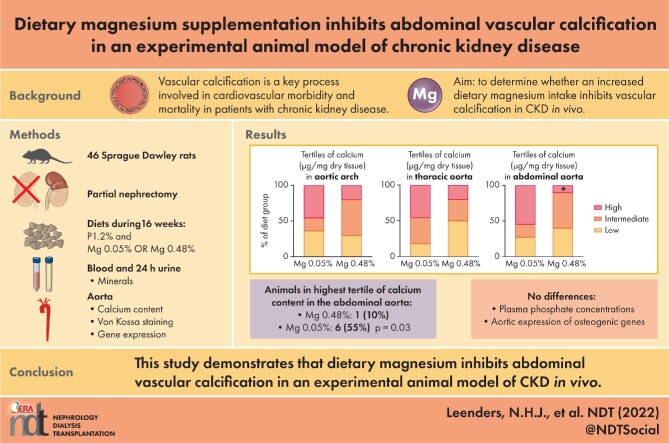
ABSTRACT
Background
Vascular calcification is a key process involved in cardiovascular morbidity and mortality in patients with chronic kidney disease (CKD). Magnesium supplementation may counteract vascular calcification. In this study we aimed to determine whether increased dietary magnesium intake inhibits vascular calcification in CKD in vivo and explore the mechanisms underlying these effects.
Methods
Sprague Dawley rats were partially nephrectomized and fed a diet with high phosphate and either high or normal magnesium content for 16 weeks. The primary outcome was the tissue calcium content of the aorta in the high versus normal dietary magnesium group. In addition, we analysed plasma mineral concentrations, aortic vascular calcification identified with von Kossa staining, calcium apposition time and aortic expression of genes related to vascular calcification.
Results
The number of animals in the highest tissue calcium content tertile was significantly lower in the abdominal aorta [1 (10%) versus 6 (55%); P = .03] in the high versus normal dietary magnesium group, but did not differ in the aortic arch and thoracic aorta. Von Kossa staining and calcium apposition time corresponded to these results. The median tissue calcium content was not significantly different between the groups. Serum phosphate concentrations and expression of osteogenic markers in the aorta did not differ between the groups.
Conclusions
This study demonstrates that increased dietary magnesium inhibits abdominal vascular calcification in an experimental animal model of CKD in vivo. These are promising results for CKD patients and further study is needed to identify the mechanisms involved and to determine the clinical relevance in patients.
Keywords: chronic kidney disease (CKD), magnesium, vascular calcification
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Patients with chronic kidney disease (CKD) are at an increased risk for all-cause and cardiovascular mortality.
Vascular calcification is a key process involved in cardiovascular morbidity and mortality in patients with CKD.
Magnesium may counteract vascular calcification.
What this study adds?
This study demonstrates that increased dietary magnesium inhibits abdominal vascular calcification in an established model of CKD in vivo.
Plasma magnesium concentration and urinary magnesium excretion were substantially increased in the high dietary magnesium-treated group, indicating that dietary magnesium was absorbed and available for endogenous effects.
The effect was not mediated by the phosphate-binding actions of magnesium in the gastrointestinal tract and was not explained by inhibition of osteogenic transformation of vascular smooth muscle cells, indicating that magnesium may exert inhibiting effects more downstream in the pathogenesis of calcification.
What impact this may have on practice or policy?
Magnesium supplementation, a safe and inexpensive intervention, may inhibit vascular calcification in CKD patients, similar to our animal study, and may potentially decrease cardiovascular morbidity and mortality.
Further study is needed to identify the mechanisms involved and to determine the clinical relevance in patients.
INTRODUCTION
Patients with chronic kidney disease (CKD) are at increased risk for all-cause and cardiovascular mortality [1]. Vascular calcification is an important pathophysiological process involved in cardiovascular disease in these patients and a major contributor to the increased all-cause and cardiovascular mortality [2, 3]. Calcification of the vascular wall is characterized by deposition of crystalline calcium-phosphate in the extracellular matrix of the tunica media of arteries. In CKD, this occurs in a milieu of imbalance between procalcifying factors and calcification inhibitors [4–6]. The process involves, among other factors, the formation of amorphous nanoparticles containing proteins and calcium and phosphate and the transformation of these primary calciprotein particles (CPPs) into crystalline or secondary CPPs [7]. In addition, vascular smooth muscle cells (VSMCs) undergo osteogenic transformation with increased expression of osteogenic differentiation-related genes [8, 9]. These VSMCs secrete matrix vesicles and apoptotic bodies containing calcium/phosphate nanocrystals and are depleted from mineralization inhibitors, which provide a nidus for mineral nucleation and maturation [10].
In patients with CKD, plasma magnesium concentrations are inversely associated with all-cause and cardiovascular mortality [11]. In addition to anti-arrhythmic effects of magnesium, inhibition of vascular calcification by magnesium may contribute to these associations [12–14]. Plasma magnesium concentrations have also been inversely associated with vascular calcification in patients with CKD [15–17]. This suggests that an increase of magnesium concentrations may retard vascular calcification in the clinical setting and benefit patients with CKD. Explorative studies with magnesium-based interventions in patients with CKD, both dialysis dependent and independent, have indeed shown beneficial effects on several markers of calcification, including carotid intima-media thickness, coronary artery calcification score and calcification propensity score [18–21]. Studies that used various animal models of CKD, induced by the toxicity of adenine or with high-dose calcitriol, showed beneficial effects of magnesium supplementation on the development of vascular calcification [22–24]. In vitro, magnesium is able to inhibit phosphate-induced calcification in the extracellular medium of VSMC cultures, in conjunction with inhibited expression of osteogenic differentiation-related genes [25, 26]. However, the mechanisms by which magnesium inhibits vascular calcification in vitro are incompletely understood.
