Abstract
Background
CD103+CD8+ tissue-resident memory T (TRM) cells, associated with better overall survival among various malignancies, are thought to activate anti-tumour immune response and affect therapeutic sensitivity including both immunotherapy and adjuvant chemotherapy (ACT).
Methods
Totally 650 muscle-invasive bladder cancer (MIBC) patients from three independent cohorts were included in this study for survival and cisplatin-based ACT response analysis. Another public data set consisting of 195 patients from IMvigor210 trial receiving PD-L1 blockade were involved in the assessment of immunotherapeutic response. Fifty-nine fresh tumour tissues were used to evaluate immune infiltration of CD103+CD8+ TRM cells.
Results
Patients with high CD103+CD8+ TRM cells infiltration, but not CD8+ T cells, are more likely to benefit from immunotherapy and ACT. The presence of TRM cells is highly associated with an enhanced IFNγ-enriched and T cell-inflamed anti-tumour microenvironment. Elevated CD103+CD8+ TRM cells infiltration correlated with superior ACT response in mismatch repair (MMR), homologous recombination (HR), PIK3CA/AKT and RAS/RAF pathway proficient or histone modification and cell cycle pathway deficient patients.
Conclusions
CD103+CD8+ TRM cells played a crucial role in anti-tumour immunity and served as an ideal prognostic biomarker. It could be treated as a superior companion predictor for treatment response to PD-L1 inhibitor and ACT within MIBC patients.
Subject terms: Bladder cancer, Cancer microenvironment, Chemotherapy, Cancer immunotherapy
Introduction
Bladder cancer is a heterogeneous disease with diverse clinical outcomes [1]. Tumours that invade the muscular layer are defined as muscle-invasive bladder cancer (MIBC), which has a higher propensity to spread to other organs with worse clinical outcomes. MIBC represents approximately 20% of newly diagnosed cases of bladder cancer. Despite the application of radical cystectomy (RC) plus adjuvant chemotherapy (ACT) or neoadjuvant chemotherapy (NAC), almost half of patients ultimately develop metastatic disease because of disseminated micrometastases [2]. Currently, novel immune checkpoint inhibitor (ICI) is a key emerging treatment strategy for advanced MIBC patients. Programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors have already been approved as first- and second-line settings in clinical practice, but the therapeutic benefit is limited to only a small subset of patients and the prediction of patients’ clinical responsiveness remains a major challenge [2]. Therefore, efficacious predictive biomarkers for patients’ therapeutic response are urgently needed in MIBC.
The titling balance between anti-tumour immunity and immune evasion could be one of the crucial factors of the unsatisfactory systemic therapy response [3, 4]. As the main anti-tumour immune cells, CD8+ T cells have a decisive influence on anti-tumour immunity [5]. However, increasing studies revealed that intratumoural CD8+ T cells remained highly heterogeneous [6]. The phenotypes of intratumoural CD8+ T cells could determine the orientation of anti-tumour immune response [7, 8], which might largely affect the therapeutic sensitivity.
Recent studies have revealed that CD103 is a marker of T cell activation on CD8+ tissue-resident memory (TRM) T cells. CD103+CD8+ TRM cells could reside in tumour tissue and possess a mighty anti-tumour effector function by secreting effector molecules [9, 10]. Established evidence denotes that the enrichment of CD103+CD8+ TRM cells could indicate favourable prognoses in various human cancers including breast cancer [11, 12], ovarian cancer [13], lung cancer [14], melanoma [15], head and neck cancer [16] and bladder cancer [17]. Although the direct cytotoxic abilities of CD103+ CD8+ TRM cells were well elucidated [18], the impact of CD103+CD8+ TRM cells on global anti-tumour immune response and its association with the sensitivity to systematic therapies remained further exploration.
In this study, we reported that in patients with MIBC, CD103+CD8+ TRM cells possessed superior prognostic value for clinical survival than CD8+ T cells and could be treated as an ideal predictor for the treatment response to PD-L1 immunotherapy and ACT. Besides, the enrichment of CD103+CD8+ TRM cells correlated with a T cell-inflamed and activated anti-tumour microenvironment. These results suggested that CD103+CD8+ TRM cells took a pivotal role in anti-tumour immunity in MIBC, aiding decision-making for patient stratification and translational research in systematic therapies involving ACT and immunotherapies.
