Highlights
-
•
Ultrasound-assisted chitosan grafted caffeic acid coating (USG) was applied in Trachinotus ovatus preservation.
-
•
The microbiota community of iced Trachinotus ovatus was altered by USG treatment.
-
•
USG inhibited the microbial growth and retarded the quality deterioration of Trachinotus ovatus.
Keywords: Ultrasound, Chitosan grafted caffeic acid, High-throughput sequencing technology, Microbial composition
Abstract
The effects of ultrasound-assisted chitosan grafted caffeic acid coating on the quality and microbial composition of fresh pompano (Trachinotus ovatus) fillets during ice storage for 24 days were evaluated. Samples were treated by distilled water (CK), ultrasound (US), chitosan grafted caffeic acid coating (G), and chitosan grafted caffeic acid coating with ultrasound-assisted (USG). Results showed that samples treated with USG could inhibit the formation of corrupt substances such as TVB-N, TBA, biogenic amines (BAs), hypoxanthine (Hx), and hypoxanthine riboside (HxR) when compared to the CK group. The results of high-throughput sequencing technology observed that the major bacteria genus of fresh samples was Acinetobacter. The diversity of bacterial communities at the initial stage was more diverse than that at the end of stage. With the extension of storage time, the USG treatment could maintain the microbial diversity. The dominant microbiota was Shewanella and Brochothrix in the CK group after 24 days of storage. In addition, Brochothrix in treated groups was effectively decreased. The microbial communities of samples in all treatments were changed during storage. At the end of storage, there was a significant difference in bacterial composition between the CK and treated samples, indicating that the treatment can effectively inhibit the growth of microorganisms, especially spoilage microorganisms, and reduce the quality deterioration caused by bacteria.
1. Introduction
Pompano (Trachinotus ovatus), belongs to the Carangidea family, which is one of the important commercial fish species in China, Japan, Australia and other countries [1]. It has the characteristics of delicious meat, excellent quality, fast growth, and the fact its fillet has few small bones. It is an important variety of aquaculture in China [2]. However, due to a series of microbial and endogenous biochemical reactions in fish after death, it is vulnerable to microbial corruption, which could easily lead to the deterioration of quality and shorten the shelf-life of fish [3]. The challenge of fillets during storage is how to delay and evaluate their quality change.
At present, bio-based edible coating technology is considered to be an effective and eco-friendly protection method for food preservation, and has been widely used [4]. Chitosan (CS) is an amino-polysaccharide that could be obtained by partial deacetylation of chitin [5]. Due to its natural non-toxic properties, biodegradable, bio-compatible and nontoxic, as a bio-based coating, chitosan has great application prospects in food industry [6]. However, CS coating could not be widely used because of its low antioxidant activity because there is no phenol-like functional group or conjugated structure in the molecule [7]. Caffeic acid (CA) is one of the most commonly used phenolic acids, which has been reported as a kind of phenolic acid with a high antioxidant capacity [8]. Recently, the combination of phenolic acid and CS effectively enhanced the antioxidant and antibacterial activities of CS coatings, and further used CS graft copolymer for food preservation [9], [10]. In our previous study, CS-g-CA was attained by 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydro (EDC)/N-hydroxy succinimide (NHS) coupling reaction, which displayed superior antioxidant and antimicrobial activities [11].
At present, static coating method of immersion or impregnation is mostly used to preserve fish, but the coating efficiency is low. Ultrasound (US) is a eco-friendly and non-thermal technology to reach a variety of uses, such as defoaming, crystallization, thawing and so on [4]. Meanwhile, US technology has broad application prospects in food processing, preservation and safety [12]. US could inhibit some enzymes and inactivate microorganisms in food preservation. Compared to conventional method, ultrasound induces mechanical, physical and biochemical changes through cavitation, so as to improve the quality of aquatic products, destroy microbial cells and alter the structural and functional properties of proteins [13]. At present, the application of US to prolong the shelf-life of food has received extensive attention. Wang et al. [14] reported that US treatment could significantly promote the mass transfer of water and improved the salted-dried grass carp's appeal for customers. Lan et al. [15] found that US incorporated with slightly acidic electrolyzed water could prolong the shelf life of sea bass for another 4 days during cold storage. Sun et al. [16] showed that chitosan nanocomposite water retaining agent combined with ultrasonic treatment can significantly reduce the quality deterioration of crayfish during frozen storage. There is little information on united technologies to extend the shelf-life of aquatic products. Therefore, the aim of this research was to research the effects of ultrasound-assisted chitosan grafted caffeic acid coating on the quality attributes and microbial composition of pompano (Trachinotus ovatus) in terms of TVB-N, TBA, nucleotides, biogenic amines, microbial enumeration and high-throughput sequencing.
2. Materials and methods
2.1. Sample preparation
The synthesis of G was performed by EDC/NHS coupling according to the previous study [11]. The grafting ratios of CS-g-CA were 93.68 mg/g.
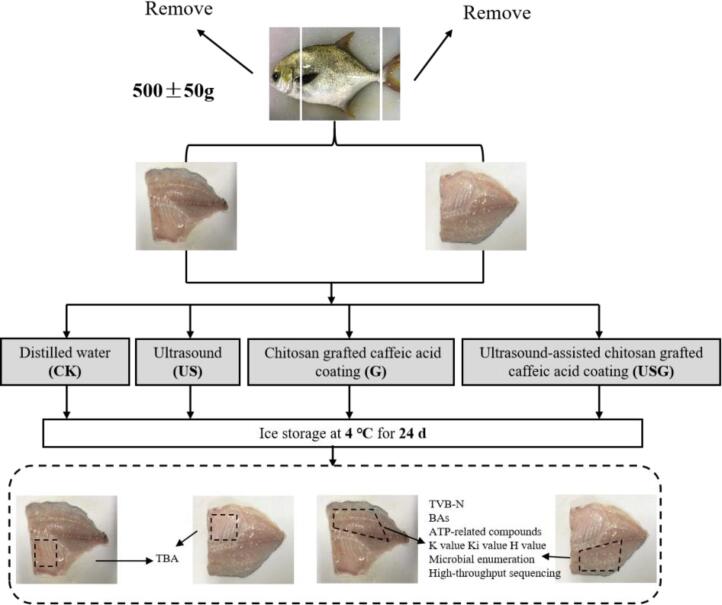
Lived pompano were bought from the local market (Shanghai, China) and transported to the laboratory alive within 30 min. The mean ± SD weight and body length of fish was 500 ± 50 g and 30 ± 5 cm, respectively. The experimental design was shown in Fig. 1. Next, the head, tail and guts were removed. Four groups of samples were prepared and the treatments were applied to the fillets with a fillet/solution ration of nearly 1:5 (m/v): (1) Distilled water (CK); (2) 20 kHz 600 W ultrasound (US); (3) 1% CS-g-CA in the 1% acetic acid solution (G); (4) 1% CS-g-CA in the 1% acetic acid solution with 20 kHz 600 W ultrasound (USG).
