Abstract
In veterinary medicine, blood transfusion is commonly performed on companion animals. The common marmoset is a small nonhuman primate with increasing popularity as an animal model in biomedical research. Because of its small whole blood volume, the marmoset is at high risk of exsanguination, and blood transfusion is required to care for life-threatening bleeding. However, few clinical evaluations exist on transfusions for marmosets. This study performed whole blood transfusion with cross-matching on nine marmosets and surveyed the therapeutic effects. Recipients included clinical cases with persistent bleeding, anemia, and coma, as well as animals subjected to postoperative bleeding prophylaxis. Donors were selected from healthy marmosets, including littermates. Cross-match assay before transfusion were all negative, and recipients showed no visible signs of transfusion-related adverse reactions. Whole blood transfusions caused hemostasis and successful recovery in bleeding marmosets, including long-term improvement of anemia cases. Our results indicated that blood transfusion is effective for marmosets with severe anemia and persistent hemorrhage from both non-experimental and surgical causes. Furthermore, DNA sequencing for blood-group classification revealed that all subject marmosets were type A, suggesting that the risk of blood type mismatch may be low in this species.
Keywords: blood, blood transfusion, common marmoset, cross-matching, veterinary medicine
Introduction
The common marmoset (marmoset, Callithrix jacchus) is a New World primate species frequently used for biomedical research and preclinical studies because it is closely related to humans, small (300–500 g), and reproduces rapidly for a primate [1]. Recent advances in genetic modification technologies have expanded the species’ research usability as a translational nonhuman primate model [2,3,4,5]. Many of these new research techniques require surgical treatments for analyzing physiological status, such as two-photon calcium imaging [6], multielectrode arrays [7], or awake MRI with attached head post [8]. Sophisticated veterinary management techniques are indispensable for performing these advanced analyses while maintaining marmoset well-being [4, 9, 10].
Due to their small size, marmosets have a relatively high risk of fatal blood loss. An adult of average weight (350 g) has an estimated circulating blood volume of 24.5 ml, meaning it can only lose 4.9 ml before reaching the acute bleeding volume that causes hemorrhagic shock (20% circulating blood volume) [11]. In veterinary medicine, including of non-human primates, blood transfusion is common and generally applied to anemia, hemorrhage [12], hemolysis, or ineffective erythropoiesis to increase oxygen-carrying capacity [9,10,11,12,13,14]. However, no detailed research on the clinical effects and safety of blood transfusions has been reported for the marmoset.
Therefore, this study performed whole blood transfusion on nine marmoset cases. We included a cross-matching assay to avoid adverse reactions. We then surveyed evaluated the therapeutic and negative effects of transfusion.
Materials and Methods
Animal facilities
This study was conducted at the animal facility in the Central Institute for Experimental Animals (CIEA), Kawasaki, Japan. The experimental protocol was approved (approval number 17051) by the Institutional Animal Care and Use Committee, according to CIEA Regulations for Animal Experiments, based on Guidelines for the Proper Conduct of Animal Experiments from the Science Council of Japan (2006). Marmosets were kept in cages with family (820 × 610 × 1,578 mm), in pairs (409–820 × 610 × 728–1,578 mm), or alone (409–820 × 610 × 728–1,578 mm) depending on experimental and veterinary care reasons. Cages were positioned facing each other, allowing subjects to communicate visually and vocally. Each cage is equipped with a sleeping area, wooden perches, and hammocks for environmental enrichment. The animal rooms were conditioned at 26–28°C and 40–60% humidity with a 12:12 h light/dark cycle. Animals were negative for Salmonella spp., Shigella spp., and Yersinia pseudotuberculosis in yearly fecal examinations.
Blood-transfusion recipients
Recipients were 9 marmosets (5 females and 4 males, R1–9) aged 2.3–9.3 years (Table 1). Their weights ranged from 315–432 g at the time of transfusion. Before transfusion, 0.2–0.3 ml of blood was collected from the femoral vein and used for blood tests, except in a fatal case (R1). In R1, 0.1 ml of blood was collected only for the hematocrit test with a capillary tube and cross-matching assay. A complete blood count (CBC) was performed for all cases using Sysmex XT-2000i (Sysmex Corp., Kobe, Japan), except for R1. Blood biochemical tests were performed using DRI-CHEM 7000 (Fujifilm Corp., Tokyo, Japan), depending on the amount of plasma that could be collected.
