Abstract
In this study, a novel nanobiocomposite was synthesized using graphene oxide, lignin, silk fibroin and ZnO and used in biological fields. To synthesize this structure, after preparing graphene oxide by the Hummer method, lignin, silk fibroin, and ZnO nanoparticles (NPs) were added to it, respectively. Also, ZnO NPs with a particle size of about 18 nm to 33 nm was synthesized via Camellia sinensis extract by green methodology. The synthesized structure was examined as anti-biofilm agent and it was observed that the Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite has a significant ability to prevent the formation of P. aeruginosa biofilm. In addition, due to the importance of the possibility of using this structure in biological environments, its toxicity and blood compatibility were also evaluated. According to the obtained results from MTT assay, the viability percentages of Hu02 cells treated with Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite after 24, 48, and 72 h of incubation were 89.96%, 89.32%, and 91.28%. On the other hand, the hemolysis percentage of the synthesized structure after 24 h and 72 h of extraction was 9.5% and 11.76% respectively. As a result, the synthesized structure has a hemolysis percentage below 12% and its toxicity effect on Hu02 cells is below 9%.
Subject terms: Biochemistry, Chemistry, Materials science
Introduction
Composites as one of the most important multicomponent materials are a group of substances composed of continuous matrix phase and uncontinuous reinforcement material1. These composites are created by at least two portion that have new properties compared to the raw material2. However, the features of the raw material are also preserved in the final structure. Composites are usually classified into three groups based on size of the reinforcement part, which includes macrocomposites, microcomposites, and nanocomposites1. Nanocomposites are a group of composites in which their components, especially their reinforcement parts, has a particle size of less than 100 nm1. In addition, if biocompatible and/or eco-friendly components are added to the mentioned structure, nanobiocomposite is obtained3–5. This materials are used in various fields including remediation of heavy metals6, sensor7, food packaging8, catalyst and organic synthesis9–12, and adsorption of textile dye13. Furthermore, nanobiocomposites with properties such as nontoxicity, hemocompatibility, and improved mechanical properties are appropriate candidates for utilizing in biological applications and in this regard, various reports have been presented in fields of drug delivery14, pharmaceutics15, wound healing16, antibacterial materials17, cancer therapy3,18,19, and tissue engineering20. A wide range of materials has been used to synthesize biological nanobiocomposites. Among them, carbon-based materials, especially graphene oxide have received special attention due to their properties such as high surface area21 and remarkable mechanical strength22, their ability to help for create schafford23, and used as filler24 in nanobiocomposites. graphene oxide with individual layer structure prepared via oxidation of graphite and these sheet have functional groups including active oxygen such as carboxylic acid (COOH), epoxy (-O-), and hydroxyl (OH)22. The special structure of graphene oxide with the negative charge in its carboxylate groups has made it known as a structure with high colloidal stability and hydrophilicity, which makes graphene oxide suitable for biological applications25. It should be noted that reports of graphene oxide toxicity have limited its use in biological applications alone and usually utilized in combination with safe material as a nanobiocomposite26. In this regard, various natural biorenewable resources such as starch, chitin, chitosan, cellulose, alginate, hyaluronic acid, gelatin, collagen, silk fibroin, and lignin have been used1. Lignin is an organic biopolymer with a cross-linked polyphenolic structure and it is found as support tissue in the vast majority of the plants27,28. Lignin has received considerable attention in health care due to its non-toxicity and biocompatibility. Its use has been studied as antioxidant, antimicrobial, anti-tumor, and antiviral agents29. It can also be utilized in fields of antidiabetic materials, drug delivery and, tissue engineering29. In addition to lignin, silk fibroin as an important biomaterial is a major component of silk protein with polypeptide chains with a molecular weight of 200 to 350 kDa30. Silk fibroin with properties such as biocompatibility, blood compatibility, non-carcinogenicity, non-toxicity, and suitable mechanical properties has been highly regarded by researchers17. Based on previous studies, the combination of silk fibroin with materials such as graphene oxide, natural polymers, and metal/metal oxide/metal hydroxide NPs enhances the mechanical and antibacterial properties of a composite made from silk fibroin17,31–34. Metal NPs play an important role in a variety of biological applications, including anti-microbial and anti-bacterial materials, biosensing, drug delivery, bio-imaging, and etc.4,35–38. There are various methods for the synthesis of metal oxide NPs, which can be referred to mechanical milling, laser ablation, ion sputtering, physical vapor deposition, chemical vapor deposition, sol–gel, chemical reduction, hydrothermal, solvothermal, spray pyrolysis, laser pyrolysis, and flame pyrolysis39. These methods also have drawbacks such as need the large amounts of energy, long reaction time, high cost, low efficiency of NPs production, use of toxic and corrosive substances, difficult reaction conditions, and production of impurities39. The mentioned negative points caused the introduction of alternative methods. Green synthesis of metal/metal oxide NPs by plant extract has been highly regarded as a new method in recent years. During this method, the plant extract can act as a reducing and stabilizing agent and convert metal ions into metal NPs40,41. Herein, a novel nanobiocomposite base on graphene oxide, lignin, silk fibroin, and ZnO was synthesized and its application as a substance with antibiofilm properties was evaluated. Also, due to the importance of the possibility of using this substance in biological environments, its toxicity and blood compatibility were investigated and it was observed that this new antibiofilm substance is not significantly toxic and is also compatible with blood.
Experimental
General
In this study, all reagents, chemical materials, and solvents were purchased from Merck and Flucka except the silkworm cocoons that are taken from local stores. Also, the 14,000 Da dialysis tubing cellulose membrane was purchased from Sigma-Aldrich Company. The structure of synthesized nanobiocomposite was evaluated from different points of view by using Fourier-transform infrared (FT-IR) spectroscopy, X-Ray Diffraction (XRD) Analysis, Thermogravimetric Analysis (TGA), Energy Dispersive X-Ray (EDX) Analysis, and Field-Emission Scanning Electron Microscopy (FE-SEM). FT-IR analysis was performed using AVATAR Thermo device in the range of 450 cm−1 to 4500 cm−1 and using the potassium bromide pellets method. EDX and FE-SEM analysis was carried out using EM8000 KYKY apparatus and ZEISS SIGMA VP model, respectively. XRD analysis was evaluated using PANalytical X-PERT-PRO MPD at 2θ, 5° to 90°. TGA was performed using STA504 analyzer in a temperature range of 50 °C to 550 °C with a temperature rate of 10 °C/min in air.
