Abstract
The methanol metabolite that causes hepatotoxicity is formic acid, generating reactive oxygen radical formation and cell damage. Carvacrol is an antioxidant monoterpenic phenol produced from Thymus vulgaris. This study aimed to investigate the effects of carvacrol on methanol-induced oxidative liver damage in rats. Eighteen rats were divided into three groups. Methotrexate was administered orally for 7 days to methotrexate+methanol (MTM) and methotrexate+methanol+carvacrol (MMC) groups. Methotrexate was given before methanol to cause methanol poisoning. Distilled water was given to the healthy group (HG) as a solvent. At the end of the 7th day, 20% methanol was administered orally at a dose of 3 g/kg to the MTM and MMC groups. Four hours after methanol administration, 50 mg/kg carvacrol was injected intraperitoneally into the MMC group. Animals were sacrificed 8 h after carvacrol injection. Biochemical markers were studied in the excised liver tissue and blood serum samples, and histopathological evaluations were made. Severe hemorrhage, hydropic degeneration, pycnosis, and mononuclear cell infiltration were observed in the liver of the MTM group. Additionally, the levels of malondialdehyde (MDA), total oxidant status (TOS), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were significantly higher, and total glutathione (tGSH) and total antioxidant status (TAS) were significantly lower in the MTM group compared to HG (P<0.001). Carvacrol prevented the increase in MDA, TOS, ALT and AST levels with methanol and the decrease in tGSH and TAS levels (P<0.001), and alleviated the histopathological damage. Carvacrol may be useful in the treatment of methanol-induced liver damage.
Keywords: carvacrol, experimental models, liver injury, methanol, oxidative damage
Introductıon
Methanol is a toxic alcohol used in industrial production [1]. Methanol poisoning is observed during the consumption of moonshine or as a means of committing suicide [2]. Intoxication due to methanol can result in acidosis, coma, and even death [3]. This toxic effect arises from the metabolism of methanol by alcohol-dehydrogenase to formaldehyde and formic acid, which are toxic [4]. Due to formic acid formation, metabolic acidosis is the main biological feature of poisoning [5]. Formic acid has been reported as the metabolite causing liver toxicity [6]. Formic acid leads to anaerobic glycolysis and lactic acidosis by inhibiting the cytochrome c oxidase complex [7]. Acidosis causes reactive oxygen species (ROS) formation, lipid peroxidation (LPO), and cell membrane and mitochondrial damage [8]. Methanol causes an increase in malondialdehyde (MDA), the last toxic product of LPO, and a decrease in the endogenous antioxidant glutathione (GSH) in the liver [9]. Kurcer et al. stated that methanol poisoning causes hepatic toxicity in rats, which is likely a result of ROS induction [10]. Rats have a higher liver folic acid content than humans, and formic acid metabolism occurs quickly. Thus, methanol poisoning patterns do not develop in rats. Folate-dependent formate metabolism is impaired by methotrexate treatment [11].
Carvacrol, whose protective effect against methanol-related oxidative liver damage was investigated in this study, is a monoterpenic phenol produced from Origanum vulgare and Thymus vulgaris [12]. Carvacrol is known to have various biological and pharmacological properties, such as antioxidant, anti-inflammatory, hepatoprotective, spasmolytic, vasorelaxant, antibacterial, antifungal, and anticancer properties [13]. Carvacrol is known to suppress the excessive production of proinflammatory cytokines and MDA and the consumption of antioxidants [14], suggesting that carvacrol may be useful in treating methanol-related oxidative liver injury. No studies investigating the protective effect of carvacrol against methanol-induced oxidative liver injury were found in the literature. Therefore, the aim of this study was to investigate the effect of carvacrol on methanol-induced oxidative liver damage in rats biochemically and histopathologically.
Materials and Methods
Animals
The animals we used in the study were supplied from Ataturk University Medical Experiments Application and Research Center. A total of 18 albino Wistar male rats weighing between 250–265 g. were used for the experiment. Animals were kept and fed in the laboratory at the room temperature (22°C). Animal experiments were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and were approved by the local animal ethics committee of Ataturk University, Erzurum, Turkey (Ethics Committee No.: 2021/133, dated May 27, 2021).
