Abstract
Pseudomonas aeruginosa accumulates polyphosphates in response to nutrient limitations. To elucidate the function of polyphosphate in this microorganism, we have investigated polyphosphate metabolism by isolating from P. aeruginosa 8830 the genes encoding polyphosphate kinase (PPK) and exopolyphosphatase (PPX), which are involved in polyphosphate synthesis and degradation, respectively. The 690- and 506-amino-acid polypeptides encoded by the two genes have been expressed in Escherichia coli and purified, and their activities have been tested in vitro. Gene replacement was used to construct a PPK-negative strain of P. aeruginosa 8830. Low residual PPK activity in the ppk mutant suggests a possible alternative pathway of polyphosphate synthesis in this microorganism. Primer extension analysis indicated that ppk is transcribed from a ςE-dependent promoter, which could be responsive to environmental stresses. However, no coregulation between ppk and ppx promoters has been demonstrated in response to osmotic shock or oxidative stress.
Inorganic polyphosphates (polyP) are linear polymers in which inorganic orthophosphate (Pi) residues are linked by energy-rich phosphoanhydride bonds (21). Their chain lengths may vary from two to several hundred Pi residues. PolyP are widely distributed in nature and have been detected in bacteria, fungi, protozoa, plants, and mammals (22, 24); however, uncertainty still remains regarding their exact physiological role.
Depending on the species, the cellular localization, and the physiological conditions, polyP may have a variety of functions (19), including serving as an ATP substitute and energy source, a phosphate reservoir, and a chelator for divalent cations (16) and in the inhibition of RNA degradation (6) and regulatory responses to stresses and nutritional deficiencies (30). Moreover, it can be a structural element in DNA entry and transformation (32).
Several polyP-metabolizing enzymes have been purified, and their genes have been cloned. Accumulation of this polymer is known to occur in microorganisms catalyzed by the enzyme polyphosphate kinase (PPK); PPK catalyzes the reversible transfer of the terminal phosphate from ATP to polyP (2, 13, 15, 36). PolyP degradation and utilization is mediated by exopolyphosphatases (PPX) (3, 38), endopolyphosphatases (23), and specific kinases (37).
Extensive accumulation of polyP has been detected in the Pseudomonas aeruginosa mucoid strain 8830, particularly during the stationary phase (18) and in response to phosphate and amino acid limitations (5), yet essentially nothing is known about the genes and their regulation in the synthesis of polyP. We report here the cloning and sequencing of the P. aeruginosa ppk and ppx genes and the characterization of their promoters and gene products.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains and plasmids used in this work are described in Table 1. Escherichia coli and P. aeruginosa were routinely grown at 37°C on Luria-Bertani (LB) solid or liquid medium (Difco). For antibiotic selection of E. coli, ampicillin was used at 50 or 100 μg/ml, tetracycline was used at 12.5 μg/ml, kanamycin was used at 50 μg/ml, and chloramphenicol was used at 34 μg/ml. For selection of P. aeruginosa, carbenicillin, chloramphenicol, and tetracycline were used at 300 μg/ml in solid media and at 100 μg/ml in liquid media. Triparental mating was performed by using pRK2013 as the helper plasmid (12), and exconjugants were isolated on Pseudomonas isolation agar (Difco) with appropriate antibiotic selection. The broad-host-range plasmid pQF50 and its constructs were introduced into P. aeruginosa via electroporation (28).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype and/or description | Reference or source |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| 8830 | his-1 ppk+ | 9 |
| 8830K::Tc | his-1 ppk mutant | This study |
| E. coli | ||
| DH5α | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 ΔlacU169 φ80lacZΔM15 | 27 |
| BL21(DE3)(pLysS) | F−ompT hsdSB (rB− mB−) gal dcm (λDE3) pLysS | Novagen |
| Plasmids | ||
| pCR2.1 | Ampr Kmr; vector for TA cloning of PCR products | InVitrogen |
| pBR322 | Ampr Tcr; E. coli plasmid cloning vector | 7 |
| pUC19 | Ampr; E. coli plasmid cloning vector | 39 |
| pNOT19 | pUC19 derivative; 10-nucleotide NotI oligonucleotide inserted into NdeI site | 33 |
| pMOB2 | Kmr; mobilization cassette | 33 |
| pET24a(+) | Kmr; T7lac promoter, His · Tag C-terminal fusion | Novagen |
| pET28a(+) | Kmr; T7lac promoter, His · Tag N-terminal fusion | Novagen |
| pQF50 | Ampr; broad-host-range promoter probe vector | 11 |
| pBBR1MCS | Cmr; broad-host-range plasmid cloning vector | 20 |
| pCR-F2R5 | 1.7-kb PCR probe cloned in pCR2.1 | This study |
| pPPKB | 3.5-kb SphI fragment containing the ppk gene cloned into pBR322 | This study |
| pPPX5 | 3-kb SalI fragment containing the ppx gene cloned into pUC19 | This study |
| pPPK-His | ppk PCR-generated cloned into EcoRI and HindIII sites of pET24a(+) | This study |
| pPPX-His | ppx PCR-generated cloned into NdeI and HindIII sites of pET28a(+) | This study |
| pNotK::Tc | pNot19 derivative, 1.6-kb KpnI-HindIII PCR fragment and Tcr cassette in BamHI site | This study |
| pQF-376K | 376-bp XhoI fragment containing the ppk promoter cloned into SalI site of pQF50 | This study |
| pQF-649X | 649-bp SphI fragment containing the putative ppx promoter cloned into pQF50 | This study |
| pCR-5 | 2,488-bp PCR fragment containing the ppk gene and its promoter cloned into pCR2.1 | This study |
| pBBR-K | HindIII-EcoRV fragment derived from pCR-5 cloned into pBBR1MCS | This study |
DNA manipulations.