Preparation of graphene oxide
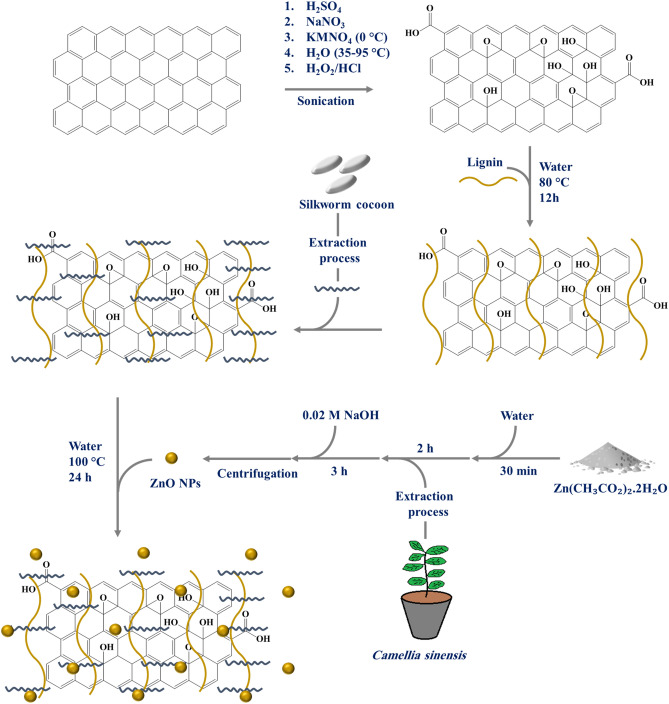
Graphene oxide was prepared using the modified Hummer method42. In this regard, 1 g of graphite and 23 ml of sulfuric acid (98%) were poured into a 1 L beaker and they were mixed for 5 min. Then, 0.5 g of sodium nitrate was added to the blend and they were mixed for 20 min at 65 °C. The beaker was then placed in an ultrasonic bath at room temperature for 20 min to completely dissolve the components. Afterwards, 3.5 g of potassium permanganate was added to the mixture during one hour until a sludge-like substance formed. This process was performed while the components were mixing in an ultrasonic bath with plenty of ice particles. In the next step, mixture was kept for further 30 min in an ultrasonic bath (25 °C) to complete the reaction and subsequently, 50 ml of distilled water was added to the beaker and mixed for 30 min at 98 °C. 700 ml of distilled water and 12 ml of hydrogen peroxide were added to the obtained mixture to observed a significant amount of foam. The pH of the mixture was then set using a 2% HCl solution (2 ml HCl in 100 ml distilled water). The mixture was remained stationary for one day and after the precipitate settles. After the mentioned time, the containers water was changed and elution process was repeated for 3 times. Finally, the precipitate was placed in an oven at 60 °C for 24 h to dry (Fig. 1).
Figure 1.
Graphical representation of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite synthesis procedure.
Extraction of silk fibroin
Extraction of silk fibroin was performed using the method reported in literatures22,43. Initially, three silk worm cocoons with a perfectly clean appearance were divided into small pieces and allowed to boil in 0.21% w/v sodium carbonate aqueous solution for 2 h. After the mentioned time, the fibers were separated and washed 6 times with distilled water. The filaments should be well separated from each other's during washing so that the pollutants are washed well. After washing, the fibers were dried at room temperature for 12 h. The dried fibers were weighed (0.644) and then, 9.3 M lithium bromide solution was made by using 6.44 g of water (10 times of the dry fibers weight) and it was kept under stirring at 60 °C for 2 h. Then, to remove the remaining lithium bromide, the obtained solution enters the dialysis cellulose membrane and is placed in the presence of distilled water, and this process continues for 3 days at room temperature. Finally, the obtained silk fibroin was stored at − 4 °C for later use.
Preparation of NPs
Preparation of Camellia sinensis extract
First, 10 g of dried leaves of Camellia sinensis were mixed with 200 ml of deionized water and heated at 70 °C for 10 min. The resulting solution was then passed through filter paper after cooling.
Preparation of ZnO NPs
0.11 g Zn(CH3CO2)2·2H2O and 25 ml of deionized water mixed with a magnetic stirrer for 30 min. Then 1 ml of the extract was added to the container and it was stirred for 2 h. In the next step, the pH of the solution was adjusted to 12 by using the 0.02 M NaOH solution. After the yellow color was observed, the solution was stirred vigorously for 3 h. The obtained NPs were separated by using a centrifuge (10,000 r/min) and after washing with distilled water, they were dried in an oven at 50 °C for 12 h.
Preparation of graphene oxide-lignin
0.4 g of graphene oxide and 45 ml of distilled water were mixed and placed in an ultrasonic bath for 30 min. Then 0.4 g of lignin was added to the previous mixture and the mixture was placed in an ultrasonic bath for another 30 min. The resulting mixture was stirred at 80 °C for 12 h. After this time, the reaction mixture was stored in the refrigerator for later use.
Preparation of graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite
At this stage, 10 ml of silk fibroin solution was added to 30 ml of Graphene oxide-lignin solution and refluxed for 12 h. After the mentioned time, 0.03 g of zinc oxide powder was dispersed in 10 ml of distilled water and added to the previous blend. Subsequently the mixture was stirred for 12 h under reflux conditions. Finally, the synthesized nanobiocomposite was freeze dried for 48 h and stored in a cool and dry place.
