Abstract
The trimethylammonium compound glycine betaine (N,N,N-trimethylglycine) can be accumulated to high intracellular concentrations, conferring enhanced osmo- and cryotolerance upon Listeria monocytogenes. We report the identification of betL, a gene encoding a glycine betaine uptake system in L. monocytogenes, isolated by functional complementation of the betaine uptake mutant Escherichia coli MKH13. The betL gene is preceded by a consensus ςB-dependent promoter and is predicted to encode a 55-kDa protein (507 amino acid residues) with 12 transmembrane regions. BetL exhibits significant sequence homologies to other glycine betaine transporters, including OpuD from Bacillus subtilis (57% identity) and BetP from Corynebacterium glutamicum (41% identity). These high-affinity secondary transporters form a subset of the trimethylammonium transporter family specific for glycine betaine, whose substrates possess a fully methylated quaternary ammonium group. The observed Km value of 7.9 μM for glycine betaine uptake after heterologous expression of betL in E. coli MKH13 is consistent with values obtained for L. monocytogenes in other studies. In addition, a betL knockout mutant which is significantly affected in its ability to accumulate glycine betaine in the presence or absence of NaCl has been constructed in L. monocytogenes. This mutant is also unable to withstand concentrations of salt as high as can the BetL+ parent, signifying the role of the transporter in Listeria osmotolerance.
In the early 1980s a number of major outbreaks of human listeriosis established Listeria monocytogenes as an important foodborne pathogen (13). Even allowing for improvements in diagnostic techniques and greater awareness, the incidence of listeriosis appears to be increasing (26). This is extremely significant given that mortality rates of 23% have been reported for the organism (36). L. monocytogenes can survive a variety of environmental stresses, growth having been reported at NaCl concentrations as high as 10% (30) and at temperatures as low as −0.1°C (39). The ability of the organism to withstand hostile environments is illustrated by an outbreak of listeric septicemia which was linked to consumption of salted mushrooms (7.5% NaCl) stored at low temperatures (17). The ability of the organism to survive both high salt concentrations and low temperatures is attributed mainly to the accumulation of the compatible solute glycine betaine. This trimethylamino acid, which occurs at high concentrations in sugar beets and other foods of plant origin, has been shown to stimulate growth of L. monocytogenes at between 0.3 and 0.7 M NaCl (2), resulting in a 2.1-fold increase in the growth rate at 0.7 M NaCl (3) and a 1.8-fold increase at 4°C (20). Patchett et al. (32) described glycine betaine uptake in L. monocytogenes as a highly specific, constitutive, energy-dependent system which was subsequently shown to be Δψ-driven via cotransport with Na+ (11) and regulated at the protein level by a novel osmolyte-sensing mechanism (37). On the other hand, a recent report suggests that at least a component of the glycine betaine uptake system in Listeria is ςB dependent, since a ςB knockout mutant was affected in its ability to accumulate glycine betaine (4).
While much information regarding the physiological characterization of glycine betaine transport is available, genetic analysis of the uptake systems in L. monocytogenes has been largely ignored. In contrast, the genetic basis of glycine betaine uptake in other gram-positive bacteria has been studied extensively. Bacillus subtilis has been shown to possess three transport systems for glycine betaine: the secondary uptake system opuD (18) and two binding-protein-dependent transport systems, opuA (19) and opuC (proU) (25). The secondary transport system betP, isolated by Peter et al. (33), is involved in glycine betaine accumulation in Corynebacterium glutamicum.
In this communication, we describe the isolation, characterization, and disruption of betL, a gene which plays an important role in glycine betaine uptake in L. monocytogenes and which exhibits high homologies to the secondary glycine betaine uptake systems of other gram-positive bacteria.