Table 1. The pairs of recipients and donors for blood transfusion and results of cross-match assays.
| Recipients |
Donors |
Cross match assay |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Sex/Agea | ABO genotyping |
BWb (g) |
No. | Sex/Agea | ABO genotyping |
BWb (g) |
Relationships between recipients |
Blood volume (ml) |
Prec | Postd |
| R1 | M/3.2 y | A | 301 | D1e | F/4.5 y | A | 491 | Unrelated | 2 | Negative | Negative |
| R2 | F/8.5 y | A | 295 | D1e | F/5.0 y | A | 443 | Unrelated | 2 | Negative | Negative |
| R3 | F/3.3 y | A | 310 | D2 | M/3.3 y | A | 318 | Littermate | 2 | Negative | Negative |
| R4 | M/8.6 y | A | 330 | D3 | M/9.3 y | A | 342 | Littermate | 2 | Negative | Negative |
| R5 | M/9.3 y | A | 373 | D4 | F/5.4 y | - | 442 | Unrelated | 2 | Negative | Negative |
| R6 | M/7.0 y | A | 327 | D5e | M/2.8 y | A | 420 | Unrelated | 2 | Negative | Negative |
| R7 | F/2.3 y | A | 437 | D5e | M/3.0 y | A | 361 | Unrelated | 1.7 | Negative | Negative |
| R8 | F/8.3 y | A | 410 | D6 | M/8.3 y | A | 435 | Littermate | 2 | Negative | Negative |
| R9 | F/6.2 y | A | 401 | D7 | M/8.9 y | A | 413 | Unrelated | 2 | Negative | Negative |
aAge (in years) at transfusion, bBW: body weight, cPre: just before transfusion, dPost: 8–41 weeks after transfusion, eUsed twice as donors.
Reference CBC values were acquired using 46 two-year-old marmosets (22 females, 24 males) kept at CIEA. These individuals, had no history of experimental use and no outward health abnormalities. Blood samples were collected from the femoral vein without anesthesia and tested using Sysmex XT-2000iV. Mean ± 2SD of each value was calculated as reference range.
Donor selection
Donors were 7 animals (2 females and 5 males, D1–7) aged 2.8–9.3 years and with a body weight of at least 310 g (Table 1). Animals that had not donated blood within the past month were selected as donors. D1 and D5 donated twice in this study after 27- and 9-week intervals, respectively. Three donor animals were littermates of their recipients (D2, 3, 6). The remainder were not related to their recipients within the second degree of consanguinity. To minimize adverse reactions, recipient littermates were prioritized as donors because their hematopoietic chimerism confers immunotolerance [15,16,17]. Prior to transfusion, 0.3–0.4 ml of blood was sampled from the femoral vein of candidate donors. These were used for cross-matching and CBC.
Cross-matching assay
The standardized tube test was used to perform major (donor red blood cells [RBC] and recipient plasma) and minor (donor plasma and recipient RBCs) cross-matching, as previously described [18]. Briefly, heparin-anticoagulated recipient and donor blood were centrifuged for 15 min at 1,000 × g and 4°C to collect plasma, which was stored in a 1.5 ml tube. Next, RBCs were washed with 1 ml of physiological saline and centrifuged for 15 min at 1,000 × g and 4°C. After washing two to three times with physiological saline, 1–2% RBC saline suspension was prepared. This suspension was mixed with plasma (20 µl each) in a 1.5 ml tube and left standing for 30 min at room temperature. A drop of the mixture was placed on a slide and coverslipped for microscopic observation of RBC aggregations. The suspension and plasma (donor-recipient and recipient-donor) were both tested. Self-combinations were negative controls.
Blood transfusions and follow-up
After confirming negative cross-match results, whole blood transfusions were performed. Blood (1.7–2 ml) was taken from the femoral vein of each donor and immediately mixed with Citrate-Phosphate-Dextrose-Adenine (CPDA-1) solution (Terumo Corp., Tokyo, Japan) to a final concentration of 0.15% for anticoagulation. For case R1, heparin sodium (Mochida Pharmaceutical, Tokyo, Japan) with a final concentration of 1.2 U/ml was used instead. In following the good practice guidelines of the European Center for the Validation of Alternative Methods and the European Federation of Pharmaceutical Industries and Associations, the amount of blood drawn was kept below 15% of the circulating blood volume. The maximum volume of blood collected during this study was 2 ml, which is equivalent to 400 ml in humans.