Chemical agents
Carvacrol, thiopental sodium, methotrexate and methanol used in the experiment was supplied from Sigma-Aldrich, Inc. (St. Louis, MO, USA), IE Ulagay (Istanbul, Turkey), Med-Drug (Istanbul, Turkey), Binali Yıldırım University Faculty of Medicine Pharmacology Laboratory (Erzincan, Turkey), respectively. The carvacrol used in this study was 98% pure. Methanol-anhydrous of 99.8% purity was used. Thiopental sodium was administered intraperitoneally as a 2.5% solution (500 mg in 20 ml).
Experimental procedure
Rats were divided into three groups, with six in each group: healthy group (HG), methotrexate+methanol group (MTM), and methotrexate+methanol+carvacrol group (MMC). Methotrexate dissolved in distilled water was administered orally at a dose of 0.3 mg/kg body weight by gavage to the MTM and MMC groups for seven days. Distilled water was given to the HG as a solvent in the same way. At the end of the 7th day, the MTM and MMC groups received 20% methanol orally at a dose of 3 g/kg body weight. Four hours after the administration of methanol, the MMC group was injected intraperitoneally with carvacrol at a dose of 50 mg/kg body weight. Carvacrol was administered by dissolving it in 25% dimethyl sulfoxide [DMSO] solution in normal saline. Carvacrol was used at this dose in another study and it was found to be effective [15]. Eight hours after carvacrol injection, all rats were sacrificed with thiopental anesthesia at a high dose (50 mg/kg body weight). The MDA and total glutathione (tGSH) levels and total oxidant (TOS) and total antioxidant status (TAS) were measured in the excised liver tissues. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were also measured in blood serum samples taken from the tail veins of all animals before they were sacrificed. Liver tissues were examined histopathologically. All biochemical and histopathological results obtained from the MTM group were compared with the MMC and HG groups. This method has been used previously to induce oxidative liver damage [2]. Rats have a higher liver folic acid content than humans, and formic acid metabolism occurs quickly. Therefore, methanol poisoning patterns do not develop in rats. Folate-dependent formate metabolism is impaired by methotrexate treatment [11]. In this study, methotrexate was given before methanol to cause methanol poisoning.
Biochemical analyses
After resection, all tissues were rinsed with phosphate-buffered saline solution. The tissues were homogenized in ice-cold phosphate buffers (50 mM, pH 7.4) that were appropriate for the variable to be measured. The tissue homogenates were centrifuged at 5,000 rpm for 20 min at 4°C, and the supernatants were extracted to analyze tGSH and MDA. All tissue results were expressed by dividing to g protein. All spectrophotometric measurements were performed via microplate reader (Bio-Tek, Winooski, VT, USA).
Tissue MDA and tGSH determinations
MDA measurements are based on the method used by Ohkawa et al., which includes the spectrophotometric measurement of the absorbance of the pink-colored complex formed by thiobarbituric acid (TBA) and MDA [16]. The principle of tGSH measurement is based on measuring the color intensity of dark yellow 5-thio 2-nitrobenzoic acid (TNB), which is released by the reduction of Ellman’s reagent [5,5-dithiobis (2-nitrobenzoic acid); DTNB] by free thiol groups, at a wavelength of 412 nm [17].
TOS and TAS determinations
TOS and TAS levels of tissue homogenates were determined using a novel automated measurement method and commercially available kits (Rel Assay Diagnostics, Gaziantep, Turkey), both developed by Erel [18, 19].
ALT analysis
Quantitative determination of serum ALT was performed by spectrophotometric method with a Roche Cobas 8000 autoanalyzer. In the method with pyridoxal-5’-phosphate, according to the International Federation of Clinical Chemistry (IFCC), 3,4 ALT catalyzes the reaction between L-alanine and 2-oxoglutarate. The pyruvate formed is reduced by NADH in a reaction catalyzed by lactate dehydrogenase (LDH), where L-lactate and NAD+ are formed. Pyridoxal phosphate acts as a coenzyme in the amino transfer reaction ensuring the complete enzyme activation. L-Alanine + 2-oxoglutarate yields (ALT) pyruvate + L-glutamate. The rate of oxidation of pyruvate + NADH + H+ yields (LDH) L-lactate + NAD+. The rate of NADH is directly proportional to the catalytic ALT activity.