Steps involved in the cloning of the P. aeruginosa 8830 ppk and ppx genes are described in the Results section. Genomic DNA was prepared with a GNome Kit (Bio 101, Inc.). Restriction endonucleases (Gibco BRL), T4 DNA ligase (Gibco BRL) and calf intestinal phosphatase (Pharmacia) were used as specified by the manufacturers. Plasmid DNA was isolated on a small scale as described by Zhou et al. (41) and on a large scale by using the Qiagen Plasmid Kit (Qiagen).
DNA probes for Southern blotting were internally labeled with [α-32P]dCTP by using the Megaprime DNA labeling system (Amersham) as described by the manufacturer. Southern blotting was performed by capillary transfer of DNA fragments to positively charged nylon membranes (Hybond-N+). Membranes were incubated in Rapid-hyb buffer (Amersham) at 65°C and washed under high-stringency conditions. PCR were performed with Pfu polymerase (Stratagene); oligonucleotides (Table 2) were purchased from Gibco BRL. DNA sequencing was done by the Genetic Engineering Laboratory of the University of Illinois at Urbana-Champaign. Sequence analysis was performed with a Sequencer 3.0. Homology searches were performed by using BLASTP and BLASTX (4) provided by the National Center for Biotechnology Information. Amino acid alignments were performed with CLUSTAL X, an updated version of the general-purpose multiple alignment program CLUSTAL W (35).
TABLE 2.
Oligonucleotides used in this work
| Primer | Sequence 5′→3′a |
|---|---|
| PPK5R | GTTGCGGATCATCCAGTC(C/G)GC |
| PPK2F | GT(G/C)CGCTTCGC(G/C)GA(G/A)CT(G/C)AA(G/A) |
| PPK5F | GGTATCGCGGCGGTACCGGATC*TCAACGAGACCC |
| Tc1 | GATCAGATCTGAATAAGGGCGACACGGAAA |
| Tc2 | GATCAGATCTCTCAACGACAGGAGCACGAT |
| K1 | TCCTTGCATGCCCTACC |
| K2 | CGGGGTACCTCAATGATGATGATGATGATGACGTGCGGTAAGC |
| PPK-N | CGGAATTCGTGGATGACAGC |
| PPK-C | CCCAAGCTTACGTGCGGT |
| PPX-N | CGGAATTCATATGGACTTGC |
| PPX-C | CCCAAGCTTATTACCGCACGTTG |
| P1 | CACGCGGATGTTGAACTGCAACTG |
Underlined sequences are complementary to sequences deposited in GenBank. An asterisk (*) indicates a missing C to eliminate a BamHI site.
To isolate the ppk gene, a 1.7-kb probe was obtained by PCR by using the degenerate oligonucleotides PPK5R and PPK2F (Table 2). PCR was carried out under low-stringency annealing conditions (40°C) with P. aeruginosa genomic DNA as a template. The 1.7-kb PCR product was cloned into PCR2.1 (pCR-F2R5) and sequenced.
The tetracycline (Tc) cassette used to knock out the ppk gene was constructed by PCR amplification of the tetracycline resistance gene of pBR322 by using oligonucleotides Tc1 and Tc2. The oligonucleotides were designed to create BglII sites at the ends of the tetracycline cassette. This cassette was inserted into the unique BamHI restriction site of a 1.6-kb PCR product amplified with the primers PPK5R and PPK5F and with pCR-F2R5 as the template. PPK5F has been designed internally to the 1.7-kb PCR probe to eliminate one of the two BamHI sites (Table 2) and to generate the shorter 1.6-kb PCR product. The fragment was first cloned into the PCR2.1 vector and then ligated into the HindIII and KpnI sites of pNOT19. To complement the ppk::Tc mutant, the ppk gene and its promoter were amplified by using the oligonucleotides K1 and K2 and pPPKB as a template. The PCR product was ligated into pCR2.1 (pCR-5). This construct was digested with HindIII and EcoRV, and the 2.5-kb fragment containing the ppk gene was cloned into pBBR1MCS (pBBR-K).
Cloning of ppk and ppx genes for overexpression and protein purification.
The ppk gene was amplified by using oligonucleotides PPK-N and PPK-C corresponding to the 5′ and 3′ ends of the gene. The plasmid DNA extracted from PPKB was used as template DNA. EcoRI and HindIII sites were designed in the two oligonucleotides to allow the PCR product to be cloned into the expression vector pET24a(+) (Novagen). In PPK-C the stop codon at the 3′ end of the ppk coding sequence has been omitted to allow an in-frame fusion with six histidine residues encoded in the vector.