Fig. 1.
Schematic diagram showing the experimental design.
2.2. Total volatile basic nitrogen (TVB-N)
According to Lan et al. [17], TVB-N was measured by micro Kjeldahl nitrogen meter (FOSS, Shanghai, China). The value of TVB-N was expressed as mgN/100 g muscle.
2.3. Thiobarbituric acid reactive substances (TBA)
According to Feng et al. [18], the TBA of samples was done. 5.0 g of samples were homogenized with 10 mL of 5% trichloroacetic acid (TCA) for 2 min. The homogenate was centrifuged twice at 8500×g at 4 °C for 10 min and the filtrates were gathered and then transferred into a volumetric flask and diluted with TCA to a final volume of 50 mL. Then, 5 mL of filtrate and 0.02 mol/L TBA solution were mixed, followed by boiling in a water bath for 20 min. After cooling to room temperature, the absorbance of the final colored solution was measured at 532 nm. The results were represented by mg of malonaldehyde (MDA)/g of sample.
2.4. Adenosine triphosphate (ATP)-related compounds
ATP and its related compounds were extracted according to the previous study[19], which were measured by HPLC (LC-2010C HT, Shimadzu Corporation). The pH value of phosphate buffer as mobile phase was 6.5 and the flow rate was 1.0 mL/min. The injection volume was 10 µL and selects 254 nm as the detection wavelength. The amounts of ATP-related compounds were defined and analyzed on the basis of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), inosine monophosphate (IMP), hypoxanthine riboside (HxR), and hypoxanthine (Hx) standards (Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China). Ki, K, and H values were determined as follow:
2.5. Biogenic amines (BAs)
BAs were determined according to the method of Zhao et al. [20]. 5 g of minced flesh were homogenized with 10 mL perchloric acid (0.6 M), and the homogenate was centrifuged at 10,000×g for 5 min. The sediment was washed with 10 mL perchloric acid and centrifuged as before. The combined supernatants were adjusted to 25 mL and used for further analysis. BAs were derived with dansyl chloride (Sigma-Aldrich Trading Co., Ltd., Shanghai, China) and analyzed by HPLC. Putrescine and cadaverine were identified by comparing to external standards (Sigma-Aldrich Trading Co., Ltd., St. Louis, USA).
2.6. Microbial enumeration
All microbiological enumeration were measured by previous study [11]. Fish flesh (5 g) was put in a sterile bag and homogenized 5 min in 45 mL of 0.85% (w/v) sterilized normal saline with an Interscience Bag Mixer (HaiBo Biological Technology Co., Ltd, Qingdao, China) and then the homogenate was serially diluted. Total viable count (TVC) and Pseudomonas bacteria count were determined using plate count agar and Pseudomonas CFC Selective Agar (HaiBo Biological Technology Co., Ltd, Qingdao, China) at 30 ± 1 °C for 72 ± 3 h, and then enumerated. Psychrophilic bacteria count (PBC) was determined using the plate count agar and then enumerated after 10 days of incubation at 4 ± 1 °C. The H2S-producing bacteria count was determined using Iron Agar (HaiBo Biological Technology Co., Ltd) at 30 ± 1 °C for 72 ± 3 h. All counts were performed in triplicate and are expressed as log CFU/g.
2.7. High-throughput sequencing
Bacterial DNA was extracted using the bacterial genome extraction kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu) and high-throughput sequencing was done according to the previous method[17].
2.8. Statistical analysis
Results were analyzed with SPSS software (Version 18.0, Inc., Chicago, IL) and done with triplicate. All analysis used the least significant difference (LSD) method and Duncan’s test at a significance level of 0.05. All the results were showed as “average value ± standard deviation”.
3. Results and discussion.
2.9. TVB-N and TBA analysis
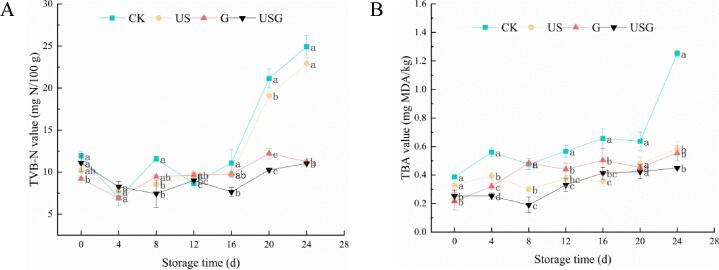
TVB-N could represent the quality of fishery products. Fig. 2 showed that the TVB-N values of fresh samples were 10.26–11.92 mg N/100 g at day 0. The increase of TVB-N values in all the samples was less visible at the first 16 days of storage. Since then, TVB-N values increased rapidly, which was consistent with the results of other studies[21]. Significant decrease of TVB-N was found after 20 days of storage in G and USG groups compared to the CK group. According to the research of Liu et al.[19], the TVB-N value of 30 mg N/100 g could be used as an acceptable threshold for the spoilage of marine fish. At day 20, the TVB-N value of CK group came up to 21.14 mg N/100 g. The TVB-N values of the US, G, and USG groups were only 19.1, 12.2 and 10.26 mg N/100 g, respectively. However, TVB-N values showed a rapid upward trend in the later storage, which was similar to the results of other studies[22]. The increase of TVB-N value is due to the increase of endogenous enzymes, spoilage bacteria and the subsequent biochemical reactions leading to the increase of microbial degradation substances. The lower content of the treated samples was mainly due to the presence of bioactive phenolic compounds in the coating, which significantly inhibited the ability of bacteria to oxidize and deaminate non-protein nitrogen compounds. It could be inferred, as an antibacterial coating, chitosan grafted caffeic acid coating could more effectively inhibit the growth of TVB-N value, suggesting that ultrasound could be used as an auxiliary means to enhance the inhibitory effect of grafts on microbial growth. Similar results showed that chitosan based coating could inhibit the production of TVB-N, and ultrasonic assisted treatment could further enhance this inhibition[4].