All transfusions were performed in veterinary intensive care units (ICU, Dear M, Fukuda ME Kogyo, Tokyo, Japan) maintained at 30–34°C, 40% humidity, and 25–35% oxygen (Fig. 1). Before transfusion, a recipient animal was sedated via intramuscular injection of a solution containing 0.04 mg/kg medetomidine (Domitor, Kyoritsu Seiyaku, Tokyo, Japan), 0.4 mg/kg midazolam (Dormicum, Astellas Pharma, Tokyo, Japan), and 0.5 mg/kg butorphanol (Vetorphale, Meiji Seika Pharma, Tokyo, Japan) (R5). Two recipients were administered of 0.15 mg/kg midazolam and 0.15 mg/kg butorphanol (R6, 7). Recipients (R2, 4) under no anesthesia were held in a restrainer (CL-4532; CLEA Japan, Tokyo, Japan) when necessary.
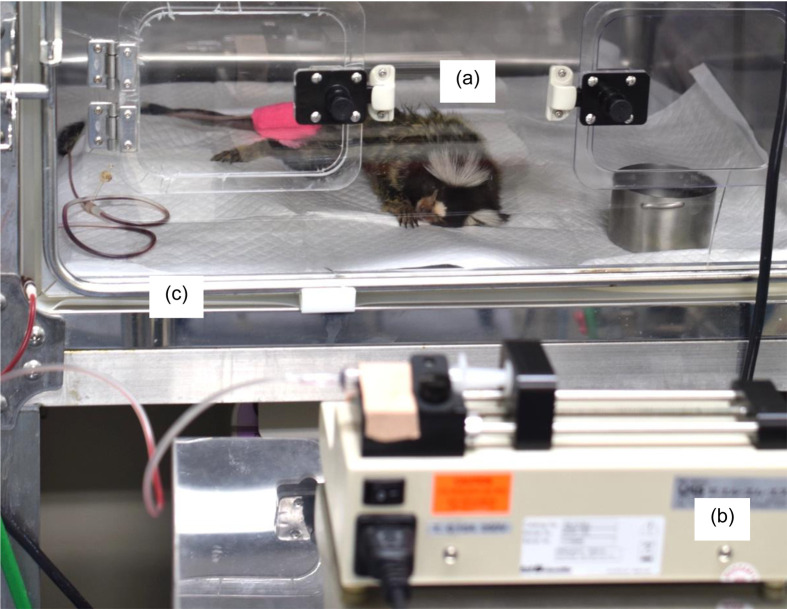
Fig. 1.
Recipient marmoset. (a) Individuals received whole blood transfusion using syringe pump (b) in an animal ICU unit (c). Donated blood was administered through the tail vein.
Blood was transfused through intravenous injection of the tail vein secured with an indwelling catheter (24G, Top Corp., Tokyo, Japan), butterfly needle (21G, Top Corp), and PRN adapter (BD, Franklin Lakes, NJ, USA). Flow rate was controlled with a syringe pump (KDS100, KD Scientific, Holliston, MA, USA) (Fig. 1). The flow rate was set at 3 ml/kg/h until 15 min from the start of transfusion, then decreased to 1 ml/kg/h for the first 3 min before increasing to 5 ml/kg/h. This rate was maintained until transfusion was complete, and then up to 8 ml of saline was injected at the same flow rate to flush the line. From the start of transfusion to 60 min after the end, recipients were carefully monitored for adverse reactions, including high fever, shock, and anaphylaxis. They were followed for a year after transfusion to record monthly body weights and clinical incidents. Re-blood tests and re-cross-match assays between the same donor/recipient pairs were performed at 8–4 weeks post-transfusion.
DNA sequencing for blood types
To investigate the risk of transfusion due to blood group mismatch, the ABO blood group of the marmosets was tested after transfusion. All recipients, 6 donors (D1–3, D5–7), and 3 other marmosets (1 male, 2 females aged 0, 5.1, and 8.7 years old) were tested. D4 was transported out of the facility immediately after transfusion, so its sample could not be obtained.
Genomic DNA was extracted from hair roots of upper arms or skin of the thigh using the phenol-chloroform-isoamyl alcohol method [19]. A partial region (321 bp) in exon 7 of the ABO gene was PCR-amplified using existing primers (forward: TCAGCGACTTCTGCGAGCGG, reverse: GGCCAATGGCATCGAGGCCG). This region contains a single nucleotide polymorphism that distinguishes blood types. The reaction volume (20 µl) was prepared with KOD One enzyme solution (Toyobo, Osaka, Japan), according to manufacturer protocol. The thermocycling schedule was as follows: 94°C for 2 min, 5 cycles of 98°C for 10 s and 74°C for 30 s, 5 cycles of 98°C for 10 s and 72°C for 30 s, 5 cycles of 98°C for 10 s and 70°C for 30 s, 25 cycles of 98°C for 10 s and 68°C for 30 s, and finally 68°C for 5 min. Purified amplicons (1/20th of reaction volume) were directly sequenced using the Big Dye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) and the 3730xl DNA Analyzer (Thermo Fisher Scientific).
