Abstract
Purpose
The goal of this study is to investigate the relationship between joint inflammation and damage of the wrists and hands, measured by semiquantitative ultrasound and magnetic resonance imaging scoring systems, with functional disability and handgrip strength (HGs).
Materials and methods
Consecutive adult RA patients with active disease, as defined by a Disease Activity Score 28 joints C-reactive protein (DAS28-CRP) > 3.2, underwent a cross-sectional evaluation comprehensive of a clinimetric assessment, an HGs evaluation, an ultrasound assessment aimed at calculating the UltraSound-CLinical ARthritis Activity (US-CLARA), and a magnetic resonance imaging scored according to the modified Simplified Rheumatoid Arthritis Magnetic Resonance Imaging Score (mod SAMIS). The Spearman’s rho correlation coefficient was used to test the correlations.
Results
Sixty-six patients with RA were investigated (age 55.6 ± 12.2 years). The mod SAMIS total score and the US-CLARA had a weak but significant correlation (rho = 0.377, p = 0.0018). Among the mod SAMIS sub-scores, there was a significant relationship between mod SAMIS bone edema (SAMIS-BME) and US-CLARA (rho = 0.799, p < 0.001) and mod SAMIS synovitis (SAMIS synovitis) and US-CLARA (rho = 0.539, p < 0.001). There were also significant negative relationships between the HGs score and the mod SAMIS total score and US-CLARA (rho = − 0.309, p = 0.011 and rho = − 0.775, p < 0.0001, respectively).
Conclusions
BME and synovitis have an influence on the function of the upper extremities. The US-CLARA and the mod SAMIS total score are intriguing options for semiquantitative assessment of joint inflammation and damage in RA.
Keywords: Rheumatoid arthritis, Handgrip strength, Disease activity, Magnetic resonance imaging, Ultrasound
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that mostly affects the hands, resulting in pain, deformity, and functional limitations. Inflammation of small joints can lead to articular abnormalities and muscle weakness, with consequent impairment of activities of daily living (ADLs) [1, 2]. It is estimated that 70% of RA patients show some hand dysfunction during their disease [3]. An adequate handgrip strength (HGs) is fundamental for the majority of ADLs [4]. A number of studies have shown that chronic, low-grade inflammation has a role in the deterioration of HGs [5]. On the other hand, the traditional disease activity scores such as the Disease Activity Score with 28-joint count (DAS28) may not accurately reflect the regional impact of RA on the hands. The majority of research in this topic has focused on individuals with advanced RA who were not treated according to contemporary guidelines in the early stages of the disease [6, 7].
Nowadays, imaging modalities such as ultrasound (US) and magnetic resonance imaging (MRI) play a significant role in the care of RA patients, in addition to clinical assessment. The great sensitivity of US to detect joint abnormalities is recognized in current European League Against Rheumatism (EULAR) recommendations on the use of imaging modalities in RA [8], advising that this technique should be used for an accurate assessment of patient’s disease activity [9]. In particular, the sensitivity of power Doppler ultrasound (PDUS) in detecting inflammatory flow at the microvascular level has proven to be a useful technique for quantifying the degree of inflammation in RA [10, 11]. For the detection of early bone damage, MRI has shown to be more sensitive than traditional radiography [12]. Furthermore, MRI has the ability to visualize synovitis and bone marrow edema (BME), which has been demonstrated to be predictive of radiologic development [13–15]. Treatment benefits on joint inflammation have been observed using periodic MRI examination in studies lasting 3–6 months [13, 16, 17]. The Food and Drug Administration and the European Medicines Agency guidance documents admit that MRI measures may be useful for assessing RA joint damage in randomized controlled trials (RCTs), but they also state that MRI methodologies are not properly validated [18, 19].
The standardized scoring systems for quantifying inflammatory signs and/or joint damage in RA using different imaging techniques are rapidly evolving, with the development of the Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) [20] and Simplified Rheumatoid Arthritis Magnetic Resonance Imaging Score (SAMIS) systems for MRI [21], and the EULAR-Outcome Measures in Rheumatology Clinical Trials (OMERACT) system for US [22, 23]. According to the OMERACT filter, RAMRIS and SAMIS synovitis, osteitis, and erosions seen with 1.5 T MRI, are valid and useful for assessing joint inflammation and damage in RA of the wrist/hand [24, 25].
US scoring systems have been also implemented in multimodal disease activity indices. The construct validity and reliability of the UltraSound-CLinical ARthritis Activity (US-CLARA) index, which combines the values of the Recent-Onset Arthritis Disability (ROAD) questionnaire, self-administered tender joint count (TJC) scores, and the US assessment into a single measure of disease activity for RA have been investigated. US-CLARA demonstrated a strong correlation with the traditional disease activity indices [DAS28, Simplified Disease Activity Index (SDAI), and Clinical Disease Activity Index (CDAI)] [26, 27].
Starting from these considerations, the goal of this research is to investigate the relationship between joint inflammation and damage of the wrists and hands, measured by semiquantitative US and MRI scoring systems, with functional disability and HGs.
Methods
Design and study population
This pilot study included consecutive adult RA patients, defined according to the 2010 American of College of Rheumatology (ACR)/EULAR criteria [28], with an active disease, as defined by DAS28 C-reactive protein (DAS28-CRP) > 3.2, independently of current therapy. Exclusion criteria were represented by the coexistence of comorbid conditions able to interfere with the clinical and HGs assessment: coexisting fibromyalgia, hearth failure, severe chronic obstructive pulmonary disease, multiple sclerosis, Alzheimer disease, extracorporeal dialysis, active neoplasms, or persistent infectious diseases.
Demographics, clinical and composite disease activity assessment
Data on demographic characteristics and all core-set variables were extrapolated from the internal center database. These details included age, gender, and the length of the illness (defined as time since diagnosis). For each patient was collected the presence of rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), and CRP.
The following items of disease activity indices were used in clinical assessments: 28-joint counts for swollen and tender joints (SJC and TJC, respectively), patient self-administered tender joint count (self-TJC), numerical rating scale (NRS) of pain, evaluator, and patient assessments of disease activity (EGA and PGA, respectively) by NRS, patient assessment of general health status by NRS (GH). Composite disease activity indices, such as the DAS28, the CDAI, and the SDAI, were calculated using these variables [29–32].
Assessment of physical functioning with patient-reported outcome measures (PROs)
All patients completed the shortened Disability of Arm, Shoulder and Hand questionnaire (QuickDASH) [33], the hand and finger function subscale of the Arthritis Impact Measurement Scale (AIMS2-HFF) [34, 35], and the upper extremity function of the ROAD questionnaire [36, 37].