Materials and methods
Study population
Totally, four independent cohorts of patients were included for analyses in this study.
The IMvigor210 (NCT02108652) trial [19] is a single-arm phase II study investigating atezolizumab (anti-PD-L1) in patients with metastatic urothelial carcinoma (mUC). Both expression data and relevant clinical data were downloaded from http://research-pub.gene.com/IMvigor210CoreBiologies/. One hundred and ninety-five patients out of 348 patients were included as bladder-derived UC in this study based on the publicly available data. Besides, the Cancer Genome Atlas bladder cancer (TCGA-BLCA) cohort consists of 391 MIBC patients with clinical information. Twenty-one patients were excluded from the initial cohort (n = 412) due to the lack of survival data (n = 3), lack of mRNA data (n = 4), previous application of neoadjuvant therapy (n = 10) and non-muscle-invasive bladder cancer pathologic diagnoses (n = 4). The mRNA sequencing data of bladder cancer in TCGA-BLCA cohort were downloaded from the TCGA program acquired via TCGA-Assembler 2.0.7 in July 2021.
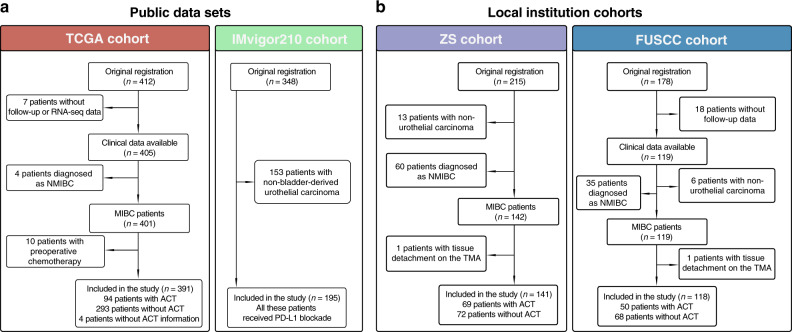
Meanwhile, this study also involved 178 patients and another 215 MIBC patients underwent RC at Fudan University Shanghai Cancer Center (FUSCC cohort, surgery date: 2008–2012) and Zhongshan Hospital, Fudan University (ZS cohort, surgery date: 2002–2014), respectively. Among them, a total of 132 patients were excluded due to unavailable clinical or follow-up data (n = 18) and not diagnosed with MIBC (n = 114). Therefore, we finally enrolled 118 patients from FUSCC cohort and 141 patients from ZS cohort. Detailed selecting procedures and the treatment information of the four cohorts included in this study were shown in Fig. 1. Additional clinicopathological characteristics of these cohorts were also listed in Supplementary Table 1.
Fig. 1. Flow chart of cohort selection.
a The inclusion and exclusion process of two public data sets included in this study. The TCGA-BLCA cohort consisting of 391 patients was included for the analyses of overall survival (OS), recurrence-free survival (RFS) and response to adjuvant chemotherapy (ACT). The IMvigor210 cohort consisting of 195 patients treated with PD-L1 blockade was included for the analyses of immunotherapeutic response. b The inclusion and exclusion process of two local institution cohorts included in this study. Both ZS and FUSCC cohorts were included for the analyses of OS, RFS and response to ACT of MIBC patients.
Immunostaining and evaluation
Tumour tissues resected by RC were embedded in paraffin after the surgery and stored in each hospital. All samples were reviewed histologically by hematoxylin and eosin staining, and representative areas were marked on the paraffin blocks away from necrotic and haemorrhagic materials. Then, the 4-μm thick slides were sliced from each paraffin-embedded tissue block to construct the tissue microarrays (TMAs) in 2015. As to each TMA block, one of the sections was stained with haematoxylin–eosin for histological verification to ensure the arrayed tumour tissues were adequately established. Subsequently, qualified TMAs slides were stored at −20 °C and allowed for further immunostaining. For immunofluorescence (IF), TMAs were dewaxed in oven, washed in xylene, hydrated in ethanol, and heated in 0.01 M sodium citrate buffer (pH = 6.0) for antigen retrieval. Sections then were incubated in normal goat serum for 20 min at 37 °C. After antigen blocking, two primary antibodies were incubated for 30 min at 37 °C. Then, two secondary antibodies conjugated with FITC (111-095-003, 1:100, Jackson ImmunoResearch) and TRITC (115-025-003, 1:100, Jackson ImmunoResearch) were incubated for 30 min at 37 °C. Finally, sections were stained with DAPI (4083S, Cell Signaling Technology) and mounted using an anti-fade medium (H1700, VectorLab). Sections were then observed with Leica SP5 confocal microscopy, and images were captured. For double-staining immunohistochemistry (IHC), it was performed according to the protocols detailed previously [20]. Digital images of TMAs were taken scanned by NanoZoomer-XR (Hamamatsu) under high-power magnification field (HPF, ×200 magnification). Antibodies used for immunostaining were listed in Supplementary Table 2. The density of CD103+CD8+ TRM cells and other interested immune cells in both ZS and FUSCC cohorts were recorded as the mean number of cells/HPF from 3 randomised fields counted by two urologic pathologists who were blinded to the clinicopathological information.