AST analysis
Quantitative determination of serum AST was performed by a spectrophotometric method with a Roche Cobas 8000 autoanalyzer. In the method with pyridoxal-5’-phosphate, according to the International Federation of Clinical Chemistry (IFCC), 3,4 AST in the sample catalyzes the transfer of an amino group between L-aspartate and 2-oxoglutarate to form oxaloacetate and L-glutamate. Oxaloacetate then reacts with NADH in the presence of malate dehydrogenase (MDH) to form NAD+. Pyridoxal phosphate acts as a coenzyme in the amino transfer reaction. L-Aspartate + 2-oxoglutarate yields (AST) oxaloacetate + L-glutamate. Oxaloacetate + NADH + H+ yields (MDH) L-malate + NAD+. The rate of NADH oxidation is directly proportional to the catalytic AST activity.
Histopathological examination
Necropsies were performed on the rats, and liver tissues were sampled and placed into a 10% buffered formalin solution. The samples were then subjected to routine follow-up processes and embedded in paraffin blocks. Five µm sections were examined with hematoxylin-eosin staining in light microscopy in terms of histopathological findings. Evaluations of hemorrhage, hydropic degeneration, pycnosis, and mononuclear cell infiltration were performed semi-quantitatively and scored as no (0), mild (1), moderate (2), and severe (3). Histopathological assessment was carried out by a pathologist who was blind to the study groups.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences for Windows version 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp., Armonk, NY, USA). Descriptive statistics for each variable were determined. The results for continuous variables were recorded as the mean ± SD. The significance of differences between the groups was determined using the one-way analysis of variance (ANOVA) test followed by Tukey’s analysis. A P value <0.05 was considered significant. In the histopathological examination, the differences between the groups were determined by Kruskal-Wallis test, and groups that exhibited differences were determined by the Mann-Whitney U test.
Results
Biochemical results
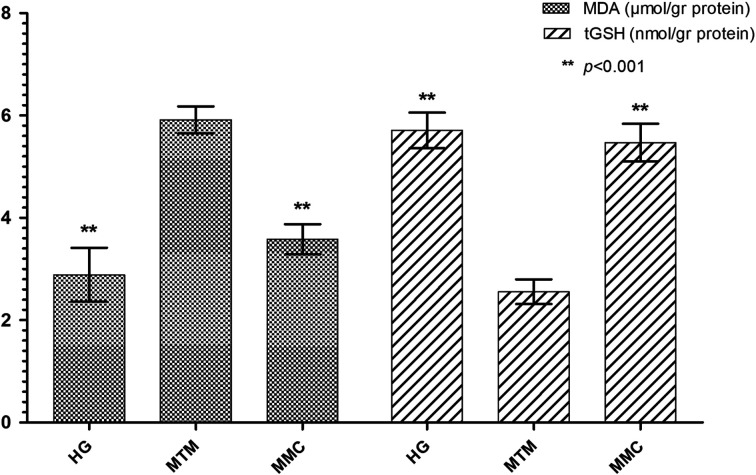
As shown in Fig. 1, MDA levels were significantly higher in the MTM group compared to the HG and MMC groups (P<0.001). While the amount of tGSH in the MTM group was significantly lower (P<0.001), there was no significant difference between the HG and MMC groups (P>0.05). Carvacrol significantly inhibited the increase in the MDA content and decrease in tGSH levels by methanol in the liver tissue (P<0.001; Fig. 1). When the HG and MMC groups were compared, there was no significant difference in terms of MDA and tGSH levels (P>0.05).
Fig. 1.
The effects of carvacrol on MDA and tGSH levels in the liver tissues of rats administered methanol. Bars are the mean±standard deviation (SD). The methanol group (MTM) was compared with the HG and MMC groups. MDA: malondialdehyde; tGSH: total glutathione; HG: healthy group; MTM: methotrexate+methanol group; MMC: methotrexate+methanol+carvacrol group.