The shortened Disability of Arm, Shoulder and Hand questionnaire (QuickDASH)
The QuickDASH is a patient-based outcome instrument for assessing upper extremity function [33]. It is a shortened version of the original DASH outcome measure [38], which assesses a person's capacity to execute tasks, absorb pressures, and the severity of their symptoms [39]. Compared to the original DASH outcome measure, which included 30 elements, the QuickDASH only has 11 items. The QuickDASH tool is made of 5-point Likert scales, with the patient selecting a number that corresponds to his or her severity/function level [40–42]. The given values for all completed replies are simply added together and averaged to get a five-point score. After removing one and multiplying by 25, this result is converted to a score out of 100. This adjustment is used to make the score more comparable to other 0–100 scaled measurements. A higher score implies a higher level of impairment. For this study was employed the Italian QuickDASH validated version [43].
The Hand and Finger Function subscale of the Arthritis Impact Measurement Scale (AIMS2-HFF)
The AIMS2-HFF was created with the goal of evaluating physical function in individuals with rheumatic diseases. The AIMS2 is an updated and expanded version of the AIMS, is a self-administered questionnaire that assesses three areas of one’s health: physical, psychological, and social [34]. For the purposes of this study, it has been used only the physical domain questions, namely ones concerning hand and finger function. On a 5-point Likert scale, the patients were asked how often they experienced impaired hand and finger function when completing five particular tasks: writing with a pen or pencil; buttoning up a shirt; turning a key; tying knots or shoelaces; and opening a jar within the preceding four weeks. Each of the item’s scores, which ranged from 1 (every day) to 5 (never), were combined to create a total score, which ranged from 0 (indicating excellent function) to 10 points (representing poor function). The Italian validated version of AIMS2 demonstrated good metrologic properties [35, 44].
The Recent-Onset Arthritis Disability (ROAD) questionnaire
The ROAD is a valid, reliable, and responsive instrument for assessing physical function in RA patients [36, 37]. The ROAD is a 12-item questionnaire and contains questions about fine upper extremity movements, lower extremity activities, and tasks that include both upper and lower extremities. Patients are asked to rate the amount of difficulty during the previous week on a 5-point scale ranging from 0 (no difficulty) to 4 (extreme difficulty, unable to do). The ROAD score has a range of 0 to 48, with a simple mathematical normalizing process the score is converted to a 0–10 scale (higher scores indicating worse physical function). A previous study using both classical test theory and Rasch analysis methods supported the use of separate sub-scores for upper limb function, lower limb function, and activities of daily living/work [45]. In this study, only the ROAD upper extremity function sub-score was calculated.
Assessment of handgrip strength (HGs)
HGs was assessed using a cylindrical-shape grip device with five force sensors (FSR-402, interlink electronics and connected to an Arduino Mega 2560). This instrument records peak force data as a continuous acquisition within a frame of 30 s with one measurement per sensor every 5 s, providing information on maintained grip rather than a single-time peak of grip force [26, 46]. HGs was measured twice in the dominant hand, with the average of the two results utilized, and with a 5 min interval between the two measurements to recover from muscle weariness [47]. For subject placement, the American society of hand therapist’s instructions were followed [48] (Fig. 1).
Fig. 1.
Assessment of handgrip strength (HGs). In the seated position, HGs was measured with the shoulder adducted and neutrally rotated. The wrist was slightly extended, the elbow was flexed to roughly 90 degrees, and the forearm was in neutral. Instructions were presented in a consistent manner
Joint inflammation and damage assessment
Semiquantitative assessments of joint inflammation and damage were carried out using US and MRI scoring techniques such as US-CLARA [27] and mod SAMIS [21].
The UltraSound-CLinical ARthritis Activity index (US-CLARA)
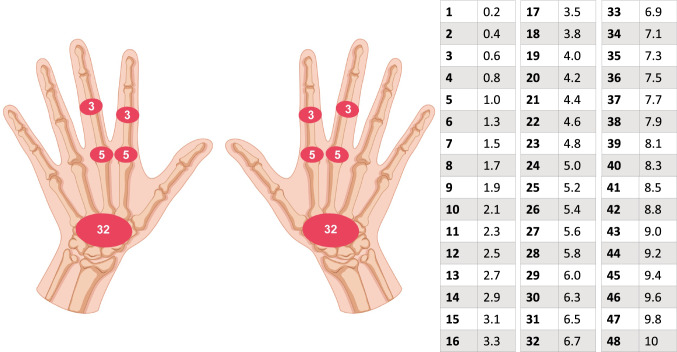
The US-CLARA is a composite index that combines the ROAD [36, 37, 45], a self-administered TJC, and the US semiquantitative evaluation into a single disease activity measure. Its total score ranges from 0 to 10 and was calculated by adding the scores of the three distinct metrics and dividing by three [27]. The self-administered TJC is that of RA Disease Activity Index (RADAI) [49]. The US examination includes multiplanar gray scale (GS) and power Doppler (PD) dorsal scans of both wrists and hands, examining the following joints: radiocarpal, 2nd and 3rd metacarpophalangeal (MCP), and 2nd and 3rd proximal interphalangeal (PIP) (Fig. 2). The results of the US exams are weighted by joint area according to Thompson’s articular index [50] and then normalized on a scale of 0–10. For US-CLARA, the following interpretability cutoff values have been proposed: remission (REM) US-CLARA < 2.0; low disease activity (LDA) 2.0 ≤ US-CLARA < 3; moderate disease activity (MDA) 3 ≤ US-CLARA ≤ 4.8; high disease activity (HDA) US-CLARA > 4.8. The detailed description of the index is provided in the original paper [27].
Fig. 2.
The UltraSound-CLinical ARthritis Activity (US-CLARA) scoring spreadsheet. Weight of each joint according to the Thompson's articular index and nomogram. The US final score is the sum of the weights of the joints of both hands divided by two (range 0–48), the value is normalized from a 0–48 scale to a 0–10 scale, using the nomogram
MRI scanning and modified SAMIS simplified score (mod SAMIS)
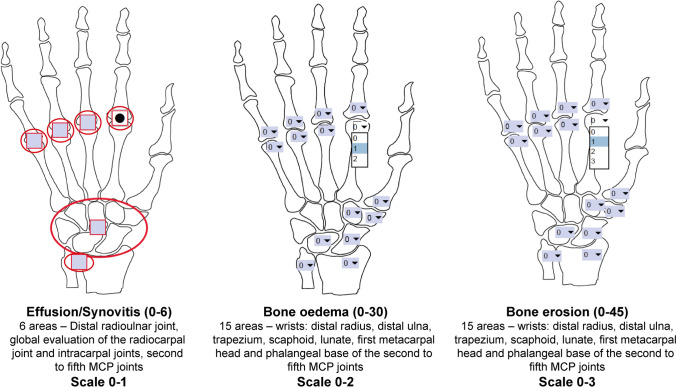
The MRI was performed using a 1.5 T Impact MRI device (Achieva Philips Medical Systems, Best, The Netherlands) with four phased-arrived coils that were exclusively received. The sequences were taken without the use of contrast and are detailed in Table 1. The SAMIS was realized to reduce MRI scoring time while maintaining correlation with the OMERACT RA-MRI scoring system and similar or superior intra- and inter-reader reliability [21, 51]. SAMIS evaluates only one hand and was based on the radiographic Simple Erosion Narrowing Score (SENS) [52], reducing the number of study areas from 116 to 36. Erosions were scored with a scale from 1 to 10. Edema and synovitis were scored with scales from 0 to 2 and 0 to 1, respectively. The scoring system can be distinct in three sub-scores evaluating the presence/absence of synovitis (SAMIS synovitis), semiquantitative ratings for bone erosion (SAMIS-ERO), and bone marrow edema (SAMIS-BME), without contrast injection (Fig. 3).
