Abstract
Coenzyme Q10 is an important molecule for mitochondrial respiration and as an antioxidant. Maintenance of the ovum in a good condition is considered to be important for successful fertilization and development, which has been reported to be promoted by coenzyme Q10. In this study, we investigated the level of coenzyme Q10 during ovum fertilization and maturation. We attempted to analyze coenzyme Q10 levels during ovum development in species that use coenzyme Q10 but not coenzyme Q9. It was shown that medaka produces coenzyme Q10. We then measured the amount of coenzyme Q10 after fertilization of medaka ovum and found that it increased. The amount of free cholesterol biosynthesized from acetyl CoA as well as coenzyme Q10 increased during development, but the increase in coenzyme Q10 was more pronounced. The mRNA expression level of coq9 also increased during embryonic development, but the mRNA expression levels of other coenzyme Q10 synthases did not. These results suggest that the coq9 gene is upregulated during the development of medaka ovum after fertilization, resulting in an increase in the amount of coenzyme Q10 in the ovum. Medaka, which like humans has coenzyme Q10, is expected to become a model animal for coenzyme Q10 research.
Keywords: coenzyme Q10, medaka, mitochondrial DNA, egg development, Oryzias latipes
Introduction
Coenzyme Q10 (CoQ10) is an essential lipid in the mitochondrial respiratory chain. It is also an important lipid-soluble antioxidant. CoQ10 has two forms: a reduced form (ubiquinol, CoQ10H2) and an oxidized form (ubiquinone, CoQ10). Only ubiquinol exhibits strong antioxidant activity.(1)
Although CoQ10 is important as an energy producer and as an antioxidant, the cellular CoQ10 level has been reported to decrease with aging. In 1989, Kalén et al.(2) reported that CoQ levels decrease with age. In addition, Battino et al.(3) reported that CoQ content in nonsynaptic mitochondria from mouse brain decreased between 2 and 18 months of age. Kamzalov et al.(4) also reported that CoQ content in mitochondria decreases with age in liver, heart, and kidney. CoQ level in rat skeletal muscle mitochondria has also been reported to decrease.(5)
Female fertility is one of the first physiological functions adversely affected by aging. Female reproductive capacity declines with aging as a result of decreases in oocyte quality and quantity.(6) Ben-Meir et al.(7) reported the diminished expression of the enzymes responsible for CoQ production, Pdss2 and Coq6, in oocytes of older females in both mouse and human.
Several animal studies have demonstrated that CoQ10 improves ovarian response and embryo quality. Özcan et al.(8) showed that CoQ10 supplementation prevents oxidative stress-induced ovarian damage. They also evaluated the protective effect of CoQ against oxidative stress-related DNA damage. Xu et al.(9) reported that pretreatment with CoQ10 improves ovarian response to stimulation and embryo quality in young women with poor ovarian reserve. Furthermore, in the clinical setting, CoQ10 supplementation has been reported to lead to a better response to ovulation induction and decreased odds of fetal aneuploidy in 35–43-year-old women.
El Refaeey et al.(10) reported that the combination of CoQ10 and clomiphene citrate in the treatment of clomiphene-citrate-resistant PCOS patients improved ovulation and clinical pregnancy rates.
Thus, CoQ10 is important in ovum fertilization and subsequent development. However, there are still many unanswered physiological questions, such as how much CoQ10 is present in the egg and post-fertilization processes, and how the amount of CoQ10 fluctuates.
The side-chain length of CoQ differs among species. Humans biosynthesize CoQ10, but mice and rats, which are frequently used as laboratory animals, have a CoQ side-chain length of 9. It is desirable to analyze the behavior of CoQ in the post-fertilization process using species that biosynthesize CoQ10 instead of CoQ9.
In this study, it was found that medaka uses CoQ10. We then analyzed CoQ10 in medaka ovum after fertilization and the variation in mRNA expression of CoQ synthase during the fertilization process.
Materials and Methods
Animal husbandry and fertilization
The species of medaka used was Oryzias latipes. The young medakas (medaka born in early spring, and at the time of the study in fall, around 6 months) and the old medakas (medaka that survived the winter, around 18 months) used in this study were obtained from a dealer. Medakas were kept in fresh water in plastic aquaria under artificial reproductive conditions (14 h light, 10 h dark; 26 ± 1°C). A measured amount of a powdered diet was supplied at least three times daily.(11) Fertilized eggs were incubated in suitable medium (6.5 mg/ml NaCl, 0.4 mg/ml KCl, and 0.15 mg/ml CaCl2·2H2O adjusted with NaHCO3 to pH 7.3) at 26 ± 1°C in a six-well plate.(12) The morphological changes during egg development were then observed under a light microscope (×100) (#CKX41; Olympus, Tokyo, Japan).