In this study we used partially nephrectomized rats as an animal model of CKD to study the effects of an increased dietary magnesium content on the development of aortic vascular calcification. We aimed to determine whether increased dietary magnesium intake inhibits vascular calcification in CKD in vivo. Besides retarding mineralization per se, possible effects of magnesium may be inhibition of gene expression involved in osteogenic differentiation or a reduction of absorption and concentration of other minerals, including calcium and phosphate. Therefore we measured concentrations and excretion of electrolytes and messenger RNA (mRNA) expression levels of genes involved in osteogenic differentiation in the aorta in order to further explore the mechanisms underlying effects of magnesium supplementation on vascular calcification.
MATERIALS AND METHODS
Ethical statement and study design
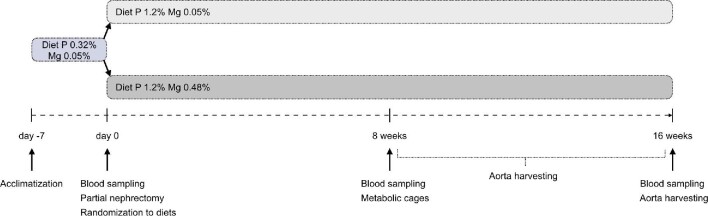
The experiments were conducted in accordance with European Directive 2010/63 EU on the protection of animals used for scientific research, and were approved by the Radboud University Animals Experiments Committee and the Netherlands Central Authority for Scientific Procedures on Animals (RU-DEC 2016-0096/AVD1030020172225). The study design is shown in Figure 1. After 1 week of acclimatization, 46 Sprague Dawley rats at 6 weeks of age underwent a partial nephrectomy as described below. Following this induction of CKD, rats were randomly allocated to a diet with high phosphate content (1.2% w/w) that was either magnesium enriched (0.48% w/w) or with normal magnesium (0.05% w/w). Blood was drawn from the tail vein at baseline and after 8 and 16 weeks. Animals were housed individually for 24 h for sampling of urine, as described in detail below. Rats were sacrificed by cardiac puncture under general anaesthesia with isoflurane after 16 weeks or when a humane endpoint was reached, and blood was collected. Aortas were harvested from all animals that had completed a study period of at least 8 weeks.
FIGURE 1:
Experimental design. After 1 week of acclimatization, 46 Sprague Dawley rats at 6 weeks of age underwent a partial nephrectomy and were randomly allocated to a diet that was magnesium (Mg) enriched (0.48% w/w) or with normal magnesium (0.05% w/w). In both groups, diets after nephrectomy had a high dietary phosphate (P) content. Blood was drawn from the tail vein at baseline and after 8 and 16 weeks. Five animals per group were housed individually for 24 h in metabolic cages at week 8. Rats were sacrificed after 16 weeks or when a humane endpoint was reached, and blood was collected. Aortas were harvested from all animals that had completed a study period of at least 8 weeks.
Experimental animals, housing and husbandry
Sprague Dawley rats [Crl: CD(SD)], 23 males and 23 females, were purchased at 5 weeks of age from the Charles River Laboratories, Erkrath, Germany. Animals were housed with maximally two animals per cage in individually ventilated cages (Greenline) with corn cob bedding and a polycarbonate rat retreat for environmental enrichment in a temperature-controlled room with a 12-h light/dark cycle. Drinking water and food were supplied ad libitum. Five animals per group were housed individually in metabolic cages for 24 h at week 8 for the collection of urine for measurements of mineral excretion. Animals were monitored daily during the entire experimental period and weighed daily in the first 3 weeks after surgery and twice weekly thereafter. In the case of abnormal activity, abnormal appearance or inadequate recovery from surgery that could not be resolved or in the case of a 20% decrease in body weight, a humane endpoint was reached and the animal was euthanized.
Experimental procedures
Nephrectomy surgery
In one surgical procedure under general anaesthesia with isoflurane, a reduction of in-between 3/4 and 5/6 of total functional kidney tissue was performed. First, the left kidney was exposed and decapsulated, and one or more branches of the left renal artery were ligated to induce infarction of minimal 1/2 and maximal 2/3 of this kidney. In the same procedure, the right kidney was exposed, decapsulated and removed in total after ligation of the hilar structures (artery, vein and ureter). For analgesia, buprenorphine was administered intramuscularly (30 μg/kg bodyweight; Astfarma, Oudewater, The Netherlands) during surgery and at the end of the operation day, and on indication, one additional dose was given on the next day.
Dietary interventions
Rats were fed an AIN93M synthetic diet with 0.75 mg/kg vitamin K1 (phylloquinone), no vitamin K3 (menadione) and 1000 IU/kg vitamin D3 (cholecalciferol) (Ssniff Spezialdiäten, Soest, Germany), as originally described for this diet [27]. In the week before surgery, all animals received this diet with a standard phosphate (0.32% w/w) and normal magnesium (0.05% w/w) content. After surgery, phosphate in the AIN93M diet was increased to 1.2% w/w to induce vascular calcification. In the intervention group, the diet after surgery was magnesium enriched (0.48% w/w) and the control group received a diet with normal magnesium (0.05% w/w).