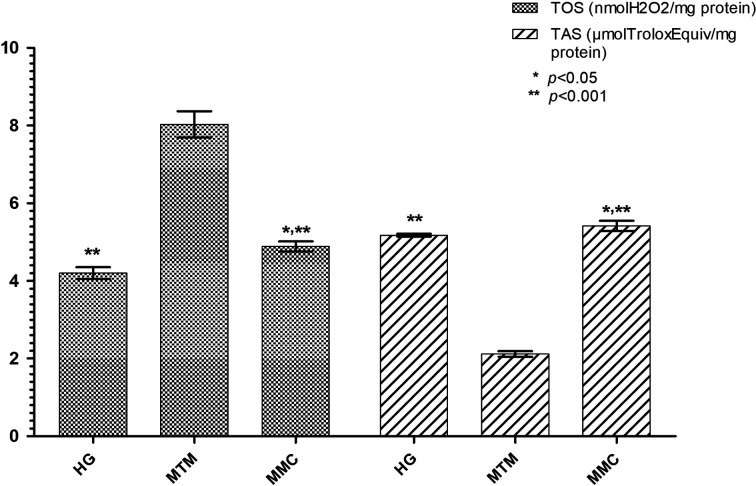
TOS levels were higher in the liver tissue of the rats that received methanol compared to the HG and MMC groups (P<0.001). TAS levels were significantly lower in the MTM group compared to the HG and MMC groups (P<0.001). When the HG and MMC groups were compared, a statistically significant difference was found in terms of TOS and TAS levels (P<0.05). However, carvacrol significantly inhibited the increase in TOS and decrease in TAS levels by methanol in the liver tissue (P<0.001; Fig. 2).
Fig. 2.
The effects of carvacrol on TOS and TAS levels in the liver tissues of rats administered methanol. Bars are the mean±standard deviation (SD). The methanol group (MTM) was compared with the HG and MMC groups. (*: P<0.05, the methotrexate+methanol+carvacrol group (MMC) was compared with the HG). TOS: total oxidant status; TAS: total antioxidant status; HG: healthy group; MTM: methotrexate+methanol group; MMC: methotrexate+methanol+carvacrol group.
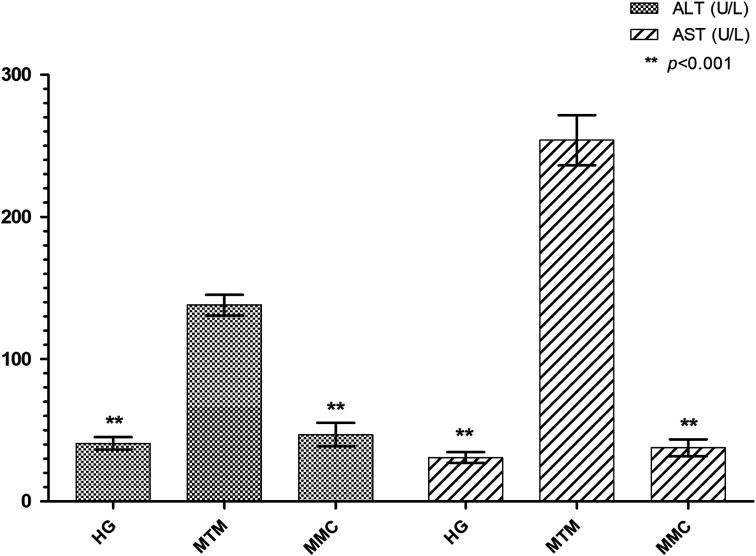
Serum ALT and AST activities in the MTM group were significantly higher compared to the other groups (P<0.001). However, there was no significant difference in ALT and AST activities between the HG and MMC groups (P>0.05; Fig. 3).
Fig. 3.
The effects of carvacrol on ALT and AST serum levels of rats administered methanol. Bars are the mean±standard deviation (SD). The methanol group (MTM) was compared with the HG and MMC groups. ALT: alanine aminotransferase; AST: aspartate aminotransferas; HG: healthy group; MTM: methotrexate+methanol group; MMC: methotrexate+methanol+carvacrol group.