MATERIALS AND METHODS
Media, chemicals, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5α was grown at 37°C in Luria-Bertani (LB) medium (29). E. coli MKH13 was grown at 37°C in either LB medium or M9 minimal medium (GIBCO/BRL, Eggenstein, Federal Republic of Germany [FRG]) containing 0.5% glucose, 0.04% arginine, 0.04% isoleucine, and 0.04% valine. L. monocytogenes strains were grown in brain heart infusion (BHI) broth or in tryptone soy broth (Sigma Chemical Co., St. Louis, Mo.) supplemented with 0.6% yeast extract. Glycine betaine (Sigma) was added to M9 as a filter-sterilized solution to a final concentration of 1 mM. Radiolabelled [1-14C]glycine betaine (55 mCi/mmol) was purchased from American Radiolabelled Chemicals Inc. (St. Louis, Mo.). Erythromycin, ampicillin, and chloramphenicol were made up as described by Maniatis et al. (29) as concentrated stocks and added to media at the required levels. Where necessary, medium osmolarity was adjusted by the addition of NaCl.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| L. monocyto-genes | ||
| LO28 | Serotype 1/2c | P. Cossart, Insti-tut Pasteur |
| LO28G | LO28 containing pVE6007 | This study |
| LO28B | LO28 betL::pCPL2, BetL− | This study |
| E. coli | ||
| DH5α | supE44 ΔlacU169(φ80lacZΔM15)R17 recA1 endA1 gyrA96 thi-1 relA1 | 12 |
| MKH13 | MC4100Δ(putPA)101Δ(proP)2Δ(proU) | 19 |
| Plasmids | ||
| pUC18 | Apr ColE1 ori | 38 |
| pCPL1 | pUC18 containing 2.5 kb of L. mono-cytogenes genomic DNA | This study |
| pVE6007 | Cmr Ts derivative of pWV01 | 28 |
| pORI19 | Emr Ori+ RepA−lacZ′ | 24 |
| pCPL2 | pORI19 containing DNA from betL | This study |
| PCPL3 | pCPL1 cut with EcoRI | This study |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Emr, erythromycin resistance.
DNA manipulations and sequence analysis.
Restriction enzymes, RNase, shrimp alkaline phosphatase, and T4 DNA ligase were obtained from Boehringer GmbH (Mannheim, FRG) and were used according to the manufacturer’s instructions. Genomic DNA was isolated from L. monocytogenes as described by Hoffman and Winston (16). Plasmid DNA was isolated with the Qiagen QIAprep spin miniprep kit (Qiagen, Hilden, FRG). E. coli was transformed by standard methods (29), while electrotransformation of L. monocytogenes was achieved by the protocol outlined by Park and Stewart (31). Restriction fragments were isolated with the Qiaex II gel extraction kit (Qiagen). PCR reagents (Taq polymerase and deoxynucleoside triphosphates dNTPs) were purchased from Boehrnger and used according to the manufacturer’s instructions with a Hybaid (Middlesex, United Kingdom) PCR express system. Oligonucleotide primers for PCR and sequence purposes were synthesised on a Beckman Oligo 1000M DNA synthesizer (Beckman Instruments, Inc., Fullerton, Calif.). Nucleotide sequence determination was performed on an ABI 373A automated sequencer with the Dye Terminator sequence kit (Applied Biosystems, Warrington, United Kingdom). Nucleotide and protein sequence analyses were done by using Lasergene (DNASTAR Ltd., London, United Kingdom). Homology searches were performed with the BLAST program (1).
Construction of an L. monocytogenes genomic library.
A genomic DNA preparation from L. monocytogenes was partially digested with Sau3A and ligated to plasmid pUC18 DNA, which had been digested with BamHI and dephosphorylated with shrimp alkaline phosphatase. The resulting recombinant plasmids were transformed in restriction-deficient E. coli DH5α, and colonies were selected on LB plates containing ampicillin (50 μg/ml), IPTG (isopropyl-1-thio-β-d-galactopyranoside) (1 mM), and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml). Approximately 70% of the plasmids in the bank (30,000 CFU) carried inserts, as judged from their LacZ− phenotypes. Transformants were pooled and grown for 2 h in LB medium with ampicillin and stocked at −80°C. Plasmid DNA was extracted and used to transform the glycine betaine uptake mutant E. coli MKH13. Transformants were selected on M9 minimal medium containing 4% NaCl and 1 mM glycine betaine.