Table 1.
MRI sequence details
| Sequence plane | Parameters |
|---|---|
| STIR coronal | FOV = 160 × 140, Matrix = 232 × 147, NEX = 3, slice thickness = 3 mm, gap = 0,3 mm, TR = 2500 ms, TE = 60 ms, TI = 140 ms |
| T1 TSE coronal | FOV = 260 × 140, Matrix = 520 × 220, NEX = 3, slice thickness = 3 mm, gap = 0,3 mm, TR = 566 ms, TE = 10 ms, flip angle = 90° |
| T1 TSE sagittal | FOV = 260 × 110, Matrix = 520 × 170, NEX = 3, slice thickness = 3 mm, gap = 0,3 mm, TR = 566 ms, TE = 10 ms, flip angle = 90° |
| STIR axial | FOV = 100 × 138, Matrix = 124 × 132, NEX = 2, slice thickness = 4 mm, gap = 0,4 mm, TR = 3684 ms, TE = 60 ms, TI = 140 ms |
| T2 TSE axial | FOV = 100 × 140, Matrix = 200 × 190, NEX = 3, slice thickness = 4 mm, gap = 0,4 mm, TR = 2760 ms, TE = 90 ms, flip angle = 90° |
| T2 GRE coronal | FOV = 260 × 160, Matrix = 520 × 257, NEX = 1, slice thickness = 3 mm, gap = 0,3 mm, TR = 450 ms, TE = 12 ms, flip angle = 90° |
FOV field of view; NEX number of excitations; SE spin echo, TR repetition time; TE echo time; TI inversion time; T1 TSE T1-weighted turbo spin echo; STIR short tau inversion recovery; T2 GRE T2-weighted gradient echo
Fig. 3.
The modified simplified SAMIS magnetic resonance score (mod SAMIS) scoring spreadsheet. The MRI was graded for the presence/absence of synovitis and semiquantitative ratings of bone marrow edema and bone erosion, without contrast injection
Only one hand was assessed by MRI. If both hands were equally painful it was evaluated the dominant one. According to van der Heijde radiographic scoring system, several bones were ruled out for ERO and BME [53]. The metacarpal head and phalangeal base from the 2nd to the 5th MCP joints, as well as the first metacarpal base, trapezium, scaphoid, lunate, and distal end of both the ulna and radius, were also investigated. ERO was scored in proportion of eroded bone to the measured bone volume on a 0–3 scale: (0) no erosion; (1) 11 to 33% of bone eroded; (2) 33 to 66% of bone eroded; (3) more than 66% of bone eroded. A BME scale ranging from 0 to 2 was applied to rate the fraction of bone that was edematous: 0 for normal bone, 1 for mild BME, and 2 for severe BME. Synovitis manifests itself as a region in the synovial compartment with an elevated signal on T2-weighted fat-suppressed images and a thickness higher than the typical synovium’s breadth. The distal radioulnar joint, the radiocarpal and intracarpal joints, and the 2nd to 5th MCP joints were all evaluated for the presence or absence of synovitis without being rated.
To determine interobserver variability, two radiologists (L.C., M.C.), who were blinded to clinical information and the other reader’s scoring, independently evaluated 22 cases within the same period by using MR imaging definitions of synovitis, BME and ERO, in accordance with the OMERACT RAMRIS recommendations [20].
Statistical analysis
The data was input into a Microsoft Excel database and analyzed with MedCalc® 64-bit version 19.0.1.0. (MedCalc Software, Mariakerke, Belgium). The sample size for a pilot trial like this one should be at least 40 patients, according to standards (54). Where available, median and interquartile ranges, as well as means and standard deviations, are displayed (SD). Considering that data were not normally distributed (Kolmogorov–Smirnov test for normal distribution), nonparametric procedures have been used in order to provide a more cautious estimate of statistical significance.
Data deriving from US and MRI have been compared with the other clinimetric measurements employed in the study (DAS28-CRP, SDAI, CDAI, ROAD upper extremity function, AIMS2-HFF, QuickDASH, HGs). To quantify the correlations, it has been used the Spearman’s rho correlation coefficient, interpreted as follows: below 0.19 very weak; 0.20–0.39 weak; 0.40–0.59 moderate; 0.60–0.79 strong, above 0.79 very strong.
Intraclass correlation coefficient (ICC) average values with 95% confidence intervals (CIs) were used to report interobserver agreement. The ICC was considered as excellent if above 0.75, as fair to good if between 0.4 and 0.75, and as poor if below 0.4 [54].
Results
Demographic and pharmacological data
The study included 66 RA patients. The case study was composed mostly by middle-aged females [58 (87.9%), mean ± SD age 55.5 ± 12.2 years], with 10.6 ± 3.6 years of formal education, a mean disease duration of 4.4 ± 3.0 years. Fifty-seven (86.4%) patients were RF positive and 54 (81.8%) ACPA positive. The mean number of comorbidities was 1.7 comorbidities. All of the patients received at least one conventional disease-modifying anti-rheumatic drug (csDMARD) (methotrexate, leflunomide, sulfasalazine, or hydroxychloroquine) and/or a biological DMARD [39 (59.1%) patients, respectively, 15 (38.5%) adalimumab, 12 (30.8%) etanercept, 5 (12.8%) abatacept, 4 (10.2%) golimumab, and 3 (7.7%) tocilizumab]. Twenty-nine (43.9%) patients were taking oral corticosteroids, with a mean prednisone or equivalent dosage of 3.9 mg/day (range 2.5–25).