The ppx gene was amplified by using oligonucleotides PPX-N and PPX-C. Plasmid DNA extracted from PPX5 was used as a template. NdeI and HindIII enzymes whose sites were designed into the two oligonucleotides were used to digest and clone this PCR product into pET28a(+) (Novagen). This strategy created an N-terminal fusion of PPX with a histidine tag encoded in the vector.
These constructs were amplified in DH5α and subsequently transformed into E. coli BL21(DE3)(pLys) (Novagen). E. coli transformants freshly streaked on LB agar plates were inoculated into 500 ml of LB broth (Difco) supplemented with appropriate antibiotics. When this culture reached an optical density at 600 nm (OD600) at 37°C of ca. 0.3, expression of the genes from the T7 promoters of the expression vectors was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were grown for 4 h before being harvested. Cells were resuspended in 8 ml of 1× binding buffer of the His · Bind Buffer Kit (Novagen) and sonicated three times for 1 min with a Bronson Sonifier 450 at an output of 3.5 (25 W) with a 50% duty cycle. Purification under nondenaturing conditions was performed by using Ni2+ affinity chromatography with His · Bind resin (Novagen) as described by the manufacturer. Protein concentration was determined with Coomassie Plus protein assay reagent (Pierce). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of proteins was conducted according to the methods described by Laemmli (26). Protein bands were visualized by Coomassie blue staining.
Assay for PPK activity.
PPK activity was measured in membrane preparations as the production of acid-insoluble 32P-labeled polyP, as previously described (1). Membranes were prepared by centrifugation of total cell extracts in a Beckman ViTi65 rotor at 45,000 × g for 1 h after sonication.
The activity of purified PPK was tested in 20 μl of the same reaction mixture. After incubation for 1 h at 37°C, the product was purified by using Glassmilk as described by Ault-Riché et al. (5).
Assay for PPX activity.
The activity of purified PPX was tested in 20 μl of 50 mM Tris-HCl buffer (pH 8)–1 mM MgCl2–175 mM KCl–250 μM polyP. The synthesis and purification of the [32P]polyP substrate was done as described for the PPK assay. The PPX reaction was performed at 37°C. Samples (2 μl) were taken periodically and loaded on a polyethyleneimine plate. Polyphosphates remaining at the origin were separated from orthophosphate by thin-layer chromatography (TLC) with 0.75 M KH2PO4 (pH 3.5). Dried plates were visualized with a PhosphorImager (Molecular Dynamics).
Primer extension analysis.
Total RNA was isolated by using TRIzol reagent (Gibco BRL) from P. aeruginosa 8830 culture at an OD600 of 1.0. Samples of 30 μg of RNA were hybridized overnight with 10 pmol of P1 primer labeled with the MaxKinase kit (Ambion). After precipitation, RNA was resuspended in 10 μl of water, and the primer extension reaction was performed with the First Strand cDNA synthesis kit (Pharmacia) at 37°C. Sequencing was performed by use of the dideoxynucleotide chain termination reaction method with T7 DNA polymerase (Sequenase 2.0 kit; USB Corp.).
β-Galactosidase assay.
P. aeruginosa 8830 cells harboring the promoter probe constructs were grown overnight in LB with ampicillin (100 μg/ml). These overnight cultures were used to inoculate fresh LB (1%). P. aeruginosa 8830 cells were harvested in mid-log phase and stationary phase and then divided into two parts. One part was treated with NaCl (added to a 2 M final concentration) or H2O2, while the other was used as a control. Samples (50 ml) were centrifuged, and the cell pellets were resuspended in the assay buffer and sonicated. After centrifugation to eliminate cell debris, the cell extract was assayed for β-galactosidase activity. Quantitative determination of β-galactosidase activity was performed by the method of Miller (29). Each experiment was performed in triplicate.
Nucleotide sequence accession numbers.
The nucleotide sequences of the P. aeruginosa 8830 ppk and ppx genes have been deposited in the GenBank nucleotide sequence database under accession numbers AF087931 and AF053463, respectively.
RESULTS
Cloning of P. aeruginosa 8830 ppk and ppx genes.
On the basis of the homology alignment of amino acid sequences of PPKs from Neisseria meningitidis, Klebsiella aerogenes, and E. coli, we designed degenerate primers that corresponded to highly conserved amino acid sequences. These oligonucleotides were used to perform PCR as described in Materials and Methods. The PCR product had the expected size of 1.7 kb. This product was sequenced, and the sequence homology to known ppk genes suggested it could be the amplification of a portion of the ppk gene.