MTT assay
First, the synthesized Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite was extracted. In this way, 50 mg of it was dispersed in 1 ml of PBS using shaker incubator for 24 h at 37 °C44. Then, in order to measure the survival rate of Hu02 cell line (human skin fibroblast cells) in the vicinity of the Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite, MTT assay was performed. For this purpose, 1 × 105 cell/well was cultured in 96-well plates at optimal conditions (37 °C, 5% CO2) in humidified incubator. Next, the growth media (10% FBS) was removed and the cells were washed twice with PBS. New maintenance RPMI (Roswell Park Memorial Institute) medium including nanobiocomposite extract was added and the cells were incubated for 24, 48, and 72 h. Also, attached RPMI without nanobiocomposite extract and cells in each well, were considered as negative control. Following, 10 μl solution of freshly prepared 5 mg/ml MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) in PBS was added to each well and allowed to incubate at 37 °C for 4 h. Thereafter, the media with MTT solution was removed and 2-propanol was added at 100 µL/well. Next, the plates were shaken gently to facilitate formazan crystal solubilization22. The absorbance was measured at 590 nm using a microplate reader (STAT FAX 2100, BioTek, Winooski, USA). Finally, the percentage of cell toxicity and cell viability was calculated as follows45:
| 1 |
| 2 |
Hemolysis assay
This study was performed in accordance with the principles outlined in the Declaration of Helsinki. Also, the experimental methods and the procedure for taking informed satisfaction were approved by Semnan University of Medical Sciences, Ethics Research Committee. First, 50 mg of nanobiocomposite was dissolved in 1 ml PBS by shaker incubator at 37 °C with two extraction time, 24 h and 72 h44. Next, hemolytic assay was performed to measure the potential lytic effects of the Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite on human red blood cells (RBCs). Then, Fresh blood sample was taken from a volunteer with the O negative blood type. A subsequent blood sample was diluted in PBS (1:20). After that, 100 μl of the solution was added in triplicate to 100 μl of each Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite extract (24 h and 72 h) in a 96-well plate. 1% Triton X-100 solution, which lyses 100% of RBCs and sterile 0.9% NaCl solution were also used as positive control and negative control, respectively. Then, the plate was incubated at 37 °C for 1 h and samples were regained and centrifuged at 3000 rpm for 15 min46. The absorbance of each sample was measured by photometric analysis of supernatant at 414 nm using a microplate reader (STAT FAX 2100, BioTek, Winooski, USA). Eventually, the hemolysis percentage of the samples was calculated using the following formula47:
| 3 |
Anti-biofilm assay
The antimicrobial properties of the Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite, were studied using a tissue culture plate (TCP) anti-biofilm assay. First, 1 cm2 pieces of nanobiocomposite and a polystyrene (as a positive control), were sterilized in 70% ethanol aqueous solution, and then dried in a sterilized incubator at 37 °C. Next, each piece was placed in a sterilized tube containing selected bacteria (Pseudomonas aeruginosa ATCC 27853) at concentration of 107 colony-forming unit (CFU)/ml in Nutrient Broth (NB) culture medium. Tubes were then incubated at 150 rpm in a shaker incubator at 37 °C for 24 h. Afterward, samples were removed from the tubes and washed twice by PBS solution. To measure the anti-biofilm properties of the nanobiocomposite, both samples were stained by 0.1% crystal violet solution for 5 min and then washed by 33% acetic acid solution to separate the bacteria from their surface. Finally, using a microplate reader (STAT FAX 2100, BioTek, Winooski, USA), the absorbance of the resulting solutions was evaluated at 570 nm16,48.
Result and Discussion
Characterization of the of graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite
FT-IR analysis
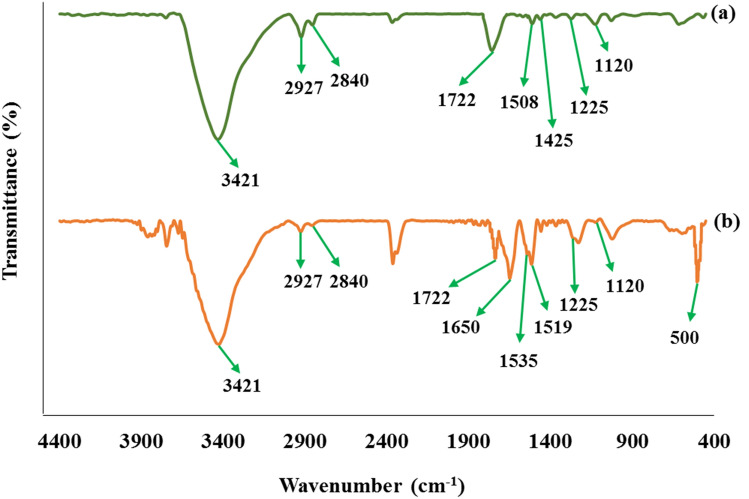
Figure 2 shows the FT-IR spectrum of Graphene oxide-lignin and Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite. As shown in Fig. 2a, spectrum represents a mixture of graphene oxide and lignin. The broad peak observed in region 3421 cm−1 is related to the stretching vibration of the hydroxyl group of graphene oxide and lignin22,31,49. Peaks in the 1722 cm−1 and 1225 cm−1 regions may represent the stretching modes of carbonyl and C–O–C groups in the graphene oxide structure, respectively22,50. In addition, the presence of lignin is confirmed according to the peaks observed in areas 2927 cm−1, 2840 cm−1, and 1120 cm−151. These peaks are related to the stretching vibration of aliphatic C–H in methyl and methylene, symmetric stretching of CH3 in methoxy, and stretching vibration mode of C–O in alcohol, respectively51. On the other hand, the peaks in the 1508 cm−1 and 1425 cm−1 indicate the vibrations of the aromatic skeleton in the lignin structure51. Figure 2b shows the structure of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite. Lignin and graphene oxide peaks are maintained in this spectrum. Also, the peak of ZnO NPs is observed in the region of about 500 cm−152. The presence of silk fibroin is also confirmed by observing peaks in 1519 cm−1 and 1535 cm−1 which are related to the N–H bending vibration of amide II22. In addition, the peak observed around 1650 cm−1 could demonstrate the presence of amides carbonyl in the structure of silk fibroin22.
Figure 2.
FT-IR spectra of (a) Graphene oxide-lignin and (b) Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite.