Lipid analysis by HPLC
Extraction of the CoQ of one medaka and ovary was performed by homogenizing 40 times the wet weight (mg) of one fish in 2-propanol. The CoQ of medaka eggs was extracted by homogenizing three medaka eggs with 200 μl of 2-propanol. The type of CoQ synthesized by the medaka was identified by comparison with CoQ9 and CoQ10 standards. The identification of CoQ in medaka and the concentrations of CoQ and free cholesterol (FC) in medaka and medaka eggs were measured using HPLC, as previously reported with minor modifications.(13) Briefly, samples collected with 2-propanol were centrifuged and the resulting supernatant was separated on two separation columns (Ascentis® C8, 5 μm, 250 mm × 4.6 mm i.d. and SupelcosilTM LC-18, 3 μm, 5 cm × 4.6 mm i.d.; Supelco Japan, Tokyo, Japan) and passed through one reducing column (RC-10, 15 mm × 4 mm i.d.; IRICA, Kyoto, Japan) before detection by ECD and UV. The mobile phase was 50 mM sodium NaClO4 dissolved in methanol/2-propanol (85/15, v/v) at a flow rate of 0.8 ml/min. The column was kept at 25°C.
Gene expression analysis by reverse transcription quantitative polymerase chain reaction assays
Total RNA was extracted from three medaka eggs using RNAqueousTM-Micro Total RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA). The RNA quality and concentration were measured using a NanoPhotometer® NP80 (Implen, Munich, Germany). cDNA was synthesized by reverse transcription using QuantiTect Reverse Transcription Kit (QIAGEN, Venlo, The Netherlands). Housekeeping genes were selected based on published studies.(14) The expression levels of the genes in Table 1 were measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays. qPCR (95°C for 2 min followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, with a final extension step of 60°C for 30 s) was conducted using QuantStudio® 3 (Thermo Fisher Scientific). The change in gene expression was calculated by the 2−ΔΔCt method.(15)
Table 1.
List of primer sequences used in qPCR assays
| SOD1 | F | 5'-TGAAGCCTGTCTTTGCCAGA-3' |
| R | 5'-CCTTTACCCCTTACTACCCTGTAG-3' | |
| SOD2 | F | 5'-TTACCCTTCAGCCTGCTCTG-3' |
| R | 5'-GCCACAGTAGCAGCAGAGA-3' | |
| GS | F | 5'-TGGACAAGATCAGCCAGGAC-3' |
| R | 5'-TCTGCTGGTGGATCCTGAAC-3' | |
| GST | F | 5'-ACCTGCGATCACACTGTTCA-3' |
| R | 5'-TTTGGAGACTTCAGAGCCCA-3' | |
| ACTB | F | 5'-TCCACCTTCCAGCAGATGTG-3' |
| R | 5'-AGCATTTGCGGTGGACGAT-3' | |
| Catalase | F | 5'-GCGGTACAACAGCGCAGATG-3' |
| R | 5'-GGATGGACGGCCTTCAAGTT-3' | |
| FDFT-1 | F | 5'-GTTTATACTGAACCAGCG-3' |
| R | 5'-CTTCTCCTGACTCTCAGTGAAG-3' | |
| PDSS1 | F | 5'-GGTCCTTAAATGAAGTGTGGGG-3' |
| R | 5'-CTTGAAGGTCTTCTCCAGGTAG-3' | |
| PDSS2 | F | 5'-CATAGCAACAAGGCTCTGGA-3' |
| R | 5'-GTAGACATTGCACTTCCAGAGC-3' | |
| coq2 | F | 5'-GCTCATCAGGATAAGGAGGATG-3' |
| R | 5'-GATGTCCACCGAGTAAATCTGG-3' | |
| coq3 | F | 5'-GGGATGAAGACGGAGAGTTTG-3' |
| R | 5'-TACAGGATCTATGCCCAGCAC-3' | |
| coq4 | F | 5'-GATTCGTCICTCCACICTAGAC-3' |
| R | 5'-CAGCATATTCGTGGGCATTC-3' | |
| coq5 | F | 5'-GGGACATTTCTGATGGGTATCC-3' |
| R | 5'-CAAACTGGTCATCGTTGAAGGG-3' | |
| coq6 | F | 5'-CGTCCAAGTGAAGTACAGATCC-3' |
| R | 5'-CGTGGGTATCCCTAACTCTTG-3' | |
| coq7 | F | 5'-GTTGGGAAAGGAAGGAGCTATG-3' |
| R | 5'-CAAAAACCAGGCACCG.ATTC-3' | |
| coq8 | F | 5'-GGTCCTTATCCATTCACCCATC-3' |
| R | 5'-GITGACCTGACAACTCCTGT-3' | |
| coq9 | F | 5'-CTGCTTCCTCATAACATCCCAG-3' |
| R | 5'-CACATCCTGGATCCGGTTATC-3' | |
| psap | F | 5'-CAAGGAGATGGTGGATAGCTAC-3' |
| R | 5'-GATGAGACAGATCAACCTGAGG-3' |
DNA isolation and quantification by quantitative PCR
DNA was extracted from medaka eggs using NucleoSpin® Tissue (TaKaRa, Shiga, Japan). Two genes each were used for the detection of nDNA and mtDNA [nDNA: serpin family A member 1 (SERPINA1), solute carrier organic anion transporter family member 2B1 (SLCO2B1); mtDNA: mitochondrially encoded NADH dehydrogenase 1 (ND-1), mitochondrially encoded NADH dehydrogenase 5 (ND-5)]. Table 2 lists the primers used for qPCR. qPCR (95°C for 2 min followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, with a final extension step of 60°C for 30 s) was conducted using QuantStudio® 3 (Thermo Fisher Scientific). The change in mtDNA levels was calculated by a previously described method.(16)
Table 2.
