Abstract
Despite the standard of care, patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) progress to dialysis, are hospitalized for heart failure and die prematurely. Overactivation of the mineralocorticoid receptor (MR) causes inflammation and fibrosis that damages the kidney and heart. Finerenone, a nonsteroidal, selective MR antagonist, confers kidney and heart protection in both animal models and Phase II clinical studies; the effects on serum potassium and kidney function are minimal. Comprising the largest CKD outcomes program to date, FIDELIO-DKD (FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease) and FIGARO-DKD (FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease) are Phase III trials investigating the efficacy and safety of finerenone on kidney failure and cardiovascular outcomes from early to advanced CKD in T2D. By including echocardiograms and biomarkers, they extend our understanding of pathophysiology; by including quality of life measurements, they provide patient-centered outcomes; and by including understudied yet high-risk cardiorenal subpopulations, they have the potential to widen the scope of therapy in T2D with CKD.
Trial registration number: FIDELIO-DKD (NCT02540993) and FIGARO-DKD (NCT02545049)
Keywords: albuminuria, CKD, clinical trial, diabetic kidney disease, mineralocorticoid receptor antagonist
INTRODUCTION
Diabetes is the leading cause of chronic kidney disease (CKD) [1, 2], is a risk factor for the progression of CKD to end-stage kidney disease (ESKD) [3] and is costly [4]. In 2015, US Medicare expenditures were in excess of $64 billion and $34 billion for CKD and ESKD, respectively, while the total UK expenditures attributable to CKD were estimated at £1.45 billion in 2010 [4, 5]. CKD in type 2 diabetes (T2D) is also associated with reduced life expectancy. A recent study of 512 700 patients demonstrated that while diabetes itself can reduce life expectancy by 10 years and early CKD by 6 years, early CKD with T2D shortens life by 16 years [6]. Those with early CKD in T2D are much more likely to die than progress to ESKD, yet are often excluded from randomized trials of kidney failure. Furthermore, both diabetes and CKD are strongly associated with cardiovascular (CV) disease [2, 7]. In particular, albuminuria, a marker of kidney damage, is an independent predictor of CV and all-cause mortality [8, 9].
Over the last 2 decades, treatments to slow the progression of CKD in T2D have primarily focused on the improved management of hyperglycemia and hypertension and the use of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs) [10–12]. Since mid-2019, the American Diabetes Association has recommended sodium–glucose cotransporter-2 inhibitors (SGLT2is) in addition to an ACEi or ARB for the reduction of kidney and CV risk in patients with T2D with albuminuria >30 mg/g if their estimated glomerular filtration rate (eGFR) is ≥30 mL/min/1.73 m2, particularly in those with albuminuria >300 mg/g [13]. However, despite the use of ACEis or ARBs and the concomitant use of SGLT2is, there remains an unmet need to reduce both the residual risk of progression to ESKD and CV morbidity and mortality [11, 14].
There is growing evidence that pathophysiological overactivation of the mineralocorticoid receptor (MR) leads to inflammation and fibrosis and is a key driver of the progression of CKD and its associated morbidity. Therefore blockade of the MR is being investigated as a novel treatment approach to slow the progression of CKD [15, 16]. Interventions in early CKD [Kidney Disease: Improving Global Outcomes (KDIGO) stages G1A2, G2A1 or G2A2] are more effective in delaying the progression of CKD and CKD-related morbidity and mortality [12, 17]. Reducing inflammation and fibrosis at the earliest possible stage may therefore prove to be the most effective intervention. Although the steroidal hormones that activate the MR—aldosterone and cortisol—are familiar to most physicians, MR antagonists (MRAs) are not indicated for use in patients with CKD and T2D and are therefore not commonly used [18–21]. Furthermore, only limited data exist to support their use in this patient population. The available steroidal MRAs, spironolactone and eplerenone, are both effective in reducing mortality and hospitalization in the treatment of heart failure [22, 23]; however, their role in reducing the rate of progression of kidney disease to ESKD is unknown. Finerenone is a novel, nonsteroidal, selective MRA that has a high affinity for the MR and a unique binding mode that has been shown to reduce inflammation and fibrosis in animal models [16, 24–27]. In addition, Phase II trials have demonstrated significant reductions in albuminuria with finerenone and adverse events comparable with placebo, as well as less hyperkalemia compared with spironolactone [28]. The FIDELIO-DKD (FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease) and FIGARO-DKD (FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease) trials are based on both biological plausibility of cardiorenal benefit and promising data from Phase II trials [16]. Here we explore the role of these trials in providing new treatment opportunities for improving cardiorenal outcomes in patients with CKD in T2D. We highlight the use of finerenone as a new treatment approach to CKD in T2D. We explore the rationale for having a randomized clinical trial program with two independent and individually powered trials assessing the renal and CV protective effects of finerenone compared with placebo in addition to the standard background therapy.