The cut-off number of CD103+CD8+ TRM cells and CD8+ T cells were determined as the 75% FUSCC cohort value in this study, which were 23 cells/HPF and 57 cells/HPF, respectively. Then the cut-off values of FUSCC cohort were applied to ZS cohort for validation. Meanwhile, the cut-off value of CD103+CD8+ TRM signature score and CD8+T cells absolute percentage were also defined as the 75% cohort value in TCGA-BLCA cohort. Comparison of demographics and clinicopathological features according to CD8+ T cells and CD103+CD8+ TRM cells infiltration subgroups was listed in Supplementary Table 3.
Flow cytometry (FCM)
Fresh tumour and peri-tumour tissues were collected from three independent clinical centres (Fudan University Shanghai Cancer Center, Zhongshan Hospital and Ruijin Hospital). Procedures of tissue resection were performed by experienced urologic surgeons and were approved by the ethic committee of these four clinical centres, respectively. In total, 59 resected fresh tumour tissues and 13 peri-tumour tissues were obtained. All involved patients were pathologically diagnosed with MIBC, and none of them received any anti-tumour treatment prior to surgery.
After surgical resection, tumour tissues and peri-tumour tissues were immediately dissociated into single-cell suspension. Briefly, tissues were minced and digested in RPMI-1640 (Gibco) containing collagenase V (C9263, Sigma) and Golgi stop (554724, BD Bioscience) following the instruction of the manufacturers. Cell suspensions were then incubated at 200 rpm, 37 °C for 4 h. Next, the suspensions were filtered through 70-μm cell strainer (352350, Corning), and single-cell suspension was obtained. Then, cell suspensions were incubated with RBC lysis buffer (555899, BD Bioscience), and stained with Fixable Viability Dye 510 to distinguish alive cells. After Fc-receptor blocking (564219, BD Bioscience), cells were stained with surface markers for 40 min at 4 °C. For intracellular staining and transcription factor staining, cells were previously stimulated with phorbol myristate acetate (50 ng/ml) and ionomycin (1 μg/ml) in the presence of GolgiStop Protein Transport Inhibitor (1:1000) for 5 h, and then Fixation/Permeabilization Solution Kit (554715, BD Bioscience) and Transcription Factor Buffer Set (562574, BD Bioscience) were used, respectively, following the instruction of manufacturers. Antibodies used for FCM staining are listed in Supplementary Table 4. Stained cells were then washed, re-suspended in cell staining buffer, and were tested on FACSCelesta Flow Cytometer (BD Bioscience). Flow cytometry data were analysed via FlowJo (ver.10.0.7, Tree Star), and cells were gated according to the isotype control and FMO control.
RNA-seq data and processing
RNA-seq data of TCGA-BLCA cohort were also mined along with the process of acquiring clinical information and were normalised by log2(FPKM + 1) to exclude potential bias. In both TCGA-BLCA and IMvigor210 cohort, tissue-resident memory T cells (TRM) signature, Gajewski T cell-inflamed signature, interferon (IFN)-γ-related gene signature, immune cytolytic activity signature and T effector signature were calculated by the mean arithmetic value of log2(FPKM + 1) based on related genes expression as previously reported gene signature [21–25] (genes were listed in Supplementary Table 5). Immune cytolytic activity signature score in ZS cohort was calculated by the mean arithmetic value of log2(FPKM + 1) based on count of perforin 1 single positive (PRF1+) and granzyme B single positive (GZMB+) cells. Besides, the infiltration evaluation and further patients’ stratification of CD8+ T in both IMvigor210 and TCGA-BLCA cohorts were conducted by CIBERSORT [26]. Tumour mutation burden (TMB) was defined as total non-silent somatic mutation counts which were acquired from https://portal.gdc.cancer.gov/. TMB ≥ 10 mut/Mb was defined as TMBhigh group, while TMB < 10 mut/Mb was defined as TMBlow group [27].