Clinimetric and instrumental evaluation
The mean values (± SD) of DAS28, CDAI, and SDAI were, respectively, 4.54 ± 0.56, 35.92 ± 16.13, and 43.14 ± 14.91. The mean values of HGs peak grip force, ROAD upper extremity function, and QuickDASH, respectively, are 20.35 ± 9.17, 5.35 ± 2.80, and 26.66 ± 11.88. The SAMIS-ERO mean score was 18.28 ± 9.40. For the SAMIS-BME the mean score was 7.77 ± 4.90. The lunate, the capitate, the triquetrum, the hamate, the distal ulna, and the radius had the greatest SAMIS-ERO score, whereas the 2nd phalangeal base had the highest SAMIS-BME score, followed by the lunate, the capitate, the 4th metacarpal base, and the triquetrum. The SAMIS-synovitis mean score was 3.55 ± 1.87. The following bones were studied: distal radioulnar joint, global evaluation of the radiocarpal joint and intracarpal joints, second to fifth MCP joints. Mod SAMIS total score and US-CLARA mean values were determined to be 29.61 ± 11.29 and 5.76 ± 2.02, respectively. Table 2 lists the baseline characteristics of the 66 RA patients who took part in the trial.
Table 2.
Demographic, clinical, and instrumental characteristics of 66 RA patients
| Mean | Median | SD | Q25–Q75 | |
|---|---|---|---|---|
| Age (years) | 55.56 | 57.50 | 12.21 | 47.00–65.00 |
| Educational level (years) | 10.59 | 10.00 | 3.64 | 6.50–13.00 |
| Disease duration (years) | 4.43 | 4.00 | 3.00 | 3.00–7.00 |
| Number of comorbidity | 1.71 | 1.00 | 1.51 | 1.00–2.00 |
| CRP (mg/dl) | 4.47 | 3.41 | 3.56 | 2.20–4.84 |
| TJC (0–28) | 13.43 | 11.00 | 8.21 | 6.00–20.00 |
| SJC (0–28) | 9.59 | 8.00 | 6.45 | 4.00–14.00 |
| GH (0–100) | 70.07 | 80.00 | 27.25 | 50.00–90.00 |
| DAS28-CRP (0–10) | 4.54 | 4.60 | 0.56 | 4.09–4.99 |
| CDAI (0–68) | 35.92 | 33.00 | 16.13 | 23.00–49.00 |
| SDAI (0–78) | 43.14 | 42.05 | 14.92 | 30.55–55.66 |
| HGs peak grip force (kg) | 20.35 | 16.77 | 9.17 | 11.82–30.27 |
| ROAD upper extremity function (0–10) | 5.35 | 6.00 | 2.80 | 3.00–7.50 |
| QuickDASH (0–100) | 26.66 | 24.00 | 11.89 | 14.00–37.00 |
| AIMS2-HFF | 4.02 | 4.00 | 1.90 | 2.50–5.00 |
| mod SAMIS-BME (0–30) | 7.77 | 7.91 | 4.90 | 3.00–12.00 |
| mod SAMIS-ERO (0–45) | 18.28 | 18.61 | 9.40 | 12.00–22.00 |
| mod SAMIS synovitis (0–6) | 3.55 | 3.29 | 1.87 | 2.00–5.00 |
| mod SAMIS total score (0–81) | 29.61 | 30.00 | 11.29 | 21.50–36.00 |
| US-CLARA (0–10) | 5.76 | 6.53 | 2.02 | 3.76–7.30 |
CRP C-reactive protein; TJC tender joint count; SJC swollen joint count; GH general health status; DAS28 Disease Activity Score 28 joints; CDAI Clinical Disease Activity Index; SDAI Simplified Disease Activity Index; HGs handgrip strength; ROAD Recent-Onset Arthritis Disability questionnaire; QuickDASH shortened Disability of Arm, Shoulder and Hand questionnaire; AIMS2-HFF Arthritis Impact Measurement Scale Hand and Finger Function; mod SAMIS modified Simplified Rheumatoid Arthritis Magnetic Resonance Imaging Score; BME bone marrow edema; ERO erosion; US-CLARA UltraSound-CLinical ARthritis Activity index
Correlations and interobserver agreement
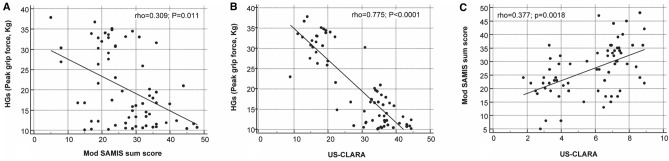
US-CLARA was strongly and negatively correlated to the HGs, more than the mod SAMIS total score (rho 0.775; p < 0.0001 vs. rho 0.309; p = 0.011) (Fig. 4a, b). The mean HGs were very strongly and inversely correlated with the mod SAMIS-BME (rho 0.815; p < 0.0001), as well as with the mod SAMIS-synovitis score (rho 0.815; p < 0.0001).
Fig. 4.
Scatterplot with linear regression lines displays the relationship between A mod SAMIS total score versus HGs, B US-CLARA versus HGs, and C US-CLARA versus mod SAMIS total score. The values of one variable appear on the horizontal axis, and the values of the other variable appear on the vertical axis. Each individual in the data appears as a point on the graph
A weak correlation was observed between mod SAMIS total score and US-CLARA (rho 0.377; p = 0.0018) (Fig. 4c).
The SAMIS-BME and SAMIS-synovitis scores were also linked with hand-specific self-report questionnaires (p < 0.05) as well as composite disease activity indicators such DAS28-CRP, CDAI, and SDAI (p < 0.0001). SAMIS-ERO had no relationship with HGs, functional impairment as determined by hand-specific self-report questionnaires, or composite disease activity indices. The mean HGs had a significant relationship with the values of hand-specific self-report questionnaires (p < 0.0001), as well as composite disease activity indices (p < 0.0001). Table 3 summarizes all the correlations studied.
Table 3.