Statistical analysis
A paired Student’s t-test was used to compare CBC values of recipients pre- and post-transfusion. Statistical analyses were performed in R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Cross-match assays
Cross-matching took approximately 60 min per donor-recipient pair. Before transfusion, neither major nor minor cross-matches of any pair showed RBC agglutination (Table 1). Re-cross match assays were negative in all animals at 8–4 weeks post-transfusion (Table 1).
Blood transfusions
Recipients did not show visible signs of transfusion-related adverse reactions. Six recipients had non-experimental persistent gingival (R1–6) or vaginal (R2) hemorrhage, despite of 2–5 days of primary treatments such as pressing, cauterization, and administering hemostatic agents (e.g., tranexamic acid, Table 2). Five out of 6 recipients exhibited hypothermia (<36.5°C), 3 were comatose, 2 had low activity, 1 had melena, and 1 was vomiting (Table 2). Blood examinations revealed severe anemia with hematocrit values of 10–22.4%, hypokalemia (<3.5 mEq/l) (3 recipients), hypercreatininemia (>0.4 mg/dl) (2), high blood urea nitrogen (BUN; 25.6 mg/dl) (3), and high alanine aminotransferase (ALT; 14 IU/l) (1) (Table 2 and Supplementary Table 1) [1]. After transfusions, any hemorrhage was not observed in 5 recipients (R1–4, R6). The 3 comatose cases (R1–3) and 2 low-activity cases (R4 and R6) recovered by the completion of transfusion (Supplementary Fig. 1). Case R5 presented mild gingival re-bleeding at 1 day post-transfusion, which was stopped through stanching with a cautery knife.
Table 2. Clinical characteristics of blood transfusion recipients.
| No. | Category | Bleeding sites | Periods of primary treatments |
Ht (%) | Other signs/remarks |
|---|---|---|---|---|---|
| R1 | Clinical cases | Gingiva | 3 days | 10* | Anemia, comatose, hypothermia, melena |
| R2 | Gingiva, vagina | 2 days | 16.8 | Anemia, comatose, hypothermia | |
| R3 | Gingiva (around first premolar) | 5 days | 13.0 | Anemia, comatose, hypothermia, K↓ | |
| R4 | Gingiva | 2 days | 12.6 | Anemia, low activity, hypothermia, BUN↑, Cre↑, K↓ | |
| R5 | Gingiva (around mandibular canine) | 2 days | 46.2 | Re-bleeding after transfusion, ALT↑ | |
| R6 | Tongue | 2 days | 22.4 | Anemia, low activity, hypothermia, vomiting, BUN↑, Cre↑, K↓ | |
| R7 | Experiment-related cases | Unidentifiable (abdomen) | Unknown | 44.5 | Post-surgery intra-abdominal hemorrhage, BUN↑ |
| R8 | Surgical wound after surgery | 3 hours | 35.0 | Cesarean section, bleeding from surgical wound | |
| R9 | No bleeding (preventive transfusion after surgery) | Not applicable | 43.8 | Cesarean section | |
Ht: hematocrit, K↓: hypokalemia (<3.5 mEq/l), BUN↑: high blood urea nitrogen (>25.6 mg/dl), Cre↑: hypercreatininemia (>0.4 mg/dl), ALT↑: high alanine aminotransferase (>14.0 IU/l), *Measurement by capillary tube.
Three recipients (R7–9) were transfused to prevent postoperative bleeding from ovum pick up (OPU) (R7) and cesarean sections (R8–9) [3, 20]. Case R7 presented no obvious preoperative clinical abnormalities. Although we observed intra-abdominal hemorrhage after OPU surgery, we could not accurately identify the bleeding site. For this subject, blood transfusion was performed after the operation to achieve hemostasis. Even though R8 presented no major abnormalities before or during surgery, it did not stop bleeding from the surgical wound post-operation, and blood transfusion became necessary for hemostasis. R9 presented no major bleeding during the perioperative period, but blood transfusion was performed to prevent weakness. All 3 surgical cases recovered with no visible hemorrhage after transfusion. The mothers (R8 and R9) were able to exhibit parental behaviors at 1 and 7 days later, respectively.