Statistical analyses
Statistical P values were computed using two-way tests and detailed statistical tests were described in corresponding figure legends. Kaplan–Meier curves were depicted to conduct survival analyses, and univariate and multivariate Cox regression models were also applied. P value of ≤ 0.05 was considered statistical significance. All statistical analyses were conducted using IBM SPSS Statistics 25.0 and R software 3.4.2 (http://www.r-project.org/).
Results
CD103+CD8+ TRM cells correlated with better therapeutic response to PD-L1 blockade in IMvigor210 trial
By analysing data from the IMvigor210 cohort, we demonstrated that patients with complete response to atezolizumab had a higher normalised CD103+CD8+ TRM signature score, calculated by the related gene expression described in the ‘Materials and methods’ section, than other response groups. High TRM signature score subgroups, defined as the top 75% of the patient population, correlated to higher disease control rate (DCR) and lower incidence of progressive disease (Fig. 2a). Patients in high TRM subgroups also had a better overall survival (OS) after treatment with atezolizumab (Fig. 2b). Notably, CD8+ T cells absolute percentage calculated by CIBERSORT [26] showed no significant association to various clinical response which indicated that this TRM cells infiltration might serve as a better predictor for immunotherapeutic sensitivity than CD8+ T cells. Considering that the heterogeneous intratumoural CD8+ T cells could largely affect the immunotherapeutic response, we further combined TRM and CD8+ T cells levels for discovering the clinical significance of TRM proportion in CD8+ T cells. We found that patients in CD8+ T high and TRM high infiltration subgroup have the best DCR and OS while patients in CD8+ T high and TRM low infiltration subgroup have the worst DCR and OS (Fig. 2c, d). This investigation indicated that high TRM proportion in CD8+ T cells could predict a better immunotherapy response and clinical outcome. The presence of CD103+CD8+ TRM cells within T cells might herald the successful generation of immune activation derived by immunotherapy.
Fig. 2. CD103+CD8+ TRM cells correlated with better therapeutic response to PD-L1 blockade in IMvigor210 trial.
a Distribution of CD8+ T cells absolute percentage (CIBERSORT) and normalized TRM signature score between different response groups. b Kaplan–Meier analyses for OS between high/low CD8+ T (HR [95% CI]: 0.845 [0.561–1.274]) and TRM (HR [95% CI]: 0.549 [0.354–0.851]) infiltration score groups after treatment by atezolizumab. c Distribution of treatment responses across four CD8+ T/TRM-stratified subgroups. d Kaplan–Meier analyses for OS according to four CD8+ T/TRM-stratified subgroups. CR complete response, PR partial response, PD progressive disease, SD stable disease, DCR disease control rate (consisting of CR, PR and SD), HR hazard ratio, CI confidence interval. The two-sided P values of Kaplan–Meier analyses were conducted by log-rank test. Cox regression analyses were also applied for survival analysis. Pearson’s chi-square test was used for studying the distribution of treatment responses across different subgroups. P ≤ 0.05 was considered statistical significance.