Spearman’s correlation coefficients among mod SAMIS scores, US-CLARA, HGs, disease composite activity indices, and hand-specific self-report questionnaires in patients with RA
| mod SAMIS-BME | mod SAMIS synovitis | mod SAMIS total score | US-CLARA | HGs peak grip force (kg) | CDAI | DAS28-CRP | SDAI | AIMS2 hand finger function | Quick DASH | ROAD upper extremity function | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mod SAMIS-ERO |
0.042 0.7335* |
− 0.174 0.1560* |
0.798 < 0.0001* |
0.054 0.6680* |
0.107 0.3925* |
− 0.010 0.9393* |
− 0.030 0.8087* |
− 0.135 0.2794* |
− 0.079 0.5290* |
− 0.119 0.3393* |
− 0.094 0.4517* |
| mod SAMIS-BME |
0.626 < 0.0001* |
0.477 < 0.0001* |
0.799 < 0.0001* |
− 0.815 < 0.0001* |
0.678 < 0.0001* |
0.715 < 0.0001* |
0.588 < 0.0001* |
0.345 0.0046* |
0.312 0.0109* |
0.809 < 0.0001* |
|
| mod SAMIS synovitis |
0.288 0.0172* |
0.539 < 0.0001* |
− 0.711 < 0.0001* |
0.489 < 0.0001* |
0.532 < 0.0001* |
0.453 0.0001* |
0.256 0.0383* |
0.395 0.0010* |
0.610 < 0.0001* |
||
| mod SAMIS total score |
0.377 0.0018* |
− 0.309 0.0116* |
0.307 0.0123* |
0.290 0.0184* |
0.146 0.2432* |
0.117 0.3487* |
0.053 0.6709* |
0.290 0.0183* |
|||
| US-CLARA |
− 0.775 < 0.0001* |
0.671 < 0.0001* |
0.750 < 0.0001* |
0.566 < 0.0001* |
0.479 < 0.0001* |
0.406 0.0007* |
0.804 < 0.0001* |
||||
| HGs peak grip force (kg) |
− 0.691 < 0.0001* |
− 0.717 < 0.0001* |
− 0.586 < 0.0001* |
− 0.397 0.0010* |
− 0.413 0.0006* |
− 0.872 < 0.0001* |
|||||
| CDAI |
0.885 < 0.0001* |
0.867 < 0.0001* |
0.391 0.0012* |
0.407 0.0007* |
0.713 < 0.0001* |
||||||
| DAS28-CRP |
0.838 < 0.0001* |
0.403 0.0008* |
0.486 < 0.0001* |
0.706 < 0.0001* |
|||||||
| SDAI |
0.311 0.0111* |
0.368 0.0023* |
0.616 < 0.0001* |
||||||||
| AIMS2-HFF |
0.211 0.0889* |
0.428 0.0003* |
|||||||||
| Quick-DASH |
0.389 0.0012* |
mod SAMIS modified Simplified Rheumatoid Arthritis Magnetic Resonance Imaging Score; BME bone marrow edema; ERO erosion; US-CLARA UltraSound-CLinical ARthritis Activity index; HGs handgrip strength; CDAI Clinical Disease Activity Index; DAS28-CRP Disease Activity Score 28 joints C-reactive protein; SDAI Simplified Disease Activity Index; AIMS2-HFF Arthritis Impact Measurement Scale Hand and Finger Function; QuickDASH shortened Disability of Arm, Shoulder and Hand questionnaire; ROAD Recent-Onset Arthritis Disability questionnaire. Legend: *=p values.
Interobserver agreement was good to excellent for each of the three characteristics (ICC = 0.71, 0.91, and 0.82, respectively, for SAMIS synovitis, SAMIS-BME, and SAMIS-ERO).
Discussion
In this study, correlations between objective measures of joint inflammation versus HGs and patient-reported measures of function have been demonstrated.
Hand function is generally impaired in the majority of patients with RA. Nevertheless, current recommendations for monitoring disease activity are limited to joint counts, without the inclusion of objective assessment of hand function. Pain and functional limitation may persist despite optimal monitoring of signs of joint inflammation and disease activity [55]. On the other hand, patient-reported measures are increasingly seen as potentially more reliable than physician-reported measures or laboratory parameters in predicting long-term disease outcomes [56–59]. In this perspective, PROs dedicated to the hand and upper limb have proven to be valid and accurate in assessing the disability of patients with RA [33–35, 45, 60]. There is also evidence that in patients with RA in DAS28 remission or with low compromise of ADLs according to the health assessment questionnaire, HGs is considerably impaired in some cases. HGs is therefore not fully included in traditional indices of disease activity. HGs has been demonstrated to correlate significantly with measures of hand function in patients with RA [61], including the DASH and the AIMS [62, 63]. Reduced HGs has been recognized as a strong predictor of multi-morbidity, disability, and mortality, and is a major contributor to frailty and sarcopenia in young old (aged ≥ 65) and very old adults (aged ≥ 85) [26, 64–67].
Studies have shown both MRI and US to be highly sensitive in assessing the inflammation of joints [68]. However, US is unable to represent BME, a robust predictor of bone damage and disease progression. It may also fail to adequately evaluate certain joint portions [69], and is operator dependent. US has the advantage of being cheaper and more readily available compared to MRI. MRI has the benefit of greater articular coverage and BME detection, but is more expensive and less accessible in a resource-constrained setting [20, 51, 70, 71]. Among patients with active early RA, high levels of objective MRI–detected inflammation at baseline are indicative of which patients are more likely to achieve clinical remission with treatment [72]. For a standardized and easily applicable MRI assessment system, it has developed a scoring system, the EULAR-OMERACT RAMRIS, which includes semiquantitative scores for bone erosion, BME, and synovitis of the wrist and MCP joints [73]. RAMRIS has been simplified in SAMIS in order to overcome the time-consuming aspects and the long learning curve [21, 74]. The modified SAMIS employed for this study graded MRI for the presence/absence of synovitis (SAMIS synovitis) and semiquantitative ratings of bone erosion (SAMIS-ERO) and bone marrow edema (SAMIS-BME), without contrast injection. To save more time, we chose to only assess the one most painful or the dominant hand. MRI, regardless of whether it covers unilateral wrist and MCP joints or bilateral wrists and MCP joints plus unilateral metatarsophalangeal joints, is significantly superior to conventional radiography for the detection of progressive joint destruction in RA [75]. Sufficient reproducibility is a prerequisite for any scoring method: the proposed mod SAMIS had excellent inter-reader reliability.
Several studies have investigated the extent to which RA joint pathologies could be reliably assessed with unenhanced MRI images rather than with gadolinium (Gd)-enhanced MRI (as the reference method) to reduce the imaging time, invasiveness, and cost. Gd is generally recommended for MRI assessment of RA joint changes, particularly synovitis. Previous studies found that the Gd contrast administration for the MRI procedure did not change the scores of the bone erosion and bone edema but decreased the reliability of the synovitis scores [76–78]. Based on these observations, in this study it has been merely looked at the presence or absence of synovitis without rating it. This could be considered a limitation of the present study, however the use of Gd markedly prolongs the examination time and increases costs, invasiveness, and patient discomfort, and thereby reduces the feasibility of MRI in RA.
There are limitations to mention regarding this study. First, the statistical power was limited by the small sample size of 66 RA patients. Second, there is theoretical concern that generalizability of the mod SAMIS score may be hampered by difficulties in scoring the foot. However, for each of the three features (synovitis, BME, and erosion), interobserver agreement was good to excellent, and this suggests that this generalizability can be applied to other joint regions as well.