List of primer sequences used in DNA quantification by qPCR
| ND-1 | F | 5'-CTTCGCTGATGGCTTAAAGC-3' |
| R | 5'-AGGCTAGATAGTGCGAGGAT-3' | |
| ND-5 | F | 5'-TGTAAGCCTTCTACCCCTCT-3' |
| R | 5'-GTTGGGGTCGTCGTG-3' | |
| SLCO2B1 | F | 5'-GTGGAAGCTCAGGGATGAG-3' |
| R | 5'-TTGAAAGAAGCCAGGATCCC-3' | |
| SERPINA1 | F | 5'-GAAGATGCGGGGTCTTTTTC-3' |
| R | 5'-GAAGATGCCAAGAGAGGAGAA-3' |
Statistical analysis
All results are presented as the mean ± SD from at least two independent experiments. Statistical analysis was performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). Statistical significance was determined by Student’s t test and one-way analysis of variance (ANOVA) to evaluate medaka aging and egg development. Group differences were considered statistically significant at *p≤0.05, **p≤0.01, and ***p≤0.001. A rejection test was conducted by the Smirnov–Grubbs outliers test.
Results
Types of CoQ biosynthesized by medaka
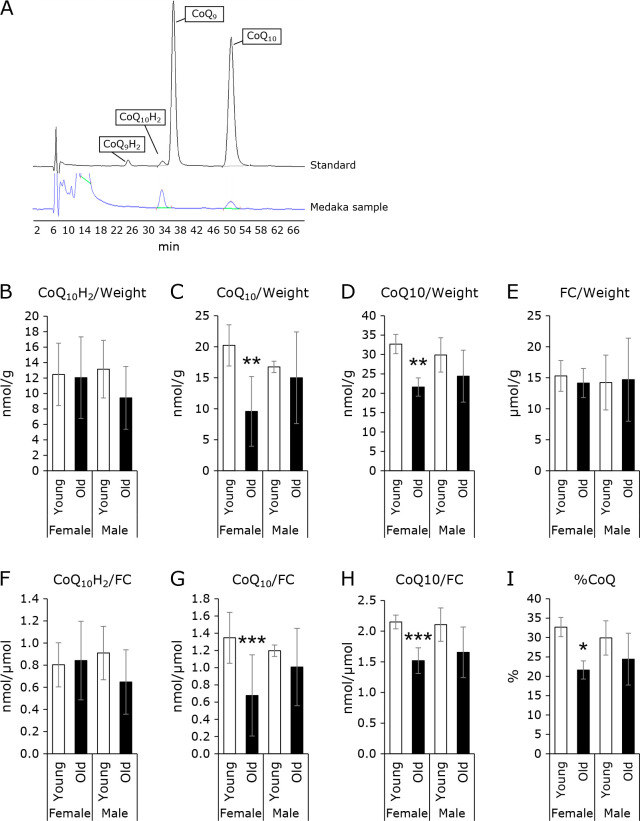
Figure 1 shows a typical example of an HPLC-ECD chromatogram. The upper line is the result of measurement of the standard specimen. Since the HPLC was performed in reverse phase, the greater the side-chain length, the longer the compound is retained in the column. In addition, between the oxidized CoQ10 (CoQ10) and the reduced CoQ10 (CoQ10H2), the reduced type flows out first, resulting in a shorter retention time. The peak at a retention time of 24.5 min is derived from reduced CoQ9 (CoQ9H2), that at a retention time of 33.7 min is derived from CoQ10H2, that at 37.5 min is derived from oxidized CoQ9 (CoQ9), and that at 50.5 min is CoQ10. The lower line is the result of the analysis of 2-propanol extract of medaka homogenates. As shown in this figure, peaks are observed at 33.7 min and 50.5 min. This shows that medaka mainly produces CoQ10. It should be noted that humans also biosynthesize CoQ10. The above suggests that medaka may be a useful animal model for CoQ10 research. Next, we measured the CoQ10 levels in male and female, young and old medakas.
Fig. 1.
Types of CoQ synthesized by medaka and CoQ levels of medaka. (A) Types of CoQ synthesized by medaka. (B–D) CoQ levels of medaka, corrected for wet weight. (B) indicates the amount of reduced CoQ, (C) indicates the amount of oxidized CoQ, and (D) indicates the amount of total CoQ. (E) FC levels of medaka, corrected for wet weight. (F–H) CoQ levels of medaka, corrected for FC levels. (F) indicates the amount of reduced CoQ, (G) indicates the amount of oxidized CoQ, and (H) indicates the amount of total CoQ. (I) Redox balance of medaka CoQ. Levels of CoQ in medaka were compared with those in young medaka using Student’s t test. Values are presented as mean ± SD (n = 5). *, **, and *** indicate significant differences (p<0.05, 0.01, and 0.001, respectively) compared with total CoQ values in young medaka.