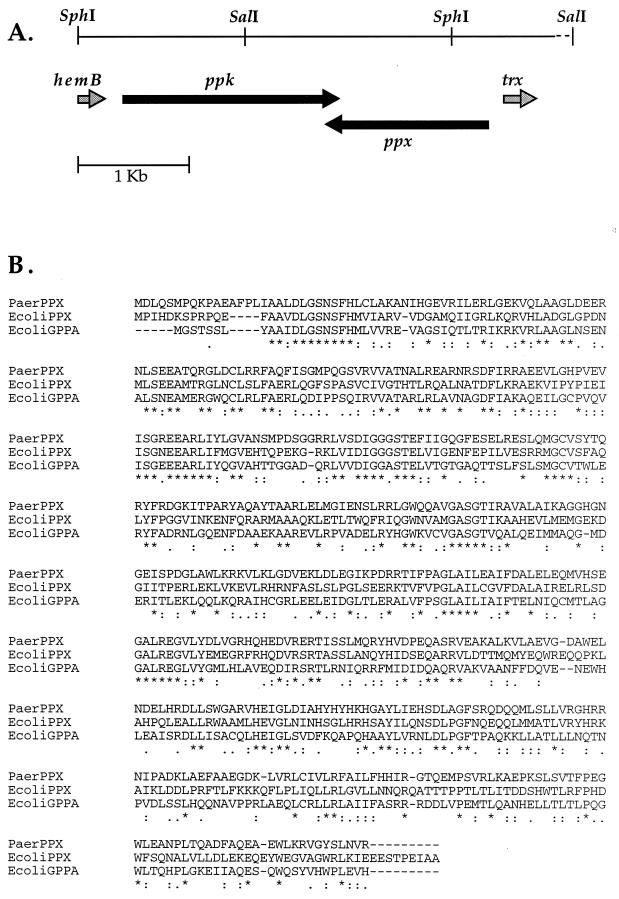
A Southern blot of P. aeruginosa 8830 DNA cleaved with various restriction enzymes was probed with the 1.7-kb PCR product. In particular, this probe hybridized to a single 3.5-kb SphI fragment. To isolate this fragment, P. aeruginosa 8830 SphI DNA fragments ranging in size from 2.5 to 5.5 kb were purified by preparative agarose gel electrophoresis. The purified DNA was ligated into the SphI restriction site of pBR322 and transformed into E. coli DH5α. One hundred tetracycline-sensitive (Tcs) and ampicillin-resistant (Ampr) colonies were screened, and one positive clone hybridizing with the 1.7-kb probe was obtained. This clone had a 3.5-kb insert (pPPKB). The sequence of this fragment showed the presence of an open reading frame (ORF) encoding a 690-amino-acid protein. The ORF begins with a GTG start codon and has a putative ribosome binding site, CGGTGG, eight nucleotides upstream of the start codon. The translated amino acid sequence of this ORF shows 58% identity with Acinetobacter calcoaceticus PPK, 50% identity with N. meningitidis PPK, 42% identity with Synechocystis sp. PPK, 36% identity with Campylobacter coli PPK, 37% identity with Helicobacter pylori PPK, and 35% identity with E. coli PPK according to an alignment performed with BLASTP. A total of 155 nucleotides upstream of the GTG start codon, the 3′ end of the hemB gene (X91820), has been identified (Fig. 1A).
FIG. 1.
(A) Organization of ppk and ppx genes. (B) Alignment of P. aeruginosa 8830 PPX with E. coli PPX (accession number L06129) and E. coli pppGpp-5′-phosphohydrolase (GppA) (accession number M83316) (CLUSTAL X). Sequences were retrieved from GenBank. ∗, amino acid identity; “:” and “.”, limited conserved substitutions.
Downstream of the ppk gene, in the 3.5-kb SphI fragment, an incomplete ORF, which resembled the gene for E. coli PPX, was detected. To isolate the entire gene, a SalI DNA fragment of ca. 3 kb was isolated that partially overlapped the 3.5-kb SphI DNA fragment. A unique SalI site was mapped in the SphI DNA fragment, and it was localized 927 bp upstream of the ppk stop codon. Southern blot hybridization with the 1.7-kb PCR probe of P. aeruginosa 8830 DNA digested with SalI showed two bands of ca. 3 and 2.5 kb. If the same probe was digested with SalI and the purified 0.7-kb fragment at the 3′ end was used as a probe, a single band of 3 kb was detected. To isolate this band, SalI DNA fragments of ca. 3 kb were cloned into pUC19 and 300 clones were screened by using the 0.7-kb DNA probe. One positive clone was detected (PPX5). With an oligonucleotide designed near the 3′ end of the ppk gene and by using sequencing by primer walking on the DNA insert, a complete ORF of 1,521 bp, coding for a 506-amino-acid protein, was identified (Fig. 1A). The translated amino acid sequence of this ORF showed considerable identity with E. coli PPX and pppGpp-5′-phosphohydrolase (GppA) (17) (Fig. 1B). This ORF is transcribed in the opposite orientation with respect to ppk, and the two genes overlap with 14 nucleotides at their 3′ ends. Located 183 bp upstream of the ppx gene, in the opposite orientation, was an ORF that encodes a protein with 72% sequence identity to E. coli thioredoxin.
Construction of ppk mutants with reduced PPK activity.
