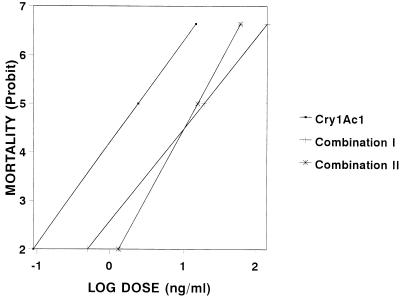
The antagonistic effect of Cyt1A1 was also evident when the regression lines from the Probit analysis were compared (Fig. 4). The line for Cry1Ac1 acting alone shifted markedly to the right when this toxin was mixed with 0.1 and 1.0 μg of Cyt1A1 per cm2.
FIG. 4.
Concentration-mortality relationship for the Cry1Ac1 toxin acting alone or mixed with Cyt1A1, as determined with first-instar T. ni larvae. The mixtures contained different concentrations of Cry1Ac1 and 1.0 μg (combination I) or 0.1 μg (combination II) of Cyt1A1 toxin per cm2.
DISCUSSION
This paper is the first report which describes the combined action of the Cry1Ac1 and Cyt1A1 toxins of B. thuringiensis both in vitro and in vivo. All three techniques employed in this study revealed that there is some antagonism between these two toxins. The Cry1Ac1 toxin was very toxic to T. ni larvae, while Cyt1A1 exhibited no obvious toxicity even at the highest concentration tested. In spite of the known lack of specificity of Cyt1A1, receptors determine the susceptibility of mosquito larvae to Cyt1A1 (9); hence, the lack of activity of Cyt1A1 against T. ni larvae may be due to the absence of specific cellular receptors for this toxin on the surfaces of columnar cells of the T. ni larval midgut. The difference in toxicity may also be related to differences in the proteases of T. ni and mosquito larvae, which may differ in their abilities to cleave the Cyt1A1 protoxin. However, no toxicity was observed even when Cyt1A1 was activated with trypsin. For the moment, these explanations are merely speculative; however, they are consistent with previous results obtained when Cyt1A1 was tested against Manduca sexta larvae (12). As a result of this work, interesting data might emerge from similar assays conducted with mosquito larvae and mosquito cell lines.
Both toxins caused cell lysis in vitro, which was expected. Unlike the in vivo test results, however, Cyt1A1 toxin exhibited much higher lytic activity than Cry1Ac1 exhibited with T. ni cells in vitro. This may have been due to the origin of the cell line, which was cloned from embryonic tissue rather than differentiated epithelial tissue, which is the principal target for this toxin (10). These results clearly show the serious limitation of using undifferentiated cell lines to assess the toxicity or the mode of action of Cry toxins. In contrast, the Cyt1A1 toxin appears to act as a nonspecific detergent with very different types of insect cells (9), as it is known that Cyt1A1 interacts with phospholipids in organized bilayers (19) and that this interaction catalyzes insertion of Cyt1A1 into the membrane without the mediation of specific membrane proteins (14).
Such effects have been studied with diverse insect cell lines, including cell lines derived from Aedes aegypti, Aedes albopictus, Anopheles stephensi, Culex quinquefasciatus, Choristoneura fumiferana, Helicoverpa zea, Spodoptera frugiperda, Mamestra brassicae, and Lymantria dispar (4, 20). Toxic activity of Cyt1A1 has not been observed previously with BTI-Tn5B1-4 cells, but the effects observed were similar to the effects observed with other insect cell lines challenged with similar concentrations of toxin (4, 20).
Additive and synergistic effects have been observed previously with the toxins of B. thuringiensis. Some studies on the interactions of toxins found in B. thuringiensis subsp. israelensis have revealed evident synergism when the toxins act in different combinations against Aedes aegypti, Culex quinquefasciatus, and Anopheles stephensi mosquito larvae; an exception is the additive effect observed with Cry4B1 and Cry11A1 (3, 16, 18). Among the lepidopteran toxins, the Cry1Ac1 toxin of B. thuringiensis subsp. kurstaki exhibited synergism in combination with the Cry1Aa1 toxin from the same subspecies when the toxins were tested against L. dispar larvae (13). Other reports of synergism between B. thuringiensis toxins are unclear (21).
In contrast, previous reports of antagonism between B. thuringiensis toxins have been scarce. A clear antagonistic effect was observed with the Cry1Aa1 and Cry1Ab1 toxins of B. thuringiensis subsp. kurstaki when they were tested against L. dispar larvae (13). However, ambiguous antagonism between the B. thuringiensis subsp. israelensis Cry4 and Cry11 toxins (the nomenclature of the toxins has changed since this study was performed) (6) was reported when they were tested against Aedes aegypti larvae (18), and the same combination was later reported to have an additive effect (16). The present study differs from the previous studies in that it assessed in vitro and in vivo interactions between toxins from different subspecies (B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. israelensis). The mechanism behind the antagonism observed in the previous studies and the present study is not known. It is possible that toxins may interact physically to form a complex, thus blocking one or more of the active sites on one or more molecules. Alternatively, antagonism may be a result of competition for space (not receptors) on the cell surface. Such hypotheses require experimental confirmation of the underlying cause(s) of the antagonism, which was observed in this study both in vitro and in vivo.