EDX analysis
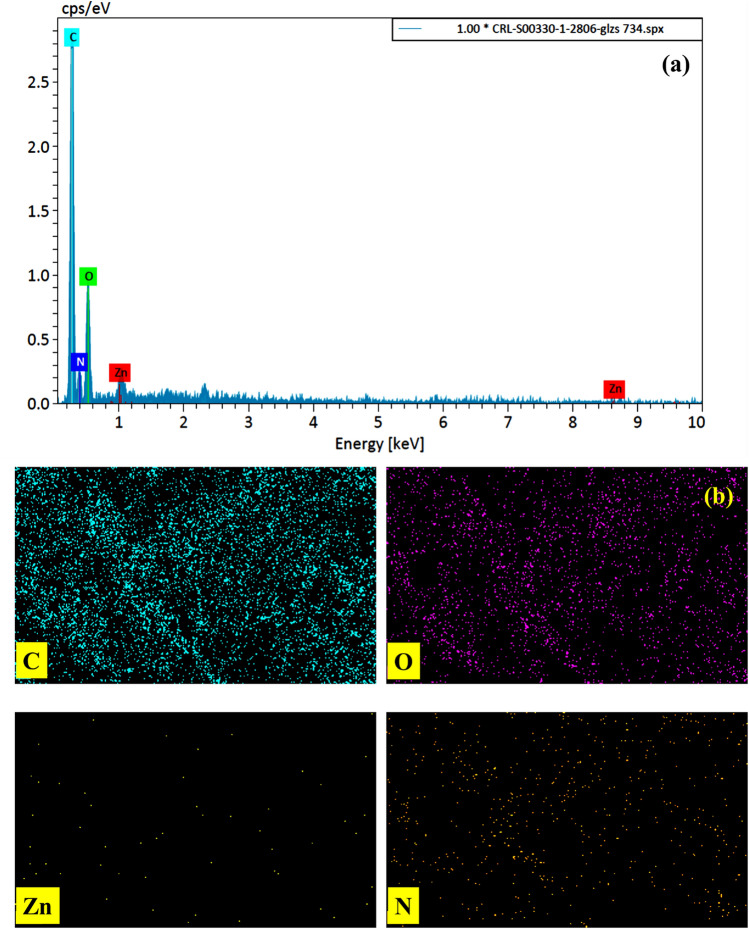
The EDX spectrum of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite can be seen in Fig. 3a. As can be seen, all the expected elements in the final structure are seen in the EDX image. The presence of zinc in the EDX spectrum confirms the presence of ZnO NPs. In addition, carbon and oxygen can be due to the presence of graphene oxide, lignin and silk fibroin. The presence of nitrogen in the EDX spectrum, confirms the existence of silk fibroin in the structure. Distribution of the elements was also evaluated by elemental mapping pictures and it was observed that the elements have acceptable distribution (Fig. 3b).
Figure 3.
(a) EDX analysis and (b) element mapping of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite.
FE-SEM imaging
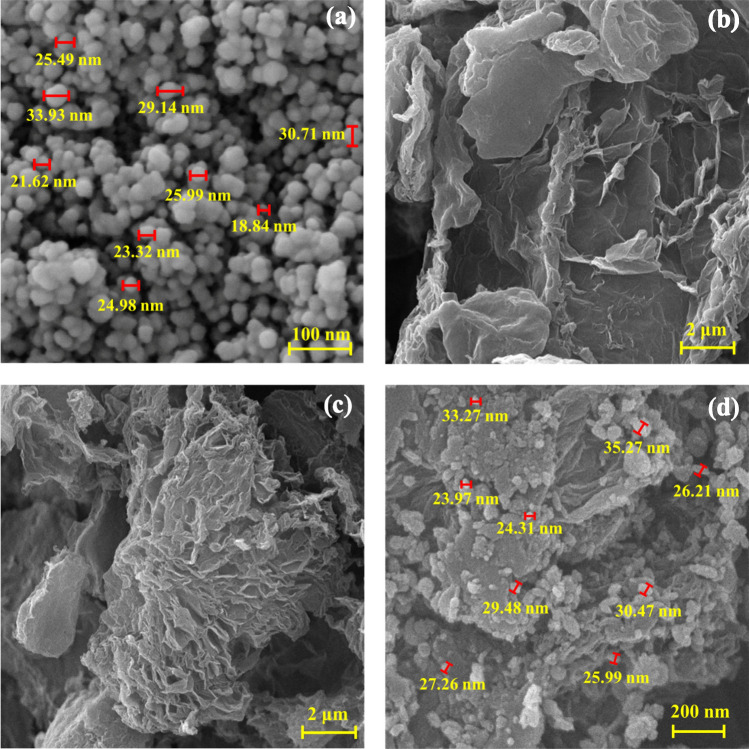
FE-SEM images were taken from Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite synthesis steps. As shown in Fig. 4a, ZnO NPs were well synthesized with spherical shapes and a particle size of about 18 to 33 nm. Figure 4b shows the morphology of the Graphene oxide-lignin composite and based on what is seen, the graphene oxide plates alongside with secondary structure (lignin) are clearly visible Fig. 4c,d shows Graphene oxide-lignin/silk fibroin and Graphene oxide-lignin/silk fibroin/ZnO respectively. Based on the FE-SEM image of the final nanobiocomposite, ZnO NPs with the same size as Fig. 4a are well dispersed in the structure of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite.
Figure 4.
FE-SEM image of (a) ZnO, (b) Graphene oxide-lignin, (c) Graphene oxide-lignin/silk fibroin, and Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite.
XRD pattern
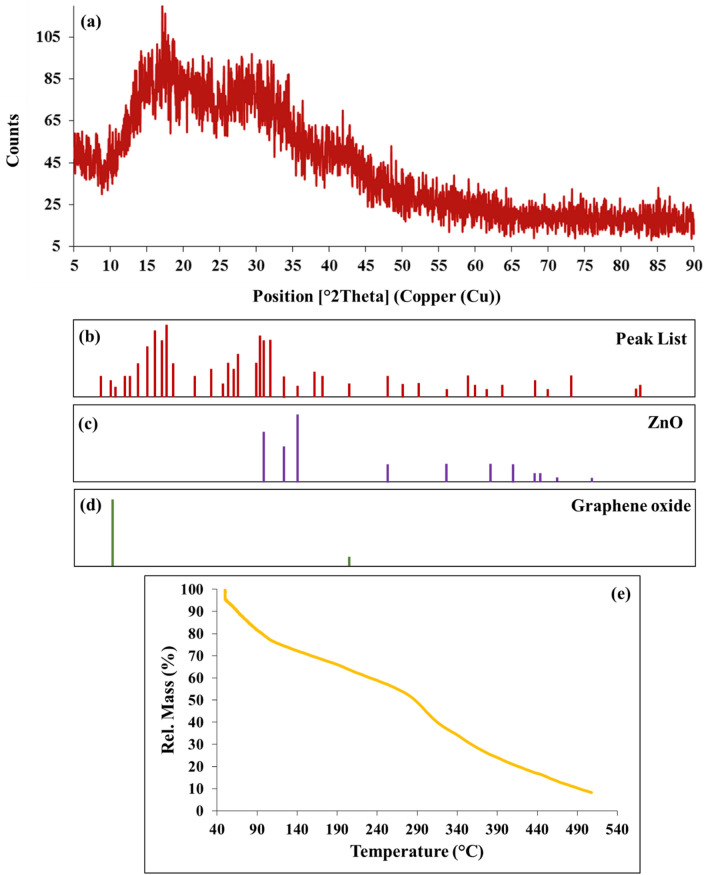
XRD analysis was performed to investigate the crystalline structure of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite. As can be seen in Fig. 5a,b, the peaks observed in 2θ around 31.29°, 33.95°, 36.04°, 47.05°, 56.09°, 62.38°, 67.45° and, 68.60° are related to the crystalline structure of ZnO NPs (Fig. 5c)53. Also, these peaks correspond to the Miller Index of (100), (002), (101), (102), (110), (103), (112), and (201) respectively53. In addition, the peak observed in 2 theta about 11.2° with Miller Index (001) indicates the presence of graphene oxide (Fig. 5d)54. The wide area below the XRD pattern could indicate the amorphous structure of the final structure, and this appears may be due to the presence of lignin and silk fibroin55,56.