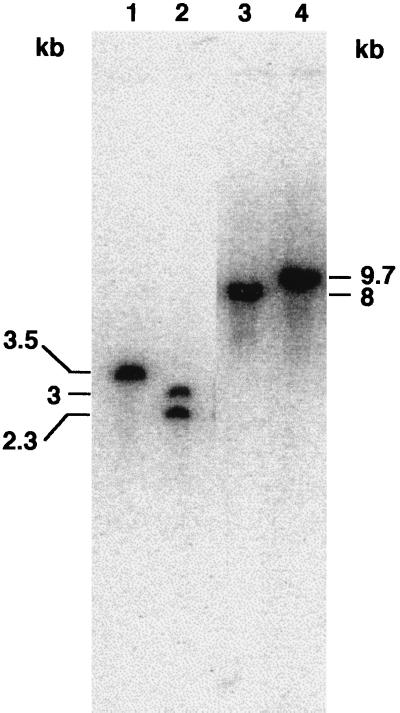
To isolate a knockout mutation in the ppk gene, the tetracycline cassette was inserted in the unique BamHI site of the 1.6-kb PCR fragment as described in Materials and Methods, and pMOB2 was inserted in the NotI site (33). The resulting plasmid (pNotK::Tc) was conjugated into P. aeruginosa 8830 by triparental mating, and several tetracycline-resistant but carbenicillin-sensitive colonies were obtained. Southern blotting of chromosomal DNA from one of these transformants digested with SphI and by using the 1.7-kb PCR probe showed two bands of ca. 2.3 and 3 kb, instead of a single 3.5-kb band. This finding was consistent with the insertion of the 1,720-bp Tc cassette which had one SphI restriction site and no KpnI site. Moreover, DNA digested with KpnI showed an increase in fragment size from 8 to ca. 9.7 kb (Fig. 2).
FIG. 2.
Southern blot analysis of ppk knockout mutant genomic DNA, determined by using the 1.7-kb PCR probe. Lanes: 1, P. aeruginosa 8830 chromosomal DNA digested with SphI; 2, ppk::Tc genomic DNA digested with SphI; 3, P. aeruginosa 8830 chromosomal DNA digested with KpnI; 4, ppk::Tc genomic DNA digested with KpnI. The different band patterns for the parental and mutant strains are consistent with the insertion of the Tc cassette of 1,720 bp having one SphI restriction site and no KpnI sites.
PPK activity was tested in the membrane preparations of mid-log phase cultures of the ppk::Tc mutant and of the parental strain P. aeruginosa 8830. A residual PPK activity was detected in the mutant (430 ± 1 U mg−1), but it was 23 times lower than in the parental strain (10,000 ± 10 U mg−1). When the mutant was complemented with the ppk gene as part of a high-copy-number vector (pBBR-K), the activity (35,700 ± 3,400 U mg−1) was 3.5 times higher than in the wild type. The activity measured in the strain containing the vector pBBR1MCS alone was 130 ± 5 U mg−1.
Purification and activity of recombinant PPK.
The His-tagged protein was purified by metal-chelate affinity chromatography on an Ni-iminodiacetic acid column. Eluted protein (3.5 μg) was loaded onto an SDS-PAGE gel and stained with Coomassie blue R250. Protein purification was homogeneous since a single 75-kDa band was detected. The purified protein was tested for its ability to synthesize polyP in vitro. For this purpose PPK was incubated with [γ-32P]ATP, and the product of the in vitro reaction was purified with Glassmilk and loaded onto a TLC plate. After TLC analysis, the product of the in vitro reaction was retained at the origin (Fig. 3, lane 2), but after digestion for 1 h with an excess of recombinant yeast PPX (rPPX1), as described by Wurst and Kornberg (38), it was completely degraded to free Pi (Fig. 3, lane 3). Similar results were observed when purified E. coli PPK (Fig. 3, lanes 4 and 5) was used. When the same product was incubated with an ATPase purified in our laboratory from Mycobacterium bovis (40), no release of free Pi was detected (Fig. 3, lanes 7 and 8). However, the ATPase activity of this enzyme was confirmed by digestion of ATP to free Pi in the PPK reaction mixture (Fig. 3, lane 6).
FIG. 3.
TLC analysis of PPK enzymatic activity product. Lanes: 1, P. aeruginosa PPK reaction mixture; 2, polyP purified with Glassmilk from the reaction mixture shown in lane 1; 3, P. aeruginosa polyP digested with rPPX1; 4, purified polyP synthesized by E. coli PPK; 5, E. coli polyP digested with rPPX1; 6, reaction mixture in lane 1 digested with an M. bovis ATPase; 7, E. coli polyP digested with M. bovis ATPase; 8, P. aeruginosa polyP digested with M. bovis ATPase.
Purification of recombinant PPX and polyP degradation.