Fig. 2.
Effects of different treatments on the variation of TVB-N (A) and TBA (B) in Trachinotus ovatus during ice storage. Within the same storage time, different lowercase superscripts indicate significant differences (P < 0.05).
As a vital index to evaluate lipid oxidation, Changes in TBA value of all treatments were shown in Fig. 3. The initial TBA value of fresh meat was 0.22–0.39 mg MDA/kg, and there was no significant change in all treated group during the first 16 days of storage. Afterward, the TBA value of CK group sharply increased from 0.64 mg MDA/kg to final value of 1.25 mg MDA/kg. This may be due to the accumulation of lipid peroxide and peroxide degradation products during the oxidation of unsaturated fatty acids[22]. However, it could be found that the growth rate of TBA value in the CK sample was significantly higher than that in the treatments. Additionally, the USG group had the lowest growth rate with being recorded at 0.45 mg MDA/kg, it may be due to its inhibitory effect on lipid oxidation, its ability to scavenge free radicals and improve the activity of antioxidant enzymes in vitro. In addition, no statistic difference (p ≥ 0.05) of TBA value between G and USG indicated ultrasonic treatment has little positive effect on delaying lipid oxidation of fish fillets. This was consistent with the report of Shokri et al.[23], who reported that EO-chitosan coating could prevent lipid oxidation in fish samples.
Fig. 3.
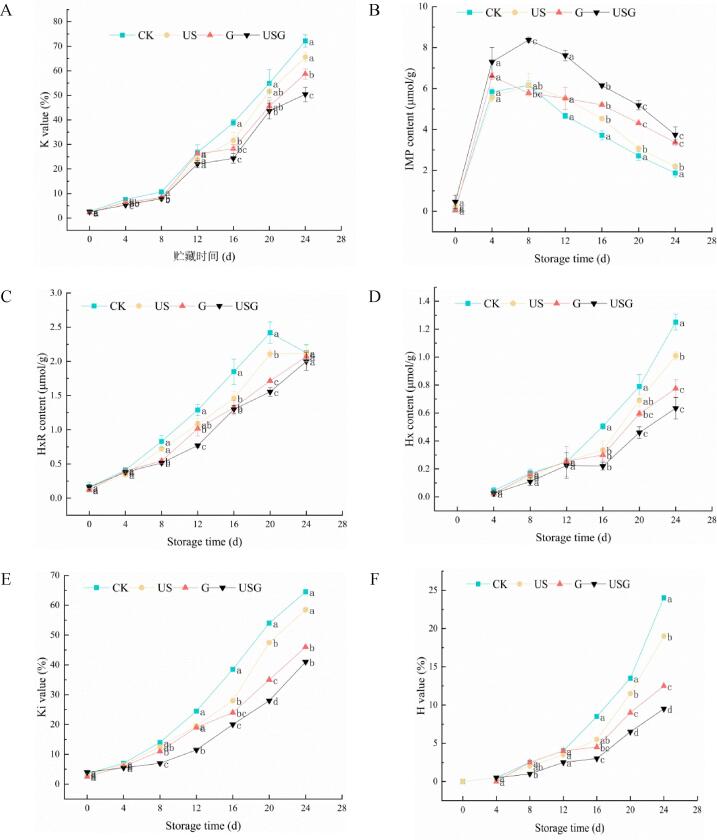
Effects of different treatments on the variation of IMP (A), HxR (B), Hx (C), Ki (D), H (E), K value (F) in Trachinotus ovatus during ice storage. Within the same storage time, different lowercase superscripts indicate significant differences (P < 0.05).
2.10. ATP-related compounds, H, Ki and K value
Generally, the important variation happened in IMP, HXR and HX, while the contents of ATP, ADP and AMP were low during the whole storage process[24]. ATP degradation could produce IMP, which is the vital flavor substance of fish and usually has a high content in early storage. With the extension of storage time, IMP was gradually degraded into bitter substances HXR and HX. As shown in Fig. 3, the initial concentrations of IMP were 0.04–0.47 μmol/g, and their concentration of all groups presented an increasing trend first and then a downward trend. IMP contents in the CK, US, and USG obtained maximum values of 6.16, 6.17, and 8.37 μmol/g at day 8, and G reached maximum value of 6.60 μmol/g at day 4. The higher IMP content in USG indicated that the active components in ultrasonic assisted chitosan grafted caffeic acid coating may reduce the activity of enzymes involved in IMP changes[24]. The remaining IMP content in the CK, US, G, and USG groups after 24 days of storage decreased to 1.87, 2.21, 3.37, and 3.75 μmol/g. The initial content of HxR was 0.13–0.16 μmol/g in fresh samples. For the CK group, the HxR content had a steep rise to 2.13 μmol/g at day 20. Meanwhile, the content of HXR decreased significantly (0.86 μmol/g) for USG compared to the CK group. The concentration of HxR decreased in the CK group at the end, because HxR decomposed into Hx. Hx of all samples presented an increasing trend during storage. Hx in the group was 0.78 and 0.64 μmol/g at day 0, which exceed 1.00 μmol/g in the CK and US groups at the end of storage.