THE MECHANISM OF ACTION OF FINERENONE
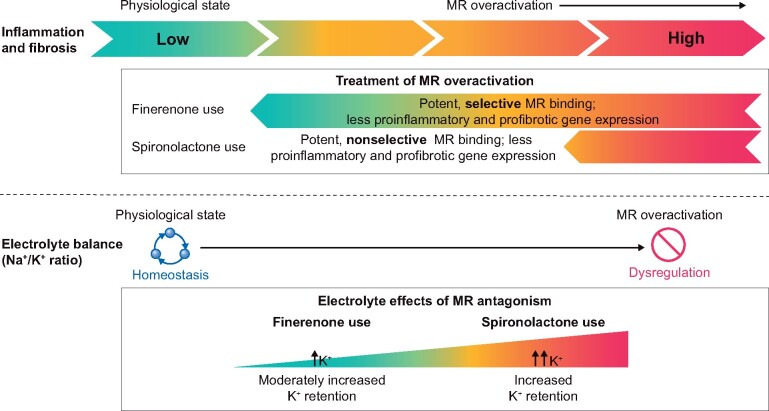
The MR belongs to the superfamily of nuclear hormone receptors and is predominantly expressed in the heart, kidneys, vasculature, brain, gut and myeloid cells [15]. It is well recognized that MR gene expression controls fluid, electrolyte and hemodynamic homeostasis [16, 29]. A lesser known fact is that overactivation of the MR, implicated in chronic pathophysiological disease states such as T2D and CKD, results in high levels of inflammation and fibrosis leading to end-organ damage in cardiorenal disease (Figure 1) [15, 16].
FIGURE 1.
Schematic representation of the pathophysiological effects of MR overactivation and the subsequent impact of MRA intervention using finerenone or spironolactone. K+, potassium; Na+, sodium
Both steroidal MRAs as well as novel, nonsteroidal MRAs can be used to inhibit MR overactivation and reduce its deleterious effects by reducing proinflammatory and profibrotic gene expression [16, 29]. Spironolactone and eplerenone have both demonstrated effective reduction in CV morbidity and mortality in patients with chronic heart failure and reduced left ventricular ejection fraction [15]. In addition, spironolactone has been demonstrated to improve endothelial dysfunction and increase nitric oxide bioavailability in patients with heart failure, thereby reducing CV mortality [30]. However, this benefit was not observed in patients with diabetes [31]. Importantly, steroidal MRAs are not indicated for the treatment of patients with CKD and T2D; their use is associated with hyperkalemia and, for the nonselective MRA spironolactone, antiandrogenic adverse effects such as gynecomastia (as indicated in the spironolactone USA and UK prescribing information) [18, 21]. Furthermore, studies have demonstrated an increase in cortisol and glycated hemoglobin (HbA1c) levels in patients receiving spironolactone [32, 33]. In contrast, no changes were observed in HbA1c with finerenone in Phase II studies [34]. Eplerenone is contraindicated in hypertensive patients with creatinine clearance <50 mL/min, any patient with creatinine clearance ≤30 mL/min or those with T2D and microalbuminuria (USA and UK prescribing information) [19, 20]. Despite the class 1A guideline recommendation in patients with heart failure, analysis of registry data reveals that MRAs remain underused in these patients, with their use decreasing with impaired renal function, even in patients with creatinine clearance of 30–<60 mL/min, where MRAs are not contraindicated [35].
Finerenone results in MR blockade that is at least as potent as spironolactone (Figure 1) and more selective than eplerenone [27]. Unlike spironolactone and eplerenone, finerenone has a nonsteroidal structure that allows it to bind to the MR with a unique mechanism to inhibit recruitment of transcriptional cofactors involved in the expression of hypertrophic, proinflammatory and profibrotic genes [36, 37]. In preclinical animal models, the kidney benefits of finerenone are manifested by the following: reduced expression of proinflammatory and profibrotic markers in the kidney, protection from glomerular, tubular and renal vascular damage and an improvement in proteinuria [24–26]. Also, when compared with an equinatriuretic dose of the steroidal MRA eplerenone, finerenone demonstrated more effective reduction in cardiac hypertrophy, proteinuria and inflammation and fibrosis in the kidneys in rodent models [24, 37, 38].
An important question that emerges is whether novel MRAs can protect the kidney while reducing the risk of hyperkalemia. Preclinical studies demonstrate that it is possible to achieve cardiorenal protection by MR blockade without causing hyperkalemia. Data from Huang et al. [29], using cell-type MR knockout mice, suggest that decreased myeloid MR signaling can protect the kidney without affecting urinary potassium levels. Preclinical studies using BR-4628, a precursor to finerenone, have also demonstrated an improvement in kidney structure and function without substantial effects on urinary sodium and potassium [39]. Finally, finerenone was shown to inhibit macrophage infiltration into inflamed renal tissue by blockade of myeloid MR in a rodent CKD model [26].
Emerging data from Phase II trials with finerenone in patients with heart failure and patients with CKD and T2D demonstrate that neither hyperkalemia nor reductions in kidney function were limiting factors to its use [15]. The exact reasons for the minimal effects of finerenone on serum potassium levels are unclear. However, it may be related to the distinct mode of MR antagonism of finerenone and subsequent transcriptional cofactor recruitment, its short plasma half-life and a lack of active metabolites and the tissue distribution characteristics of finerenone, which has an equal distribution to the heart and kidneys, in contrast to spironolactone and eplerenone, which exhibit higher drug accumulation in the kidney [24] (Figure 1).
FINERENONE PHASE II PROGRAM
The Phase II ARTS (MinerAlocorticoid Receptor antagonist Tolerability Study) program included in aggregate >2000 patients and was designed to test the safety and efficacy of finerenone in patients with T2D and CKD or with heart failure with reduced ejection fraction with T2D and/or CKD (Supplementary data, Table 1) [28, 40, 41]. The ARTS Phase IIa study demonstrated that finerenone was associated with a significantly smaller increase in serum potassium compared with spironolactone but was at least as effective in decreasing cardiac biomarkers of hemodynamic stress [B-type natriuretic peptide and N-terminal pro hormone B-type natriuretic peptide (NT-proBNP)] and albuminuria in patients with stable chronic heart failure [41]. Also, the incidence of adverse events related to worsening of renal function was lower with finerenone compared with spironolactone [41].