Post-transfusion follow-up
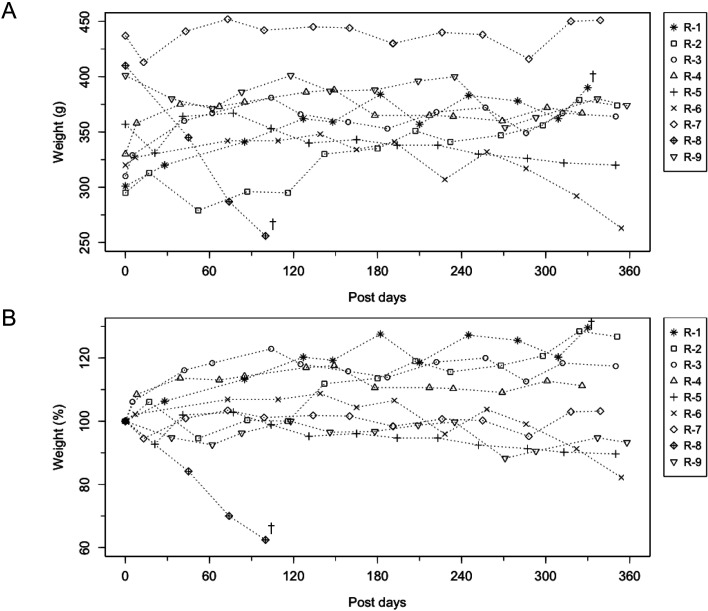
Donors were in good physical condition after transfusion. They were clinically followed for 3 months and then returned to experimental use. Recipients showed no transfusion-related disorders during the 1 year follow-up period. Except for R8, none exhibited notable post-transfusion weight loss (Fig. 2). However, 2 cases were euthanized during the follow-up period. The aforementioned R8 was euthanized 18 weeks post-transfusion due to severe weakness associated with weight loss and mild anemia, diagnosed as marmoset wasting syndrome (MWS) [21, 22]. Additionally, R1 was euthanized 4 weeks post-transfusion for acute collapse; the necropsy revealed abdominal blood coagulation adhered to the mesentery.
Fig. 2.
Bodyweight changes in the recipients for one year post transfusion period. (A) Bodyweight (g). (B) Percent weight change (%). †R-1 and 8 were dead at these time points.
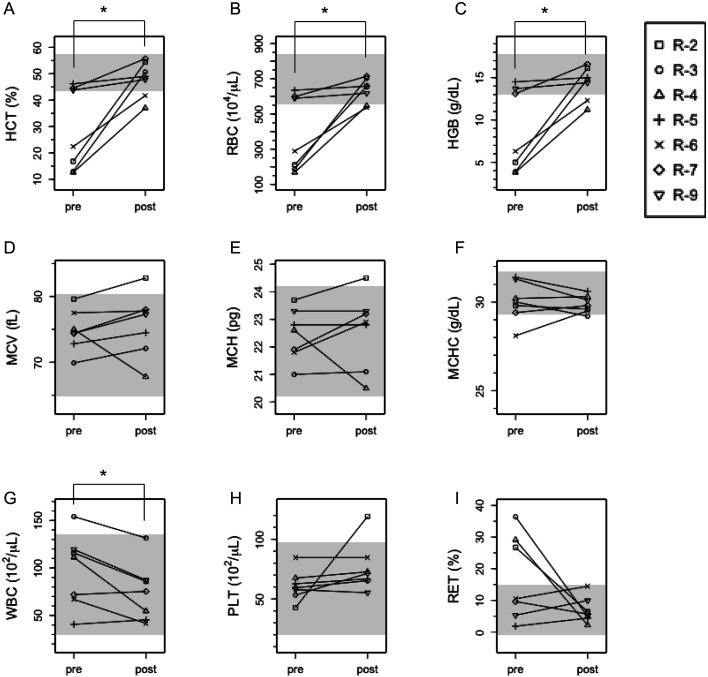
During 8–4 weeks post-transfusion, CBC tests revealed that all anemia cases recovered; hematocrit (HCT), red blood cell count (RBC), and hemoglobin (HGB) significantly increased post-transfusion (P<0.05) (Figs. 3A–C). Mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) ranged around reference values at pre- and post-transfusion (Figs. 3D and E). One exception was R2, with higher MCV and MCH than reference values after transfusion. R3 was slightly lower than reference values for mean corpuscular hemoglobin concentration (MCHC) after transfusion (Figs. 3D–F). White blood cell count (WBC) significantly decreased after transfusion (P<0.05), but all cases were within the reference value (Fig. 3G). Platelet count (PLT) was within the reference value in all recipients before transfusion, but after transfusion, R2 values were highly elevated (Fig. 3H). Reticulocyte ratio (RET (%)) in three cases with severe pre-transfusion anemia (R2–4) decreased to the reference range post-transfusion (Fig. 3I).