Medaka CoQ10 values by age and sex
Figure 1B–D shows the CoQ10 level in medaka. The medaka born in the early spring and used for experiments in the fall are called young medaka (around 6 months). The medaka that survived the winter are called old medaka (around 18 months). As shown in Fig. 1B, the level of CoQ10H2 did not differ significantly by age and sex. CoQ10 level in female was significantly reduced with age. Total CoQ10 level (CoQ10H2 + CoQ10) was also significantly reduced in females. CoQ10 level in male medaka also showed a downward trend. There was no significant difference in total CoQ10 level between males and females. It should be noted that CoQ is synthesized in vivo.(17,18) In mammalian cells, CoQ is biosynthesized from acetyl CoA via the mevalonate pathway, as with the biosynthesis of FC.(17,18) Thus, we next measured the FC level in medaka. As shown in Fig. 1E, there was no difference in FC levels by age or sex. As mentioned above, CoQ10 is synthesized by the same synthetic pathway as FC until halfway through the process, so CoQ values were often corrected for FC values in previous studies. As shown in Fig. 1F–H, CoQ10 and total CoQ10 values also decreased in the female old medaka group even after correcting for the value of FC. Figure 1I shows the proportion of oxidized CoQ10 (%CoQ10). This value is an indicator of the oxidative stress and is calculated as follows: (oxidized form of CoQ10)/(oxidized form of CoQ10 + reduced form of CoQ10) × 100. As shown in this figure, %CoQ10 decreased in old medaka.
Level of CoQ in ovary
We then measured the CoQ10 values of the ovaries. Unexpectedly, the level of CoQ10 in the ovaries varied. As shown in Fig. 2, ovarian CoQ10 levels and ovarian mass were plotted. The results showed that the ratio of CoQ10/FC in the ovaries decreased with increasing ovarian weight. This suggests that the value of CoQ10/FC decreases with ovary maturation. The physiological significance of this phenomenon is unclear and further investigation of it is anticipated.
Fig. 2.
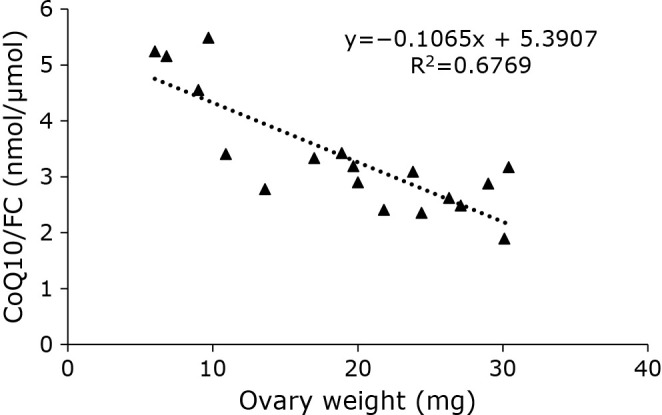
Correlation between CoQ10/FC and wet weight in medaka ovaries (n = 18).
CoQ10 level in egg after fertilization
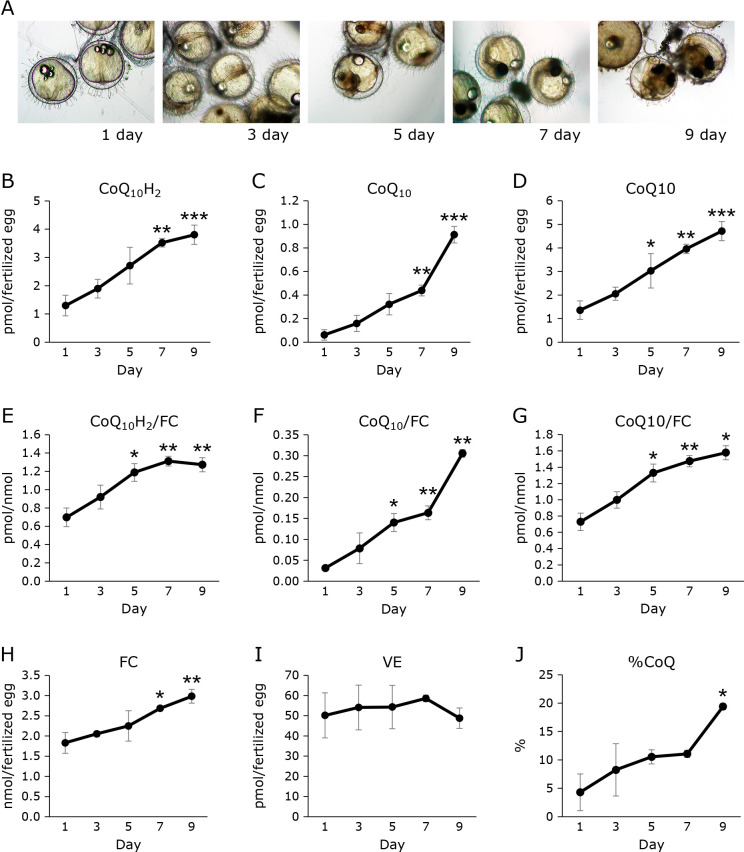
After fertilization, the eggs matured as previously reported.(11) A micrograph of an egg is shown in Fig. 3A. We confirmed that the medaka hatched in around 10 days. The concentration of CoQ10H2 in eggs increased over time (Fig. 3B). The CoQ10 level was lower than that of CoQ10H2 (Fig. 3C). CoQ10 level also increased over time (Fig. 3C). The total CoQ10 level also increased with time after fertilization (Fig. 3D). CoQ10H2, CoQ10, and CoQ10 levels increased with time, even when corrected for FC level (Fig. 3E–G). FC level increased (Fig. 3H). However, the rate of increase in FC was not as pronounced as that of CoQ10. Vitamin E (VE) is another important lipid-soluble antioxidant. We also measured VE level in egg. VE level remained unchanged during the studied period (Fig. 3I). %CoQ10 value increased (Fig. 3J).