Gao et al.[25] suggested that K value <20% indicates that the fish is very fresh, while K value >70% indicates that the fish is unacceptable. The CK showed a sharp increase of K value, exceeding the threshold at day 20, when treated groups were less than it. Besides, Ki and H value showed a similar slow increase trend in treatments. The accumulation of Hx and HxR in fish fillets would lead to a sharp increase in Ki and H values. Gao et al.[25] indicated that rosemary extract combined with nisin treatment could effectively reduce ATP loss and prolong the shelf-life of fish.
2.11. Biogenic amines
Within the same storage time, different lowercase superscripts indicate significant differences (P < 0.05).
Two vital BAs (putrescine and cadaverine) were found in fish that could test the safety and characteristics of fish. The formation of BAs in aquatic products depends on various factors, such as the free amino acid contents in fish, the presence of bacterial biogenic amine decarboxylases and favorable environmental conditions[26]. The initial putrescine and cadaverine in fresh fillets was 0.01–0.04 and 0.11–0.14 mg/kg (Table 1). With the extension of storage time, the concentration of putrescine and cadaverine rose gradually. The concentration of putrescine in the CK sample was significantly higher than those in the other samples (P < 0.05). Putrescine in the CK group increased to 3.99 mg/kg at day 24, although the concentration of USG was only 2.03 mg/kg, it was still less than that in the other samples (P < 0.05). At the beginning of storage, there was no significant difference between the treated samples and the CK sample (P > 0.05), and then the values of putrescine in the treatments was significantly lower than that in the CK sample (P < 0.05) except for day 12, which indicated that USG was more effective in the preservation of fish fillets. At the end of storage, the content of cadaverine in CK group was 2.10 mg/kg, which was significantly higher than those in treated groups (P > 0.05). However, cadaverine content in the USG group was only 0.87 mg/kg. Similarly, Zhang et al. [27] reported that putrescine and cadaverine concentrations in common carp fillets with cinnamon essential oil was lower than control group.
Table 1.
Changes in biogenic amines concentration of Trachinotus ovatus during ice storage.
| Biogenic amines (mg/kg) | Storage time(d) | CK | US | G | USG |
|---|---|---|---|---|---|
| Putrescine | 0 | 0.02 ± 0.02a | 0.01 ± 0.01b | 0.04 ± 0.03b | 0.01 ± 0.01b |
| 4 | 1.81 ± 0.09a | 1.57 ± 0.03b | 1.57 ± 0.06b | 1.54 ± 0.08b | |
| 8 | 1.89 ± 0.01a | 1.93 ± 0.08a | 1.88 ± 0.03a | 1.88 ± 0.01a | |
| 12 | 2.02 ± 0.11a | 1.80 ± 0.10a | 1.95 ± 0.06a | 1.93 ± 0.06a | |
| 16 | 2.03 ± 0.14a | 1.88 ± 0.03a | 1.93 ± 0.07a | 1.88 ± 0.06a | |
| 20 | 2.72 ± 0.09a | 2.51 ± 0.23a | 2.04 ± 0.20b | 2.04 ± 0.09b | |
| 24 | 3.99 ± 0.14a | 2.82 ± 0.23b | 2.29 ± 0.01c | 2.03 ± 0.06c | |
| Cadaverine | 0 | 0.13 ± 0.01a | 0.13 ± 0.02a | 0.14 ± 0.01a | 0.11 ± 0.02a |
| 4 | 0.17 ± 0.04a | 0.14 ± 0.01a | 0.16 ± 0.04a | 0.14 ± 0.02a | |
| 8 | 0.21 ± 0.03a | 0.20 ± 0.01ab | 0.16 ± 0.01bc | 0.15 ± 0.01c | |
| 12 | 0.26 ± 0.02a | 0.23 ± 0.01a | 0.25 ± 0.03a | 0.23 ± 0.04a | |
| 16 | 0.48 ± 0.06a | 0.36 ± 0.05b | 0.28 ± 0.01b | 0.26 ± 0.02b | |
| 20 | 1.87 ± 0.07a | 1.68 ± 0.06b | 0.60 ± 0.06c | 0.46 ± 0.04c | |
| 24 | 2.10 ± 0.12a | 1.78 ± 0.06b | 1.01 ± 0.04c | 0.87 ± 0.07c | |
2.12. Microbial enumeration
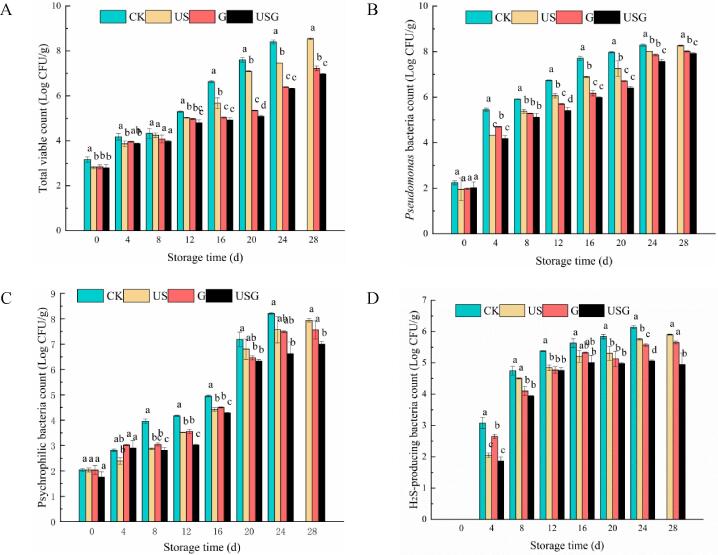
The changes in microbial enumeration of Trachinotus ovatus fillets were presented in Fig. 4. During storage, the number of microorganisms in all groups showed an increasing trend. The initial TVC was 2.80–3.17 log CFU/g. TVC reached approximately 7.60 log CFU/g in the CK group at day 20, whereas lower TVC were observed in the US, G and USG samples at the same interval (7.10, 5.35, and 5.08 log CFU/g, respectively), indicating that G was effective in delaying microbial growth, and the effects could be further enhanced using US assisted. According to TVC of 7 log CFU/g as the standard of corruption[20]. The TVC in the CK, US, and G groups reached the acceptance value at day 20 20, and 28, respectively, whereas USG was always acceptable during storage, which was showing no difference with the TVB-N and TBA value results. Therefore, the USG group had longer shelf-life (28 days) than the CK group (20 days).