In conclusion, BME and synovitis have an influence on the function of the upper extremities. The US-CLARA and the mod SAMIS total score are intriguing options for semiquantitative assessment of joint inflammation and damage in RA, and they are currently being investigated. These shorter scores may reduce the amount of time required for image processing in US and MRI-controlled RA investigations, as well as make the use of these imaging modalities in RA therapy response assessment studies more straightforward. Further longitudinal studies, with larger numbers of patients and using various MRI and US scoring systems, are needed to prove that these methods are universally useful.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by FS, MC, LC, and SF. The first draft of the manuscript was written by FS, MC, MDC, SF, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This observational study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and was approved by the local ethics committee (Comitato Unico Regionale—ASUR Marche, No 2015 0458 AS).
Informed consent
Informed consent was obtained from all participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fausto Salaffi, Email: fausto.salaffi@gmail.com.
Marina Carotti, Email: marina.carotti@gmail.com.
Marco Di Carlo, Email: dica.marco@yahoo.it.
Luca Ceccarelli, Email: luca.ceccarelli28@gmail.com.
Sonia Farah, Email: sonia.farah91@gmail.com.
Andrea Giovagnoni, Email: a.giovagnoni@univpm.it.
References
- 1.Helliwell PS, Jackson S. Relationship between weakness and muscle wasting in rheumatoid arthritis. Ann Rheum Dis. 1994;53:726–728. doi: 10.1136/ard.53.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee P, Baxter A, Dick WC, Webb J. An assessment of grip strength measurement in rheumatoid arthritis. Scand J Rheumatol. 1974;3:17–23. doi: 10.3109/03009747409165124. [DOI] [PubMed] [Google Scholar]
- 3.Trieb K. Treatment of the wrist in rheumatoid arthritis. J Hand Surg. 2008;33:113–123. doi: 10.1016/j.jhsa.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Fiebert IM, Roach KE, Armstrong T, Mandel DW, Donohue M. Dynamometric grip strength assessment of subjects sixty year and older. Phys Occup Ther Geriatr. 1995;13:27–40. doi: 10.1080/J148v13n04_03. [DOI] [Google Scholar]
- 5.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordenskiold U, Grimby G. Assessments of disability in women with rheumatoid arthritis in relation to grip force and pain. Disabil Rehabil. 1997;19:13–19. doi: 10.3109/09638289709166440. [DOI] [PubMed] [Google Scholar]
- 7.Bodur H, Yilmaz O, Keskin D. Hand disability and related variables in patients with rheumatoid arthritis. Rheumatol Int. 2006;26:541–544. doi: 10.1007/s00296-005-0023-1. [DOI] [PubMed] [Google Scholar]
- 8.Colebatch AN, Edwards CJ, Ostergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–814. doi: 10.1136/annrheumdis-2012-203158. [DOI] [PubMed] [Google Scholar]
- 9.Salaffi F, Filippucci E, Carotti M, et al. Inter-observer agreement of standard joint counts in early rheumatoid arthritis: a comparison with grey scale ultrasonography—A preliminary study. Rheumatology. 2008;47:54–58. doi: 10.1093/rheumatology/kem286. [DOI] [PubMed] [Google Scholar]
- 10.Qvistgaard E, Rogind H, Torp-Pedersen S, Terslev L, Danneskiold-Samsoe B, Bliddal H. Quantitative ultrasonography in rheumatoid arthritis: evaluation of inflammation by Doppler technique. Ann Rheum Dis. 2001;60:690–693. doi: 10.1136/ard.60.7.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carotti M, Salaffi F, Morbiducci J, et al. Colour Doppler ultrasonography evaluation of vascularization in the wrist and finger joints in rheumatoid arthritis patients and healthy subjects. Eur J Radiol. 2012;81:1834–1838. doi: 10.1016/j.ejrad.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Ten Cate DF, Luime JJ, Swen N, et al. Role of ultrasonography in diagnosing early rheumatoid arthritis and remission of rheumatoid arthritis—A systematic review of the literature. Arthritis Res Ther. 2013;15:1–9. doi: 10.1186/ar4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bøyesen P, Haavardsholm EA, Ostergaard M, van der Heijde D, Sesseng S, Kvien TK. MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis. 2011;70:428–433. doi: 10.1136/ard.2009.123950. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson RJ, O’Connor P, Moots R. MRI of rheumatoid arthritis image quantitation for the assessment of disease activity, progression and response to therapy. Rheumatology. 2008;47:13–21. doi: 10.1093/rheumatology/kem250. [DOI] [PubMed] [Google Scholar]
- 15.Sewerin P, Buchbender C, Vordenbaumen S, et al. Advantages of a combined rheumatoid arthritis magnetic resonance imaging score (RAMRIS) for hand and feet: does the RAMRIS of the hand alone underestimate disease activity and progression? BMC Musculoskelet Disord. 2014;15:104. doi: 10.1186/1471-2474-15-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haavardsholm EA, Bøyesen P, Østergaard M, Schildvold A, Kvien TK. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis. 2008;67:794–800. doi: 10.1136/ard.2007.071977. [DOI] [PubMed] [Google Scholar]
- 17.Emery P, van der Heijde D, Østergaard M, et al. Exploratory analyses of the association of MRI with clinical, laboratory and radiographic findings in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70:2126–2130. doi: 10.1136/ard.2011.154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolen JS, Avila JC, Aletaha D. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction. Ann Rheum Dis. 2012;71:687–693. doi: 10.1136/annrheumdis-2011-200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Døhn UM, Ejbjerg BJ, Hasselquist M, et al. Detection of bone erosions in rheumatoid arthritis wrist joints with magnetic resonance imaging, computed tomography and radiography. Arthritis Res Ther. 2008;10:R25. doi: 10.1186/ar2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Østergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–1386. [PubMed] [Google Scholar]