In the search for a new combination of toxins, workers generally look for potentiation of each toxin when the toxins act jointly. It is clear that a natural combination of Cyt and mosquitocidal Cry proteins significantly improves the insecticidal activity of B. thuringiensis subsp. israelensis; however, a combination consisting of Cyt proteins and lepidopteran-active Cry proteins had never been tested. Such combinations may improve the ability of B. thuringiensis to control crop pests even if the toxins are present in very different strains, as recombinant strains with combinations of toxins can be developed. However, as important as finding synergistic effects among toxins is, the discovery of antagonism is relevant, not only because new joint-action studies may help improve our understanding of the mode of action of different B. thuringiensis toxins but also because using a combination of these toxins in a bioinsecticide is now senseless.
ACKNOWLEDGMENTS
We thank Brian Federici and R. R. Granados for providing crucial material, Trevor Williams for revising the manuscript, and Javier Luévano-Borroel and Joel E. López-Meza for technical assistance.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Busvine J R. A critical review of the techniques for testing insecticides. London, England: Commonwealth Agricultural Bureaux; 1971. [Google Scholar]
- 3.Chang C, Dai S M, Frutos R, Federici B A, Gill S. Properties of a 72-kilodalton mosquitocidal protein from Bacillus thuringiensis subsp. morrisoni PG-14 expressed in B. thuringiensis subsp. kurstaki by using the shuttle vector pHT3101. Appl Environ Microbiol. 1992;58:507–512. doi: 10.1128/aem.58.2.507-512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilcott C N, Ellar D J. Comparative toxicity of Bacillus thuringiensis var. israelensis crystal proteins in vivo and in vitro. J Gen Microbiol. 1988;134:2551–2558. doi: 10.1099/00221287-134-9-2551. [DOI] [PubMed] [Google Scholar]
- 5.Chow E, Gill S S. A rapid colorimetric assay to evaluate the effects of Bacillus thuringiensis toxins on cultured insect cells. J Tissue Cult Methods. 1989;12:39–42. [Google Scholar]
- 6.Crickmore N, Zeigler D R, Feitelson J, Schnepf E, Van Rie J, Lereclus D, Baum J, Dean D H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:807–813. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Federici B A, Luthy P, Ibarra J E. Parasporal body of Bacillus thuringiensis israelensis. In: deBarjac H, Sutherland D J, editors. Bacterial control of mosquitoes & black flies. New Brunswick, N.J: Rutgers University Press; 1990. pp. 17–65. [Google Scholar]
- 8.Finney D J. Probit analysis. 3rd ed. Cambridge, United Kingdom: Cambridge University Press. Cambridge; 1977. [Google Scholar]
- 9.Gill S S, Singh G J P, Hornung J M. Cell membrane interaction of Bacillus thuringiensis subsp. israelensis cytolytic toxins. Infect Immun. 1987;55:1300–1308. doi: 10.1128/iai.55.5.1300-1308.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill S S, Cowles E A, Pietrantonio P V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 11.Guy R H, Lepla N C, Lye J R, Green C W, Barrette S L, Hollien K H. Trichoplusia ni. In: Singh P, Moore R F, editors. Handbook of insect rearing. II. Amsterdam, The Netherlands: Elsevier; 1985. pp. 487–494. [Google Scholar]
- 12.Koni P A, Ellar D J. Biochemical characterization of Bacillus thuringiensis cytolytic δ-endotoxins. Microbiology. 1994;140:1869–1880. doi: 10.1099/13500872-140-8-1869. [DOI] [PubMed] [Google Scholar]
- 13.Lee M K, Curtiss A, Alcantara E, Dean D H. Synergistic effect of the Bacillus thuringiensis toxins CryIAa and CryIAc on the gypsy moth, Lymantria dispar. Appl Environ Microbiol. 1996;62:583–586. doi: 10.1128/aem.62.2.583-586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Koni P A, Ellar D J. Structure of the mosquitocidal δ-endotoxin CytB from Bacillus thuringiensis subsp. kyushuensis and implications for membrane pore formation. J Mol Biol. 1996;257:129–152. doi: 10.1006/jmbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- 15.López-Meza J E, Ibarra J E. Characterization of a novel strain of Bacillus thuringiensis. Appl Environ Microbiol. 1996;62:1306–1310. doi: 10.1128/aem.62.4.1306-1310.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poncet S, Delécluse A, Klier A, Rapoport G. Evaluation of synergistic interactions among the CryIVA, CryIVB, and CryIVD toxic components of B. thuringiensis subsp. israelensis crystals. J Invertebr Pathol. 1995;66:131–135. [Google Scholar]
- 17.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabashnik B E. Evaluation of synergism among Bacillus thuringiensis toxins. Appl Environ Microbiol. 1992;58:3343–3346. doi: 10.1128/aem.58.10.3343-3346.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas W E, Ellar D J. Mechanism of action of Bacillus thuringiensis var. israelensis insecticidal δ-endotoxin. FEBS Lett. 1983;154:362–368. doi: 10.1016/0014-5793(83)80183-5. [DOI] [PubMed] [Google Scholar]
- 20.Thomas W E, Ellar D J. Bacillus thuringiensis var. israelensis crystal δ-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- 21.Van Frankenhuyzen K, Gringorten J L, Miine R E, Gauthier D, Pusztai M, Brousseau R, Masson L. Specificity of activated CryIA proteins from Bacillus thuringiensis subsp. kurstaki HD-1 for defoliating forest Lepidoptera. Appl Environ Microbiol. 1991;57:1650–1655. doi: 10.1128/aem.57.6.1650-1655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]