Table 1.
Baseline characteristics in the FIDELIO-DKD and FIGARO-DKD trials and in combination
| Characteristics | FIDELIO-DKD | FIGARO-DKD | Total |
|---|---|---|---|
| (n = 5674) | (n = 7354) | (N = 13 028) | |
| Age (years), mean (SD) | 65.6 (9.1) | 64.1 (9.8) | 64.8 (9.5) |
| Gender (male), n (%) | 3984 (70.2) | 5107 (69.4) | 9091 (69.8) |
| Race, n (%) | |||
| White | 3738 (65.9) | 5408 (73.5) | 9146 (70.2) |
| Asian | 1434 (25.3) | 1451 (19.7) | 2885 (22.1) |
| Black/African American | 267 (4.7) | 258 (3.5) | 525 (4.0) |
| Other | 235 (4.1) | 237 (3.0) | 472 (3.6) |
| Region, n (%) | |||
| Europe | 2358 (41.6) | 3504 (47.6) | 5862 (45.0) |
| Western | 1251 (22.0) | 1485 (20.2) | 2736 (21.0) |
| Eastern | 1107 (19.5) | 2019 (27.5) | 3126 (24.0) |
| North America | 944 (16.6) | 1109 (15.1) | 2053 (15.8) |
| Latin America | 593 (10.5) | 841 (11.4) | 1434 (11.0) |
| Asia Pacific | 1579 (27.8) | 1625 (22.1) | 3204 (24.6) |
| Other | 200 (3.5) | 275 (3.7) | 475 (3.6) |
| BMI (kg/m2), mean (SD) | 31.1 (6.0) | 31.4 (6.0) | 31.3 (6.0) |
| Duration of diabetes (years), mean (SD) | 16.6 (8.8) | 14.5 (8.5) | 15.4 (8.7) |
| HbA1c (%), mean (SD) | 7.7 (1.3) | 7.7 (1.4) | 7.7 (1.4) |
| Systolic blood pressure (mmHg), mean (SD) | 138 (14) | 136 (14) | 137 (14) |
| Diastolic blood pressure (mmHg), mean (SD) | 76 (10) | 77 (10) | 76 (10) |
| eGFR (mL/min/1.73 m2), mean (SD) | 44.3 (12.6) | 67.8 (21.7) | 57.6 (21.7) |
| eGFR (mL/min/1.73 m2), n (%) | |||
| ≥60 | 656 (11.6) | 4540 (61.7) | 5196 (39.9) |
| ≥45–<60 | 1900 (33.5) | 1535 (20.9) | 3435 (26.4) |
| ≥25–<45 | 2981 (52.5) | 1251 (17.0) | 4232 (32.5) |
| <25 | 135 (2.4) | 27 (0.4) | 162 (1.2) |
| UACR (mg/g), median (IQR) | 851 (446–1634) | 312 (110–744) | 519 (200–1147) |
| UACR category (mg/g), n (%) | |||
| <30 | 25 (0.4) | 198 (2.7) | 223 (1.7) |
| 30–300 | 685 (12.1) | 3385 (46.0) | 4070 (31.2) |
| ≥300 | 4960 (87.4) | 3762 (51.2) | 8722 (66.9) |
| Serum potassium (mEq/L), mean (SD) | 4.4 (0.5) | 4.3 (0.4) | 4.3 (0.4) |
| ACEis, n (%) | 1942 (34.2) | 3130 (42.6) | 5072 (38.9) |
| ARBs, n (%) | 3727 (65.7) | 4198 (57.1) | 7925 (60.8) |
| Beta-blockers, n (%) | 2963 (52.2) | 3524 (47.9) | 6487 (49.8) |
| Diuretics, n (%) | 3210 (56.6) | 3486 (47.4) | 6696 (51.4) |
| Loop diuretics | 1618 (28.5) | 1172 (15.9) | 2790 (21.4) |
| Thiazide diuretics | 1356 (23.9) | 1802 (24.5) | 3158 (24.2) |
| Statins, n (%) | 4213 (74.3) | 5172 (70.3) | 9385 (72.0) |
| Platelet aggregation inhibitors, n (%) | 3222 (56.8) | 4063 (55.2) | 7285 (55.9) |
| Glucose-lowering therapies, n (%) | 5526 (97.4) | 7180 (97.6) | 12 706 (97.5) |
| Insulin | 3636 (64.1) | 3977 (54.1) | 7613 (58.4) |
| Metformin | 2486 (43.8) | 5053 (68.7) | 7539 (57.9) |
| Acarbose | 323 (5.7) | 334 (4.5) | 657 (5.0) |
| Sulfonylurea | 1329 (23.4) | 2059 (28.0) | 3388 (26.0) |
| DPP-4 inhibitors | 1521 (26.8) | 1748 (23.8) | 3269 (25.1) |
| GLP-1 agonists | 395 (7.0) | 548 (7.5) | 943 (7.2) |
| SGLT2 inhibitors | 258 (4.5) | 613 (8.3) | 871 (6.7) |
| Arterial hypertension, n (%) | 5505 (97.0) | 7046 (95.8) | 12 551 (96.3) |
| Diabetic retinopathy, n (%) | 2657 (46.8) | 2265 (30.8) | 4922 (37.8) |
| History of CV disease, n (%) | 2602 (45.9) | 3400 (46.2) | 6002 (46.1) |
| Coronary artery disease | 1693 (29.8) | 2273 (30.9) | 3966 (30.4) |
| Peripheral artery disease | 920 (16.2) | 1168 (15.9) | 2088 (16.0) |
| Myocardial infarction | 765 (13.5) | 1251 (17.0) | 2016 (15.5) |
| Ischemic stroke | 685 (12.1) | 819 (11.1) | 1504 (11.5) |
| Carotid endarterectomy | 71 (1.3) | 94 (1.3) | 165 (1.3) |
| Heart failure | 423 (7.5) | 556 (7.6) | 986 (7.6) |
BMI, body mass index; DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
In the ARTS-HF (ARTS-Heart Failure) Phase IIb trial in patients treated within 7 days of a hospitalization for worsening heart failure, finerenone reduced NT-proBNP levels to a similar degree compared with eplerenone [40]. However, the exploratory composite endpoint of death from any cause, CV hospitalization or emergency presentation for worsening heart failure occurred less frequently with finerenone compared with eplerenone, with smaller mean increases in serum potassium observed (Supplementary data, Table 1) [40].