Aorta processing and tissue staining
After sacrifice, the aorta was dissected, perfused with phosphate-buffered saline, cleaned and cut into three parts of equal length: arch, thoracic and abdominal. Each anatomical part was split into three pieces. The proximal piece and distal piece of each anatomical part were frozen in liquid nitrogen and stored at –80°C, for calcium content measurements and RNA extraction, respectively. The middle pieces of each anatomical part were stored in formalin 10% v/v for at least 24 h. After dehydration and clearing in xylene, this tissue was cut into two to four rings that were embedded in paraffin positioned next to each other to assess multiple height levels of the tissue for the presence of calcification. After rehydration, tissue sections of 4 μm were stained according to the von Kossa staining method, which stains phosphate in calcium deposits. Sections were immersed in 1% w/v silver nitrate for 30 min under constant ultraviolet light exposure (365 nm), after which excessive silver was removed by 5% w/v sodium thiosulfate and sections were counterstained in nuclear fast red, cleared and mounted with Pertex (Histolab, Göteborg, Sweden).
Tissue calcium content
The proximal pieces of all three anatomical aorta parts were used for quantitative measurement of calcification expressed by tissue calcium content. Tissue was freeze dried using a vacuum freeze dryer (Christ, Osterode, Germany) for 12 h, weighed and incubated in a 10–50-fold excess of 10% w/v formic acid for 24 h. The supernatant was used for assessment of tissue calcium content by the colorimetric cresolphthalein method (Randox Laboratories, Crumlin, UK) according to the manufacturer's manual. All measurements were performed in duplicate.
RNA isolation and real-time quantitative polymerase chain reaction (qPCR)
The distal pieces of all three anatomical aorta parts were used for RNA extraction. Total RNA was extracted from the aortic tissue stored at –80°C using TRIzol (Invitrogen, Bleiswijk, The Netherlands) according to the standard protocol after tissue homogenization. Total RNA was treated with DNase (Promega, Leiden, The Netherlands) to break down genomic DNA. To obtain cDNA, M-MLV reverse transcriptase (Invitrogen) was used for reverse transcription (RT) for 1 h at 37°C. RT-qPCR was executed in duplicate using IQ SYBRGreen Mix (Bio-Rad Laboratories, Hercules, CA, USA) using a Bio-Rad thermocycler. Relative expression was determined by the Livak method and shown as a fold change compared with the control group (0.05% w/w Mg) and normalized to glyceraldehyde 3-phosphate dehydrogenase expression [28]. The primers are listed in Supplementary data, Table S1.
Minerals and kidney function
Plasma was collected from blood drawn in lithium–heparin gel tubes (Multivette 600 LH, Sarstedt, Nümbrecht, Germany) that were centrifuged at 2000 g for 5 min. The following measurements were performed: magnesium with the colorimetric xylidyl blue method; calcium with the colorimetric 5-nitro-5′-methyl-BAPTA (NM-BAPTA) method; albumin with the colorimetric bromocresol-purple method and phosphate, creatinine and urea with enzymatic methods. All measurements were performed with reagents from Roche with the Cobas 8000 automatic analyser (Roche, Basel, Switzerland). In 24-h urine samples, magnesium, phosphate and calcium were measured with the same methods.
Bones
Femurs were harvested from all animals that had completed a study period of at least 12 weeks and micro-computed tomography (micro-CT) was performed. Details are described in Supplementary data, Figure S4 and Table S2.
Outcomes
The primary outcome was the quantity of vascular calcification determined with colorimetric quantification of calcium content in μg/mg dry weight tissue for each aorta segment in the intervention group compared with the control group. Secondary outcomes included the number of animals with vascular calcification of the aortic segments determined by von Kossa staining in the intervention group compared with the control group and the expression of genes related to vascular calcification in the aorta in the intervention group compared with the control group.
Sample size
Based on the literature, in remnant kidney Sprague Dawley rats on a high phosphate diet, a calcium content in the aorta of 1.8 ± 0.3 μg/mg dry weight in this group was expected [29]. We considered a 15% decline in calcium content in the group with the high magnesium diet a relevant reduction. Setting a significance level at 5% (α = 0.05) and with a power of 80% (β = 0.80), the estimated number of animals was calculated at 16 per group. Taking into account a compensation of 30% for reported animal loss of 30–46% after 5/6 nephrectomy due to anatomical differences between animals and the complexity of the surgical procedure [30–32], the number of animals needed per group was 23. Therefore, in total, 46 animals were included in the study.
Allocation and blinding
To make sure that the animals were of equal age at the time of surgery, operations were performed at the beginning of three consecutive weeks and there was a cohort of animals for each surgery week. Per cohort, animals were randomly allocated to the two experimental groups, stratified by sex, using the website www.random.org/sequences. In addition, the order of the nephrectomy per cohort was randomized. Sample collection and outcome measurements were performed in the same order. Diets were allocated to the groups by an independent laboratory worker. Researchers and laboratory personnel involved in the experiments were blinded to the treatment. Unblinding was not performed before the analysis of the main outcome parameters was completed.