Fig. 3.
Complete blood cell count (CBC) in recipients pre- and post-transfusion. We recorded CBC just before transfusion (pre) and 2–8 months after transfusion (post). *P<0.05 (paired t-test). (A) HCT: hematocrit, (B) RBC: red blood cell, (C) HGB: hemoglobin, (D) MCV: mean corpuscular volume, (E) MCH: mean corpuscular hemoglobin, (F) MCHC: mean corpuscular hemoglobin concentration, (G) WBC: white blood cell, (H) PLT: platelet, (I) RET: reticulocyte. The reference value is indicated by gray shading.
DNA sequencing of ABO blood groups
Nucleotide sequences (280 bp) were identical across all 18 samples and matched with known marmoset ABO sequences. The SNP sequence was CTGgggGGG and presumed to be type A (Supplementary Table 2).
Discussion
In this study, we successfully performed whole blood transfusions in 9 marmosets. Immediately and for weeks after the procedure, recipients recovered from state of debility and stopped bleeding, without any noticeable incidents on either them or donors. R1–6 were clinical cases that were mainly due to spontaneous oral bleeding, which we suspect to have been caused by trauma. There were no obvious underlying diseases that could have caused continuous bleeding. R4 and R6 were cases that had traumatic hemorrhage as etiology since the bleeding was caused by obvious trauma in the mouth, although blood tests revealed that the renal function might have been impaired. In these cases of traumatic bleeding, hemostatic agents were administered until just before transfusion; however, there was no response. The hemostatic effect was observed only after transfusion. In our past experience, marmosets in a coma due to severe blood loss were euthanized because of humane endpoints. In the present study, the fatal cases were rescued by blood transfusion, which can contribute to reduce mortality in captive marmosets.
R7–9 were experiment-related cases that were transfused for bleeding associated with surgery. The underlying diseases of R7–9 are unknown. In the case of bleeding from the surgical wound (R8), the bleeding did not respond to hemostatic agents but stopped immediately after transfusion. In R7 and R9, no bleeding was observed after transfusion, and the animals were in good condition. These results suggest that blood transfusion can contribute to the improvement of animal conditions even in surgical procedures associated with bleeding. The overall positive outcome could be attributed to the hemostatic effect of whole blood transfusion, which replenishes coagulation components and platelets in plasma [23].
Our post-transfusion follow-ups (Figs. 2 and 3) revealed long-term improvements in anemia for 4 animals, with good prognosis in most cases. Case R2 showed abnormal values in post-transfusion CBC tests at 18 weeks (Fig. 3), but by 54 weeks, all parameters were within the reference range for MCV, MCH, and PLT (Supplementary Table 3). The two euthanized animals suffered from MWS (R8) and intra-abdominal bleeding (R1), both apparently unrelated to blood transfusion. R1 was not used in any prior experiment and the cause of bleeding was unknown. The results of CBC tests at 47 weeks post-transfusion indicated that intra-abdominal bleeding began a week before euthanasia (Supplementary Table 3).
Our findings suggest that the blood transfusion method we used is safe for both recipients and donors. Donated blood volume was 1.7–2 ml per animal, lower than the acceptable sampling volume of <15% body weight [11]. Immobilization with a restrainer or sedatives is necessary for safe transfusion because marmosets have high motor ability and finger dexterity. For this purpose, combinations of butorphanol, an analgesic opioid, could be a good sedative option to relieve pain in diseased animals.
Pre-transfusion blood cross-matching is recommended for both humans and companion animals to prevent adverse effects such as shock, high fever, hemolysis, and coagulation [13, 24]. However, previous marmoset studies rarely describe blood cross-match assays [9, 10]. In this study, the results of cross-matching were negative in all pairs (Table 1). Specifically, we examined ABO blood groups because they are the critical determinant of transfusion incompatibility in humans; blood type is determined from allelic variation at the ABO gene, which encodes a glycosyltransferase [25, 26]. We found only type A among our marmoset samples, corroborating previous reports at three marmoset facilities that the primates were all type A [26]. Furthermore, re-cross matches after transfusion were negative in every pair. These results imply that marmosets are less likely to have primary and secondary immune responses to transfused blood. Therefore, they may be able to have multiple blood transfusions more safely than other species. In other non-human primates, some non-ABO antigens have been reported using commercial microtyping cards [27]. Kell, Lutheran, Kidd, and Duffy antigens were detected in rhesus macaques, baboons, and squirrel monkeys. Rh antigens were detected in squirrel monkeys. Since marmosets were not examined in the exhaustive study, it is possible that other blood group systems exist in marmosets as well. Because of this possibility, there is a need to perform a pre-transfusion cross-match assay for safe transfusion even in marmosets, where only type A has been reported. For urgent cases that cannot wait for cross-matching results, using littermates as donors is theoretically acceptable because marmoset littermates are known to be allo-tolerant because of hematopoietic chimerism [28].