The ARTS-DN (ARTS-Diabetic Nephropathy) Phase IIb study of 823 patients with T2D and albuminuria [urine albumin:creatinine ratio (UACR) ≥30 mg/g] on stable therapy with an ACEi or ARB evaluated the safety and efficacy of different once-daily doses of finerenone (up to 20 mg) compared with placebo [28]. Among these T2D patients with CKD, finerenone demonstrated a dose-dependent reduction in UACR (the primary outcome) of 25–38% compared with placebo in the finerenone 10-, 15- and 20-mg once-daily groups over 90 days. Minimal adverse effects on potassium and renal function and a limited effect on reducing blood pressure were demonstrated. No meaningful correlation was observed between the dose-dependent reductions in UACR and small reductions in blood pressure or eGFR reported (that were not dose-dependent). This may suggest that the effect of finerenone on albuminuria was independent of measured hemodynamic effects [28]. In addition, no changes were observed in HbA1c levels with finerenone [34]. The findings of ARTS-DN provided the rationale for initiating a large-scale Phase III program with finerenone to investigate kidney and CV outcomes in T2D patients with all stages of CKD.
Hyperkalemia with MRAs and treatment-emergent hyperkalemia in the finerenone Phase II program
In 2014, a Cochrane Database Systematic Review reported the use of MRAs on top of ACEi or ARB for 27 studies in 1549 patients [42]. Hard endpoints such as ESKD or major adverse CV effects were not noted in these trials. Steroidal MRAs doubled the risk of hyperkalemia versus placebo (data from 11 studies, 632 patients), with a risk ratio (RR) of 2.00 [95% confidence interval (CI) 1.25–3.20] and the number needed to treat for an additional harmful outcome of 7.2 (95% CI 3.4–∞).
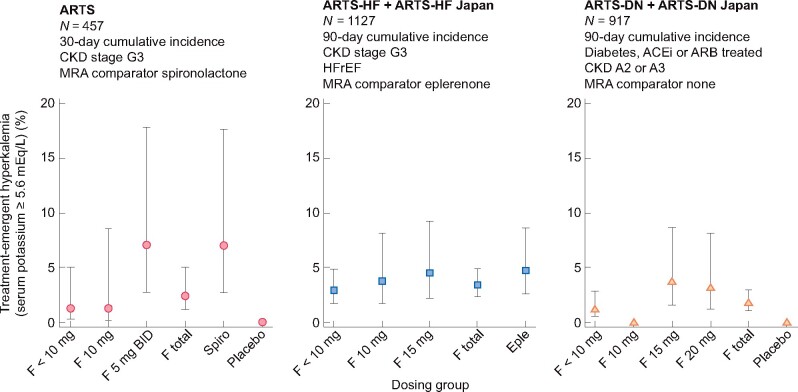
Figure 2 shows the percentage of patients with a treatment-emergent adverse effect of hyperkalemia, defined as serum potassium ≥5.6 mEq/L in Phase II finerenone trials. The event was reported as hyperkalemia, defined as serum potassium ≥5.6 mEq/L in each of the three Phase II randomized trials: ARTS, ARTS-HF pooled with ARTS-HF Japan and ARTS-DN pooled with ARTS-DN Japan. In two of the trials, an active comparator was used: spironolactone in the ARTS and eplerenone in the ARTS-HF [40, 41]. In the ARTS, compared with spironolactone, the incidence of a treatment-emergent adverse event of hyperkalemia with finerenone was lower. In the ARTS-HF, the incidence of treatment-emergent adverse event of hyperkalemia was comparable to eplerenone. Although the incidence of adverse events of hyperkalemia was low in the ARTS-DN (pooled incidence with finerenone 2.1% versus 0% with placebo), there was a statistically significant change from baseline to Day 90 in serum potassium for each of the doses of finerenone [28].
FIGURE 2.
Percentage of patients with a treatment-emergent adverse event of hyperkalemia defined as serum potassium ≥5.6 mEq/L in Phase II finerenone trials. Data are point estimates ± 95% CIs. eple: eplerenone; F: finerenone; HFrEF: heart failure with reserved ejection fraction; spiro: spironolactone.