Figure 5.
(a) XRD pattern and (b) peak list of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite (c) reference of ZnO and (d) graphene oxide. (e) TGA curve of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite.
TG analysis
TGA analysis was performed to evaluate the correct formation and thermal stability of the nanobiocomposite (Fig. 5e). The first mass reduction, which is in the temperature range of 50 °C to 100 °C, is related to the removal of trapped water, solvents and probable impurities from the Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite structure (about 20%)17. The second mass reduction occurs in the range of 150 °C to 300 and during this process, about 30% of the mass was eliminated. Thermal degradation of lignin macromolecules occurs at temperatures around 150 °C to 550 °C57. In addition, a mass reduction in the range of 200 °C to 300 °C can be related to the pyrolysis of the oxygenated portions of graphene oxide, including the carboxyl, epoxide, and hydroxyl groups22. The third mass reduction occurs at 300 °C to 500 °C and 40% of the sample weight is lost. Mass reduction in the temperature range about 250 °C to 400 °C can be related to the destruction of peptide structures in silk fibroin22. In addition, ZnO NPs do not have a significant mass reduction of up to 500 degrees, and their partial mass reduction can be due to the release of absorbed moisture58.
Biological properties of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite
Biocompatibility
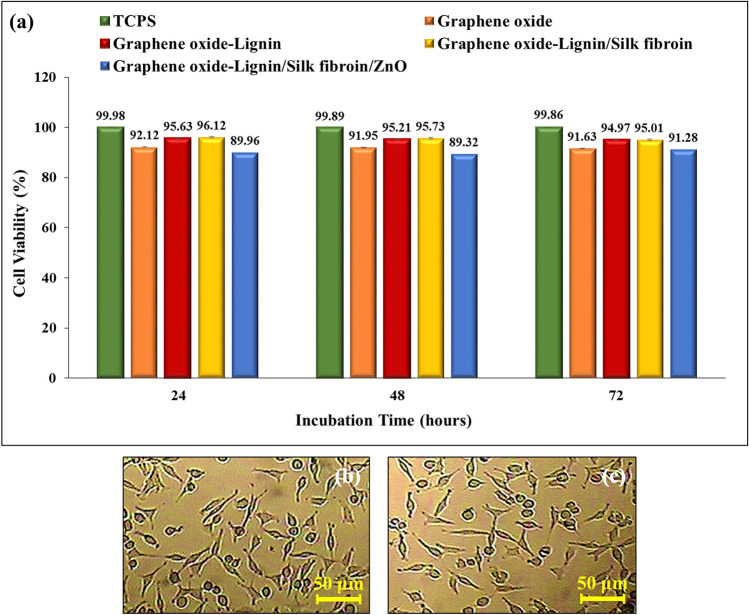
Graphene and its derivatives are very suitable filler material in biopolymers for tissue regeneration. Moreover, graphene oxide itself is nontoxic within the low concentration limit (50 μg/ml for human cells) and therefore suitable for use as an additive for the preparation of polymer composite scaffolds for clinical applications59. Lignin-based materials have a wide range of biomedical applications due to their excellent biological properties such as biocompatibility and non-toxicity60. Many studies have also shown the non-toxicity of silk fibroin and its hydrogels61. It should be noted that studies have shown that heavy metal nanoparticles based on zinc and its oxides exhibit toxicity to human cells62,63. However, due to the fact that the nanobiocomposites used in wound healing must have antimicrobial properties, the use of antibacterial nanoparticles is mandatory. As can be seen in Fig. 6a, histogram of the cell viability percentage after different incubation times of tissue culture polystyrene (TCPS), graphene oxide, Graphene oxide-Lignin, Graphene oxide-Lignin/silk fibroin and Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite was evaluated. The viability percentages of Hu02 cells treated with Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite, after 24, 48 and 72 h of incubation were 89.96%, 89.32% and 91.28%, respectively. Also, the toxicity of graphene oxide decreased after binding to lignin, because its release into the environment was reduced due to the strong covalent bond with lignin. Addition of silk fibroin to this composite also again reduced the emission of graphene oxide and thus reduced the amount of toxicity. But after adding ZnO to the biocomposite, the toxicity increased by about 4%. Therefore, in general, graphene oxide shows less toxicity in the structure of the biocomposite than itself. Also, effect of Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite on morphology and shape of Hu02 cells after 72 h of incubation was imaged with reverse microscope. (Fig. 6b,c) Hu02 cells retains their fibroblast shape after treatment with Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite. Results are the average of three independent experiments. Overall, MTT assay results showed that Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite was slightly toxic (less than 10%), but is generally biocompatible with Hu02 cells.
Figure 6.
(a) Histogram of the cell viability percentage after different incubation times of TCPS, graphene oxide, Graphene oxide-Lignin, Graphene oxide-Lignin/silk fibroin and nanobiocomposite. (b) Untreated Hu02 cell line morphology and (c) Hu02 cell line morphology after treatment with Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite after 72 h incubation.
Hemocompatibility
Various studies have shown the hemocompatibility of graphene oxide22 and lignin-based64 scaffolds. Also, silk fibroin based scaffolds do not have high hemolysis potential65. On the other hand, various studies have shown that zinc and its oxide nanoparticles have different hemolytic activity according to their morphology, size and concentration66,67. As shown in Fig. 7, hemolysis histogram of 1% Triton X-100, 0.9% NaCl, graphene oxide and Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite after different extraction times (24 h and 72 h) are shown. The hemolysis percentage of the Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite after 24 h of extraction was 9.5%. This amount increased to 11.76% after 72 h of extraction. This is while 1% triton X-100 lysed almost all RBCs. Also, the percentage of hemolysis of nanobiocomposite has increased due to the presence of ZnO nanoparticles, compared to graphene oxide. It is also worth noting that these results are the average of three separate experiments. Based on the results, it can be said that Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite lyses RBCs to some extent (below 12%).
Figure 7.