Transfusion of whole blood containing active white blood cells poses a risk of graft-versus-host disease (GVHD) [29]. Since no blood preservation method has been established for marmosets, transfusions were performed using blood collected just before transfusion. Nine patients did not experience any post-transfusion side effects or thrombocytopenia. Therefore, it is believed that GVHD did not occur. In the future, it will be necessary to study the method of leukocyte removal and appropriate radiation exposure for a safer transfusion method.
In one animal, we performed preventive transfusion immediately after cesarean section. Obstetric hemorrhage remains an important cause of maternal morbidity in humans, and blood transfusions were performed in 0.5% of pregnant women during cesarean sections [30]. Thus, we felt that preparation for a preventive blood transfusion may be necessary before cesarean section in marmosets.
In conclusion, we showed a practical technique for cross-match assay and whole blood transfusion in marmosets. Our results indicated that blood transfusion is very effective for marmosets with severe anemia and persistent hemorrhage from non-experimental and surgical causes, which was the first report of practical cases in this species. Furthermore, there was no deterioration in the recipients’ condition that could be associated with blood transfusion. Safe blood transfusion should contribute to improving the welfare of marmosets in biomedical research.
Supplementary
Acknowledgments
This research was partially supported by the Research Program for Brain Science, ‘Construction of System for Spread of Primate Model Animals (grant # 17dm0107051)’, and the Brain Mapping by ‘Integrated Neurotechnologies for Disease Studies (Brain/MINDS grant # JP19dm0207065, JP19dm0207068)’ from the Japan Agency for Medical Research and Development (AMED) to ES.
We are also thankful to Dr. Kazuhito Segawa of Azabu University, Dr. Takashi Yamaguchi of the Yutaka Animal Clinic, and Dr. Kinya Tamura of Tamura Animal Hospital for their valuable clinical advice.
References
- 1.Kramer R, Burns M. Chapter 6 − Normal clinical and biological parameters of the common marmoset (Callithrix jacchus). In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. Academic Press; 2019. pp. 93–107. [Google Scholar]
- 2.Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009; 459: 523–527. doi: 10.1038/nature08090 [DOI] [PubMed] [Google Scholar]
- 3.Sato K, Oiwa R, Kumita W, Henry R, Sakuma T, Ito R, et al. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell. 2016; 19: 127–138. doi: 10.1016/j.stem.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 4.National Academies of Sciences Engineering and Medicine (NASEM). Care, use, and welfare of marmosets as animal models for gene editing-based biomedical research: proceedings of a workshop. In: Anestidou L, Johnson AF, editors. Care, use, and welfare of marmosets as animal models for gene editing-based biomedical research. Proceedings of a Workshop. Washington DC; 2019. [PubMed] [Google Scholar]
- 5.Park JE, Sasaki E. Assisted reproductive techniques and genetic manipulation in the common marmoset. ILAR J. 2021; ilab002. doi: 10.1093/ilar/ilab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada Y, Matsumoto Y, Okahara N, Mikoshiba K. Chronic multiscale imaging of neuronal activity in the awake common marmoset. Sci Rep. 2016; 6: 35722. doi: 10.1038/srep35722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S, Wang X. Wireless multi-channel single unit recording in freely moving and vocalizing primates. J Neurosci Methods. 2012; 203: 28–40. doi: 10.1016/j.jneumeth.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaeffer DJ, Liu C, Silva AC, Everling S. Magnetic resonance imaging of marmoset monkeys. ILAR J. 2021; ilaa029. doi: 10.1093/ilar/ilaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monika Burns LW. Physical examination, diagnosis, and common clinical procedures. In: Marini R, Wachtman L, Tardif S, Mansfield K, Fox J, editors. The common marmoset in captivity and biomedical research. Academic Press; 2019. pp. 145–175. [Google Scholar]
- 10.Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med. 2003; 53: 369–382. [PubMed] [Google Scholar]
- 11.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001; 21: 15–23. doi: 10.1002/jat.727 [DOI] [PubMed] [Google Scholar]