CD103+CD8+ TRM cells predict better OS and superior response to ACT in patients with MIBC
As we confirmed that high TRM cells infiltration could predict better immunotherapy response, we further investigated the relationship between the infiltration of TRM cells and chemotherapeutic response. Both IF (Supplementary Fig. 1A) and double-staining IHC (Supplementary Fig. 1B) confirmed the presence of CD103+CD8+ TRM cells in MIBC tissues from ZS cohort. Using paired tumour and peri-tumour tissues, we found that TRM cells were less enriched in tumour tissues (Supplementary Fig. 1C). Furthermore, the results from both IHC and flow cytometry revealed that the infiltration of TRM cells was negatively correlated with disease stages (Supplementary Fig. 1D–F). For further analyses, the evaluation of CD8+ T and TRM cells was based on CIBERSORT [26] and established cell signature [22] in TCGA-BLCA cohort, respectively, while the evaluation of their infiltration was conducted by IHC in both FUSCC and ZS cohorts. Then, we separated the study population of TCGA-BLCA, ZS and FUSCC cohorts into TRM cells high and low infiltration subgroups by the cut-off value mentioned above. Better OS and recurrence-free survival (RFS) were observed in MIBC patients with elevated TRM cells, while the infiltration of CD8+ T cells showed no correlation with OS and RFS in TCGA-BLCA (n = 391), FUSCC (n = 118) and ZS (n = 141) cohorts (Fig. 3a and Supplementary Fig. 2). Additionally, patients with a high number of TRM cell infiltration have excellent survival outcomes after ACT in three cohorts rather than CD8+ T cells (Fig. 3b). However, TRM cells could not predict survival benefit in MIBC patients untreated by ACT (Supplementary Fig. 3). Taken together, these data provide evidence for the integration of TRM quantification as a robust prognostic indicator for patients survival and predictor for response to ACT in patients with MIBC.
Fig. 3. CD103+CD8+ TRM cells predict better overall survival and superior response to adjuvant chemotherapy in patients with MIBC.
a Kaplan–Meier analyses for OS of whole-cohort patients in TCGA-BLCA cohort (top; CD8+ T, HR [95% CI]: 0.845 [0.561–1.274]; TRM, HR [95% CI]: 0.802 [0.568–1.133]), FUSCC cohort (middle; CD8+ T, HR [95% CI]: 0.500 [0.241–1.038]; TRM, HR [95% CI]: 0.173 [0.062–0.486]) and ZS cohort (bottom; CD8+ T, HR [95% CI]: 0.856 [0.439–1.668]; TRM, HR [95% CI]: 0.215 [0.093–0.500]). b Kaplan–Meier analyses for OS of ACT-applied patients in TCGA-BLCA cohort (top; CD8+ T, HR [95% CI]: 0.592 [0.270–1.300]; TRM, HR [95% CI]: 0.303 [0.126–0.730]), FUSCC cohort (middle; CD8+ T, HR [95% CI]: 0.415 [0.157–1.096]; TRM, HR [95% CI]: 0.148 [0.044–0.502]) and ZS cohort (bottom; CD8+ T, HR [95% CI]: 0.388 [0.093–1.618]; TRM, HR [95% CI]: 0.025 [0.001–0.497]). HR hazard ratio, CI confidence interval. The two-sided P values of Kaplan–Meier analyses were conducted by log-rank test. Cox regression analyses were also applied for survival analysis. P ≤ 0.05 was considered statistical significance.
CD103+CD8+ TRM cells shape an anti-tumour immune microenvironment
To explore the functional characteristic of CD103+CD8+ TRM cells in the tumour microenvironment, we investigated the association between TRM cells signature score and different immune functional signatures in both TCGA-BLCA and ZS cohorts. The functional signatures were calculated based on the related gene expression mentioned above in TCGA-BLCA cohort while part of them was represented by the infiltration of related functional cells in the ZS cohort. A statistically significant and strong positive linear association was observed between the expression of the TRM cell gene signature and T cell-inflamed (Fig. 4a, right panel), IFN-γ-related (Fig. 4b, right panel), immune cytolytic (Fig. 4c, right panel) and T effector (Fig. 4d, right panel) gene signature in the TCGA-BLCA cohort. CD8+ T cells only showed a weak correlation with these four functional signatures (Fig. 4a–d, left panel). Similar results were also observed in 241 MIBC patients from the ZS cohort (Fig. 4e). Notably, TRM cells might shape a T cell-inflamed and aggressive anti-tumour microenvironment rather than the whole cluster of CD8+ T cells.
Fig. 4. CD103+CD8+ TRM cells shape an anti-tumour immune microenvironment.
Linear association between CD8+ T cells absolute percentage (left panel), TRM cell signature score (right panel) and expression of the TRM cell gene signature and a T cell inflamed signature score, b IFN-γ-related signature score, c Immune cytolytic activity signature score, d T effector signature score, respectively, in the TCGA-BLCA cohort. Linear association between relative counts of CD8+ T cells (left panel), TRM cells (right panel) and e IFN-γ+ cells, Immune cytolytic activity immune score in ZS cohort. Spearman correlation analyses were used to study the linear association between different expression scores. P ≤ 0.05 was considered statistical significance.