- 21.Cyteval C, Miquel A, Hoa D, Daures JP, Mariette X, Combe B. Rheumatoid arthritis of the hand: monitoring with a simplified MR imaging scoring method–preliminary assessment. Radiology. 2010;256:863–869. doi: 10.1148/radiol.10091759. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino MA, Terslev L, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 1: definition and development of a standardised, consensus-based scoring system. RMD Open. 2017;3:e000428. doi: 10.1136/rmdopen-2016-000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terslev L, Naredo E, Aegerter P, et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce-Part 2: reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open. 2017;3:e000427. doi: 10.1136/rmdopen-2016-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodworth TG, Morgacheva O, Pimienta OL, Troum OM, Ranganath VK, Furst DE. Examining the validity of the rheumatoid arthritis magnetic resonance imaging score according to the OMERACT filter-a systematic literature review. Rheumatology. 2017;56:1177–1188. doi: 10.1093/rheumatology/kew445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boers M, Kirwan JR, Wells G, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67:745–753. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Salaffi F, Carotti M, Farah S, Ceccarelli L, Di Carlo M. Handgrip strength features in rheumatoid arthritis patients assessed using an innovative cylindrical-shaped device: relationships with demographic, anthropometric and clinical variables. J Med Syst. 2021;45:100. doi: 10.1007/s10916-021-01778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salaffi F, Di Carlo M, Iannone F, et al. The UltraSound-CLinical ARthritis Activity (US-CLARA) index: properties of a new composite disease activity index for rheumatoid arthritis. Semin Arthritis Rheum. 2018;47:619–629. doi: 10.1016/j.semarthrit.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, disease activity score (DAS) and disease activity score with 28-joint counts (DAS28), simplified disease activity index (SDAI), clinical disease activity index (CDAI), patient activity score (PAS) and patient activity score-II (PASII), routine assessment of patient index data (RAPID), rheumatoid arthritis disease activity index (RADAI) and rheumatoid arthritis disease activity index-5 (RADAI-5), chronic arthritis systemic index (CASI), patient-based disease activity score with ESR (PDAS1) and patient-based disease activity score without ESR (PDAS2), and mean overall index for rheumatoid arthritis (MOI-RA) Arthritis Care Res. 2011;63(Suppl 11):S14–S36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- 30.Prevoo MLL, van T Hof M, Kuper HH, van Leeuwen MA, van de Putte LBA, van Riel PLCM. Modified disease activity scores that include twenty-eight joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 31.Aletaha D, Smolen J. The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(5):S100–S108. [PubMed] [Google Scholar]
- 32.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42:244–257. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 33.Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87:1038–1046. doi: 10.2106/JBJS.D.02060. [DOI] [PubMed] [Google Scholar]
- 34.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded arthritis impact measurement scales health status questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 35.Salaffi F, Piva S, Barreca C, et al. Validation of an Italian version of the arthritis impact measurement scales 2 (ITALIAN-AIMS2) for patients with osteoarthritis of the knee. Gonarthrosis and quality of life assessment (GOQOLA) study group. Rheumatology. 2000;39:720–727. doi: 10.1093/rheumatology/39.7.720. [DOI] [PubMed] [Google Scholar]
- 36.Salaffi F, Bazzichi L, Stancati A, et al. Development of a functional disability measurement tool to assess early arthritis: the recent-onset arthritis disability (ROAD) questionnaire. Clin Exp Rheumatol. 2005;23:628–636. [PubMed] [Google Scholar]
- 37.Salaffi F, Stancati A, Neri R, Grassi W, Bombardieri S. Measuring functional disability in early rheumatoid arthritis: the validity, reliability and responsiveness of the recent-onset arthritis disability (ROAD) index. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S31–S42. [PubMed] [Google Scholar]
- 38.Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the disabilities of the arm, shoulder and hand outcome measure in different regions of the upper extremity. J Hand Ther. 2001;14:128–146. doi: 10.1016/S0894-1130(01)80043-0. [DOI] [PubMed] [Google Scholar]
- 39.Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (Quick DASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:1–7. doi: 10.1186/1471-2474-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bot SD, Terwee CB, van der Windt DA, Bouter LM, Dekker J, de Vet HC. Clinimetric evaluation of shoulder disability questionnaires: a systematic review of the literature. Ann Rheum Dis. 2004;63:335–341. doi: 10.1136/ard.2003.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hervás MT, Navarro Collado MJ, Peiro S, Rodrigo Pérez JL, López Matéu P, Martínez Tello I. Spanish version of the DASH questionnaire. Cross-cultural adaptation, reliability, validity and responsiveness. Med Clin. 2006;127:441–447. doi: 10.1157/13093053. [DOI] [PubMed] [Google Scholar]
- 42.Matheson LN, Melhorn M, Mayer TG, Theodore BR, Gatchel RJ. Reliability of a visual analog version of the Quick DASH. J Bone Joint Surg Am. 2006;88:1782–1787. doi: 10.2106/JBJS.F.00406. [DOI] [PubMed] [Google Scholar]
- 43.Salaffi F, Di Carlo M, Carotti M, Farah S. Validity and interpretability of the QuickDASH in the assessment of hand disability in rheumatoid arthritis. Rheumatol Int. 2019;39:923–932. doi: 10.1007/s00296-018-4216-9. [DOI] [PubMed] [Google Scholar]
- 44.Salaffi F, Stancati A, Carotti M. Responsiveness of health status measures and utility-based methods in patients with rheumatoid arthritis. Clin Rheumatol. 2002;21:478–487. doi: 10.1007/s100670200119. [DOI] [PubMed] [Google Scholar]
- 45.Salaffi F, Franchignoni F, Giordano A, Ciapetti A, Gasparini S, Ottonello M. Classical test theory and Rasch analysis validation of the recent-onset arthritis disability questionnaire in rheumatoid arthritis patients. Clin Rheumatol. 2013;32:211–217. doi: 10.1007/s10067-012-2101-6. [DOI] [PubMed] [Google Scholar]
- 46.Salaffi F, Farah S, Di Carlo M. Force-time curve features of handgrip strength in fibromyalgia syndrome. Sci Rep. 2020;10:3372. doi: 10.1038/s41598-020-60227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haidar SG, Kumar D, Bassi RS, Deshmukh SC. Average versus maximum grip strength: which is more consistent? J Hand Surg. 2004;29:82–84. doi: 10.1016/j.jhsb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Fess EE. Clinical assessment recommendations. 2. Garner NC: American Society of Hand Therapists; 1992. Grip strength; pp. 41–45. [Google Scholar]
- 49.Stucki G, Liang MH, Stucki S, Bruhlmann P, Michel BA. A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum. 1995;38:795–798. doi: 10.1002/art.1780380612. [DOI] [PubMed] [Google Scholar]
- 50.Thompson PW, Silman AJ, Kirwan JR, Currey HL. Articular indices of joint inflammation in rheumatoid arthritis. Correlation with the acute-phase response. Arthritis Rheum. 1987;30:618–623. doi: 10.1002/art.1780300603. [DOI] [PubMed] [Google Scholar]
- 51.Østergaard M, Emery P, Conaghan PG, et al. Significant improvement in synovitis, osteitis, and bone erosion following golimumab and methotrexate combination therapy as compared with methotrexate alone: a magnetic resonance imaging study of 318 methotrexate-naive rheumatoid arthritis patients. Arthritis Rheum. 2011;63:3712–3722. doi: 10.1002/art.30592. [DOI] [PubMed] [Google Scholar]