Fig. 4.
Effects of different treatments on the variation of total viable count (A), Pseudomonas bacteria count (B), psychrophilic bacteria count(C), and H2S-producing bacteria count (D) in Trachinotus ovatus during ice storage. Within the same storage time, different lowercase superscripts indicate significant differences (P < 0.05).
The production of off-odor and harmful metabolites was related to Pseudomonas and H2S-producing bacteria[24]. The growth trend for the counts of psychrophilic bacteria, Pseudomonas bacteria and H2S-producing bacteria was agreement with TVC. The initial counts of psychrophilic bacteria and Pseudomonas bacteria were 1.76–2.04, and 2.02–2.24 log CFU/g. H2S-producing bacteria were not found in all groups at day 0. H2S-producing bacteria were first detected in all groups at day 4. The psychrophilic bacteria counts of CK group was more than 7.0 log CFU/g at day 20, but the psychrophilic bacteria counts of US, G, and USG samples were all significantly lower than the threshold of seafood. The final bacterial number in USG reduced by 0.72 log CFU/g (Pseudomonas) and 1.08 log CFU/g (H2S-producing bacteria) when compared to the CK group. All treatments evidently reduced microbial levels. Similar results were also found for the samples treated with chitosan-based coating[24]. A number of previous studies have assessed the ability of US to inhibit the growth of microorganisms in food. Garrido et al.[28] reported that samples with US treatment were significant reduction in microbial counts for both salmon and mackerel when compared to untreated groups.
2.13. Microbial composition analysis using high-throughput sequencing
The microbiota composition of different treatments was determined by high-throughput sequencing. Table 2 showed that the coverage of all groups was >0.99, meaning that almost all microbial system types were detected in the samples. The Shannon, Simpson, ACE and Chao indicators were determined. 356,953 valid sequences were detected. The number of OTUs in all treatments was lower than Chao1 and ACE index, which represented that there may be some other bacterial system types in these samples. The results of Shannon and Simpson showed that the bacterial diversity of samples at the end was lower than that at the beginning of storage, which was agreement with other study of Jia et al.[29].
Table 2.
Phylotype coverage and alpha diversity estimation of bacterial communities in pompano (Trachinotus ovatus) during ice storage.
| Storage time (d) | Sample | Number | OTUs | Shannon | Chao | Ace | Simpson | Coverage |
|---|---|---|---|---|---|---|---|---|
| 0 | CK | 36,516 | 540 | 4.22 | 556.50 | 563.43 | 0.04 | 0.9998 |
| US | 11,491 | 346 | 3.96 | 398.25 | 407.10 | 0.06 | 0.9997 | |
| G | 36,287 | 584 | 3.94 | 613.71 | 615.91 | 0.07 | 0.9999 | |
| USG | 39,975 | 568 | 3.99 | 613.31 | 615.54 | 0.05 | 0.9999 | |
| 12 | CK | 34,585 | 411 | 3.67 | 461.83 | 446.11 | 0.07 | 0.9999 |
| US | 34,474 | 340 | 3.86 | 353.64 | 352.57 | 0.05 | 0.9999 | |
| G | 42,683 | 538 | 1.53 | 576.59 | 581.19 | 0.06 | 0.9999 | |
| USG | 39,821 | 539 | 4.37 | 597.60 | 605.04 | 0.02 | 0.9998 | |
| 24 | CK | 15,074 | 33 | 1.53 | 48.000 | 87.380 | 0.38 | 0.9999 |
| US | 18,536 | 28 | 1.18 | 83.000 | 78.770 | 0.48 | 0.9999 | |
| G | 21,592 | 124 | 1.82 | 260.15 | 323.62 | 0.23 | 0.9999 | |
| USG | 25,919 | 352 | 3.3 | 420.02 | 427.23 | 0.09 | 0.9999 | |
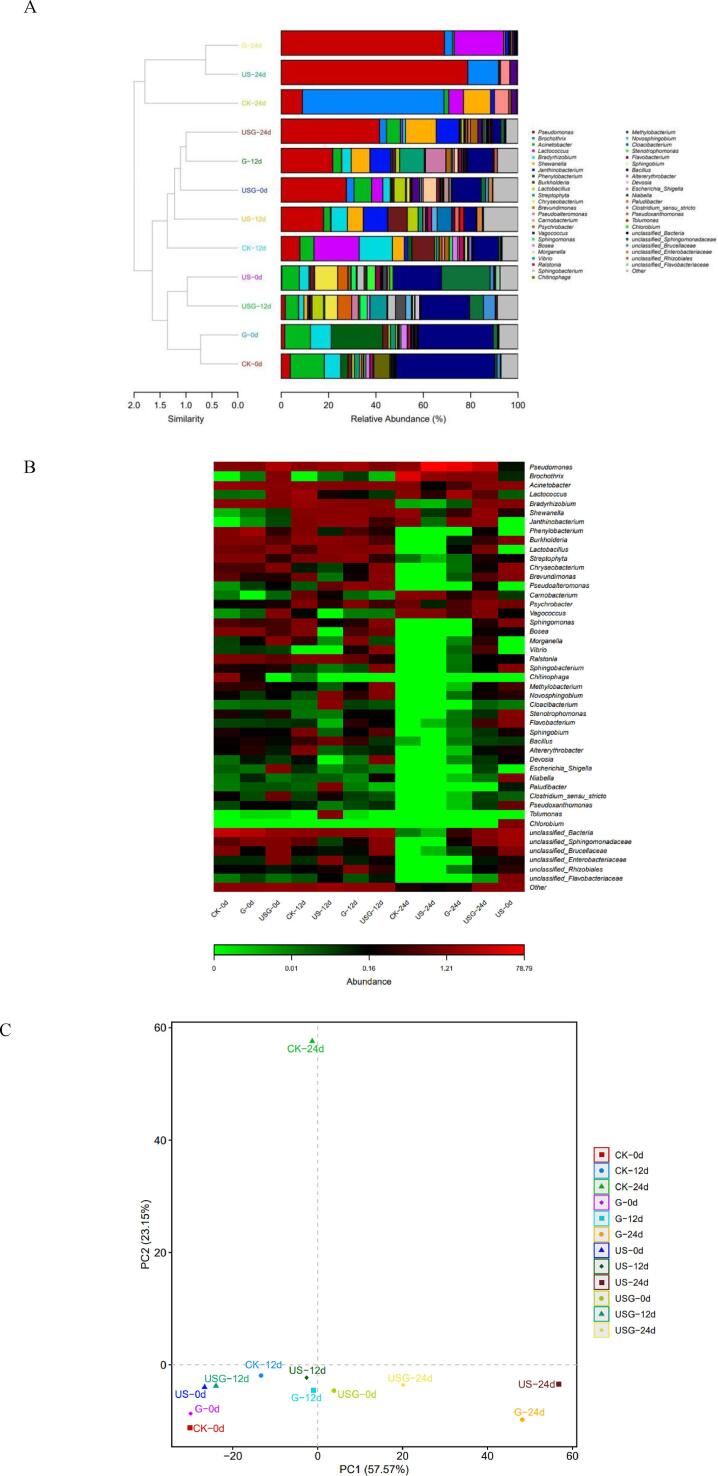
The relative abundances of the top 46 genera at the genus levels of all samples were represented by histogram. As shown in Fig. 5A, on day 0, unclassified Bacteria, Acinetobacter, and Bradyrhizobium were the dominant microbiota in the CK group, which accounted for 41.78%, 14.36%, and 6.93% of all OTU numbers, respectively. Because the ability of Acinetobacter not produce some corrupt substances, the factors leading to corruption were relatively weak[30]. However, at the initial stage, the relative abundance of microbiota in sample with different treatments were changed and then Pseudomonas, Chryseobacterium, and Phenylobacterium became the dominant microbiota in the US, G, and USG samples, respectively.
Fig. 5.
Community treebarplot analysis (A), Community heatmap (B), and PCA (C) on genus level of microbiota at the genus level of microbiota in Trachinotus ovatus during ice storage.