Histopathological findings
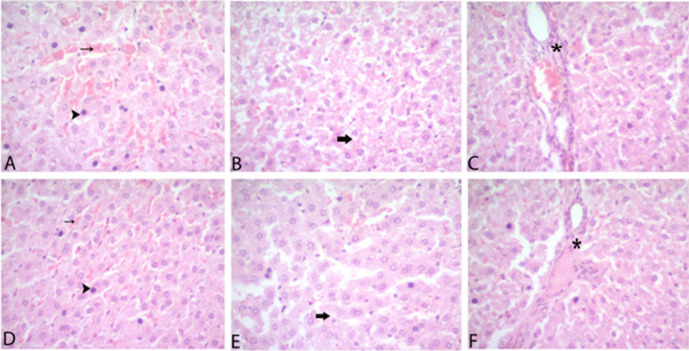
The liver tissues of the rats in the HG had a normal histological appearance (Fig. 4). However, severe hemorrhage, hydropic degeneration, pycnosis and mononuclear cell infiltration were detected in the livers of rats in the MTM group (Figs. 5A–C). In the livers of the rats treated with carvacrol, hemorrhage, hydropic degeneration, pycnosis, and mononuclear cell infiltration were found to be alleviated (Figs. 5D–F). A statistically significant difference was found between the groups in terms of hemorrhage, hydropic degeneration, pycnosis and mononuclear cell infiltration (Table 1, P<0.05).
Fig. 4.

Sections of the normal liver tissue of the healthy group.
Fig. 5.
Sections of the liver (a–f). (a) Severe pycnosis (arrowhead) and hemorrhage (thin arrow) in hepatocytes of the methanol group, (b) hydropic degeneration (thick arrow) of the methanol group, c) mononuclear cell infiltration (*) of the methanol group, (d) mild pycnosis (arrowhead) and hemorrhage (thin arrow) in hepatocytes of the carvacrol group, (e) hydropic degeneration (thick arrow) of the carvacrol group, (f) Mononuclear cell infiltration (*) of the carvacrol group.
Table 1. Results of histopathological scoring in liver tissues of groups.
| Groups | Hemorrhage | Hydropic degeneration | Pycnosis | Mononuclear cell infiltration |
|---|---|---|---|---|
| HG | 0.16 ± 0.51a | 0.33 ± 0.51a | 0.00 ± 0.00a | 0.16 ± 0.51a |
| MTM | 2.83 ± 0.40b | 2.83 ± 0.40b | 2.83 ± 0.40b | 2.66 ± 0.51b |
| MMC | 1.33 ± 0.51c | 1.16 ± 0.40c | 1.33 ± 0.51c | 1.16 ± 0.40c |
a, b, c refers to differences between groups (P<0.05). The values are mean ± SD. HG: healthy group; MTM: methotrexate+methanol group; MMC: methotrexate + methanol + carvacrol group.
Discussion
The effect of carvacrol on methanol-induced oxidative liver damage in rats was investigated biochemically and histopathologically. To induce liver damage in rats, methotrexate was administered for seven days before methanol administration to cause methanol poisoning. Methotrexate, which impairs folate-dependent formate metabolism, was administered because rats have a higher liver folic acid content and they do not develop methanol poisoning patterns. Folate-dependent formate metabolism is impaired by methotrexate treatment [11]. In the study, methotrexate was given before methanol in order to cause methanol poisoning. Our biochemical test results showed that MDA and TOS levels were higher in the liver tissue of methanol-given animals (MTM group), while tGSH and TAS levels were lower compared to carvacrol (MMC) and healthy groups (HG). This pattern revealed that oxidants were high and antioxidants were low in the MTM group. In the literature, an increase in oxidants and a decrease in antioxidants are considered oxidative stress [20]. In a previous study, it was reported that methanol caused an increase in MDA in the liver [9]. Similarly, in another study, methanol administration increased MDA levels in both liver and blood serum [21]. The increase in MDA in the methanol group indicates increased ROS production and cell membrane lipid oxidation. The increase in ROS and LPO due to methanol is associated with acidosis caused by methanol in the body [7, 22]. Acidosis occurs when methanol is metabolized to formaldehyde, formate, and formic acid [23].