Statistics
Data were analysed using SPSS for Windows (version 26; IBM, Armonk, NY, USA) and GraphPad Prism for Windows (version 8.2.1; GraphPad Software, San Diego, CA, USA). Continuous variables are expressed as mean and standard deviation (SD) for normally distributed variables or median and interquartile range (IQR) for non-parametric distributed variables. In addition, tissue calcium content for each aorta segment was categorized into tertiles for lowest, intermediate and highest calcium content. Categorical variables are presented as numbers and percentages. Analysis of differences between the experimental groups was performed. Each animal was considered an experimental unit within the groups. For the outcome tissue calcium content, in addition to the analyses per aortic segment, a pooled analysis was performed in which all aortic segments were included and each aortic piece was considered a separate experimental unit. In addition, in order to take into account the duration of exposure, calcium apposition time was calculated by dividing the tissue calcium content by the number of days of follow-up until the aorta was harvested. Significance was assessed with the Student's t-test for normally distributed continuous variables and with the Mann–Whitney U-test for non-parametrically distributed continuous variables. For categorical variables, significance was assessed with the Pearson's chi-quadrate test. Significance was assessed as two-sided in all analyses. Differences with P < .05 were considered statistically significant.
RESULTS
Animals and experimental course
The mean body weight of the animals at baseline was 192 ± 35 g. Body weight, kidney function markers creatinine and urea, and electrolytes magnesium, phosphate and calcium at baseline were comparable between the two diet groups (Table 1).
Table 1.
Baseline characteristics of 46 Sprague Dawley rats at 6 weeks of age
| Characteristics | 0.05% w/w Mg diet | 0.48% w/w Mg diet |
|---|---|---|
| Sex, n (%) | ||
| Male | 11 (48) | 12 (52) |
| Female | 12 (52) | 11 (48) |
| Body weight (g) | 189 ± 34 | 196 ± 36 |
| Urea (mmol/L) | 4.1 ± 1.4 | 4.2 ± 1.0 |
| Creatinine (µmol/L) | 10 ± 3 | 10 ± 3 |
| Magnesium (mmol/L) | 0.75 ± 0.08 | 0.75 ± 0.09 |
| Calcium (mmol/L) | 2.86 ± 0.12 | 2.84 ± 0.10 |
| Albumin (mmol/L) | 15 ± 2 | 15 ± 1 |
| Phosphate (mmol/L) | 2.5 ± 0.3 | 2.6 ± 0.3 |
Values are expressed as mean ± SD unless stated otherwise.
A minimum of 8 weeks of follow-up was reached in 11 animals in the normal magnesium group and 10 animals in the high magnesium group. Over the maximally planned 16-week experimental course, 20 animals in the normal magnesium group and 18 animals in the high magnesium group were lost because they died or a humane endpoint was reached. The median duration of follow-up was 35 days (IQR 2–84) in the normal magnesium group and 49 days (IQR 5–94) in the high magnesium group. For the animals that reached a follow-up duration of at least 8 weeks, the median follow-up was 90 days (IQR 72–107) in the normal magnesium group and 108 days (IQR 85–113) in the high magnesium group, and this difference was not statistically significant.
Renal insufficiency
The surgical procedure effectively induced renal insufficiency, as demonstrated in both groups by the ratio of plasma urea concentration at 8 weeks after nephrectomy over baseline: median 2.5 (IQR 2.0–3.3) in the normal magnesium group and 2.8 (IQR 2.2–3.8) in the high magnesium group, and there was no statistical difference in this ratio between the groups (P = .51). Also, the increase of plasma creatinine was comparable between the groups (Table 2).
Table 2.
Kidney function 8 weeks after nephrectomy in 21 Sprague Dawley rats
| Function | 0.05% w/w Mg diet | 0.48% w/w Mg diet | P-value |
|---|---|---|---|
| Urea (times increase T/T0) | 2.5 (2.0–3.3) | 2.8 (2.2–3.8) | 0.51 |
| Creatinine (times increase T/T0) | 8.4 (6.7–11.1) | 9.3 (5.5–12.0) | 0.92 |
Values are expressed as median (quartile 1–quartile 3).
Minerals
At 8 weeks, plasma magnesium concentrations were increased in the high compared with the normal magnesium group (1.27 ± 0.34 versus 0.67 ± 0.13 mmol/L; P < .01, respectively). Plasma phosphate and calcium concentrations did not differ significantly between the groups. In the rats that were housed in metabolic cages, mean urinary excretion of magnesium was substantially higher in the high compared with the normal magnesium group (0.58 ± 0.07 mmol/24 h versus 0.11 ± 0.03; P < .01). There was no statistically significant difference between groups in mean urinary calcium excretion (0.02 ± 0.01 mmol/24 h versus 0.04 ± 0.02; P = .18) or phosphate excretion (6.4 ± 1.8 mmol/24 h versus 5.4 ± 2.2; P = .48) for the high and normal magnesium groups, respectively (Table 3).
Table 3.
Electrolytes 8 weeks after nephrectomy
| Electrolytes | 0.05% w/w Mg diet | 0.48% w/w Mg diet | P-value |
|---|---|---|---|
| Week 8 (n = 21) | |||
| Plasma concentrations | |||
| Magnesium (mmol/L) | 0.67 ± 0.13 | 1.27 ± 0.34 | <0.01 |
| Calcium (mmol/L) | 2.34 ± 0.35 | 2.33 ± 0.39 | 0.94 |
| Albumin (mmol/L) | 13 ± 4 | 15 ± 3 | 0.23 |
| Phosphate (mmol/L) | 5.1 ± 1.5 | 4.3 ± 1.5 | 0.23 |
| Week 8—only animals that were housed in metabolic cages (n = 10) | |||
| Plasma concentrations | |||
| Magnesium (mmol/L) | 0.68 ± 0.17 | 1.25 ± 0.34 | 0.01 |
| Calcium (mmol/L) | 2.17 ± 0.43 | 2.39 ± 0.21 | 0.33 |
| Albumin (mmol/L) | 12 ± 5 | 15 ± 2 | 0.25 |
| Phosphate (mmol/L) | 5.7 ± 1.8 | 4.4 ± 1.0 | 0.18 |
| Urine excretion | |||
| Magnesium (mmol/24 h) | 0.11 ± 0.03 | 0.58 ± 0.07 | <0.01 |
| Calcium (mmol/24 h) | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.18 |
| Phosphate (mmol/24 h) | 5.4 ± 2.2 | 6.4 ± 1.8 | 0.48 |
Values are expressed as mean ± SD.