FINERENONE PHASE III PROGRAM
FIDELIO-DKD and FIGARO-DKD are large-scale (n = 5674 and n = 7354, respectively) international, randomized, double-blind, placebo-controlled trials investigating the efficacy and safety of finerenone in reducing CKD progression and CV mortality and morbidity in patients with CKD and T2D [43, 44]. The studies enrolled patients from 48 countries and territories across 6 continents (Figure 3 and Supplementary data, Figure 1) [43, 44].
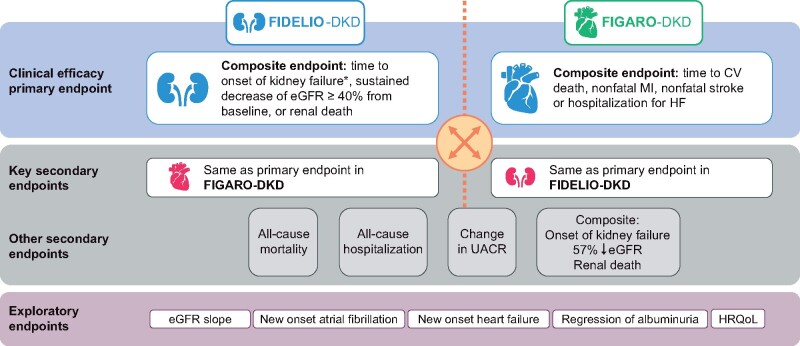
FIGURE 3.
FIDELIO-DKD and FIGARO-DKD endpoints. aKidney failure defined as occurrence of ESKD (initiation of chronic dialysis for ≥90 days or renal transplantation) or sustained eGFR <15 mL/min/1.73 m2. HF: heart failure; HRQoL: health-related quality of life; MI: myocardial infarction.
FIDELIO-DKD and FIGARO-DKD are independent event-driven trials that individually, with the total number of primary endpoints accrued, provide a minimum of 90% power to detect a 20% relative risk reduction in their respective primary endpoints with finerenone compared with placebo. The trials were designed to include common and reciprocal primary and key secondary endpoints consisting of important and relevant renal and CV outcomes (Figure 3). The FIDELIO-DKD primary endpoint is a composite of time to first occurrence of kidney failure, defined as either the initiation of chronic dialysis over 90 days or renal transplantation (ESKD) or a sustained eGFR <15 mL/min/1.73 m2over at least 4 weeks, a sustained decrease in eGFR of ≥40% from baseline over at least 4 weeks or renal death, whereas the key prespecified secondary endpoint is a composite of CV death, nonfatal myocardial infarction, nonfatal stroke or hospitalization for heart failure [43]. The FIGARO-DKD primary endpoint is the composite of CV death, nonfatal myocardial infarction, nonfatal stroke or hospitalization for heart failure, with the key prespecified secondary endpoint replicating the primary composite endpoint of FIDELIO-DKD (Figure 3) [43, 44].
Other secondary endpoints include deaths and hospitalizations from any cause. Exploratory endpoints such as annualized eGFR slope will also be studied. In addition, the trials will prospectively assess the change in health-related quality of life, which will be evaluated in all patients using the 36-item Kidney Disease Quality of Life and 5-level European Quality of Life 5-Dimensions questionnaires. Moreover, the trials will include echocardiography and biomarker substudies, thereby contributing a depth of insight that considers pathophysiology as well as patient-centered outcomes, aspects that have not been previously addressed to this extent in this patient population.
Comparing FIGARO-DKD and FIDELIO-DKD populations with other trials in CKD with T2D
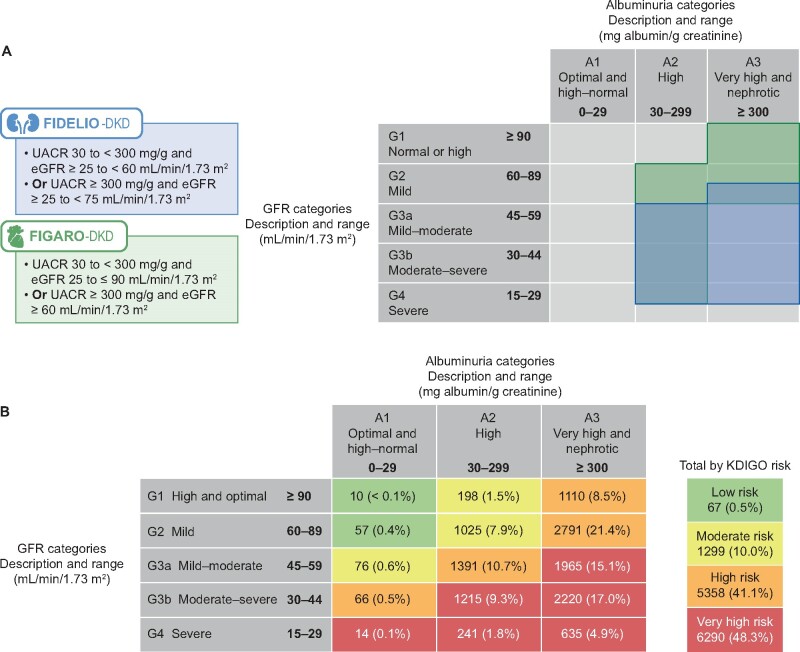
Patients with preserved eGFR are often excluded from CKD with T2D trials. Previously understudied patient groups with respect to renal and CV outcomes, such as those with high albuminuria (UACR 30–<300 mg/g) or very high albuminuria (UACR >300 mg/g) and eGFR >60 mL/min/1.73 m2(Figure 4A and Table 1) is now well represented. In total, 39.9% (5196/13 028) of patients included in both trials corresponded to this population with preserved eGFR.