Restriction deletion analysis.
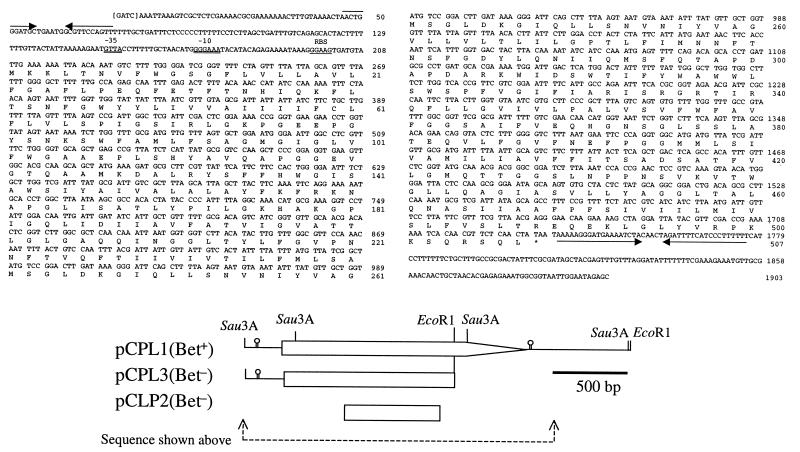
pCPL3 (Table 1) was constructed by digestion of pCPL1 with EcoRI, followed by religation (Fig. 1). The pCPL1 insert contains one EcoRI site (nucleotide [nt] 1379 [Fig. 1]), and a second site is located in the multiple cloning site. The larger EcoRI fragment of pCPL1 was gel extracted, religated, and transformed into MKH13. Removal of the smaller EcoRI fragment resulted in inactivation of betL by removing a 350-bp region from the 3′ end of the gene. The loss of the EcoRI fragment in pCPL3 was confirmed by restriction analysis. Gene inactivation was confirmed by the failure of the truncated plasmid to complement MKH13.
FIG. 1.
DNA sequence of the betL gene and deduced amino acid sequence of the BetL protein. The likely ribosome-binding site (RBS) and the putative ςB-dependent −10 and −35 sites are underlined. Inverted repeats are indicated by pairs of arrows. A graphic illustration of the cloned fragment of LO28 genomic DNA is also presented, together with constructs mentioned in the text.
Construction of an L. monocytogenes betL mutant.
A betL mutant was constructed by gene disruption with a single crossover event, as described by Law et al. (24). This system relies upon the lactococcal pWV01-derived Ori+ RepA− vector pORI19. Maintenance of pORI19 is dependent on the temperature-sensitive pGhost plasmid pVE6007 to supply RepA in trans. A 551-bp fragment (nt 703 to 1253 [Fig. 1]) from the center of the betL gene was generated by PCR with primers XbaIKO (5′ TAAGCGCCACTCTAGACC 3′) (nt 703 to 720 [Fig. 1]) and EcoRIKO (5′ GCACGAATTCACCAAGTA 3′) (nt 1236 to 1253 [Fig. 1]), modified to contain the restriction sites XbaI and EcoRI (underlined), respectively. The resulting PCR product, purified by gel extraction, was cut with XbaI and EcoRI and ligated into similarly digested pORI19 to give pCPL2 (Fig. 1), which was then transformed into L. monocytogenes LO28G (LO28 harboring pVE6007). A temperature upshift from 30°C to the nonpermissive 42°C resulted in the loss of pVE6007. Plating on erythromycin selected for chromosomal integration of pCPL2 at the point of homology with betL. PCR with primers betL F (nt 402 to 423 [Fig. 1]; 5′ AGTCCGATTGGCTCGATTCGAC 3′) and betL R (nt 1790 to 1812 [Fig. 1]; 5′ TCGCGAAATAGTCGCGGCAAAGC 3′) was used to confirm the integration event in one mutant strain, designated LO28B. A 4.6-kb product (corresponding to the length of betL plus pCPL2) was obtained for LO28B, while LO28 gave a 1.4-kb product (corresponding to betL alone).