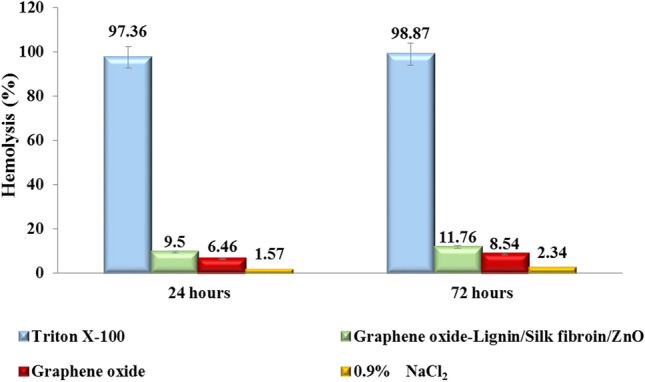
Hemolysis histogram of 1% Triton X-100 (positive control), 0.9% NaCl (negative control), graphene oxide and Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite after different extraction times (24 h and 72 h).
Anti-biofilm activity
As shown in Fig. 8, the adsorption rate of polystyrene (as a positive control) at 570 nm was 0.84, which was reduced to 0.12 for Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite. Also, the anti-biofilm activity of nanobiocomposite has increased due to the presence of ZnO nanoparticles, compared to graphene oxide. The anti-biofilm activity of ZnO NPs is reduced due to the strong bonding with the nanocomposite and its placement in the structure. In fact, the decrease in OD of the NB culture medium containing Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite at 570 nm, indicated that our scaffold could well barricade P. aeruginosa biofilm formation on its surface. The reported values are the average of three independent repetitions of the experiment.
Figure 8.
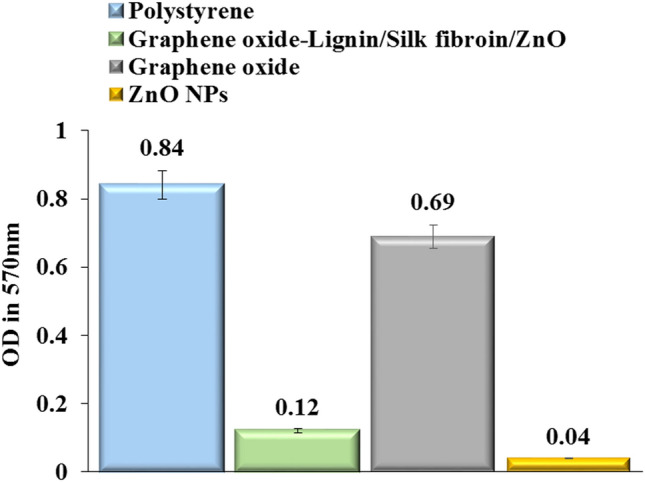
Anti-biofilm histogram of polystyrene, graphene oxide, Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite and ZnO NPs.
Conclusions
In this study, Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite was synthesized for the first time and the structure was evaluated using FT-IR, EDX, FE-SEM, XRD, and TGA. In addition, the biological characteristics of the synthesized structure were examined. According to the obtained results, this novel nanobiocomposite acted significantly as an anti-biofilm agent against P. aeruginosa. Moreover, the MTT assay shows that the viability percentages of Hu02 cells treated with Graphene oxide-lignin/silk fibroin/ZnO nanobiocomposite after 24, 48, and 72 h of incubation were 89.96%, 89.32%, and 91.28%. On the other hand, the hemolysis percentage of the synthesized structure after 24 h and 72 h of extraction was 9.5% and 11.76% respectively.
Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology. Also the authors thank of Ethics Research Committee and Biotechnology Research Center from Semnan University of Medical Sciences.
Author contributions
R.E.-K.: Substantial contributions to the conception, Design of the work, have drafted the work, Writing—Review & Editing, Analysis and interpretation of data and wrote the main manuscript. M.G.G.: Have drafted the work, Analysis and interpretation of data, substantively revised it. Wrote the main manuscript and prepared figures. E.Z.-B.: Have drafted the work, Analysis and interpretation of data, substantively revised it. Wrote the main manuscript and prepared figures. H.A.M.A.: Analysis and interpretation of data, substantively revised it, wrote the main manuscript and prepared figures. M.M.: The corresponding (submitting) author of current study. Analysis and interpretation of data, substantively revised it. A.M.: The corresponding (submitting) author of current study, Substantial contributions to the conception, Design of the work, have drafted the work, Writing—Review & Editing, substantively revised it. H.G.: The corresponding (submitting) author of current study, Substantial contributions to the conception, Design of the work, have drafted the work. H.M.: The corresponding (submitting) author of current study, Substantial contributions to the conception, Design of the work, have drafted the work, Writing—Review & Editing, substantively revised it. All authors are aware about ethical approval, informed consent, and guidelines followed in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-025-86520-y"
Change history
2/4/2025
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-025-86520-y
Contributor Information
Ali Maleki, Email: maleki@iust.ac.ir.
Hamid Madanchi, Email: hamidmadanchi@yahoo.com.
Mohammad Mahdavi, Email: momahdavi@sina.tums.ac.ir.