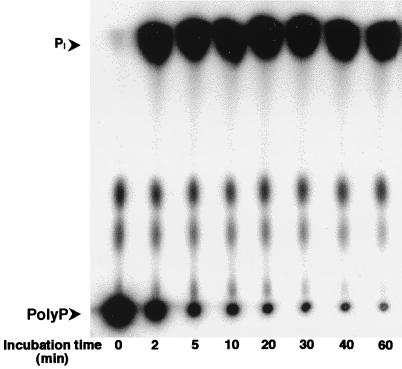
PPX with an oligohistidine domain at the N terminus was purified to homogeneity as visualized by Coomassie blue R250 staining of 6 μg of elution fraction run on an SDS-PAGE gel. The molecular mass of the denatured histidine-tagged protein was determined by SDS-PAGE to be 60 kDa, with a linear regression curve of the standard low-molecular-mass protein (14,400 to 97,400 Da) (Bio-Rad). The theoretical molecular mass calculated from the amino acid sequence encoded by the ppx gene, including six histidine terminal residues, was 58.7 kDa. The hydrolytic activity of this enzyme toward polyP was demonstrated in vitro by using polyP as a substrate (Fig. 4). Its specific activity, measured with E. coli polyP as a substrate, was 7 × 106 pmol of Pi produced min−1 mg of protein−1.
FIG. 4.
TLC analysis of P. aeruginosa PPX enzymatic activity. [32P]polyP (267 μM) was incubated for various time periods as specified with 200 ng of PPX protein in 20 μl of total reaction mixture. Samples (1 μl) were taken periodically and spotted onto a polyethyleneimine plate.
ppk and ppx promoter investigation.
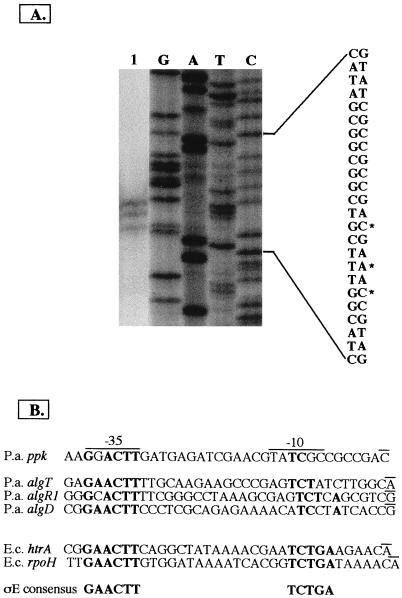
The region upstream of the ppk gene was investigated for its promoter activity through construction of a promoter-reporter gene fusion. For this purpose, a 376-bp XhoI fragment including 251 bp upstream of the GTG translational start site was cloned into the SalI site of the promoterless lacZ gene of the pQF50 plasmid in both orientations. These constructs were electroporated into P. aeruginosa 8830, and the β-galactosidase activity was tested at various stages of growth. When the fragment was cloned in the inverted orientation, the activity was 8.7 times lower (37.27 ± 1.4 Miller units) than in the direct orientation (327.4 ± 12.87 Miller units) (pQF-376K). This suggested that the promoter could be located upstream of the ppk coding sequence. Primer extension analysis identified three putative transcriptional start sites (Fig. 5A) differing by one or two bases. The start site farthest upstream is a C residue that produces the most intense transcript band. This start site is accompanied by −35 and −10 promoter sequences, which resemble the consensus promoter sequence for ςE-dependent promoters as exemplified by E. coli htrA and rpoH or P. aeruginosa algT, algR1, or algD (10, 14) (Fig. 5B). A 649-bp SphI fragment upstream of the ppx gene was cloned in the direct orientation into pQF50 upstream of the promoterless lacZ gene (pQF-649X). When two strains of P. aeruginosa 8830, which contained the promoter probe construct (pQF-649X) and the control plasmid (pQF50), respectively, were grown to log phase (OD600 = 1.50) and the β-galactosidase activity was measured, it was 7.7 times greater in pQF-649X (93 ± 0.4 Miller units) than in the control (12 ± 0.2 Miller units). To investigate a putative coregulation at the transcriptional level between ppx and ppk, the β-galactosidase activity was measured in P. aeruginosa 8830 strains containing pQF-376K or pQF-649X in response to stress conditions such as a high concentration of NaCl or oxidative stress, which are known to influence polyP synthesis in E. coli (30). β-Galactosidase levels were measured after 30 min of exposure to 2 M NaCl. Compared to the activity measured in each control, a modest increase was seen for both ppk and ppx promoters (Table 3). The increase became more significant after 2 h, but the response of the ppk promoter to such a stress was greater (3.2 times) in the stationary phase than in the log phase (Table 3). In contrast, ppx promoter activation was stronger in the log phase. The response to oxidative stress was evaluated by using 53 mM H2O2 (final concentration) and measuring the β-galactosidase activity after 10 min. Stress-induced ppx promoter activity decreased slightly during both the log and the stationary phases, while a small increase of ppk promoter activity was measured during the stationary phase.
FIG. 5.
(A) Primer extension analysis of the transcription start sites of the ppk gene. P. aeruginosa 8830 total RNA was annealed to oligonucleotide P1 and extended with murine reverse transcriptase. Lanes G, A, T, and C represent the dideoxy sequencing ladder carried out with the same primer. Lane 1, primer extension products. (B) Comparison of −35 and −10 motifs identified upstream of the transcription start site (the C residue that gave the most intense band) with ςE consensus sequences.
TABLE 3.