Fig. 3.
Changes over time in CoQ, FC, and VE levels in medaka eggs from fertilization to before hatching. (A) Observations on medaka eggs from fertilization to before hatching. (B–D) CoQ levels per medaka egg. (B) indicates the amount of reduced CoQ, (C) indicates the amount of oxidized CoQ, and (D) indicates the amount of total CoQ. (E–G) CoQ levels of medaka, corrected for FC levels. (E) indicates the amount of reduced CoQ, (F) indicates the amount of oxidized CoQ, and (G) indicates the amount of total CoQ. (H) FC Levels per medaka egg. (I) VE levels per medaka egg. (J) Redox balance of CoQ of medaka egg. Statistical analysis was conducted by ANOVA. Values are presented as mean ± SD (n = 3) of the data obtained from two independent experiments (*p<0.05, **p<0.01, ***p<0.001).
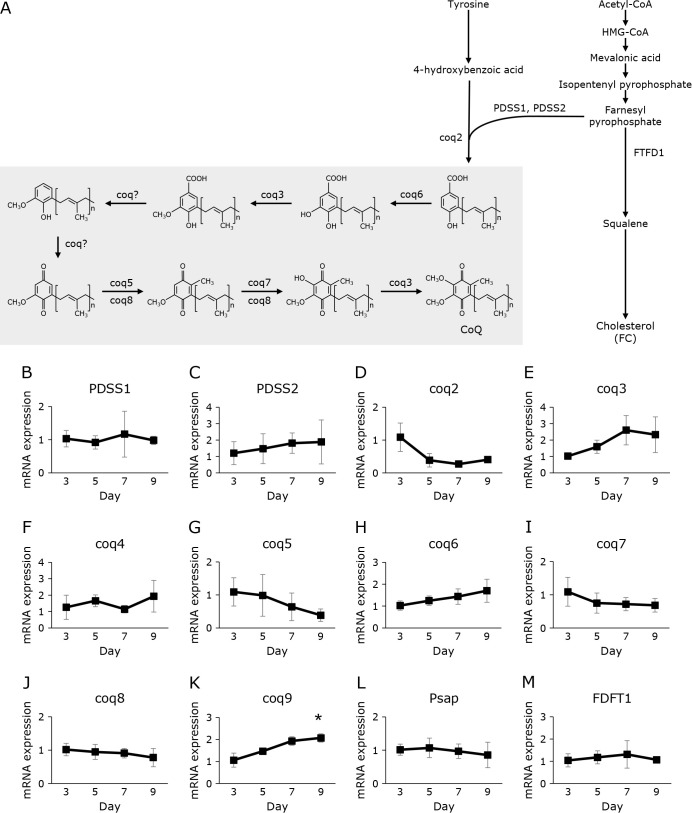
Level of mRNA expression of various CoQ10 biosynthesis genes
Figure 4A illustrates the reported mechanisms of the biosynthesis of CoQ10. We measured the mRNA expression levels of these genes (PDSS1, PDSS2, coq2–9), as shown in Fig. 4B–K. Although the CoQ10 level increased during the process of egg maturation, the levels of mRNA expression of various CoQ synthesis enzymes did not change significantly (PDSS1, PDSS2, coq2–8). Only coq9 increased significantly during this process. Prosaposin (Psap) is a CoQ10 binding protein.(19) We previously reported that transfection of Psap increased and knockdown of Psap decreased cellular CoQ10 levels in HepG2 cells.(20) Thus, the level of Psap was also measured in medaka eggs. The level of Psap mRNA expression did not change (Fig 4L). FDFT1 is an enzyme associated with the production of FC. The mRNA level of FDFT1 did not change (Fig. 4M).
Fig. 4.
Changes over time in expression of genes encoding CoQ-synthesizing enzyme, Psap, and FDFT1 in medaka eggs from fertilization to before hatching. (A) Scheme of CoQ and FC synthesis. (B–M) Gene expression of PDSS1 (B), PDSS2 (C), coq2 (D), coq3 (E), coq4 (F), coq5 (G), coq6 (H), coq7 (I), coq8 (J), coq9 (K), Psap (L), and FDFT1 (M) in medaka egg development measured using quantitative PCR; results are shown as mean ± SD of 3–5 measurements. The mean expression level was normalized to that of the 3-day samples (2−ΔΔCt method). Statistical analysis was conducted by ANOVA. *p<0.05.