Transport assays.
E. coli cells grown overnight in minimal medium (10) were inoculated into fresh minimal medium to an optical density at 600 nm (OD600) of 0.05. Cells were harvested in mid-log phase (OD600 between 0.4 to 0.6), washed twice, and suspended to an OD600 of 1.0 in minimal medium. Subsequently, the cells were incubated with shaking for 5 min at 37°C and transport was initiated by the addition of [1-14C]glycine betaine. For Km determination, the glycine betaine concentration was varied from 0.2 to 10 μM. Radioactivity was measured with a liquid scintillation counter (model 1600TR; Packard Instruments Co., Downers Grove, Ill.). To determine the ability of LO28 and LO28B to accumulate [14C]glycine betaine, log-phase cells grown in BHI broth were harvested by centrifugation, washed twice, and resuspended in 50 mM potassium phosphate buffer (pH 6.8) to an OD600 of 1.0. Glucose was added to a final concentration of 5 mM to energize the cells, and where indicated, 3% NaCl was added to subject the cells to osmotic upshock. After 20 min of incubation at 30°C, assays were initiated by the addition of [14C]glycine betaine (at a final concentration of 10 μM). Cells were collected on 0.45-μm-pore-size cellulose nitrate filters (Schleicher & Schuell GmbH, Dassell, FRG) under vacuum. Filters were then washed with 3 ml of buffer (same osmolarity as the assay buffer), and the radioactivity trapped in the cells was measured by liquid scintillation counting, as described above. In the cases of both E. coli and Listeria, protein concentrations of cell suspensions were derived from standard curves relating OD600 to protein concentration.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been submitted to GenBank and assigned accession no. AF102174.
RESULTS
Cloning of the betL gene by functional complementation of E. coli MKH13.
In contrast to the parental strain MC4100, the mutant E. coli MKH13 is unable to synthesize glycine betaine from its precursor, choline, and lacks the transport systems PutP, ProP, and ProU, rendering it unable to grow on high-osmolarity (3 to 4% NaCl) minimal media containing glycine betaine. The pUC18::LO28 genome library (see Materials and Methods) was transformed into MKH13, and transformants were selected on minimal medium containing 4% NaCl and 1 mM glycine betaine. No colonies appeared following a control transformation with pUC18 alone, while transformation efficiencies of approximately 80 CFU/μg of DNA were achieved from the plasmid bank, with colonies appearing after 36 h at 37°C. Plasmids isolated from 10 such colonies were retransformed into MKH13 to confirm complementation. Restriction analysis revealed that all 10 clones contained the same 2.5-kb insert. When clones were plated onto high-osmolarity media containing either carnitine or proline, no growth was observed, indicating that the cloned insert encodes a system specific for glycine betaine transport.
A representative plasmid, designated pCPL1, was chosen for further characterization. Analysis revealed that if pCPL1 was deleted from the internal EcoRI site to create pCPL3, no complementation of MKH13 was observed (Fig. 1). Approximately 1.9 kb of the insert was sequenced from both strands. Analysis of the sequenced region revealed a single large open reading frame spanning positions 209 to 1729. A TTG start codon was chosen as the initiation codon based on homology data. A long inverted repeat immediately downstream of betL probably functions as a rho-independent transcription termination signal with a ΔG of −28.2 kcal (34). Upstream of the TTG start codon, potential −10 and −35 regions (GTTA[16 nt]GGGAAA) which have considerable homology with the recently identified ςB-dependent consensus promoter (GTTT[15/16 nt]GGGTAA) can be identified (4). Upstream of the putative promoter site is a short inverted repeat with a ΔG of −13 kcal which may act as a terminator for upstream sequences (Fig. 1). Sequencing upstream of this inverted repeat revealed the presence of a gene homologous to the l-argininosuccinate lyase gene from Cyanobacterium synechocystis.