Characterisation of CD8+ T and CD103+CD8+ TRM cell infiltration across molecular features and prognostic value under prime mutations
Established evidence denotes that the molecular features and specific genomic alterations could serve as prognostic or predictive biomarkers for both ACT and immunotherapies, while alterations in DNA repair, cell cycle and signal transduction pathways have also been investigated as candidate biomarkers in bladder cancer therapies [28]. Thus, we further explored the association of CD8+ T cells or TRM cells infiltration subgroups with molecular classifications and gene alterations sorted by basket in the TCGA-BLCA cohort. The result demonstrated that the patients in both CD8+ T high and TRM high subgroups were mostly classified into basal or basal-squamous subtype across consensus, TCGA, the University of North Carolina (UNC) and MD Anderson Cancer Center (MDA) molecular classifications. We also observed frequent alterations of KMT2A, MSH2, BRIP1, CCND3, PIK3R1 in TRM high subgroup with higher TMB whereas KDM6A and FGFR3 mutations were more frequent in TRM low subgroup. In contrast, genes and pathways did not show significantly different alteration rates between CD8+ T high and low subgroups (Fig. 5a).
Fig. 5. Characterisation of CD8+ T and CD103+CD8+ TRM cells infiltration across molecular features and prognostic value under prime mutations.
a The landscape of molecular subtype, AJCC stage, grade, tumour mutation burden (TMB) and various genomic alterations sorted by basket across CD8+ T cells (left panel) and TRM (right panel) subgroups in TCGA-BLCA cohort. b Hazard ratio of mortality for patients with (right panel) or without (left panel) specific gene mutations according to CD8+ T cells (blue) and TRM (red) subgroups in ACT-applied patients from TCGA-BLCA cohort. WT wild type, MUT mutation. TMB ≥ 10 mut/Mb was defined as TMBhigh group, while TMB < 10 mut/Mb was defined as TMBlow group. Pearson’s chi-square test was applied for studying the relationship between different cell infiltration subgroups and molecular subtype, AJCC stage, grade, tumour mutation burden (TMB), various genomic alterations. Cox regression analyses were applied for survival analysis. P ≤ 0.05 was considered statistical significance. AJCC American Joint Committee on Cancer.
Moreover, we further investigated the predictive value of CD8+ T and TRM cells infiltration subgroups for ACT response beyond pathway alterations. TRM cells could predict a better survival benefit in ACT-applied patients from the TCGA-BLCA cohort when there were no core gene alterations in mismatch repair (MMR), homologous recombination (HR), PIK3CA/AKT and RAS/RAF pathways, whereas only genes alterations that occur in histone modification and cell cycle pathways could help TRM cell subgroups predict ACT response (Fig. 5b).
Discussion
CD103+CD8+ TRM cells were considered as resident memory CD8+ T cells which played an indispensable role in immune defence [29]. Unlike that TRM cells mostly infiltrate in epithelial regions in ovarian cancer [13] and lung cancer [14], more TRM cells are infiltrated in normal urothelial regions than the intratumoural ones in MIBC tissues. In our study, we demonstrated that TRM cells could predict therapeutic response to anti-PD-L1 or ACT treatment and better post-operative survival for MIBC patients.
As aforementioned, PD-1/PD-L1 inhibitors are emerging as the first-line and second-line settings in clinical practice. Higher response rates to pembrolizumab and atezolizumab have been reported to associate with the quantity of tumour-infiltrating lymphocytes (TILs) in advanced triple-negative breast cancer while there were no relevant studies in MIBC [22]. In our study, advanced-stage UC of bladder (UCB) patients with higher levels of TRM cells infiltration possessed an increased response rate to anti-PD-L1 therapy. These findings have motivated efforts to further explore the functional role of TRM cells in tumour microenvironment and responses to immune checkpoint inhibition. Importantly, previous studies reported that genes encoding immune checkpoint proteins, such as PD-1, CTLA-4, TIM-3 and LAG-3, are highly expressed by TRM cells isolated from multiple solid tumour types [11, 15]. It is tempting to speculate that TRM cells could not only become a companion prognostic marker for immunotherapy-sensitive patients but also serve as a target cell population for ICIs and may help restore anti-tumour T cell responses.