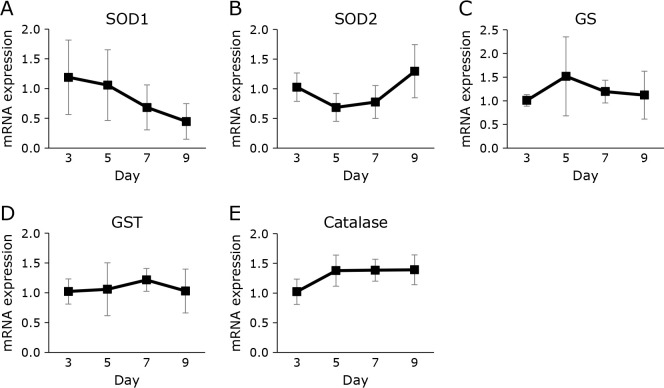
Level of mRNA of various antioxidant enzymes
Since CoQ10 is an important antioxidant and its level increased during oocyte maturation, the levels of mRNA expression of antioxidative enzymes were studied. The level of superoxide dismutase (SOD)1 decreased over time (Fig. 5A). The levels of other enzymes, such as SOD2, glutathione synthase (GS), glutathione S-transferase (GST), and catalase, were unchanged (Fig. 5B–E). As shown above, the level of VE, another lipid-soluble antioxidant, also did not change (Fig. 3I). These results indicate that only CoQ10 levels increased with egg maturation, while most antioxidant levels did not.
Fig. 5.
Changes over time in antioxidant gene expression levels in medaka eggs from fertilization to before hatching. (A–E) Gene expression of SOD1 (A), SOD2 (B), GS (C), GST (D), and catalase (E) in medaka egg development measured using quantitative PCR; results are shown as mean ± SD of 3–5 measurements. The mean expression level was normalized to that of the 3-day samples (2−ΔΔCt method). Statistical analysis was conducted by ANOVA.
Level of mitochondrial DNA copy number in oocytes after fertilization
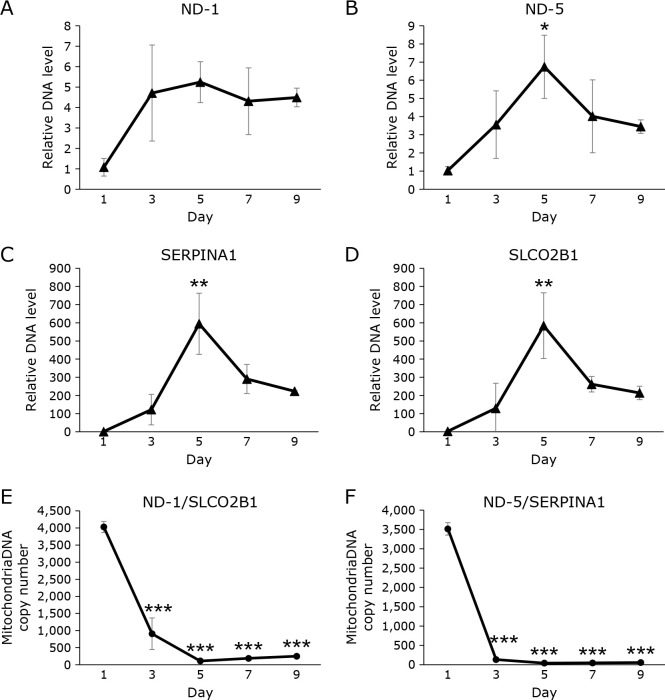
CoQ is an essential lipid in the mitochondrial electron transport chain. We measured the mitochondrial DNA copy number (mtDNAcn) in medaka egg. As shown in Fig. 6, mtDNAcn increased with time after fertilization (Fig. 6A and B). It should be noted that nuclear DNA copy number (nDNAcn) also increased in this period (Fig. 6C and D), and the ratio mtDNAcn/nDNAcn somewhat decreased (Fig. 6E and F).
Fig. 6.
mtDNA and nDNA levels in medaka eggs from fertilization to before hatching. (A–D) Changes in the amount of mtDNA [ND-1 (A) and ND-5 (B)] and nDNA [SERPINA1 (C) and SLCO2B1 (D)] corrected by the levels of the day 1 sample. mtDNA and nDNA levels were measured using quantitative PCR; results are shown as mean ± SD of triplicate measurements. (E, F) mtDNAcn was calculated by correcting each mtDNA for nuclear DNA [ND-1/SLCO2B1 (E) and ND-5/SERPINA1 (F)]. Statistical analysis was conducted by ANOVA. *p<0.05, **p<0.01, and ***p<0.001.