In our study, it was also determined that the tGSH level in the liver tissue of the methanol group (MTM) was decreased compared to the healthy (HG) and carvacrol (MMC) groups. GSH is a low molecular weight endogenous antioxidant composed of c-L-glutamyl-L-cysteinyl-glycine. The thiol group of cysteine is generally involved in reduction and conjugation reactions, which are considered the most important functions of GSH [24]. GSH has important functions, such as directly scavenging ROS in the cell [25]. However, in cases where the cellular antioxidant defense system is suppressed by ROS production, serious cell damage can occur [25]. In the literature, the correlation between the GSH content of alcoholic and non-alcoholic livers was emphasized, in addition to comparisons with other liver disease [26]. To evaluate the oxidant-antioxidant balance in methanol and other animal groups in more detail, tissue TOS and TAS levels were also measured. TOS and TAS are parameters that reflect the total effects of all oxidants and antioxidants in tissue [18, 19]. Studies showing that methanol increases TOS levels in blood serum support our experimental results [27].
Serum ALT and AST activities in the methanol group, which indicates that the oxidant antioxidant balance has changed in favor of oxidants, increased significantly compared to the carvacrol and healthy groups. Hamouda et al. reported that methanol increased rat serum AST and ALT activities [28]. ALT is concentrated only in the liver, but AST is concentrated in organs and tissues other than the liver. ALT is primarily in the cytoplasm, and 80% of AST is present in the mitochondria. Therefore, in mild hepatocellular damage, if the hepatocyte membrane is damaged but the mitochondrial membrane is intact, the ALT level in the serum is higher than that of AST [29]. However, AST secretion also increases mitochondrial membrane damage [30]. When there is alcoholic liver damage, if the AST/ALT ratio is greater than 1.5, the damage is alcohol-related, and if it is less than 1, it should not be considered related to alcohol use [31].
In our study, carvacrol, which prevented the increase of oxidant parameters and the decrease of antioxidant parameters due to methanol administration, also prevented the increase of ALT and AST activities in the blood serum of animals. No study in the literature investigated the effect of carvacrol on methanol-induced liver damage. However, 50 and 100 mg/kg body weight doses of carvacrol have been shown to reduce the severity of ethanol-related liver dysfunction and histopathological damage with its antioxidant effect [32]. In addition, methanol exposure has been reported to induce cytochrome P450 2E1, which is responsible for hepatotoxicity [33]. Carvacrol inhibits the expression of cytochrome P450 (Cyt P450) by binding to its active pocket [32]. Kim et al. reported that carvacrol suppresses alcohol-induced ALT and AST elevation [34]. In another study, it was emphasized that the protective effect of the Taraxacum Syriacum Boiss plant extract, of which the primary component is carvacrol, against acetaminophen-induced liver damage was due to its antioxidant activity [35].
Our biochemical findings in the study are consistent with histopathological findings. Severe histopathological changes were observed in the methanol group, where the oxidant-antioxidant balance changed in favor of oxidants. While grade 3 hemorrhages, hydropic degeneration, pycnosis, and mononuclear cell infiltration were detected in the livers of rats in the methanol group, these findings were evaluated as grade 1 in the group treated with carvacrol. In the literature, focal hepatocyte necrosis, micro and macrovesicular steatosis, and hydropic degeneration have been documented in the liver in cases of fatal methanol poisoning [36]. Furthermore, degeneration in liver cells, flattening of the hepatocyte surface, and disorders in the structure of cell organelles have been reported to be histopathological signs of methanol intoxication [37]. It is understood from the literature that portal inflammation is one of the pathological symptoms related to methanol [38]. The information obtained from the literature indicates that methanol causes histopathological damage to the liver.
As a result, methanol induced oxidative stress in the animal livers. Oxidative stress induced by methanol caused severe histopathological damage to the liver tissue. Carvacrol significantly attenuated methanol-related oxidative liver injury. These results show that carvacrol may be useful in the treatment of methanol-induced liver damage.
Source (s)
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interests
The authors have no conflicts of interest.