Calcification of the aorta: tissue calcium content
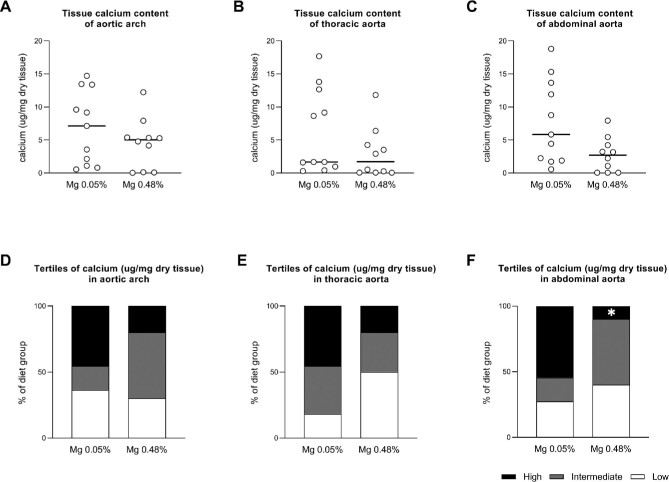
In the high magnesium group compared with the normal magnesium group, the number of animals in the highest tertile of tissue calcium content was significantly lower in the abdominal aorta [1 (10%) versus 6 (55%); P = .03] and did not differ between the groups in the aortic arch [2 (20%) versus 5 (46%); P = .22] and thoracic aorta [2 (20%) versus 5 (46%); P = .22] (Fig. 2). The median tissue calcium content was not statistically significantly lower in the high magnesium group compared with the normal magnesium group in the aortic arch [5.04 μg/mg dry weight (IQR 0.12–5.39) versus 7.15 (1.63–11.50); P = .28], thoracic aorta [1.73 (IQR 0.07–4.26) versus 1.68 (1.29–10.92); P = .15] and abdominal aorta [2.71 (IQR 0.07–4.23) versus 5.85 (2.07–12.80); P = .05] (Fig. 2). After pooling the results of all aorta segments, the median calcium content was lower in the high magnesium group compared with the normal magnesium group [3.20 μg/mg dry tissue (IQR 0.09–5.32) versus 5.85 (1.67–12.68); P < .01] (Supplementary data, Figure S1). Results for calcium apposition time in each aortic segment corresponded to the results for tissue calcium content and showed that the number of animals in the highest tertile of calcium apposition time was significantly lower in the abdominal aorta [1 (10%) versus 6 (55%); P = .03] in the high versus the normal magnesium group and did not differ between the groups in the aortic arch [2 (20%) versus 5 (46%); P = .22] and thoracic aorta [2 (20%) versus 5 (46%); P = .22] (Supplementary data, Figure S2).
FIGURE 2:
Calcium content in aortic segments of partially nephrectomized rats fed either a high or normal magnesium diet. (A–C) Calcium content in μg/mg dry tissue in the (A) aortic arch and (B) thoracic and (C) abdominal aorta. Dots represent individual animals. Horizontal lines represent medians. (D–F) The percentage of animals in each diet group, with a calcium content within the highest (black bars), intermediate (grey bars) and lowest tertile (white bars), respectively. Mg 0.05%: magnesium 0.05% w/w diet; Mg 0.48%: magnesium 0.48% w/w diet. *P < .05 for high versus normal dietary magnesium.
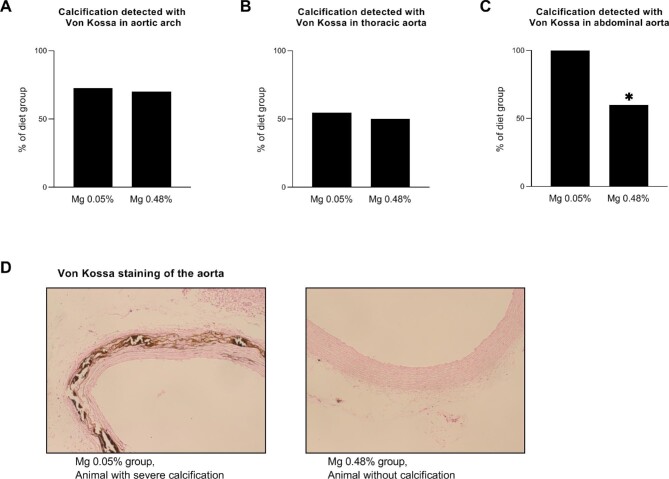
Calcification of the aorta: tissue staining
Tissue von Kossa staining confirmed the presence of calcification in the aortic wall. In the high compared with the normal magnesium group, there were fewer animals with calcification identified in the abdominal aorta [6 (60%) versus 11 (100%); P = .02], whereas there was no difference in the aortic arch [7 (70%) versus 8 (73%); P = .89] and thoracic aorta [5 (50%) versus 6 (55%); P = 0.84] (Fig. 3).