The microbiota composition at the beginning of storage was detected by high-throughput sequencing, and the results were consistent with the results of microbial count. H2S-producing bacteria were not detected by the microbial enumeration method in all groups at the initial stage of storage. The relative abundance of Shewanella was also less than 0.5% of bacterial populations in the all samples at day 0, which revealed by high throughput sequencing. In addition, Pseudomonas which had been identified as the dominant microorganism in all four groups at day 24 by microbial count was detected at 8.97%, 78.79%, 68.95%, and 41.55% in the CK, US, G, and USG groups, respectively.
As for the CK group, Lactococcus was found to be the prevalent bacteria at day 12. After 24 days, Brochothrix, Pseudomonas, and Shewanella sharply increased to 59.78%, 8.97% and 11.44% became the predominant bacteria, whereas, Lactococcus were decreased to negligible level. For US and G, Pseudomonas accounted for 0.14%, and 1.49% at day 0. Then Pseudomonas rose with the increase of storage time. At the end of the shelf-life, Pseudomonas (78.79% and 68.95%) was the dominant bacteria. Pseudomonas was the one of the main TVB-N producers[31], correspondingly, a sudden increase in TVB-N was showed in all groups at the end of storage. The abundances of Shewanella in treated groups were reduced compared with the CK group. For USG, microbial diversity was still high at the end of storage. Pseudomonas, Shewanella, and Janthinobacterium accounted for 41.55%, 12.95%, and 9.72%, respectively.
As shown in Fig. 5B, the composition and dynamics of bacterial community in all samples were analyzed using the heat map of common microorganisms at the genus level. The relative abundance of the genus was represented by the type and depth of color, the heat map showed that the diverse of microbiota in the fresh fish was the highest at day 0. Burkholderia, Streptophyta, Chryseobacterium, and Brevundimonas occupied a large proportion in fresh samples, but almost disappeared when samples deteriorated. After storage for a period, relative abundance of most microbiota decreased. Similarly, Zhang et al.[32]also identified that the bacterial diversity of grass carp fillets declined during storage. Brochothrix, Pseudomonas, and Lactococcus were abundant in spoiled fish. Pseudomonas has been reported to be a spoilage inducer and contributed to the formation of putrescine and cadaverine in various fish species from fresh and marine waters under aerobic chilled storage[3]. It is reported that Brochothrix will also affect the character of aquatic products related to corruption. Corruption will mainly produce lactic acid, ethanol and a small amount of short chain fatty acids, resulting in peculiar smell[33]. Some low abundance microbial communities are not well showed in the bar chart, but their variations are clearly reflected in the heat map.
In order to compare the microbial composition of each group, the analysis of PCA were used (Fig. 5C). The clustering results showed that the microbial community changed dynamically during storage. The similarity of microbial community structure was high in all groups at the initial stage. Meanwhile, the CK, US G, and USG groups represented two clusters at day 24. The results showed that the preservative treatment changed the microbial community structure of fish during storage. Yu et al. [24]showed that compound preservative treatment could alter the microbial community composition of fish fillets during storage.
2.14. Relationship of microflora and quality indicators
The accumulation of harmful components such as TVB-N, TBA, HX and HXR is the vital reason for the deterioration of fish quality. The correlation coefficients between 6 kinds of bacteria, and biochemical indexes for fish fillets were presented in Table 3. All quality index showed significant (P < 0.05) correlations with Carnobacterium. Most quality indicators associated with Brochothrix and Acinetobacter values significantly. Acinetobacter showed negative correlation with all quality indexes. This may be due to the relatively weak ability of Acinetobacter to cause corruption, because it would not produce some corrupt substances[30]. Shewanella was significantly correlated with putrescine value, which could cause the formation of putrescine.
Table 3.