Furthermore, it was previously uncovered that CD103+ TILs could be applied as a prognosticator for clinical outcome in UCB especially non-MIBC (Ta+T1) but not MIBC (T2-T4) [30]. Thus, we then focused on the infiltration of TRM cells in MIBC and found it could be applied as a superior prognosticator than CD8+ T cells through the analysis in a large population. Intriguingly, we also revealed that TRM cells could predict the effectiveness of ACT based on TCGA-BLCA, FUSCC and ZS cohorts. Recent studies suggested that the chemotherapeutic efficacy may depend on the immune response and high IFN-γ expression could abrogate chemoresistance [29]. In line with it, we found that TRM cells were highly associated with IFN-γ-related gene expression in the context of MIBC. Further analyses showed TRM cells retained in MIBC tumour tissues and extensively exhibited an aggressive anti-tumour and T cell-inflamed microenvironment with highly IFN-γ-related gene expression and immune cytolytic function. More exploration of the intrinsic mechanism leading to the discrepant distribution may give new insights into TRM cells in MIBC. Since granzyme and perforin were typically found in cytotoxic lymphocytes that can kill target tumour cells when released, the strong correlation between TRM cells infiltration and immune cytolytic signature heralded that TRM cells have the potential to show a tumouricidal capacity and thus control tumour growth. Therefore, we assumed that the activated anti-tumour immune response induced by TRM cells might promote the efficacy of ACT in MIBC patients.
Of note, TRM cells could predict better clinical outcomes in ACT-applied patients from the TCGA-BLCA cohort only when MMR, HR, PIK3CA/AKT and RAS/RAF pathways proficient or histone modification and cycle pathway deficient rather than other conditions. Established studies denote that alteration in cell cycle genes and overexpression in PIK3CA/AKT pathway genes were found in association with platinum resistance [31, 32]. Based on our research, TRM cells infiltration subgroups could distinguish a pack of patients who might get benefit from ACT among these platinum resistance patients. Lamentably, how these pathway alteration or the TRM cell infiltration influence the response to ACT remained under investigation, which was also worthy for future study.
Considering the retrospective design and clinical sample size of our study, further validation is required to confirm our results within the framework of more extensive, multi-centred clinical cohorts. As an IHC-based examination of TRM cells infiltration in both ZS and FUSCC cohorts, several common IHC problems also occurred in our study. A loss of antigenicity can occur on cut paraffin sections that have been stored for varying lengths of time [33]. Besides, although our pathological evaluation of immune cells infiltration was excellent, there were still some empirical differences between the two pathologists. Indeed, we have noticed the application of machine learning in automatically detecting lymphocytes on IHC slides during these years, which we would like to apply to get a more accurate evaluation in future research [34]. Meanwhile, recent studies indicated that TRM cells had multiple subgroups, and played a different role in human cancers [35, 36]. The identification and intervention of the TRM cell subtypes remained under further investigation and elucidation in the following studies.
Conclusions
Overall, our findings highlighted that CD103+CD8+ TRM cells taking a pivotal role in anti-tumour immunity correlated with facilitated IFN-γ-enriched and immune cytolytic activated anti-tumour immune responses. Further survival analyses demonstrated the superior prognostic value of TRM cells for clinical survival than CD8+ T cells. It could be treated as an ideal companion predictor for the treatment response to PD-L1 immunotherapy and ACT within MIBC patients.
Supplementary information
Acknowledgements
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Yunyi Kong (Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China) for their excellent pathological technology help.
Author contributions
KJ: acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; YY, H Zeng, ZL, RY, H Zhang, CL, XS, SY, YC and LX: technical and material support; JX, YZ and ZW: study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding
This study was funded by grants from the National Natural Science Foundation of China (31770851, 81872082, 82002670, 82103408), Shanghai Municipal Natural Science Foundation (19ZR1431800), Shanghai Sailing Program (18YF1404500, 21YF1407000), Shanghai Municipal Commission of Health and Family Planning Program (201840168) and Fudan University Shanghai Cancer Center for Outstanding Youth Scholars Foundation (YJYQ201802). All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Data availability
All data relevant to the study are included in the article or uploaded as Supplementary Information. Other data are available upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University (No. B2015-030) and the Ethics Committee of Fudan University Shanghai Cancer Center (No. 050432-4-1212B). Written informed consent was obtained from each patient.