References
- 1.Ates, B., Koytepe, S., Ulu, A., Gurses, C. & Thakur, V. K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev.120, 9304–9362 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Black, J. T. & Kohser, R. A. DeGarmo’s Materials and Processes in Manufacturing (Wiley, 2020). [Google Scholar]
- 3.Eivazzadeh-Keihan, R. et al. A novel biocompatible core-shell magnetic nanocomposite based on cross-linked chitosan hydrogels for in vitro hyperthermia of cancer therapy. Int. J. Biol. Macromol.140, 407–414 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Eivazzadeh-Keihan, R. et al. Pectin-cellulose hydrogel, silk fibroin and magnesium hydroxide nanoparticles hybrid nanocomposites for biomedical applications. Int. J. Biol. Macromol.192, 7–15 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Kaushik, A., Solanki, P. R., Ansari, A. A., Ahmad, S. & Malhotra, B. D. Chitosan–iron oxide nanobiocomposite based immunosensor for ochratoxin-A. Electrochem. Commun.10, 1364–1368 (2008). [Google Scholar]
- 6.Varghese, L. R. & Nilanjana, D. Application of nano-biocomposites for remediation of heavy metals from aqueous environment: An overview. Int. J. Chemtech. Res.8, 566–571 (2015). [Google Scholar]
- 7.Kaushik, A. et al. Iron oxide-chitosan nanobiocomposite for urea sensor. Sens. Actuators B138, 572–580 (2009). [Google Scholar]
- 8.Cava, D., Giménez, E., Gavara, R. & Lagaron, J. M. Comparative performance and barrier properties of biodegradable thermoplastics and nanobiocomposites versus PET for food packaging applications. J. Plast. Film Sheeting.22, 265–274 (2006). [Google Scholar]
- 9.Maleki, A., Firouzi-Haji, R. & Hajizadeh, Z. Magnetic guanidinylated chitosan nanobiocomposite: A green catalyst for the synthesis of 1, 4-dihydropyridines. Int. J. Biol. Macromol.116, 320–326 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Taheri-Ledari, R. et al. Facile route to synthesize Fe3O4@acacia–SO3H nanocomposite as a heterogeneous magnetic system for catalytic applications. RSC Adv.10, 40055–40067 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maleki, A., Panahzadeh, M. & Eivazzadeh-keihan, R. Agar: A natural and environmentally-friendly support composed of copper oxide nanoparticles for the green synthesis of 1, 2, 3–triazoles. Green Chem. Lett. Rev.12, 395–406 (2019). [Google Scholar]
- 12.Asgharnasl, S., Eivazzadeh-Keihan, R., Radinekiyan, F. & Maleki, A. Preparation of a novel magnetic bionanocomposite based on factionalized chitosan by creatine and its application in the synthesis of polyhydroquinoline, 1, 4-dyhdropyridine and 1, 8-dioxo-decahydroacridine derivatives. Int. J. Biol. Macromol.144, 29–46 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Darvishi Cheshmeh Soltani, R. et al. Response surface methodological evaluation of the adsorption of textile dye onto biosilica/alginate nanobiocomposite: Thermodynamic, kinetic, and isotherm studies. Desalin. Water Treat.56, 1389–1402 (2015). [Google Scholar]
- 14.Rahim, M., Haris, M. R. & Saqib, N. U. An overview of polymeric nano-biocomposites as targeted and controlled-release devices. Biophys. Rev.12, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang, Z. et al. Chitosan-based nano-biocomposites and their applications in medicine and pharmaceutics. Curr. Org. Chem.22, 628–640 (2018). [Google Scholar]
- 16.Eivazzadeh-Keihan, R. et al. Hybrid bionanocomposite containing magnesium hydroxide nanoparticles embedded in a carboxymethyl cellulose hydrogel plus silk fibroin as a scaffold for wound dressing applications. ACS Appl. Mater. Interfaces13, 33840–33849 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Eivazzadeh-Keihan, R. et al. Chitosan hydrogel/silk fibroin/Mg(OH)2 nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Sci. Rep.11, 1–3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Eivazzadeh-Keihan, R. et al. A natural and eco-friendly magnetic nanobiocomposite based on activated chitosan for heavy metals adsorption and the in-vitro hyperthermia of cancer therapy. J. Mater. Res. Technol.9, 12244–12259 (2020). [Google Scholar]
- 19.Bani, M. S. et al. Casein-coated iron oxide nanoparticles for in vitro hyperthermia for cancer therapy. Spin9, 1940003 (2019). [Google Scholar]
- 20.Naskar, D., Bhattacharjee, P., Ghosh, A. K., Mandal, M. & Kundu, S. C. Carbon nanofiber reinforced nonmulberry silk protein fibroin nanobiocomposite for tissue engineering applications. ACS Appl. Mater. Interfaces9, 19356–19370 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Eivazzadeh-Keihan, R. et al. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. J. Adv. Res.18, 185–201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eivazzadeh-Keihan, R., Radinekiyan, F., Madanchi, H., Aliabadi, H. A. & Maleki, A. Graphene oxide/alginate/silk fibroin composite as a novel bionanostructure with improved blood compatibility, less toxicity and enhanced mechanical properties. Carbohydr. Polym.248, 116802 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Song, J. et al. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon95, 1039–1050 (2015). [Google Scholar]
- 24.Ahmad, H., Fan, M. & Hui, D. Graphene oxide incorporated functional materials: A review. Compos. B. Eng.145, 270–280 (2018). [Google Scholar]
- 25.Bei, H. P. et al. Graphene-based nanocomposites for neural tissue engineering. Molecules24, 658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seabra, A. B., Paula, A. J., de Lima, R., Alves, O. L. & Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol.27, 159–168 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Zhang, Y. & Naebe, M. Lignin: A review on structure, properties, and applications as a light-colored UV absorber. ACS Sustain. Chem. Eng.9, 1427–1442 (2021). [Google Scholar]
- 28.Saake, B. & Lehnen, R. Lignin. Ullmann’s Encycl. Ind. Chem.21, 21–36 (2000). [Google Scholar]
- 29.Spiridon, I., Poni, P. & Ghica, G. Biological and pharmaceutical applications of lignin and its derivatives: A mini-review. Cellul. Chem. Technol.52, 543–550 (2018). [Google Scholar]
- 30.Nguyen, T. P. et al. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers11, 1933 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eivazzadeh-Keihan, R. et al. Investigation of the biological activity, mechanical properties and wound healing application of a novel scaffold based on lignin–agarose hydrogel and silk fibroin embedded zinc chromite nanoparticles. RSC Adv.11, 17914–17923 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, S. D., Ma, Q., Wang, K. & Chen, H. W. Improving antibacterial activity and biocompatibility of bioinspired electrospinning silk fibroin nanofibers modified by graphene oxide. ACS Omega3, 406–413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calamak, S. et al. Ag/silk fibroin nanofibers: Effect of fibroin morphology on Ag+ release and antibacterial activity. Eur. Polym. J.67, 99–112 (2015). [Google Scholar]
- 34.Guang, S. et al. Chitosan/silk fibroin composite scaffolds for wound dressing. J. Appl. Polym. Sci.132, 42503 (2015). [Google Scholar]