Regression correlation coefficients between microbial enumerations and biochemical indicators (TVB-N, TBA, Putrescine, Cadaverine, K value, and TVC) in pompano (Trachinotus ovatus) during ice storage.
| Parameters | Shewanella | Vagococcus | Pseudomonas | Brochothrix | Acinetobacter | Carnobacterium |
|---|---|---|---|---|---|---|
| K | 0.489 | 0.492 | 0.652* | 0.630* | −0.792** | 0.772** |
| TVBN | 0.202 | 0.715** | 0.374 | 0.846** | −0.453 | 0.950** |
| Putrescine | 0.596* | 0.325 | 0.403 | 0.657* | −0.806** | 0.755** |
| Cadaverine | 0.358 | 0.655* | 0.547 | 0.808** | −0.645* | 0.929** |
| TBA | 0.538 | 0.395 | 0.121 | 0.924** | −0.493 | 0.897** |
| TVC | 0.564 | 0.435 | 0.54 | 0.662* | −0.782** | 0.801** |
Note. Levels of significance and defined as *p < 0.05 and **p < 0.01.
3. Conclusions
The current research indicated that ultrasound-assisted chitosan grafted caffeic acid coating effectively inhibit the increase of components including TVB-N, TBA, HxR and Hx, and decreased K value, microbial reproduction and the reduction of IMP in pompano (Trachinotus ovatus) during ice storage. Meanwhile, the results of high throughput sequencing analysis presented that the microbial diversity of all samples at the end of storage was lower than that of fresh sample. Acinetobacter was the major microorganism in fresh pompano. Shewanella, and Brochothrix became the major microbiota in the CK samples after 24 days of storage. In addition, Brochothrix in treatments were effectively controlled. Treatment changed the microbial communities of Trachinotus ovatus fillets during storage. All of the indicators represented that the ultrasound-assisted chitosan-based coating retarded the bacterial spoilage and extend the shelf-life of Trachinotus ovatus.
CRediT authorship contribution statement
Weiqing Lan: Conceptualization, Writing - review & editing, Supervision, Visualization. Yuqing Sun: Investigation, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Software. Shucheng Liu: Project administration, Resources. Yuan Guan: Investigation, Methodology. Shengyun Zhu: Investigation, Methodology. Jing Xie: Project administration, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was financially supported by China Agriculture Research System of MOF and MARA (CARS-47), Guangdong Provincial Key Laboratory of Aquatic Product Processing and Safety (GDPKLAPPS1802), Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (ZJW-2019-06), Guangdong Innovation Team of Seafood Green Processing Technology (2019KCXTD011).
Contributor Information
Weiqing Lan, Email: wqlan@shou.edu.cn.
Yuqing Sun, Email: sunyuqing98@126.com.
Shucheng Liu, Email: Lsc771017@163.com.
Yuan Guan, Email: gy13687947696@163.com.
Shengyun Zhu, Email: wonder_hei@163.com.
Jing Xie, Email: jxie@shou.edu.cn.
References
- 1.Liu M.-J., Guo H.-Y., Zhu K.-C., Liu B.-S., Liu B., Guo L., Zhang N., Yang J.-W., Jiang S.-G., Zhang D.-C. Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus) Aquat. Toxicol. 2021;240 doi: 10.1016/j.aquatox.2021.105969. [DOI] [PubMed] [Google Scholar]
- 2.Guo H., Chen C., Yan X., Li Y., Wen X., You C., Monroig Ó., Tocher D.R., Wang S. Effects of different dietary oil sources on growth performance, antioxidant capacity and lipid deposition of juvenile golden pompano Trachinotus ovatus. Aquaculture. 2021;530:735923. doi: 10.1016/j.aquaculture.2020.735923. [DOI] [Google Scholar]
- 3.Zhang J., Li Y., Liu X., Lei Y., Regenstein J.M., Luo Y. Characterization of the microbial composition and quality of lightly salted grass carp (Ctenopharyngodon idellus) fillets with vacuum or modified atmosphere packaging. Int. J. Food Microbiol. 2019;293:87–93. doi: 10.1016/j.ijfoodmicro.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Yu D., Zhao W., Yang F., Jiang Q., Xu Y., Xia W. A strategy of ultrasound-assisted processing to improve the performance of bio-based coating preservation for refrigerated carp fillets (Ctenopharyngodon idellus) Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128862. [DOI] [PubMed] [Google Scholar]
- 5.Ebadi Z., Khodanazary A., Hosseini S.M., Zanguee N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019;139:94–102. doi: 10.1016/j.ijbiomac.2019.07.204. [DOI] [PubMed] [Google Scholar]
- 6.Fang Z., Lin D., Warner R.D., Ha M. Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chem. 2018;260:90–96. doi: 10.1016/j.foodchem.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Xie J., Li L., Xue B., Li X., Gan J., Shao Z., Sun T. Properties of phenolic acid-chitosan composite films and preservative effect on Penaeus vannamei. J. Mol. Struct. 2021;1239 [Google Scholar]
- 8.İlyasoğlu H., Nadzieja M., Guo Z. Caffeic acid grafted chitosan as a novel dual-functional stabilizer for food-grade emulsions and additive antioxidant property. Food Hydrocolloids. 2019;95:168–176. [Google Scholar]
- 9.Jun L., Shuang L., Xin Z., Juan K., Changhai J. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus) Postharvest Biol. Technol. 2019;147:39–47. [Google Scholar]
- 10.Zhang X., Liu J., Qian C., Kan J., Jin C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocolloids. 2019;89:1–10. [Google Scholar]
- 11.Sun Y., Lan W., Liu S., Guan Y., Zhu S., Xie J. Preparation of chitosan grafted caffeic acid coating and its effect on pompano (Trachinotus ovatus) preservation. J. Sci. Food Agric. 2021 doi: 10.1002/jsfa.11624. [DOI] [PubMed] [Google Scholar]
- 12.Sae-leaw T., Benjakul S., Vongkamjan K. Retardation of melanosis and quality loss of pre-cooked Pacific white shrimp using epigallocatechin gallate with the aid of ultrasound. Food Control. 2018;84:75–82. [Google Scholar]