- 52.Dias EM, Lukas C, Landew R, et al. Reliability and sensitivity to change of the Simple Erosion Narrowing Score compared with the Sharp-van der Heijde method for scoring radiographs in rheumatoid arthritis. Ann Rheum Dis. 2008;67:375–379. doi: 10.1136/ard.2007.072785. [DOI] [PubMed] [Google Scholar]
- 53.van der Heijde DM, van Leeuwen MA, van Riel PL, et al. Biannual radiographic assessments of hands and feet in a three-year prospective follow-up of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26–34. doi: 10.1002/art.1780350105. [DOI] [PubMed] [Google Scholar]
- 54.Steiner S, Norman G. Health measurement scales. A practical guide to their development and use. 5. Oxford: Oxford University Press; 2003. [Google Scholar]
- 55.Ahlstrand I, Thyberg I, Falkmer T, Dahlstrom O, Bjork M. Pain and activity limitations in women and men with contemporary treated early RA compared to 10 years ago: the Swedish TIRA project. Scand J Rheumatol. 2015;44:259–264. doi: 10.3109/03009742.2014.997285. [DOI] [PubMed] [Google Scholar]
- 56.Verhoeven AC, Boers M, Van Der Linden S. Responsiveness of the core set, response criteria, and utilities in early rheumatoid arthritis. Ann Rheum Dis. 2000;59:966–974. doi: 10.1136/ard.59.12.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salaffi F, Di Carlo M, Carotti M, Farah S. The patient-reported outcomes thermometer-5-item scale (5T-PROs): validation of a new tool for the quick assessment of overall health status in painful rheumatic diseases. Pain Res Manag. 2018;2018:3496846. doi: 10.1155/2018/3496846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salaffi F, Sarzi-Puttini P, Ciapetti A, Atzeni F. Clinimetric evaluations of patients with chronic widespread pain. Best Pract Res Clin Rheumatol. 2011;25:249–270. doi: 10.1016/j.berh.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franchignoni F, Ferriero G, Giordano A, Sartorio F, Vercelli S, Brigatti E. Psychometric properties of QuickDASH—A classical test theory and Rasch analysis study. Man Ther. 2011;16:177–182. doi: 10.1016/j.math.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Rydholm M, Book C, Wikström I, Jacobsson L, Turesson C. Course of grip force impairment in patients with early rheumatoid arthritis over the first five years after diagnosis. Arthritis Care Res. 2018;70:491–498. doi: 10.1002/acr.23318. [DOI] [PubMed] [Google Scholar]
- 62.Adams J, Burridge J, Mullee M, Hammond A, Cooper C. Correlation between upper limb functional ability and structural hand impairment in an early rheumatoid population. Clin Rehabil. 2004;18:405–413. doi: 10.1191/0269215504cr732oa. [DOI] [PubMed] [Google Scholar]
- 63.Vliet Vlieland TP, van der Wijk TP, Jolie IM, Zwinderman AH, Hazes JM. Determinants of hand function in patients with rheumatoid arthritis. J Rheumatol. 1996;23:835–840. [PubMed] [Google Scholar]
- 64.Saadeddine D, Itani L, Rossi AP, Pellegrini M, El Ghoch M. Strength and performance tests for screening reduced muscle mass in elderly Lebanese males with obesity in community dwellings. Diseases. 2021;9:23. doi: 10.3390/diseases9010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salaffi F, De Angelis R, Farah S, Carotti M, Di Carlo M. Frailty as a novel predictor of achieving comprehensive disease control (CDC) in rheumatoid arthritis. Clin Rheumatol. 2021;40:4869–4877. doi: 10.1007/s10067-021-05744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salaffi F, Di Carlo M, Carotti M, Farah S, Giovagnoni A. Frailty prevalence according to the survey of health, ageing and retirement in europe-frailty instrument (SHARE-FI) definition, and its variables associated, in patients with symptomatic knee osteoarthritis: findings from a cross-sectional study. Aging Clin Exp Res. 2021;33:1519–1527. doi: 10.1007/s40520-020-01667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salaffi F, Farah S, Di Carlo M. Frailty syndrome in rheumatoid arthritis and symptomatic osteoarthritis: an emerging concept in rheumatology. Acta Biomed. 2020;91:274–296. doi: 10.23750/abm.v91i2.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–2967. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 69.McQueen F. Imaging in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2013;27:499–522. doi: 10.1016/j.berh.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 70.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, et al. Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum. 2005;52:3860–3867. doi: 10.1002/art.21493. [DOI] [PubMed] [Google Scholar]
- 71.Hirota T, Suzuki T, Ogishima H, et al. Evaluation of changes in magnetic resonance images following 24 and 52 weeks of treatment of rheumatoid arthritis with infliximab, tocilizumab, or abatacept. Mod Rheumatol. 2016;26:29–35. doi: 10.3109/14397595.2015.1069471. [DOI] [PubMed] [Google Scholar]
- 72.Ahmad HA, Baker JF, Østergaard M, et al. Baseline objective inflammation by magnetic resonance imaging as a predictor of therapeutic benefit in early rheumatoid arthritis with poor prognosis. Arthritis Care Res. 2020;72:959–964. doi: 10.1002/acr.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ejbjerg B, McQueen F, Lassere M, et al. The EULAR-OMERACT rheumatoid arthritis MRI reference image atlas: the wrist joint. Ann Rheum Dis. 2005;64(Suppl 1):i23–i47. doi: 10.1136/ard.2004.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lassere M, McQueen F, Østergaard M, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Exercise 3: an international multicenter reliability study using the RA-MRI score. J Rheumatol. 2003;30:1366–1375. [PubMed] [Google Scholar]
- 75.Ejbjerg BJ, Vestergaard A, Jacobsen S, Thomsen HS, Østergaard M. The smallest detectable difference and sensitivity to change of magnetic resonance imaging and radiographic scoring of structural joint damage in rheumatoid arthritis finger, wrist, and toe joints: a comparison of the OMERACT rheumatoid arthritis magnetic resonance imaging score applied to different joint combinations and the Sharp/van der Heijde radiographic score. Arthritis Rheum. 2005;52:2300–2306. doi: 10.1002/art.21207. [DOI] [PubMed] [Google Scholar]
- 76.Stomp W, Krabben A, van der Heijde D, et al. Aiming for a simpler early arthritis MRI protocol: can Gd contrast administration be eliminated? Eur Radiol. 2015;25:1520–1527. doi: 10.1007/s00330-014-3522-1. [DOI] [PubMed] [Google Scholar]
- 77.Ostergaard M, Conaghan PG, O'Connor P, et al. Reducing invasiveness, duration, and cost of magnetic resonance imaging in rheumatoid arthritis by omitting intravenous contrast injection–does it change the assessment of inflammatory and destructive joint changes by the OMERACT RAMRIS? J Rheumatol. 2009;36:1806–1810. doi: 10.3899/jrheum.090350. [DOI] [PubMed] [Google Scholar]
- 78.Lee KA, Min SH, Kim TH, Lee SH, Kim HR. Magnetic resonance imaging-assessed synovial and bone changes in hand and wrist joints of rheumatoid arthritis patients. Korean J Intern Med. 2019;34:651–659. doi: 10.3904/kjim.2016.271. [DOI] [PMC free article] [PubMed] [Google Scholar]