FIGURE 4.
(A) Overlap of patient populations according to inclusion values for UACR and eGFR in the FIDELIO-DKD and the FIGARO-DKD trials. (B) Pooled data showing the baseline KDIGO risk categories of patients included in the FIDELIO-DKD and FIGARO-DKD trials (KDIGO score risk category data were missing for 4 patients in the FIDELIO-DKD trial and 10 patients in the FIGARO-DKD trial). Values are presented as n (%).
To better quantify cardiorenal risk in the FIDELIO-DKD and FIGARO-DKD patient populations we used a validated tool. The KDIGO CKD working group uses a combination of eGFR and UACR categories in its risk stratification tool for predicting CKD and CV outcomes [12]. The results are shown in Figure 4B. Across the two trials, moderate, high and very high KDIGO risk scores were noted in 10, 41.1 and 48.3% of patients, respectively; ∼90% had at least a high risk of a major clinical outcome.
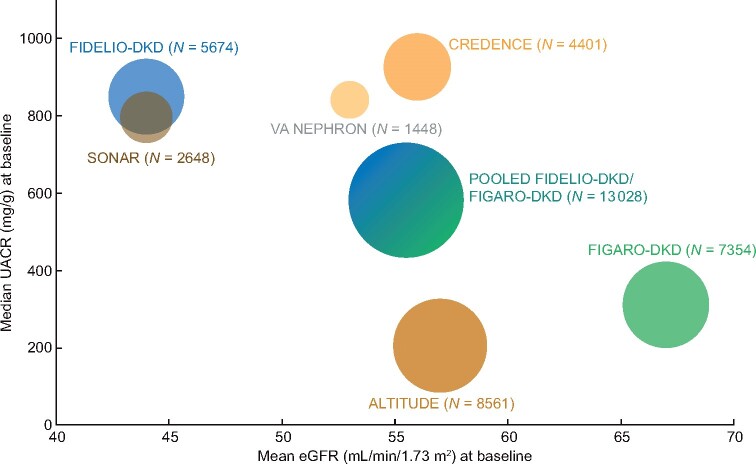
To further compare the FIDELIO-DKD and FIGARO-DKD patient populations with other trials we plotted the summary estimates of baseline UACR and eGFR data enrolled in recent CKD in T2D trials investigating CV and/or renal outcomes (Figure 5; Supplementary data, Figure 2 and Supplementary data, Table 2). The high albuminuria population is underrepresented in other trials but is well represented in the FIDELIO-DKD and FIGARO-DKD program, where 4070 (31.2%) patients have high albuminuria at baseline (Figure 5; Supplementary data, Figure 2 and Supplementary data, Table 2). Albuminuria is an independent risk marker for CV and all-cause mortality, even at levels within the upper normal range [8, 9]. Accordingly, this high-risk CV population should allow for greater insight into the possible benefit of finerenone for reducing CV outcomes in this group of patients with earlier stages of CKD.
FIGURE 5.
Comparison of baseline median UACR (mg/g) and mean eGFR (mL/min/1.73 m2) of patients enrolled in recent trials investigating cardiovascular and/or renal outcomes in patients with CKD in T2D (circle sizes are proportional to the population sizes of the trials.) [12, 14, 43–49].
Despite the pooled trial population being at high risk of CV events and having a lower mean eGFR at baseline compared with the CREDENCE (Canagliflozin and Renal Events in Diabetes and Nephropathy Clinical Evaluation) trial of canagliflozin in patients with T2D and CKD (Table 1; Supplementary data, Table 2 and Supplementary data, Figure 2), the FIDELIO-DKD and FIGARO-DKD population was well controlled relative to the CREDENCE trial with respect to lower baseline HbA1c and systolic blood pressure (Table 1;Supplementary data, Table 2). Evaluating a well-controlled patient population minimizes variation and confounding factors within the trial outcomes. Furthermore, it allows validation of the potential cardiorenal benefit of finerenone in addition to the best standard of care for glycemic control and CV risk.
The FIDELIO-DKD and FIGARO-DKD trials are designed to investigate the effect of finerenone on reducing the risk of CV disease in FIGARO-DKD as well as CKD progression in FIDELIO-DKD, but cross-validating the findings within one trial program. No previous trial program has been designed this way. As with other trials, FIDELIO-DKD and FIGARO-DKD investigated patients receiving the approved standard of care with the maximum tolerated labeled doses of ACEi or ARB (Table 1). In addition, ∼7% of patients included also received SGLT2is and glucagon-like peptide-1 receptor agonists at baseline (Table 1). Although the population receiving SGLT2is in the FIDELIO-DKD and FIGARO-DKD trials is comparatively small, this subgroup may provide insights into the effects of receiving both finerenone and SGLT2i.