The betL gene encodes a 507-residue protein (designated BetL) with a calculated molecular mass of 55.27 kDa. A search for related proteins in the databases revealed significant similarity to the gram-negative choline transporter BetT (22) from E. coli (38% identity) and two gram-positive secondary transporters, OpuD from B. subtilis (57% identity) and BetP from C. glutamicum (41% identity). Both OpuD (18) and BetP (33) are members of the trimethylammonium transporter family, whose substrates possess a fully methylated quaternary ammonium group. In the case of OpuD, BetP, and BetL, this substrate is glycine betaine. Hydropathy analysis of BetL, according to the method of Kyte and Doolittle (21), predicts that BetL is an integral membrane-bound protein containing 12 transmembrane domains. In fact, the entire hydropathy profile is very similar to that of OpuD (data not shown). Multiple alignments of the three proteins—BetL, OpuD, and BetP—show a high degree of relatedness over the entire lengths of their sequences, but one region in particular, a 37-amino-acid segment stretching from amino acids 310 to 346, which includes the eighth transmembrane segment and the connecting cytoplasmic loop to the ninth transmembrane segment, is highly conserved. While it has been speculated that this region may function in substrate binding and membrane translocation in B. subtilis (18), its actual function is as yet unknown.
Analysis of BetL kinetics in E. coli MKH13.
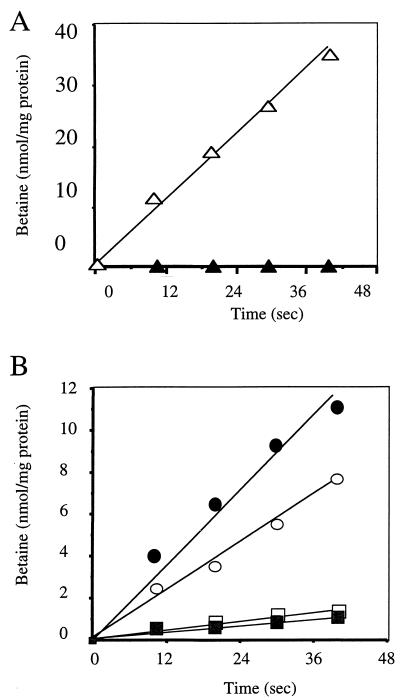
Uptake studies using [14C]glycine betaine confirmed that growth of the strain carrying pCPL1 (BetL+), when subjected to high osmolarity, was the direct result of glycine betaine accumulation mediated by BetL. Maximum uptake rates of 134 nmol min−1 mg of protein−1 were determined by Michaelis-Menten kinetics. The Km value of 7.9 μM observed following heterologous expression of betL in E. coli MKH13(pCPL1) correlates with the Km value of 10 μM observed for L. monocytogenes in another study (37). Since no measurable uptake of [14C]glycine betaine was observed for MKH13 clones carrying pUC18 alone (Fig. 2A), uptake of the compatible solute could be solely ascribed to the cloned insert on pCPL1. Given that the cloned gene is expressed, we assume that either the ςB-dependent Listeria promoter is recognized in E. coli or transcription was initiated from another, undetermined site.
FIG. 2.