Consent for publication
Consent for publication was obtained from each author.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiejie Xu, Email: jjxufdu@fudan.edu.cn.
Yu Zhu, Email: yuzhu10@fudan.edu.cn.
Zewei Wang, Email: zwwang12@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01725-6.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70:404–23.. doi: 10.3322/caac.21631. [DOI] [PubMed] [Google Scholar]
- 3.Gide TN, Quek C, Menzies AM, Tasker AT, Shang P, Holst J, et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell. 2019;35:238.e6–55.e6. doi: 10.1016/j.ccell.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–34.. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 5.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71.. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 6.Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–85.. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 7.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–9. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 8.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–36.. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688–97. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amsen D, van Gisbergen K, Hombrink P, van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat Immunol. 2018;19:538–46.. doi: 10.1038/s41590-018-0114-2. [DOI] [PubMed] [Google Scholar]
- 11.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24:986–93.. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. CD103 and intratumoral immune response in breast cancer. Clin Cancer Res. 2016;22:6290–7. doi: 10.1158/1078-0432.CCR-16-0732. [DOI] [PubMed] [Google Scholar]
- 13.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res. 2015;3:926–35.. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 14.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, et al. CD8+ CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–86. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 15.Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res. 2018;24:3036–45.. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 16.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, et al. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol. 2015;194:556–62. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 18.Banchereau R, Chitre AS, Scherl A, Wu TD, Patil NS, de Almeida P, et al. Intratumoral CD103+ CD8+ T cells predict response to PD-L1 blockade. J Immunother Cancer. 2021;9:e002231.
- 19.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin K, Cao Y, Gu Y, Fang H, Fei Y, Wang J, et al. Poor clinical outcomes and immunoevasive contexture in CXCL13+CD8+ T cells enriched gastric cancer patients. Oncoimmunology. 2021;10:1915560. doi: 10.1080/2162402X.2021.1915560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Investig. 2017;127:2930–40.. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne A, Savas P, Sant S, Li R, Virassamy B, Luen SJ, et al. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat Rev Clin Oncol. 2020;17:341–8. doi: 10.1038/s41571-020-0333-y. [DOI] [PubMed] [Google Scholar]
- 23.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 26.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto DT, Mouw KW, Feng FY, Shipley WU, Efstathiou JA. Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol. 2018;19:e683–95.. doi: 10.1016/S1470-2045(18)30693-4. [DOI] [PubMed] [Google Scholar]
- 29.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 30.Lin C, He H, Liu H, Li R, Chen Y, Qi Y, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68:1764–73.. doi: 10.1136/gutjnl-2018-316324. [DOI] [PubMed] [Google Scholar]
- 31.Hahne JC, Honig A, Meyer SR, Gambaryan S, Walter U, Wischhusen J, et al. Downregulation of AKT reverses platinum resistance of human ovarian cancers in vitro. Oncol Rep. 2012;28:2023–8. doi: 10.3892/or.2012.2041. [DOI] [PubMed] [Google Scholar]
- 32.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 33.Wester K, Wahlund E, Sundstrom C, Ranefall P, Bengtsson E, Russell PJ, et al. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8:61–70. [PubMed] [Google Scholar]
- 34.Swiderska-Chadaj Z, Pinckaers H, van Rijthoven M, Balkenhol M, Melnikova M, Geessink O, et al. Learning to detect lymphocytes in immunohistochemistry with deep learning. Med Image Anal. 2019;58:101547. doi: 10.1016/j.media.2019.101547. [DOI] [PubMed] [Google Scholar]
- 35.Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O, et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med. 2019;216:2128–49.. doi: 10.1084/jem.20190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Workel HH, Lubbers JM, Arnold R, Prins TM, van der Vlies P, de Lange K, et al. A transcriptionally distinct CXCL13(+)CD103(+)CD8(+) T-cell population is associated with B-cell recruitment and neoantigen load in human cancer. Cancer Immunol Res. 2019;7:784–96.. doi: 10.1158/2326-6066.CIR-18-0517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supplementary Information. Other data are available upon reasonable request.