FIGURE 3:
Calcification of the aorta, identified by von Kossa staining, in partially nephrectomized rats fed either a high or normal magnesium diet. (A–C) The percentage of animals within each group in which calcification was detected with von Kossa staining in the (A) aortic arch and (B) thoracic and (C) abdominal aorta. (D) Representative image of von Kossa staining of the aorta in an animal with severe calcification in the normal dietary magnesium group and an animal without calcification in the high dietary magnesium group. Mg 0.05%: magnesium 0.05% w/w diet; Mg 0.48%: magnesium 0.48% w/w diet. *P < .05 for high versus normal dietary magnesium.
mRNA expression of osteochondrogenic and contractile markers
The mRNA expression levels of the osteochondrogenic markers RUNX2, ALPL and OCN did not differ between the diet groups, nor did the expression of the smooth muscle genes SM22 and ACTA2. The expression of transcription factor Mycdn but not SRF, both involved in activation of the contractile phenotype of smooth muscle cells, was higher in the high magnesium group in the abdominal aorta and did not differ in the other segments. Expression of the contractile phenotype repressive factor KLF4 did not differ between the groups (Supplementary data, Figure S3).
Bone quality
Micro-CT showed abnormal high cortical porosity or cortical trabecularization and dense trabecular bone with an abnormal appearance in femurs from rats of both diet groups. A normal morphological appearance of femurs was observed in some of the animals in the high dietary magnesium group (43%) but not in the normal magnesium group (Supplementary data, Figure S4 and Table S2).
DISCUSSION
Summary of principal findings
This study demonstrates that high dietary magnesium inhibits abdominal aorta calcification in CKD in vivo, which was shown here in a rat model of nephrectomy-induced CKD and high dietary phosphate.
This protective effect was shown in this aortic segment by a smaller number of animals with calcium content in the highest tertile in the high dietary magnesium-treated group. There was no difference in serum phosphate concentrations or urinary phosphate excretion between the dietary magnesium groups, indicating that this beneficial effect is not mediated by phosphate-binding actions of dietary magnesium in the gastrointestinal tract. Serum magnesium concentration and urinary magnesium excretion were substantially increased in the high dietary magnesium–treated group, indicating that dietary magnesium was absorbed and available for endogenous effects. The effect could not be explained by inhibition of osteogenic transformation of VSMCs, as there was no difference in the expression of osteogenic markers in vessel wall cells between the dietary magnesium groups.
Relation with previous studies and current theory
Other studies have also demonstrated inhibiting effects of an increased dietary intake of magnesium on the development of aortic vascular calcification in various animal models of CKD with hyperphosphatemia [22–24]. Information from other studies on differential effects on specific aorta segments is limited, but another study that analysed different aorta segments separately found a significant effect only in the abdominal part of the aorta [22]. The effects of magnesium in our study were also only in the abdominal aorta. Although numerically more calcification in the other segments of the aorta was noted as well in our study, this was not significant, possibly due to limited power as a consequence of the higher than expected dropout. However, another study that analysed the thoracic segment only demonstrated that the effects are not limited to the abdominal aorta [23].
In some studies, magnesium (added as magnesium citrate intragastrically or magnesium carbonate in the diet) attenuated hyperphosphatemia and therefore phosphate-binding actions of magnesium in the gastrointestinal tract may have been at least partially responsible for the effects on vascular calcification in those experiments [24, 33]. In our data and another study, there were no differences in plasma phosphate concentration or 24-h urinary phosphate excretion between the groups, suggesting that magnesium can inhibit vascular calcification independent of phosphate binding in the gastrointestinal tract [22]. Moreover, in another study that supplemented magnesium sulphate by intraperitoneal injection, vascular calcification was decreased, a setup that precludes an additional protective mechanism by binding of dietary phosphate [23].
Previous studies used animal models that included an adenine diet or the administration of calcitriol [22–24]. In these models, other mechanisms may have influenced the effects, like more pronounced inflammation in the adenine models, and non-physiological manipulation of calcium and phosphate homeostasis in the high calcitriol models. We investigated partially nephrectomized rats without the addition of any other potentially toxic substances.
The mechanisms by which absorbed magnesium inhibits vascular calcification are still incompletely understood.
In vitro, high phosphate concentrations induce calcification of the extracellular compartment surrounding human VSMC cultures, along with an increased expression of osteogenic differentiation–related genes, which can be inhibited by the addition of magnesium [25, 26]. The protective effect of magnesium disappeared after inhibition of the magnesium transporter TRPM7 by 2-aminoethoxydiphenylborate (2-APB), suggesting that magnesium influx is required and intracellular mechanisms are involved [25, 26, 34]. However, in another experiment in bovine VSMCs, the protective effect of magnesium persisted after inhibition by 2-APB, suggesting that magnesium also has protective effects on the crystallization of calcium–phosphate complexes in vitro that do not involve intracellular effects, although it cannot be excluded that magnesium entered the cells via other pathways in this experiment [35]. In animals with kidney failure, a reduction of osteogenic expression was seen in studies in which magnesium caused a reduction of serum phosphate concentrations, but that effect might have been mediated by the lower phosphate concentration itself [23, 24]. In our study and another study, in which the increased magnesium intake did not affect serum phosphate concentrations, no reduction of expression of bone-related genes was observed [22].
In a previous study from our group, osteomalacia was observed in Klotho knockout mice on a high magnesium diet [36]. The current data show that in partially nephrectomized rats on a high phosphate diet, bone abnormalities occurred in both the high and normal magnesium groups and bone quality was not lower in the high dietary magnesium group. This suggests that these bone abnormalities resulted from renal osteodystrophy per se and that the high magnesium diet did not have any harmful effects on bone quality.