(A) BetL-mediated glycine betaine uptake in E. coli MKH13. Uptake of [1-14C]glycine betaine was assayed in low-osmolarity cultures at a final substrate concentration of 10 μM. E. coli MKH13(pCPL1) (BetL+) was grown in M9 medium to mid-log phase and assayed for glycine betaine uptake (▵). Strain MKH13(pUC18) (▴) was used as a control. Each point represents the mean value from at least two independent experiments. (B) Betaine accumulation in L. monocytogenes LO28 and the BetL− mutant LO28B. Mid-log-phase cells (OD600, 0.4 to 0.6) were harvested, washed twice, and resuspended in potassium phosphate buffer. Cells were energized by the addition of glucose and then divided into two equal volumes, and sodium chloride to a final concentration of 3% was added to one of the samples. After a 20-min incubation at 30°C, [14C]glycine betaine (at a final concentration of 10 μM) was added to each sample and aliquots were removed at 10-s intervals, filtered through 0.45-μm filters, and counted by scintillation counting. ○, LO28; ●, LO28 plus 3% NaCl; □, LO28B; ■, LO28B plus 3% NaCl. Each point represents the mean value from at least two independent experiments.
Analysis of a BetL− mutant of L. monocytogenes LO28.
A BetL− mutant of L. monocytogenes LO28 (LO28B) was constructed by homologous recombination, as described in Materials and Methods. PCR analysis confirmed the disruption of the betL gene in strain LO28B (data not shown). The ability of LO28B to accumulate radiolabelled glycine betaine was significantly impaired in comparison with the parent strain (Fig. 2B). However, uptake was not completely abolished. In the presence of 3% NaCl, uptake of glycine betaine by LO28 was enhanced as expected but no increase in the level of uptake was observed for the mutant, suggesting that the enhanced uptake observed in the parent is due to activation of BetL rather than the induction of a separate system.
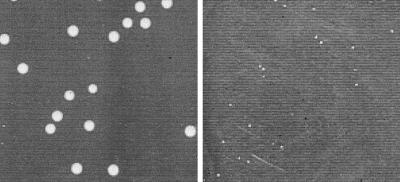
That glycine betaine uptake due to BetL may be linked to the salt tolerance of L. monocytogenes was confirmed in a simple plating experiment. LO28 and LO28B were grown to stationary phase in BHI broth, serially diluted in Ringers, and plated on BHI agar containing an additional 4% NaCl. While LO28 gave large colonies within 48 h at 37°C, LO28B was able only to form pinpoint colonies under the same conditions (Fig. 3).
FIG. 3.
Growth of L. monocytogenes LO28 (left) and the BetL− mutant LO28B (right) on BHI agar containing an additional 4% NaCl after 48 h at 37°C.
DISCUSSION
Adaptation of bacteria to high solute concentrations involves intracellular accumulation of organic compounds called osmolytes (6, 40). Osmolytes (often referred to as compatible solutes because they can be accumulated to high intracellular concentrations without adversely affecting cellular processes) can be either taken up from the environment or synthesized de novo, and they act by counterbalancing external osmotic strength, thus preventing water loss from the cell and plasmolysis. Synthesized in relatively large quantities by plants (14), glycine betaine is the preferred compatible solute for the majority of bacteria (8, 9). While precursor molecules such as choline or glycine betaine aldehyde confer considerable osmotic stress tolerance to B. subtilis and E. coli in high-osmolarity media (5, 23), L. monocytogenes cannot synthesize glycine betaine from these molecules; thus, accumulation must occur via a transport system (3).
Many microorganisms possess two or more glycine betaine transport systems. Salmonella typhimurium, for example, possesses two genetically distinct pathways, a constitutive low-affinity system (ProP) and an osmotically induced high-affinity system (ProU) (7), while B. subtilis has three glycine betaine transport systems, OpuD, OpuA, and OpuC (18, 19, 25). Generally these transport systems can be divided into two groups. The first of these are the multicomponent, binding-protein-dependent transport systems which belong to the superfamily of prokaryotic and eucaryotic ATP-binding cassette transporters or traffic ATPases (15). Members of this family, including OpuA (19) and OpuC (25) of B. subtilis and ProU of E. coli (27), couple hydrolysis of ATP to substrate translocation across biological membranes. The second group belongs to a family of secondary transporters involved in the uptake of trimethylammonium compounds. Members of this family, including OpuD of B. subtilis and BetP of C. glutamicum, form single-component mechanisms which couple proton motive force to solute transport across the membrane.