Discussion
As shown above, CoQ10 level increased during embryogenesis (Fig. 3). CoQ10 is a lipid-soluble antioxidant. Another important lipid-soluble antioxidant is VE.(21) VE, apart from CoQ10, is a nutrient and is not synthesized in vivo.(22) The level of VE was not altered during embryogenesis (Fig. 3). Furthermore, mRNA expression of several antioxidant enzymes, such as SOD and catalase, was not increased (Fig. 5). These result imply that not many antioxidant systems increased during embryogenesis, while only CoQ10 increased significantly. CoQ10 is also an essential lipid in the mitochondrial respiratory chain. The level of mtDNAcn was also measured in embryogenesis, albeit semi-quantitatively. This level increased on days 3 and 5, and decreased thereafter. This result is consistent with previous reports.(23–25) Thus, the level of CoQ10 does not parallel mtDNAcn in embryogenesis. It would be interesting to know why only the CoQ10 level increased with time during embryogenesis. The above results imply that the level of CoQ10 increase is somehow important to medaka embryogenesis. Precise control of CoQ10 levels in egg may be necessary for egg fertilization and embryogenesis.
Interestingly, the level of CoQ10 increased during egg maturation. CoQ10 is biosynthesized from mevalonate.(18) FC is also produced from mevalonate.(18) It should be noted that the level of FC does not significantly increase during the embryogenesis of medaka. If changes in the synthase are involved in the increase in CoQ content, the amount of PDSS and coq after the biosynthetic junction between CoQ10 and cholesterol may change. The coq genes are a group of genes whose deletion results in low intracellular CoQ levels. As shown in Fig. 4A, several coqs have been found to be involved in chemical reactions involved in the chemical modification of the benzene ring of CoQ10. FDFT1 is involved in the synthesis of FC. Therefore, we analyzed the mRNA expression of these genes. Analysis on the mRNA expression of CoQ biosynthesis genes revealed that the expression of many genes such as PDSS1, PDSS2, coq2–8 is unaltered. The mRNA level of FDFT1, which is associated with the synthesis of FC, was also unaltered. Only the mRNA level of coq9 was significantly increased. COQ9 is considered as an important protein for maintaining the CoQ biosynthetic complex. The enzymes in the terminal phase of CoQ biosynthesis, which are shown with a gray background in Fig. 5A, are considered to form a biosynthetic complex termed complex Q. Complex Q is located on the matrix face of the inner mitochondrial membrane. Mutation in COQ9 in mice causes selective and significant deletion of numerous CoQ proteins.(26) Therefore, coq9 is important for maintaining the CoQ biosynthetic complex, although the mechanism by which this protein stabilizes complex Q remains unknown. Lohman et al.(26) reported that COQ9 has a lipid binding pocket, but the endogenous lipid ligand is still unknown. COQ9 is reported to interact with COQ7 protein and enhance its activity. COQ9 is also reported to enhance coq6 activity.(27) These findings suggest that the level of coq9 is important for determining the cellular concentration of CoQ10, although the full extent of the physiological effects of coq9 remains unknown. The increase in coq9 level during embryogenesis might accelerate the biosynthesis of CoQ10. The precise mechanism underlying the increase in CoQ10 level in fertilized eggs requires further investigation.
As shown above, medaka biosynthesizes and utilizes CoQ10, as humans do. However, the length of the polyprenyl side-chain differs among different species: S. cerevisiae has CoQ6, Escherichia coli has CoQ8, and rat and mouse produce CoQ9. These organisms are all common experimental animal models. It is expected that the different side-chain lengths of CoQ affect its metabolism and reaction. It would be desirable to analyze CoQ10 in organisms that have the same CoQ10 as humans. This report shows that medaka utilizes CoQ10, so further research on CoQ10 using medaka is anticipated.
The level of CoQ10 has been reported to decrease with age in human and mouse. The lifespan of medaka is thought to be 2 to 4 years. As shown above, female medaka around 18 months old had lower CoQ10 than medaka aged around 6 months. In the present experiment, no significant difference in this regard was identified in male medaka, although a decreasing trend was observed. Further investigation of this issue by increasing the sample size is expected. Our results show that the CoQ10 level also had a tendency to decrease in aged medaka. The mechanism behind the decrease in CoQ10 level with aging has not yet been elucidated, but it is expected that studies of medaka, which produces CoQ10 like humans, will contribute to the elucidation of this mechanism as an experimental animal.
In conclusion, in this study, we found that medaka biosynthesizes and utilizes CoQ10. Since medaka produces the same type of CoQ10 as humans, it is expected that medaka will be a useful experimental animal for shedding light on CoQ10. In this study, we also found that the amount of CoQ10 increases during the maturation of eggs. Further analysis of the role of CoQ10 in egg fertilization and maturation, the mechanism controlling this, and its therapeutic application is expected.
Author Contributions
MK, YY, and AF conceived the project and designed the experiments. MO, AN, AM, YK, KS, MT, MO, EM, and NI performed the experiments. MO, AN, and MK wrote the paper. MK coordinated and directed the project.
Acknowledgments
The authors are grateful to Professors Yasumasu S. at Sophia University and Dr. Taguchi M. for their helpful discussions. The authors would like to thank Enago (www.enago.jp) for the English language review.