References
- 1.Prabhakaran V, Ettler H, Mills A. Methanol poisoning: two cases with similar plasma methanol concentrations but different outcomes. CMAJ. 1993; 148: 981–984. [PMC free article] [PubMed] [Google Scholar]
- 2.Yazgan ÜC, Elbey B, Kuş S, Baykal B, Keskin I, Yılmaz A, et al. Effect of caffeic acid phenethyl ester on oxidant and anti-oxidant status of liver and serum in a rat model with acute methanol intoxication. Ir J Med Sci. 2017; 186: 519–523. doi: 10.1007/s11845-016-1462-2 [DOI] [PubMed] [Google Scholar]
- 3.Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002; 40: 415–446. doi: 10.1081/CLT-120006745 [DOI] [PubMed] [Google Scholar]
- 4.Hayreh MS, Hayreh SS, Baumbach GL, Cancilla P, Martin-Amat G, Tephly TR, et al. Methyl alcohol poisoning III. Ocular toxicity. Arch Ophthalmol. 1977; 95: 1851–1858. doi: 10.1001/archopht.1977.04450100153022 [DOI] [PubMed] [Google Scholar]
- 5.Hantson PE. [Acute methanol intoxication: physiopathology, prognosis and treatment]. Bull Mem Acad R Med Belg. 2006; 161: 425–434, discussion 434–436. (in French) [PubMed] [Google Scholar]
- 6.Liesivuori J, Kosma VM, Naukkarinen A, Savolainen H. Kinetics and toxic effects of repeated intravenous dosage of formic acid in rabbits. Br J Exp Pathol. 1987; 68: 853–861. [PMC free article] [PubMed] [Google Scholar]
- 7.Erecińska M, Wilson DF. Inhibitors of cytochrome c oxidase. Pharmacol Ther. 1980; 8: 1–20. doi: 10.1016/0163-7258(80)90057-1 [DOI] [Google Scholar]
- 8.Bralet J, Bouvier C, Schreiber L, Boquillon M. Effect of acidosis on lipid peroxidation in brain slices. Brain Res. 1991; 539: 175–177. doi: 10.1016/0006-8993(91)90703-X [DOI] [PubMed] [Google Scholar]
- 9.Skrzydlewska E, Farbiszewski R. Decreased antioxidant defense mechanisms in rat liver after methanol intoxication. Free Radic Res. 1997; 27: 369–375. doi: 10.3109/10715769709065776 [DOI] [PubMed] [Google Scholar]
- 10.Kurcer MA, Kurcer Z, Koksal M, Baba F, Ocak AR, Aksoy N, et al. Effect of lycopene on caspase-3 enzyme activation in liver of methanol-intoxicated rats: comparison with fomepizole. J Med Food. 2010; 13: 985–991. doi: 10.1089/jmf.2009.0166 [DOI] [PubMed] [Google Scholar]
- 11.Eells JT, Henry MM, Lewandowski MF, Seme MT, Murray TG. Development and characterization of a rodent model of methanol-induced retinal and optic nerve toxicity. Neurotoxicology. 2000; 21: 321–330. [PubMed] [Google Scholar]
- 12.Karadayi M, Yildirim V, Güllüce M. Antimicrobial activity and other biological properties of oregano essential oil and carvacrol. Anatol J Biol. 1: 52–68. [Google Scholar]
- 13.Suntres ZE, Coccimiglio J, Alipour M. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr. 2015; 55: 304–318. doi: 10.1080/10408398.2011.653458 [DOI] [PubMed] [Google Scholar]
- 14.Ezz-Eldin YM, Aboseif AA, Khalaf MM. Potential anti-inflammatory and immunomodulatory effects of carvacrol against ovalbumin-induced asthma in rats. Life Sci. 2020; 242: 117222. doi: 10.1016/j.lfs.2019.117222 [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Zhang ZL, Chen J, Pei A, Hua F, Qian X, et al. Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PLoS One. 2012; 7: e33584. doi: 10.1371/journal.pone.0033584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 17.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968; 25: 192–205. doi: 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- 18.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004; 37: 112–119. doi: 10.1016/j.clinbiochem.2003.10.014 [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 38: 1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 20.Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Eurasian J Med. 2013; 45: 47–49. doi: 10.5152/eajm.2013.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skrzydlewska E, Farbiszewski R. Liver and serum antioxidant status after methanol intoxication in rats. Acta Biochim Pol. 1997; 44: 139–145. doi: 10.18388/abp.1997_4451 [DOI] [PubMed] [Google Scholar]