- 13.Zhao X., Lan W., Zhai Y., Xie J. Multi-frequency ultrasound: A potential method to improve the effects of surface decontamination and structural characteristics on large yellow croaker (Pseudosciaena crocea) during refrigerated storage. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T., Ning Z., Wang X., Zhang Y., Zhang Y. Effects of ultrasound on the physicochemical properties and microstructure of salted-dried grass carp (Ctenopharyngodon idella) J. Food Process Eng. 2018;41(1) [Google Scholar]
- 15.Lan W., Lang A., Zhou D., Xie J. Combined effects of ultrasound and slightly acidic electrolyzed water on quality of sea bass (Lateolabrax Japonicus) fillets during refrigerated storage. Ultrason. Sonochem. 2021;81 doi: 10.1016/j.ultsonch.2021.105854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y., Zhang M., Bhandari B., Yang C.-H. Ultrasound treatment of frozen crayfish with chitosan Nano-composite water-retaining agent: Influence on cryopreservation and storage qualities. Food Res. Int. 2019;126 doi: 10.1016/j.foodres.2019.108670. [DOI] [PubMed] [Google Scholar]
- 17.Lan W., Sun Y., Zhang N., Xie J. Effects of epsilon-polylysine and rosemary extract on quality attributes and microbial communities in vacuum-packaged large yellow croaker (Pseudosciaena crocea) during ice storage. Food Sci. Biotechnol. 2021;30:465–474. doi: 10.1007/s10068-021-00880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng H., Lan W., Sun X., Xie J. Effects of slightly acidic electrolyzed water pretreatment combined with biopreservatives on the shelf life of refrigerated obscure pufferfish (Takifugu obscurus) J. Food Sci. 2021;86:484–494. doi: 10.1111/1750-3841.15596. [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Lan W., Pu T., Zhou Y., Xie J. Combining slightly acidic electrolyzed water and slurry ice to prolong the shelf-life of mackerel (Pneumatophorus japonicus) J. Food Process. Preserv. 2021;45(9) [Google Scholar]
- 20.Zhao Y., Lan W., Shen J., Xu Z., Xie J. Combining ozone and slurry ice treatment to prolong the shelf-life and quality of large yellow croaker (Pseudosciaena crocea) LWT. 2022;154 doi: 10.1002/jsfa.12066. [DOI] [PubMed] [Google Scholar]
- 21.Guan W., Ren X., Li Y., Mao L. The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. Lwt. 2019;101:25–31. [Google Scholar]
- 22.Sun X., Guo X., Ji M., Wu J., Zhu W., Wang J., Cheng C., Chen L., Zhang Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019;272:643–652. doi: 10.1016/j.foodchem.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Shokri S., Parastouei K., Taghdir M., Abbaszadeh S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. Int. J. Biol. Macromol. 2020;153:846–854. doi: 10.1016/j.ijbiomac.2020.03.080. [DOI] [PubMed] [Google Scholar]
- 24.Dawei Y., Regenstein J.M., Jinhong Z., Qixing J., Wenshui X., Yanshun X. Inhibition of microbial spoilage of grass carp (Ctenopharyngodon idellus) fillets with a chitosan-based coating during refrigerated storage. Int. J. Food Microbiol. 2018;285:61–68. doi: 10.1016/j.ijfoodmicro.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Gao M., Feng L., Jiang T., Zhu J., Fu L., Yuan D., Li J. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control. 2014;37:1–8. [Google Scholar]
- 26.Hu Y., Huang Z., Li J., Yang H. Concentrations of biogenic amines in fish, squid and octopus and their changes during storage. Food Chem. 2012;135:2604–2611. doi: 10.1016/j.foodchem.2012.06.121. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Li D., Lv J., Li Q., Kong C., Luo Y. Effect of cinnamon essential oil on bacterial diversity and shelf-life in vacuum-packaged common carp (Cyprinus carpio) during refrigerated storage. Int. J. Food Microbiol. 2017;249:1–8. doi: 10.1016/j.ijfoodmicro.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Pedrós-Garrido S., Condón-Abanto S., Beltrán J.A., Lyng J.G., Brunton N.P., Bolton D., Whyte P. Assessment of high intensity ultrasound for surface decontamination of salmon (S. salar), mackerel (S. scombrus), cod (G. morhua) and hake (M. merluccius) fillets, and its impact on fish quality. Innov. Food Sci. Emerg. Technol. 2017;41:64–70. [Google Scholar]
- 29.Jia S., Huang Z., Lei Y., Zhang L., Li Y., Luo Y. Application of Illumina-MiSeq high throughput sequencing and culture-dependent techniques for the identification of microbiota of silver carp (Hypophthalmichthys molitrix) treated by tea polyphenols. Food Microbiol. 2018;76:52–61. doi: 10.1016/j.fm.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Sun X., Hong H., Jia S., Liu Y., Luo Y. Effects of phytic acid and lysozyme on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. Food Microbiol. 2020;86 doi: 10.1016/j.fm.2019.103313. [DOI] [PubMed] [Google Scholar]
- 31.Zhao W., Yu D., Xia W. Vacuum impregnation of chitosan coating combined with water-soluble polyphenol extracts on sensory, physical state, microbiota composition and quality of refrigerated grass carp slices. Int. J. Biol. Macromol. 2021;193:847–855. doi: 10.1016/j.ijbiomac.2021.10.190. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Li Y., Yang X., Liu X., Hong H., Luo Y. Effects of oregano essential oil and nisin on the shelf life of modified atmosphere packed grass carp (Ctenopharyngodon idellus) LWT. 2021;147 [Google Scholar]
- 33.Mohan C.O., Ravishankar C.N., Srinivasa Gopal T.K., Lalitha K.V., Asok Kumar K. Effect of reduced oxygen atmosphere and sodium acetate treatment on the microbial quality changes of seer fish (Scomberomorus commerson) steaks stored in ice. Food Microbiol. 2010;27:526–534. doi: 10.1016/j.fm.2010.01.005. [DOI] [PubMed] [Google Scholar]