The betL gene isolated in this study encodes a 507-residue protein (BetL). BetL possesses 12 transmembrane domains, a structural feature common in secondary transport systems (35). The BetL protein thus represents the newest member of the prokaryotic secondary trimethylammonium transporter family. As with OpuD and BetP, BetL is highly specific for glycine betaine and fails to transport other trimethylammonium compounds such as carnitine or choline. An interesting feature of the betL gene is the presence of −10 and −35 promoter binding sites showing similarity to recently characterized ςB-dependent promoters (4). This is significant given that Becker et al. (4) have recently shown that a ςB mutant of L. monocytogenes is affected in its ability to accumulate glycine betaine. BetL thus may represent this predicted ςB-mediated sodium or osmotically inducible component of glycine betaine transport in L. monocytogenes. While it has been proposed that glycine betaine uptake in L. monocytogenes is controlled by activation of a constitutive enzyme (20) regulated by a novel osmolyte-sensing mechanism (37), the presence of putative ςB-dependent promoter binding sites suggests that BetL-mediated uptake of glycine betaine may be regulated, at least in part, at the level of transcription. As with the OpuD system in B. subtilis, maximal uptake activity by BetL thus may result from a combination of de novo synthesis of BetL and activation of preexisting BetL (18).
The Km value of 7.9 μM for BetL synthesized in E. coli MKH13 is similar to the value of 10 μM observed in L. monocytogenes (37) and is indicative of a high-affinity uptake system, allowing Listeria to scavenge glycine betaine from the environment. BetL thus may represent an important component of the glycine betaine-mediated salt and chill stress response in Listeria (20). This is further evidenced by the dramatic decrease in the rate of glycine betaine uptake observed following disruption of betL. While nonspecific uptake or passive diffusion cannot be ruled out, uptake rates of approximately 19% of that of the wild type observed for the BetL− mutant LO28B may suggest the presence of at least one other glycine betaine transporter in L. monocytogenes. Nonetheless, the important role of BetL in Listeria salt tolerance was established by a simple plate assay. Even though this assay was performed on a complex medium (and thus presumably in the presence of both carnitine and peptides which could act as osmolytes), the growth of LO28B was severely restricted. This preliminary confirmation of the importance of BetL will have to be characterized in more detail in further experiments.
In conclusion, while previous physiological investigations established the existence of a constitutive, highly specific mechanism for glycine betaine uptake in Listeria (11, 20, 37), this study represents the first genetic analysis of compatible solute transport in Listeria. Interestingly, the presence of a putative ςB-dependent promoter suggests that high osmolarity may stimulate increased transcription of betL in addition to the activation of already synthesized BetL proteins.
ACKNOWLEDGMENTS
We thank Erhard Bremer (Universitat Marburg) for providing E. coli MKH13 and John O’Callaghan for expert technical assistance.