CONCLUSIONS
The FIDELIO-DKD and FIGARO-DKD studies comprise the largest CKD outcomes program to date and will determine the effect of a novel approach to the treatment of CKD in T2D that targets the underlying disease processes. This approach extends the CKD patient reach by including previously understudied and high-risk cardiorenal subgroups. As such, the trials deliberately included patients with high and very high albuminuria at high cardiorenal risk despite the best standard of care for glycemic control and control of CV risk factors (Table 1). Also, the trials are powered to demonstrate both efficacy and safety on the major kidney and CV outcomes in this high-risk population. Finally, the FIDELIO-DKD and FIGARO-DKD trials are prespecified superiority studies as opposed to safety trials and evaluate a treatment that does not have a glucose-lowering effect. These trials have the potential to reduce CKD progression and afford cardiorenal protection in patients with T2D across the CKD continuum. The trials are anticipated to complete in 2020 and 2021, respectively.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The FIDELIO-DKD and FIGARO-DKD trials were sponsored by Bayer AG, the manufacturer of finerenone. Medical writing assistance was provided by Cindy Jenner, PhD, of Chameleon Communications International, and was funded by Bayer AG.
CONFLICT OF INTEREST STATEMENT
R.A. is a member of data safety monitoring committees for AstraZeneca and Ironwood Pharmaceuticals; a member of steering committees of randomized trials for Akebia, Bayer, Janssen, GlaxoSmithKline, Relypsa, Sanofi and Genzyme; a member of adjudication committees for Bayer, Boehringer Ingelheim and Janssen; and a member of a scientific advisory board or a consultant for Eli Lilly, Merck, Relypsa, Lexicon and Reata; has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; and has received research grants from the US Veterans Administration and the National Institutes of Health. S.D.A. has received research support from Abbott Vascular and Vifor International and personal fees from Boehringer Ingelheim, Bayer, AstraZeneca, Novartis, Vifor International, Impulse Dynamics, Respicardia and St Jude Medical. G.B. reports research funding, paid to the University of Chicago Medicine, from Bayer, Janssen and Vascular Dynamics; has acted as a consultant for Merck, Vascular Dynamics, Relypsa, Boehringer Ingelheim, Sanofi, Pfizer, Novo Nordisk, Ionis and AstraZeneca and is an editor of the American Journal of Nephrology and Nephrology and Hypertension, section editor of UpToDate and associate editor of Diabetes Care and Hypertension Research. G.F. is a committee member of trials and registries sponsored by Bayer, Novartis, Servier, Vifor, Medtronic and Boehringer Ingelheim, is associate editor of European Heart Journal and has received research support from the European Union. B.P. reports consultant fees for Bayer, AstraZeneca, Sanofi/Lexicon, scPharmaceuticals, SQ Innovation, G3 Pharmaceuticals, Sarfez, Phasebio, Vifor/Relypsa, Cereno Scientific, Ardelyx, KBP Biosciences, Boehringer Ingelheim, Brainstorm Medical and Tricida; has stock options with KBP Biosciences, SQ Innovation, Sarfez, scPharmaceuticals, Cereno Scientific G3 Pharmaceuticals, Vifor/Relypsa, Brainstorm Medical and Tricida and holds a patent for site-specific delivery of eplerenone to the myocardium (US patent 9931412) and a provisional patent for histone acetylation–modulating agents for the treatment and prevention of organ injury (US patent 63/045784). P.R. reports giving lectures for AstraZeneca, Bayer, Novo Nordisk, Merck and Boehringer Ingelheim; serving as a consultant for AbbVie, AstraZeneca, Bayer, Eli Lilly, Boehringer Ingelheim, Astellas, Gilead, Mundipharma and Novo Nordisk, with all fees given to Steno Diabetes Center Copenhagen; and having an equity interest in Novo Nordisk. L.R. has served as an advisor/speaker for AstraZeneca, Bayer, Daiichi Sankyo, Medtronic, Novartis and Recordati. A.J., P.K., C.N. and M.G. are full-time employees of Bayer AG, Division Pharmaceuticals, Germany.
DATA AVAILABILITY STATEMENT
Anonymized data will be made publicly available at a future date.
REFERENCES
- 1. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60: 850–886 [DOI] [PubMed] [Google Scholar]
- 2. International Diabetes Federation. IDF Diabetes Atlas, 9th edn.Brussels: International Diabetes Federation, 2019 [Google Scholar]
- 3. Abbasi M, Chertow G, Hall Y.. End-stage renal disease. Am Fam Physician 2010; 82: 1512. [PubMed] [Google Scholar]
- 4. Luyckx VA, Tonelli M, Stanifer JW.. The global burden of kidney disease and the sustainable development goals. Bull World Health Org 2018; 96: 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Health Service. Chronic Kidney Disease in England: The Human and Financial Cost. 2012. https://www.england.nhs.uk/improvement-hub/publication/chronic-kidney-disease-in-england-the-human-and-financial-cost/ (9 November 2020, date last accessed)
- 6. Wen CP, Chang CH, Tsai MK. et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int 2017; 92: 388–396 [DOI] [PubMed] [Google Scholar]
- 7. Lovre D, Shah SJ, Sihota A. et al. Managing diabetes and cardiovascular risk in chronic kidney disease patients. Endocrinol Metab Clin 2018; 47: 237–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon SD, Lin J, Solomon CG. et al. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation 2007; 116: 2687–2693 [DOI] [PubMed] [Google Scholar]
- 9. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 10. Pavkov ME, Collins AJ, Coresh J. et al. Kidney disease in diabetes. In: Cowie CC, Casagrande SS, Menke A. et al. (eds). Diabetes in America, 3rd edn. Publication 17-1468. Bethesda, MD, National Institutes of Health, 2018:22-1–22-80. [Google Scholar]
- 11. Alicic RZ, Rooney MT, Tuttle KR.. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes. Diabetes Care 2020; 43(Suppl 1): S135–S151 [DOI] [PubMed] [Google Scholar]
- 14. Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 15. Kolkhof P, Jaisser F, Kim SY. et al. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol 2017; 243: 271–305 [DOI] [PubMed] [Google Scholar]
- 16. Agarwal R, Kolkhof P, Bakris G. et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2020; 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whaley-Connell A, Nistala R, Chaudhary K.. The importance of early identification of chronic kidney disease. Mo Med 2011; 108: 25–28 [PMC free article] [PubMed] [Google Scholar]
- 18. Pfizer. Aldactone (spironolactone) tablets for oral use, prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=520 (9 November 2020, date last accessed)
- 19.Upjohn UK Ltd. 2020. Eplerenone 25 mg film-coated tablets, summary of product characteristics. https://www.medicines.org.uk/emc/product/1915/smpc (09 November 2020, date last accessed)
- 20.Pfizer Inc. 2020. Inspra (eplerenone) tablets for oral use, prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=599 (09 November 2020, date last accessed).