- 22.Eells JT, Salzman MM, Lewandowski MF, Murray TG. Formate-induced alterations in retinal function in methanol-intoxicated rats. Toxicol Appl Pharmacol. 1996; 140: 58–69. doi: 10.1006/taap.1996.0197 [DOI] [PubMed] [Google Scholar]
- 23.Lanigan S. Final report on the safety assessment of Methyl Alcohol. Int J Toxicol. 2001; 20:(Suppl 1): 57–85. doi: 10.1080/109158101750300955 [DOI] [PubMed] [Google Scholar]
- 24.Pizzorno J. Glutathione! Integr Med (Encinitas). 2014; 13: 8–12. [PMC free article] [PubMed] [Google Scholar]
- 25.Vairetti M, Di Pasqua LG, Cagna M, Richelmi P, Ferrigno A, Berardo C. Changes in glutathione content in liver diseases: an update. Antioxidants. 2021; 10: 364. doi: 10.3390/antiox10030364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012; 2012: 736837. doi: 10.1155/2012/736837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak HH, Kılınç İ, Özkürkçüler A. Biochemical evaluation of the effects of quercetin on experimental acute methanol intoxication in rats. J Pharm Res Int. 2020; 32: 195–201. doi: 10.9734/jpri/2020/v32i2030743 [DOI] [Google Scholar]
- 28.Hamouda F, Nadia N, El-Kersh M. Study on Agarwood and caspase-3 activation in rat liver toxicity induced by methanol. Pharm Pharmacol Int J. 2018; 6: 127–130. [Google Scholar]
- 29.Wallach JB. Interpretation of diagnostic tests: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 30.Fickert P, Zatloukal K. Pathogenesis of alcoholic liver disease. In: Handbook of alcoholism. CRC Press; 2000. pp. 341–348. [Google Scholar]
- 31.Desai SP. Clinician’s guide to laboratory medicine: a practical approach. Lexi-Comp; 2004. [Google Scholar]
- 32.Khan I, Bhardwaj M, Shukla S, Min SH, Choi DK, Bajpai VK, et al. Carvacrol inhibits cytochrome P450 and protects against binge alcohol-induced liver toxicity. Food Chem Toxicol. 2019; 131: 110582. doi: 10.1016/j.fct.2019.110582 [DOI] [PubMed] [Google Scholar]
- 33.Allis JW, Brown BL, Simmons JE, Hatch GE, McDonald A, House DE. Methanol potentiation of carbon tetrachloride hepatotoxicity: the central role of cytochrome P450. Toxicology. 1996; 112: 131–140. doi: 10.1016/0300-483X(96)03366-5 [DOI] [PubMed] [Google Scholar]
- 34.Kim E, Choi Y, Jang J, Park T. Carvacrol protects against hepatic steatosis in mice fed a high-fat diet by enhancing SIRT1-AMPK signaling. Evid Based Complement Alternat Med. 2013; 2013: 290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazari A, Fanaei H, Dehpour AR, Hassanzadeh G, Jafari M, Salehi M, et al. Chemical composition and hepatoprotective activity of ethanolic root extract of Taraxacum Syriacum Boiss against acetaminophen intoxication in rats. Bratisl Lek Listy. 2015; 116: 41–46. [DOI] [PubMed] [Google Scholar]
- 36.Akhgari M, Panahianpour MH, Bazmi E, Etemadi-Aleagha A, Mahdavi A, Nazari SH. Fatal methanol poisoning: features of liver histopathology. Toxicol Ind Health. 2013; 29: 136–141. doi: 10.1177/0748233711427050 [DOI] [PubMed] [Google Scholar]
- 37.Dobrzyñska I, Skrzydlewska E, Kasacka I, Figaszewski Z. Protective effect of N-acetylcysteine on rat liver cell membrane during methanol intoxication. J Pharm Pharmacol. 2000; 52: 547–552. doi: 10.1211/0022357001774183 [DOI] [PubMed] [Google Scholar]
- 38.Kurcer Z, Oğuz E, Iraz M, Fadillioglu E, Baba F, Koksal M, et al. Melatonin improves methanol intoxication-induced oxidative liver injury in rats. J Pineal Res. 2007; 43: 42–49. doi: 10.1111/j.1600-079X.2007.00441.x [DOI] [PubMed] [Google Scholar]