This work has been supported by funding from the National Food Biotechnology Centre, BioResearch Ireland.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Amezaga M R, Davidson I, McLaggan D, Verheul A, Abee T, Booth I R. The role of peptide metabolism in the growth of Listeria monocytogenes ATCC 23074 at high osmolarity. Microbiology. 1995;141:41–49. doi: 10.1099/00221287-141-1-41. [DOI] [PubMed] [Google Scholar]
- 3.Amezaga M R. The adaptation of Listeria monocytogenes to osmotic stress. Ph.D. thesis. Aberdeen, Scotland: University of Aberdeen; 1996. [Google Scholar]
- 4.Becker L A, Çetin M S, Hutkins R W, Benson A K. Identification of the gene encoding the alternative sigma factor ςB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boch J, Kempf B, Bremer E. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J Bacteriol. 1994;176:5364–5371. doi: 10.1128/jb.176.17.5364-5371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth I R, Pourkomailian B, McLaggan D, Koo S-P. Mechanisms controlling compatible solute accumulation: a consideration of genetics and physiology of bacterial osmoregulation. J Food Eng. 1994;22:381–397. [Google Scholar]
- 7.Cairney J, Booth I R, Higgins C F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985;164:1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;55:476–511. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csonka L N, Hanson A D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 10.Davis B D, Mingioli E S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950;60:17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhart P N M, Smith L T, Smith G M. Sodium-driven, osmotically activated glycine betaine transport in Listeria monocytogenes membrane vesicals. J Bacteriol. 1996;178:6105–6109. doi: 10.1128/jb.178.21.6105-6109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibco-BRL. Product catalogue and reference guide: 1995–1996. Grand Island, N.Y: Gibco-BRL; 1995. pp. R40–R41. [Google Scholar]
- 13.Gill P. Is listeriosis often a foodborne illness? J Infect. 1988;17:1–5. doi: 10.1016/s0163-4453(88)92212-8. [DOI] [PubMed] [Google Scholar]
- 14.Hansen A D, Rathinasabapathi B, Rivoal J, Burnet M, Dillon M O, Gage D A. Osmoprotective compounds in the plumbaginacease: a natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci USA. 1994;91:306–310. doi: 10.1073/pnas.91.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins C F. ABC transporters: from micro-organisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman C S, Winston F. Rapid DNA extraction procedure. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 17.Junttila A, Brander M. Listeria monocytogenes septicaemia associated with consumption of salted mushrooms. Scand J Infect Dis. 1989;21:339–342. doi: 10.3109/00365548909035707. [DOI] [PubMed] [Google Scholar]
- 18.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempf B, Bremer E. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J Bacteriol. 1995;28:16701–16713. doi: 10.1074/jbc.270.28.16701. [DOI] [PubMed] [Google Scholar]
- 20.Ko R, Smith L T, Smith G M. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;177:6874–6880. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 22.Lamark T, Kassen I, Eshoo M W, Falkenberg P, McDougall J, Strøm A R. DNA sequence and analysis of the bet genes encoding the osmoregulatory choline-glycine betaine pathway of Escherichia coli. Mol Microbiol. 1991;5:1049–1064. doi: 10.1111/j.1365-2958.1991.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 23.Landfald B, Strøm A R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986;165:849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Hansen J N. Characterization of a chimeric proU operon in a subtilin-producing mutant of Bacillus subtilis 168. J Bacteriol. 1994;177:6874–6880. doi: 10.1128/jb.177.23.6874-6880.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low J C, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet J. 1997;153:9–29. doi: 10.1016/s1090-0233(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 27.Lucht J H, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Lett. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 28.Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 30.McClure P J, Roberts T A, Oguru P O. Comparison of the effects of sodium chloride, pH and temperature on the growth of Listeria monocytogenes on gradient plates and liquid medium. Lett Appl Microbiol. 1989;9:95–99. [Google Scholar]
- 31.Park S F, Stewart G S A B. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin treated cells. Gene. 1990;94:129–132. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- 32.Patchett R A, Kelly A F, Kroll R G. Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl Environ Microbiol. 1992;58:3959–3963. doi: 10.1128/aem.58.12.3959-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter H, Burkovski A, Krämer R. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J Bacteriol. 1996;178:5229–5234. doi: 10.1128/jb.178.17.5229-5234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981;24:10–32. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- 35.Saier M H., Jr Computer-aided analysis of transport protein sequences: gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol Rev. 1994;58:71–93. doi: 10.1128/mr.58.1.71-93.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schucant A, Swaminathan B, Broome C V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4:169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 39.Walker S J, Archer P, Banks J G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 40.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]