Transcriptional response of ppk and ppx promoters to osmotic stress (2 M NaCl) after 30 min and after 2 h
| Promoter type and stress condition | β-Galactosidase sp act (mean ± SD) after:
|
|||
|---|---|---|---|---|
| 30 min
|
2 h
|
|||
| Log phase | Stationary phase | Log phase | Stationary phase | |
| ppk without NaCl | 197 ± 5 | 225 ± 22 | 201 ± 6 | 265 ± 47 |
| ppk with NaCl | 305 ± 9 | 287 ± 17 | 351 ± 4 | 851 ± 138 |
| ppx without NaCl | 125 ± 5 | 132 ± 2 | 134 ± 9 | 191 ± 11 |
| ppx with NaCl | 235 ± 3 | 172 ± 3 | 542 ± 2 | 447 ± 3 |
DISCUSSION
Accumulation of polyP has been detected in P. aeruginosa in response to nutrient limitation (5, 18), but the implications of this physiological response to such stress conditions remain unclear. The pathway of synthesis and degradation of polyP in P. aeruginosa has never been investigated. We isolated the P. aeruginosa genes encoding the major enzymes involved in polyP metabolism, PPK and PPX. In contrast to E. coli, where the ppk and ppx genes are organized in an operon, in P. aeruginosa the two genes are transcribed divergently (Fig. 1A). Investigation of the two promoters did not show any coregulation between the expression of these two genes, at least under the conditions we analyzed, such as low or high Pi levels (data not shown), osmotic shock, or oxidative stress. This observation is in agreement with the observation that polyP accumulation is regulated at the enzymatic level through PPX inhibition by the stress response nucleotides ppGpp and pppGpp (25) without any modulation of the transcription rate of these two genes.
After ppk gene inactivation, the ppk knockout mutant does not show a growth defect compared to the parental strain. However, a residual low PPK activity, measured as polyP synthesis, in membrane preparations has been detected in the mutant. This may represent a separate mechanism of polyP synthesis, as suggested also by the observations that a small amount of polyP remains in the cells of E. coli (8) and N. meningitidis (36) where the ppk gene has been inactivated.
Downstream of the ppk gene in P. aeruginosa is an ORF encoding a putative thioredoxin. The ppx gene product has been shown to be able to hydrolyze polyP to Pi with a specific activity comparable to that of E. coli PPX (3). Moreover, this enzyme exhibits significant sequence similarity with E. coli PPX and E. coli GppA. Both of these enzymes belong to a large superfamily that includes sugar kinases, actin, and hsp70 proteins (31). Since we have now cloned both the genes with their respective promoters, we should be able to define various environmental parameters that might modulate the expression of either or both of the genes that allow the cells to accumulate polyP.
An interesting observation from our studies is that the ppk gene of P. aeruginosa appears to be expressed from a ςE-responsive promoter (Fig. 5B). The ςE-responsive promoters have been reported to regulate alginate synthesis through activation of critical alginate biosynthetic genes such as algR1, algT, algD, and algC (34). It is interesting to note that both polyP accumulation and alginate synthesis are coregulated in P. aeruginosa such that alginate-positive mucoid cells accumulate much more polyP than their nonmucoid counterparts (18). Indeed, a mutation in the algR2 gene that results in reduced alginate production also results in reduced polyP accumulation (18). Whether a common regulator controls the expression of key ςE-responsive promoters such as algT/algR1 and ppk is not known at present. Further investigations looking for a common link in the genetic expression of two stress-inducible polymers, such as polyP and alginate, presumably through ςE-responsive promoters, may provide important insights into the mode of regulation of the biosynthesis of these two polymers.
ACKNOWLEDGMENTS
We are grateful to A. Kornberg for the kind gift of yeast rPPX and E. coli PPK. We are also very grateful to H. P. Schweizer for providing pMOB2.
This research was supported by Public Health Service grant AI16790-18 from the National Institutes of Health.