Strengths and weaknesses
The strength of this study was the randomized allocation and blinding and this resulted in comparable groups with no difference in kidney function. Calcification was measured quantitatively by the amount of tissue calcium and, in addition, the calcification was confirmed visually by von Kossa staining. We defined the study endpoints that were set before the study was initiated.
This study also has several limitations. A substantial number of animals did not reach the 16-week endpoint because of a humane endpoint. Kidney function was markedly reduced in the experiment, which has probably contributed to animals reaching the humane endpoint. As a result, the risk for type II error is relatively high. Indeed, point estimates for the calcification endpoints were all in the direction of suggesting a benefit for high dietary magnesium. We included animals in the analysis that had completed at least 8 weeks of follow-up, but several had different durations of follow-up. However, the duration of follow-up did not differ significantly between the two dietary magnesium groups and we also analysed calcium apposition time, thereby taking into account the variable duration of follow-up. This analysis demonstrated corresponding results and therefore we do not expect that the variable duration of follow-up between the groups biased the results. The degree of calcification between animals within each group was highly variable. However, in the model used in this study, a variable incidence of calcification is not unusual [31, 37].
Another limitation of this study is that details of molecular mechanisms involved in the protective effects of higher dietary magnesium could not be explored beyond the phenotyping of arterial wall cellular components.
Translation to the patient level and implications for practice
The partial nephrectomy rat model that was used in this study is an established model of CKD, including uremia and high serum phosphate, and with CKD-related complications similar to the human condition. Research with this model has demonstrated remarkable consistency between the animal and human phenotypes [38]. We therefore consider it reasonable to assume that the effects demonstrated in this study can be translated to the human condition as well. Before implementation of this dietary intervention in clinical practice, the beneficial effect of magnesium needs to be confirmed in patients. Arterial calcification is an important pathophysiological process leading to cardiovascular disease in patients with CKD and a strong predictor of mortality [39]. In observational studies in patients with CKD, plasma magnesium concentrations are inversely associated with all-cause and cardiovascular mortality [11]. Explorative studies with magnesium-based interventions in patients with and without dialysis have shown positive effects on several markers of calcification, including carotid intima-media thickness, coronary artery calcification score and calcification propensity score [18–21]. These are promising results, indicating that magnesium supplementation may indeed inhibit vascular calcification in CKD patients, identical to our animal study, and may potentially decrease cardiovascular morbidity and mortality.
Future studies and unanswered questions
Further clinical intervention studies in patients with CKD are needed to determine the appropriate and safe dose of magnesium supplementation and confirm its effects on vascular calcification and subsequent clinically relevant endpoints. In addition, in vitro and animal studies may further help to reveal underlying mechanisms of the processes involved. Then, a further challenge is to identify which mechanisms are key in the protective role of magnesium within the complex interplay between local and systemic effects on the calcification process in vivo.
Supplementary Material
ACKNOWLEDGEMENTS
We thank B.L., K.L., M.P., K.d.H. and A.t.B. for their help with the animal experiments, E.K. for sharing his expertise on the animal model and H.v.E. and Prof. v.R for their help with the micro-CT.
Contributor Information
Nicoline H J Leenders, Department of Nephrology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Caro Bos, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
Tiny Hoekstra, Department of Nephrology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Leon J Schurgers, Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, Maastricht, The Netherlands.
Marc G Vervloet, Department of Nephrology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Joost G J Hoenderop, Department of Physiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
FUNDING
This work was supported by the Dutch Kidney Foundation (PhD grant 15OP02) and the PPP Allowance made available by Top Sector Life Sciences & Health to the Dutch Kidney Foundation to stimulate public–private partnerships (grant LSHM17034-HSGF).
AUTHORS’ CONTRIBUTIONS
N.H.J.L., M.G.V. and J.G.J.H. conceived the study. N.H.J.L., C.B., M.G.V. and J.G.J.H. designed the experiments. N.H.J.L. and C.B. performed the experiments L.J.S. performed the tissue calcium measurements. N.H.J.L. performed the data analysis with the advice of T.H. N.H.J.L., T.H., M.G.V. and J.G.J.H. interpreted the data. Each author contributed during manuscript drafting or revision and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
M.G.V. received research grants from Vifor, Amgen and Fresenius and acted as a consultant for Medice, AstraZeneca, Vifor, Amgen, Fresenius, Otsuma and Kyowa Kirin. L.J.S. received research grants from Bayer, Boehringer Ingelheim, Nattopharma and IDS. The other authors declare no conflicts of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
DATA AVAILABILITY STATEMENT
The datasets used during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Matsushita K, van der Velde M, Astor BCet al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. London GM, Guérin AP, Marchais SJet al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003; 18: 1731–1740 [DOI] [PubMed] [Google Scholar]
- 3. Okuno S, Ishimura E, Kitatani Ket al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis 2007; 49: 417–425 [DOI] [PubMed] [Google Scholar]
- 4. Goodman WG, Goldin J, Kuizon BDet al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000; 342: 1478–1483 [DOI] [PubMed] [Google Scholar]