- 35.Nolan, H. et al. Metal nanoparticle-hydrogel nanocomposites for biomedical applications: An atmospheric pressure plasma synthesis approach. Plasma Process. Polym.15, 1800112 (2018). [Google Scholar]
- 36.Eivazzadeh-Keihan, R., Radinekiyan, F., Maleki, A., Bani, M. S. & Azizi, M. A new generation of star polymer: Magnetic aromatic polyamides with unique microscopic flower morphology and in vitro hyperthermia of cancer therapy. J. Mater. Sci.55, 319–336 (2020). [Google Scholar]
- 37.Eivazzadeh-Keihan, R. et al. Magnetic copper ferrite nanoparticles functionalized by aromatic polyamide chains for hyperthermia applications. Langmuir37, 8847–8854 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Dahaghin, A. et al. A numerical investigation into the magnetic nanoparticles hyperthermia cancer treatment injection strategies. Biocybern. Biomed. Eng.41, 516–526 (2021). [Google Scholar]
- 39.Jamkhande, P. G., Ghule, N. W., Bamer, A. H. & Kalaskar, M. G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol.53, 101174 (2019). [Google Scholar]
- 40.Nasrollahzadeh, M., Mahmoudi-Gom Yek, S., Motahharifar, N. & Ghafori Gorab, M. Recent developments in the plant-mediated green synthesis of Ag-based nanoparticles for environmental and catalytic applications. Chem. Rec.19, 2436–2479 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Makarov, V. V. et al. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat.6, 35–44 (2014). [PMC free article] [PubMed] [Google Scholar]
- 42.Shahabuddin, S., Sarih, N. M., Afzal Kamboh, M., Rashidi Nodeh, H. & Mohamad, S. Synthesis of polyaniline-coated graphene oxide@SrTiO3 nanocube nanocomposites for enhanced removal of carcinogenic dyes from aqueous solution. Polymers8, 305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atrian, M., Kharaziha, M., Emadi, R. & Alihosseini, F. Silk-LAPONITE® fibrous membranes for bone tissue engineering. Appl. Clay Sci.174, 90–99 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Chang, Y. N. et al. Synthesis of magnetic graphene oxide–TiO2 and their antibacterial properties under solar irradiation. Appl. Surf. Sci.343, 1–10 (2015). [Google Scholar]
- 45.Eivazzadeh-Keihan, R. & Maleki, A. Design and synthesis of a new magnetic aromatic organo-silane star polymer with unique nanoplate morphology and hyperthermia application. J. Nanostruct. Chem.11, 1–7 (2021). [Google Scholar]
- 46.Eivazzadeh-Keihan, R. et al. Alginate hydrogel-polyvinyl alcohol/silk fibroin/magnesium hydroxide nanorods: A novel scaffold with biological and antibacterial activity and improved mechanical properties. Int. J. Biol. Macromol.162, 1959–1971 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Jaganathan, S. K., Mani, M. P., Ayyar, M., Krishnasamy, N. P. & Nageswaran, G. Blood compatibility and physicochemical assessment of novel nanocomposite comprising polyurethane and dietary carotino oil for cardiac tissue engineering applications. J. Appl. Polym. Sci.135, 45691 (2018). [Google Scholar]
- 48.Haney, E. F., Trimble, M. J., Cheng, J. T., Vallé, Q. & Hancock, R. E. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules8, 29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyvazzadeh-Keihan, R., Bahrami, N., Taheri-Ledari, R. & Maleki, A. Highly facilitated synthesis of phenyl (tetramethyl) acridinedione pharmaceuticals by a magnetized nanoscale catalytic system, constructed of GO, Fe3O4 and creatine. Diam. Relat. Mater.102, 107661 (2020). [Google Scholar]
- 50.Zolghadr, S., Kimiagar, S. & Davarpanah, A. M. Magnetic property of α -Fe2O3–GO nanocomposite. IEEE Trans. Magn.53, 1–6 (2017). [Google Scholar]
- 51.Nandanwar, R. A., Chaudhari, A. R. & Ekhe, J. D. Nitrobenzene oxidation for isolation of value added products from industrial waste lignin. J. Chem. Biol. Phys. Sci.6, 501–513 (2016). [Google Scholar]
- 52.Eivazzadeh-Keihan, R. et al. Fe3O4/GO@melamine-ZnO nanocomposite: A promising versatile tool for organic catalysis and electrical capacitance. Colloids Surf. A587, 124335 (2020). [Google Scholar]
- 53.Ramesh, M., Anbuvannan, M. & Viruthagiri, G. J. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A136, 864–870 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Seyedi, N., Nejad, M. S., Saidi, K. & Sheibani, H. Evaluation of functionalized reduced graphene oxide upgraded with gold nanoparticles as a hybrid nanocatalyst for the solvent-free oxidation of cyclohexene by molecular oxygen. C. R. Chim.23, 63–75 (2020). [Google Scholar]
- 55.Gomide, R. A. et al. Development and characterization of lignin microparticles for physical and antioxidant enhancement of biodegradable polymers. J. Polym. Environ.28, 1326–1334 (2020). [Google Scholar]
- 56.Madhu Kumar, R. et al. Gamma radiation assisted biosynthesis of silver nanoparticles and their characterization. Adv. Mater. Lett.6, 1088–1093 (2015). [Google Scholar]
- 57.Klapiszewski, Ł, Bula, K., Sobczak, M. & Jesionowski, T. Influence of processing conditions on the thermal stability and mechanical properties of PP/silica-lignin composites. Int. J. Polym. Sci.2016, 1–9 (2016). [Google Scholar]
- 58.Chunduri, L. A. et al. Streptavidin conjugated ZnO nanoparticles for early detection of HIV infection. Adv. Mater. Lett.8, 472–480 (2017). [Google Scholar]
- 59.Chaudhuri, B. Fullerens, Graphenes and Nanotubes 57–544 (William Andrew Publishing, 2018). [Google Scholar]
- 60.Ravishankar, K. et al. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C.102, 447–457 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Zuluaga-Vélez, A. et al. Silk fibroin hydrogels from the Colombian silkworm Bombyx mori L.: Evaluation of physicochemical properties. PLoS ONE14, e0213303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyszka-Czochara, M. et al. Zinc and propolis reduces cytotoxicity and proliferation in skin fibroblast cell culture: Total polyphenol content and antioxidant capacity of propolis. Biol. Trace Elem. Res.160, 123–131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Papavlassopoulos, H. et al. Toxicity of functional nano-micro zinc oxide tetrapods: Impact of cell culture conditions, cellular age and material properties. PLoS ONE9, e84983 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, Y. et al. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C.104, 110002 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Andiappan, M. et al. Electrospun eri silk fibroin scaffold coated with hydroxyapatite for bone tissue engineering applications. Prog. Biomater.2, 1–11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preedia Babu, E. et al. Size dependent uptake and hemolytic effect of zinc oxide nanoparticles on erythrocytes and biomedical potential of ZnO-ferulic acid conjugates. Sci. Rep.7, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajapriya, M. et al. Synthesis and characterization of zinc oxide nanoparticles using Cynara scolymus leaves: Enhanced hemolytic, antimicrobial, antiproliferative, and photocatalytic activity. J. Clust. Sci.31, 791–801 (2020). [Google Scholar]