Abbreviations
- CoQ10
coenzyme Q10
- CoQ10
oxidized form CoQ10
- CoQ10H2
reduced form CoQ10
- FC
free cholesterol
- GS
glutathione synthase
- GST
glutathione S-transferase
- mtDNAcn
mitochondrial DNA copy number
- ND-1
mitochondrially encoded NADH dehydrogenase 1
- ND-5
mitochondrially encoded NADH dehydrogenase 5
- nDNAcn
nuclear DNA copy number
- SERPINA1
serpin family A member 1
- SLCO2B1
solute carrier organic anion transporter family member 2B1
- SOD
superoxide dismutase
- VE
vitamin E
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Yamamoto Y, Komuro E, Niki E. Antioxidant activity of ubiquinol in solution and phosphatidylcholine liposome. J Nutr Sci Vitaminol (Tokyo) 1990; 36: 505–511. [DOI] [PubMed] [Google Scholar]
- 2.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989; 24: 579–584. [DOI] [PubMed] [Google Scholar]
- 3.Battino M, Gorini A, Villa RF, et al. Coenzyme Q content in synaptic and non-synaptic mitochondria from different brain regions in the ageing rat. Mech Ageing Dev 1995; 78: 173–187. [DOI] [PubMed] [Google Scholar]
- 4.Kamzalov S, Sohal RS. Effect of age and caloric restriction on coenzyme Q and alpha-tocopherol levels in the rat. Exp Gerontol 2004; 39: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 5.Lass A, Kwong L, Sohal RS. Mitochondrial coenzyme Q content and aging. Biofactors 1999; 9: 199–205. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald C, Zimon AE, Jones EE. Aging and reproductive potential in women. Yale J Biol Med 1998; 71: 367–381. [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015; 14: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Özcan P, Fıçıcıoğlu C, Kizilkale O, et al. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet 2016; 33: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Nisenblat V, Lu C, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: a randomized controlled trial. Reprod Biol Endocrinol 2018; 16: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Refaeey A, Selem A, Badawy A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reprod Biomed Online 2014; 29: 119–124. [DOI] [PubMed] [Google Scholar]
- 11.Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech Dev 2004; 121: 605–618. [DOI] [PubMed] [Google Scholar]
- 12.Iwamatsu T. Studies on oocyte maturation in the medaka, Oryzias latipes. Improvement of culture medium for oocytes in vitro. Jpn J Ichthyol 1973; 20: 218–224. [Google Scholar]
- 13.Nagase M, Yamamoto Y, Mitsui J, Tsuji S. Simultaneous detection of reduced and oxidized forms of coenzyme Q10 in human cerebral spinal fluid as a potential marker of oxidative stress. J Clin Biochem Nutr 2018; 63: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu M, Shariat-Madar B, Haron MH, Wu M, Khan IA, Dasmahapatra AK. Ethanol-induced attenuation of oxidative stress is unable to alter mRNA expression pattern of catalase, glutathione reductase, glutathione-S-transferase (GST1A), and superoxide dismutase (SOD3) enzymes in Japanese rice fish (Oryzias latipes) embryogenesis. Comp Biochem Physiol C Toxicol Pharmacol 2011; 153: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto M, Shimogishi M, Nakamura A, et al. Differentiation of THP-1 monocytes to macrophages increased mitochondrial DNA copy number but did not increase expression of mitochondrial respiratory proteins or mitochondrial transcription factor A. Arch Biochem Biophys 2021; 710: 108988. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Hekimi S. Molecular genetics of ubiquinone biosynthesis in animals. Crit Rev Biochem Mol Biol 2013; 48: 69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta MJ, Vazquez Fonseca L, Desbats MA, et al. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta 2016; 1857: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 19.Jin G, Kubo H, Kashiba M, et al. Saposin B is a human coenzyme q10-binding/transfer protein. J Clin Biochem Nutr 2008; 42: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiba M, Oizumi M, Suzuki M, et al. Prosaposin regulates coenzyme Q10 levels in HepG2 cells, especially those in mitochondria. J Clin Biochem Nutr 2014; 55: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med 2014; 66: 3–12. [DOI] [PubMed] [Google Scholar]
- 22.Niki E, Traber MG. A history of vitamin E. Ann Nutr Metab 2012; 61: 207–212. [DOI] [PubMed] [Google Scholar]
- 23.St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update 2010; 16: 488–509. [DOI] [PubMed] [Google Scholar]
- 24.Spikings EC, Alderson J, St John JC. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod 2007; 76: 327–335. [DOI] [PubMed] [Google Scholar]
- 25.Cree LM, Samuels DC, de Sousa Lopes SC, et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet 2008; 40: 249–254. [DOI] [PubMed] [Google Scholar]
- 26.Lohman DC, Forouhar F, Beebe ET, et al. Mitochondrial COQ9 is a lipid-binding protein that associates with COQ7 to enable coenzyme Q biosynthesis. Proc Natl Acad Sci U S A 2014; 111: E4697–E4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He CH, Black DS, Allan CM, Meunier B, Rahman S, Clarke CF. Human COQ9 rescues a coq9 yeast mutant by enhancing coenzyme Q biosynthesis from 4-hydroxybenzoic acid and stabilizing the CoQ-synthome. Front Physiol 2017; 8: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]