- 12.Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg. 2004; 6: 139–148. doi: 10.1016/j.jfms.2004.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidow B. Transfusion medicine in small animals. Vet Clin North Am Small Anim Pract. 2013; 43: 735–756. doi: 10.1016/j.cvsm.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Bohm RPGM. Emergency medicine and critical care for nonhuman primates. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman primates in biomedical research. Academic Press; 2012. pp. 359e389. [Google Scholar]
- 15.Benirschke K, Anderson JM, Brownhill LE. Marrow chimerism in marmosets. Science. 1962; 138: 513–515. doi: 10.1126/science.138.3539.513 [DOI] [PubMed] [Google Scholar]
- 16.Ross CN, French JA, Ortí G. Germ-line chimerism and paternal care in marmosets (Callithrix kuhlii). Proc Natl Acad Sci USA. 2007; 104: 6278–6282. doi: 10.1073/pnas.0607426104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney CG, Curran E, Westmoreland SV, Mansfield KG, Vallender EJ. Quantitative molecular assessment of chimerism across tissues in marmosets and tamarins. BMC Genomics. 2012; 13: 98. doi: 10.1186/1471-2164-13-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blais MC, Berman L, Oakley DA, Giger U. Canine Dal blood type: A red cell antigen lacking in some Dalmatians. J Vet Intern Med. 2007; 21: 281–286. doi: 10.1111/j.1939-1676.2007.tb02961.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh H, Takabayashi S, Itoh T. Development of microsatellite DNA markers and their chromosome assignment in the common marmoset. Am J Primatol. 2009; 71: 912–918. doi: 10.1002/ajp.20729 [DOI] [PubMed] [Google Scholar]
- 20.Kurotaki Y, Sasaki E. Practical reproductive techniques for the common marmoset. J Mamm Ova Res. 2017; 34: 3–12. doi: 10.1274/032.034.0103 [DOI] [Google Scholar]
- 21.Baxter VK, Shaw GC, Sotuyo NP, Carlson CS, Olson EJ, Zink MC, et al. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS One. 2013; 8: e82747. doi: 10.1371/journal.pone.0082747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan AC, Khan KN. Clinical pathologic changes in two marmosets with wasting syndrome. Toxicol Pathol. 1996; 24: 707–709. doi: 10.1177/019262339602400605 [DOI] [PubMed] [Google Scholar]
- 23.Mohr R, Martinowitz U, Lavee J, Amroch D, Ramot B, Goor DA. The hemostatic effect of transfusing fresh whole blood versus platelet concentrates after cardiac operations. J Thorac Cardiovasc Surg. 1988; 96: 530–534. doi: 10.1016/S0022-5223(19)35204-3 [DOI] [PubMed] [Google Scholar]
- 24.Hall TC, Pattenden C, Hollobone C, Pollard C, Dennison AR. Blood transfusion policies in elective general surgery: How to optimise cross-match-to-transfusion ratios. Transfus Med Hemother. 2013; 40: 27–31. doi: 10.1159/000345660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng ZH, Yu Q, Liang YL, Wang DM, Su YQ, Wu GG. Characterization of a novel B(A) allele with BBBA type at the ABO blood group. J Hum Genet. 2006; 51: 732–736. doi: 10.1007/s10038-006-0013-5 [DOI] [PubMed] [Google Scholar]
- 26.Ségurel L, Thompson EE, Flutre T, Lovstad J, Venkat A, Margulis SW, et al. The ABO blood group is a trans-species polymorphism in primates. Proc Natl Acad Sci USA. 2012; 109: 18493–18498. doi: 10.1073/pnas.1210603109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramis G, Martínez-Alarcon L, Quereda JJ, Mrowiec A, Funes C, Ríos A, et al. Non-ABO blood group systems phenotyping in non-human primates for blood banking laboratory and xenotransplantation. Lab Anim. 2013; 47: 100–105. doi: 10.1177/0023677213475439 [DOI] [PubMed] [Google Scholar]
- 28.Sweeney C, Ward J, Vallender EJ. Naturally occurring, physiologically normal, primate chimeras. Chimerism. 2012; 3: 43–44. doi: 10.4161/chim.20729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopolovic I, Ostro J, Tsubota H, Lin Y, Cserti-Gazdewich CM, Messner HA, et al. A systematic review of transfusion-associated graft-versus-host disease. Blood. 2015; 126: 406–414. doi: 10.1182/blood-2015-01-620872 [DOI] [PubMed] [Google Scholar]
- 30.Balki M, Dhumne S, Kasodekar S, Carvalho JCA, Seaward G. Blood transfusion for primary postpartum hemorrhage: a tertiary care hospital review. J Obstet Gynaecol Can. 2008; 30: 1002–1007. doi: 10.1016/S1701-2163(16)32994-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.