- 5. Westenfeld R, Krueger T, Schlieper Get al. Effect of vitamin K2 supplementation on functional vitamin k deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis 2012; 59: 186–195 [DOI] [PubMed] [Google Scholar]
- 6. Ketteler M, Bongartz P, Westenfeld Ret al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 2003; 361: 827–833 [DOI] [PubMed] [Google Scholar]
- 7. Pasch A, Farese S, Graber Set al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 2012; 23: 1744–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sage AP, Lu J, Tintut Yet al. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int 2011; 79: 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tyson KL, Reynolds JL, McNair Ret al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol 2003; 23: 489–494 [DOI] [PubMed] [Google Scholar]
- 10. Reynolds JL, Joannides AJ, Skepper JNet al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 2004; 15: 2857–2867 [DOI] [PubMed] [Google Scholar]
- 11. Leenders NHJ, Vermeulen EA, van Ballegooijen AJet al. The association between circulating magnesium and clinically relevant outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Clin Nutr 2021; 40: 3133–3147 [DOI] [PubMed] [Google Scholar]
- 12. Leenders NHJ, Vervloet MG.. Magnesium: a magic bullet for cardiovascular disease in chronic kidney disease? Nutrients 2019; 11: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Roij van Zuijdewijn CL, Grooteman MP, Bots MLet al. Serum magnesium and sudden death in european hemodialysis patients. PLoS One 2015; 10: e0143104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsuji H, Venditti FJ Jr, Evans JCet al. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham Heart Study). Am J Cardiol 1994; 74: 232–235 [DOI] [PubMed] [Google Scholar]
- 15. Okamoto T, Hatakeyama S, Hosogoe Set al. Proton pump inhibitor as an independent factor of progression of abdominal aortic calcification in patients on maintenance hemodialysis. PLoS One 2018; 13: e0199160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakaguchi Y, Hamano T, Nakano Cet al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS One 2016; 11: e0163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salem S, Bruck H, Bahlmann FHet al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol 2012; 35: 31–39 [DOI] [PubMed] [Google Scholar]
- 18. Mortazavi M, Moeinzadeh F, Saadatnia Met al. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol 2013; 69: 309–316 [DOI] [PubMed] [Google Scholar]
- 19. Sakaguchi Y, Hamano T, Obi Yet al. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol 2019; 30: 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bressendorff I, Hansen D, Schou Met al. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity—a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2017; 2: 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bressendorff I, Hansen D, Schou Met al. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial. Clin J Am Soc Nephrol 2018; 13: 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zelt JG, McCabe KM, Svajger Bet al. Magnesium modifies the impact of calcitriol treatment on vascular calcification in experimental chronic kidney disease. J Pharmacol Exp Ther 2015; 355: 451–462 [DOI] [PubMed] [Google Scholar]
- 23. Diaz-Tocados JM, Peralta-Ramirez A, Rodriguez-Ortiz MEet al. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int 2017; 92: 1084–1099 [DOI] [PubMed] [Google Scholar]
- 24. Yao Z, Xu Y, Ma Wet al. Magnesium citrate protects against vascular calcification in an adenine-induced chronic renal failure rat model. J Cardiovasc Pharmacol 2018; 72: 270–276 [DOI] [PubMed] [Google Scholar]
- 25. Louvet L, Buchel J, Steppan Set al. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant 2013; 28: 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montes de Oca A, Guerrero F, Martinez-Moreno JMet al. Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One 2014; 9: e89525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939–1951 [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT Method. Methods 2001; 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 29. Martin-Pardillos A, Sosa C, Millan Aet al. Effect of water fluoridation on the development of medial vascular calcification in uremic rats. Toxicology 2014; 318: 40–50 [DOI] [PubMed] [Google Scholar]
- 30. Wu-Wong JR, Chen YW, Wong JTet al. Preclinical studies of VS-505: a non-absorbable highly effective phosphate binder. Br J Pharmacol 2016; 173: 2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mizobuchi M, Ogata H, Hatamura Iet al. Up-regulation of Cbfa1 and Pit-1 in calcified artery of uraemic rats with severe hyperphosphataemia and secondary hyperparathyroidism. Nephrol Dial Transplant 2006; 21: 911–916 [DOI] [PubMed] [Google Scholar]
- 32. Eraranta A, Tormanen S, Koobi Pet al. Phosphate binding reduces aortic angiotensin-converting enzyme and enhances nitric oxide bioactivity in experimental renal insufficiency. Am J Nephrol 2014; 39: 400–408 [DOI] [PubMed] [Google Scholar]
- 33. Callera GE, He Y, Yogi Aet al. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J Hypertens 2009; 27: 155–166 [DOI] [PubMed] [Google Scholar]
- 34. Montezano AC, Zimmerman D, Yusuf Het al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010; 56: 453–462 [DOI] [PubMed] [Google Scholar]
- 35. Ter Braake AD, Tinnemans PT, Shanahan CMet al. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep 2018; 8: 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ter Braake AD, Smit AE, Bos Cet al. Magnesium prevents vascular calcification in Klotho deficiency. Kidney Int 2020; 97: 487–501 [DOI] [PubMed] [Google Scholar]
- 37. Shibata M, Shigematsu T, Hatamura Iet al. Reduced expression of perlecan in the aorta of secondary hyperparathyroidism model rats with medial calcification. Ren Fail 2010; 32: 214–223 [DOI] [PubMed] [Google Scholar]
- 38. Shobeiri N, Adams MA, Holden RM. Vascular calcification in animal models of CKD: a review. Am J Nephrol 2010; 31: 471–481 [DOI] [PubMed] [Google Scholar]
- 39. Rennenberg RJ, Kessels AG, Schurgers LJet al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag 2009; 5: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author upon reasonable request.