REFERENCES
- 1.Ahn K, Kornberg A. Polyphosphate kinase from Escherichia coli. J Biol Chem. 1990;265:11734–11739. [PubMed] [Google Scholar]
- 2.Akiyama M, Crooke E, Kornberg A. The polyphosphate kinase gene of Escherichia coli. J Biol Chem. 1992;267:22556–22561. [PubMed] [Google Scholar]
- 3.Akiyama M, Crooke E, Kornberg A. An exopolyphosphatase of Escherichia coli. J Biol Chem. 1993;268:633–639. [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ault-Riché D, Fraley C D, Tzeng C, Kornberg A. Novel assay reveals multiple pathways regulating stress induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum E, Py B, Carpousis A J, Higgins C F. Polyphosphate kinase is a component of the E. coli RNA degradosome. Mol Microbiol. 1997;26:387–398. doi: 10.1046/j.1365-2958.1997.5901947.x. [DOI] [PubMed] [Google Scholar]
- 7.Bolivar F, Rodriguez R L, Greene P J, Betlach M G, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 8.Crooke E, Akiyama M, Rao N N, Kornberg A. Genetically altered levels of inorganic polyphosphate in E. coli. J Biol Chem. 1994;269:6290–6295. [PubMed] [Google Scholar]
- 9.Darzins A, Chakrabarty A M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984;159:9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high-temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 11.Farinha M A, Kropinski A M. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J Bacteriol. 1990;172:3496–3499. doi: 10.1128/jb.172.6.3496-3499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figurski D, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissdörfer W, Ratajczak A, Hillen W. Transcription of ppk from Acinetobacter sp. strain ADP1, encoding a putative polyphosphate kinase, is induced by phosphate starvation. Appl Environ Microbiol. 1998;64:896–901. doi: 10.1128/aem.64.3.896-901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershberger C D, Ye R W, Parsek M R, Xie Z, Chakrabarty A M. The algT (algU) gene of Pseudomonas aeruginosa, a key regulator involved in alginate biosynthesis, encodes an alternative ς factor (ςE) Proc Natl Acad Sci USA. 1995;92:7941–7945. doi: 10.1073/pnas.92.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato J, Yamamoto T, Yamada K, Ohtake H. Cloning, sequence and characterization of the polyphosphate kinase-encoding gene (ppk) of Klebsiella aerogenes. Gene. 1993;137:237–242. doi: 10.1016/0378-1119(93)90013-s. [DOI] [PubMed] [Google Scholar]
- 16.Keasling J D, Hupf G A. Genetic manipulations of polyphosphate metabolism affect cadmium tolerance in E. coli. Appl Environ Microbiol. 1996;62:743–746. doi: 10.1128/aem.62.2.743-746.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keasling J D, Bertsch L, Kornberg A. Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long chain exopolyphosphatase. Proc Natl Acad Sci USA. 1993;90:7029–7033. doi: 10.1073/pnas.90.15.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H-Y, Schlictman D, Shankar S, Xie Z, Chakrabarty A M, Kornberg A. Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol Microbiol. 1998;27:717–725. doi: 10.1046/j.1365-2958.1998.00702.x. [DOI] [PubMed] [Google Scholar]
- 19.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. pBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:801–802. [PubMed] [Google Scholar]
- 21.Kulaev I S. The biochemistry of inorganic polyphosphates. New York, N.Y: John Wiley & Sons, Inc.; 1979. [Google Scholar]
- 22.Kulaev I S, Vagabov V M. Polyphosphate metabolism in micro-organisms. Adv Microb Physiol. 1983;24:83–159. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- 23.Kumble K D, Kornberg A. Endopolyphosphatases form chain of inorganic polyphosphates in yeast and mammals. J Biol Chem. 1996;271:27146–27151. doi: 10.1074/jbc.271.43.27146. [DOI] [PubMed] [Google Scholar]
- 24.Kumble K D, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda A, Murphy H, Cashel M, Kornberg A. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J Biol Chem. 1997;272:21240–21243. doi: 10.1074/jbc.272.34.21240. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.McFall S M, Parsek M R, Chakrabarty A M. 2-Chloromuconate and ClcR-mediated activation of the clcABD operon: in vitro transcriptional and DNase I footprint analyses. J Bacteriol. 1997;179:3655–3663. doi: 10.1128/jb.179.11.3655-3663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 30.Rao N N, Kornberg A. Inorganic polyphosphate supports resistance and survival of stationary phase Escherichia coli. J Bacteriol. 1996;178:1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reizer J, Reizer A, Saier H, Bark P, Sander C. Polyphosphate phosphatase and guanosine pentaphosphate phosphatase belong to the sugar kinase/actin/hsp70 superfamily. Trends Biochem Sci. 1993;18:247–248. doi: 10.1016/0968-0004(93)90172-j. [DOI] [PubMed] [Google Scholar]
- 32.Reusch R N, Sadoff H L. Putative structure and functions of a poly-β-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci USA. 1988;85:4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweizer H P. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol. 1992;6:1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 34.Shankar S, Ye R W, Schlictman D, Chakrabarty A M. Exopolysaccharide alginate synthesis in Pseudomonas aeruginosa: enzymology and regulation of gene expression. Adv Enzymol Relat Areas Mol Biol. 1995;20:221–255. doi: 10.1002/9780470123164.ch4. [DOI] [PubMed] [Google Scholar]
- 35.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinsley C R, Gotschlich E C. Cloning and characterization of the meningococcal polyphosphate kinase gene: production of polyphosphate synthesis mutants. Infect Immun. 1995;61:1624–1630. doi: 10.1128/iai.63.5.1624-1630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood H G, Clark J E. Biological aspects of inorganic polyphosphates. Annu Rev Biochem. 1988;57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]
- 38.Wurst H, Kornberg A. A soluble exopolyphosphatase of Saccharomyces cerevisiae. J Biol Chem. 1994;269:10996–11001. [PubMed] [Google Scholar]
- 39.Yanisch-Perron C, Vieira J, Messing J. Improved M15 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 40.Zaborina, O., X. Li, G. Cheng, V. Kapatral, and A. M. Chakrabarty. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol. Microbiol., in press. [DOI] [PubMed]
- 41.Zhou C, Yang Y, Jong A Y. Mini-Prep in ten minutes. BioTechniques. 1990;8:172. [PubMed] [Google Scholar]