- 21. Pfizer Ltd. 2019. Aldactone 25 mg film-coated tables, summary of product characteristics. https://www.medicines.org.uk/emc/product/1619/smpc (9 November 2020, date last accessed)
- 22. Pitt B, Zannad F, Remme WJ. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717 [DOI] [PubMed] [Google Scholar]
- 23. Zannad F, McMurray JJ, Krum H. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21 [DOI] [PubMed] [Google Scholar]
- 24. Kolkhof P, Delbeck M, Kretschmer A. et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol 2014; 64: 69–78 [DOI] [PubMed] [Google Scholar]
- 25. Lattenist L, Lechner SM, Messaoudi S. et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension 2017; 69: 870–878 [DOI] [PubMed] [Google Scholar]
- 26. Barrera-Chimal J, Estrela GR, Lechner SM. et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int 2018; 93: 1344–1355 [DOI] [PubMed] [Google Scholar]
- 27. Kolkhof P, Barfacker L.. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol 2017; 234: T125–T140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakris GL, Agarwal R, Chan JC. et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 2015; 314: 884–894 [DOI] [PubMed] [Google Scholar]
- 29. Huang LL, Nikolic-Paterson DJ, Han Y. et al. Myeloid mineralocorticoid receptor activation contributes to progressive kidney disease. J Am Soc Nephrol 2014; 25: 2231–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farquharson CA, Struthers AD.. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000; 101: 594–597 [DOI] [PubMed] [Google Scholar]
- 31. Davies JI, Band M, Morris A. et al. Spironolactone impairs endothelial function and heart rate variability in patients with type 2 diabetes. Diabetologia 2004; 47: 1687–1694 [DOI] [PubMed] [Google Scholar]
- 32. Yamaji M, Tsutamoto T, Kawahara C. et al. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A1c levels in patients with chronic heart failure. Am Heart J 2010; 160: 915–921 [DOI] [PubMed] [Google Scholar]
- 33. Zhao JV, Xu L, Lin SL. et al. Spironolactone and glucose metabolism, a systematic review and meta-analysis of randomized controlled trials. J Am Soc Hypertens 2016; 10: 671–682 [DOI] [PubMed] [Google Scholar]
- 34. Bayer AG. Data on file. 2015
- 35. Savarese G, Carrero JJ, Pitt B. et al. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail 2018; 20: 1326–1334 [DOI] [PubMed] [Google Scholar]
- 36. Amazit L, Le Billan F, Kolkhof P. et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem 2015; 290: 21876–21889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grune J, Beyhoff N, Smeir E. et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertension 2018; 71: 599–608 [DOI] [PubMed] [Google Scholar]
- 38. Grune J, Benz V, Brix S. et al. Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol 2016; 67: 402–411 [DOI] [PubMed] [Google Scholar]
- 39. Ma FY, Han Y, Nikolic-Paterson DJ. et al. Suppression of rapidly progressive mouse glomerulonephritis with the non-steroidal mineralocorticoid receptor antagonist BR-4628. PLoS One 2015; 10: e0145666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filippatos G, Anker SD, Bohm M. et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J 2016; 37: 2105–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pitt B, Kober L, Ponikowski P. et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013; 34: 2453–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bolignano D, Palmer SC, Navaneethan SD. et al. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2014; 4: CD007004 [DOI] [PubMed] [Google Scholar]
- 43. Bakris GL, Agarwal R, Anker SD. et al. Design and baseline characteristics of the finerenone in reducing kidney failure and disease progression in diabetic kidney disease trial. Am J Nephrol 2019; 50: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruilope LM, Agarwal R, Anker SD. et al. Design and baseline characteristics of the finerenone in reducing cardiovascular mortality and morbidity in diabetic kidney disease trial. Am J Nephrol 2019; 50: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ingelheim Boehringer . EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin). https://clinicaltrials.gov/ct2/show/NCT03594110 (date last accessed, 21 April 2020)
- 46. Fried LF, Emanuele N, Zhang JH. et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369: 1892–1903 [DOI] [PubMed] [Google Scholar]
- 47. Heerspink HJL, Parving HH, Andress DL. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393: 1937–1947 [DOI] [PubMed] [Google Scholar]
- 48. Heerspink HJL, Stefansson BV, Chertow GM. et al. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 2020; 35: 274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parving HH, Brenner BM, McMurray JJ. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be made publicly available at a future date.