Abstract
Background
The optimal number of neoadjuvant chemotherapy (NAC) cycles remains to be established for treating oesophageal squamous cell carcinoma (ESCC). We compared two versus three courses of NAC for treating locally advanced ESCC in a multi-institutional, randomised, Phase II trial.
Methods
We randomly assigned 180 patients with locally advanced ESCC at 6 institutions to either two (N = 91) or three (N = 89) courses of DCF (docetaxel 70 mg/m2, cisplatin 70 mg/m2 i.v. on day 1, fluorouracil 700 mg/m2 continuous infusion for 5 days) every 3 weeks, prior to surgery. The primary endpoint was 2-year progression-free survival (PFS) with an intention-to-treat analysis.
Results
Patient background parameters were well-balanced. The R0 resection rates were 98.9 and 96.5% in the two- and three-course groups, respectively (P = 0.830). In resected cases, the two- and three-course groups had comparable pN0 rates (P = 0.225) and histological responses (P = 0.898). The 2-year PFS rate was also comparable between the two groups (71.4 vs. 71.1%, P = 0.669). Among subgroups based on baseline characteristics, only patients aged under 65 years old showed a tendency for better survival with the three-course treatment (hazard ratio = 2.612, 95% confidence interval: 1.012–7.517).
Conclusions
Two courses of a DCF regimen showed potential as an optional NAC treatment for locally advanced ESCC.
Clinical trial registration
University Hospital Medical Information Network Clinical Trials Registry of Japan (identification number UMIN 000015788).
Subject terms: Chemotherapy, Chemotherapy
Introduction
Oesophageal squamous cell carcinoma (ESCC) is a common digestive tract malignancy, but it is the most refractory of all cancers [1, 2]. Surgery alone has failed to improve the dismal prognosis of ESCC. Therefore, a multimodal treatment approach, including chemotherapy and chemoradiation, has been explored in the past two decades to improve the prognosis of ESCC [3]. Based on the CROSS trial, in western countries, chemoradiation therapy (NCRT) has often been used as a neoadjuvant therapy for oesophageal cancer or junctional cancer [4, 5]. In contrast, in Asian countries, neoadjuvant chemotherapy (NAC) is the preferred approach for ESCC to ensure early control of microscopic metastatic disease, to down-stage the tumour, and to increase resectability [6, 7].
A previous large randomised clinical trial (JCOG99076) compared postoperative adjuvant chemotherapy to preoperative chemotherapy for locally advanced oesophageal squamous cell carcinoma. Based on those results, two courses of NAC with cisplatin and 5-fluorouracil (CF) has become the standard of care for locally advanced oesophageal squamous cell carcinoma. More recently, to enhance the response rate to NAC and improve the dismal survival rates, triplet NAC regimens were introduced, which included CF plus Adriamycin (ACF) or CF plus Docetaxel (DCF). Both these regimens were shown to be effective for locally advanced ESCC [7–12]. Moreover, a previous multicenter randomised Phase II trial also demonstrated that DCF chemotherapy prolonged recurrence-free survival (RFS) and overall survival (OS) for patients with resectable advanced ESCC. Those findings indicated that DCF chemotherapy could potentially serve as a standard NAC for resectable ESCC [7].
All these trials administered two courses of NAC, and additionally, two courses of NAC are often administered in daily clinical practice, regardless of the response. In contrast, the MAGIC Phase III trial demonstrated that three courses of perioperative chemotherapy (CF plus epirubicin) provided a survival benefit in resectable gastro-oesophageal adenocarcinoma [13]. In locally advanced gastric cancer, a randomised Phase II trial compared two vs. four courses of NAC followed by a D2 gastrectomy and demonstrated that two courses of NAC improved survival [14]. More recently, an ongoing randomised Phase III trial, focused on preoperative treatments for Stages II–III ESCC (JCOG1109), compared three courses of DCF, two courses of CF (standard arm), and two courses of CF plus CRT [15]. However, the optimal number of NAC cycles has not been established for ESCC, and it remains unclear whether an additional course of NAC could enhance the response rate or improve survival, compared to the standard two-course regimen.
Herein, we conducted a multi-institutional, randomised, Phase II trial to compare two versus three courses of NAC with a DCF regimen to determine the optimal number of NAC cycles for treating resectable advanced ESCC. In our previous report, which focused on short-term outcomes, two- and three-course DCF regimens in the NAC setting seemed to be equally feasible. We also found that an additional DCF course led to a better clinical response to NAC without increasing the incidence of adverse events or postoperative morbidity in patients with locally advanced ESCC [16]. In this study, we compared the survival rates between the two arms of this randomised Phase II study.
Patients and methods
Patients
The full details of the eligibility criteria and the pre-treatment evaluation were reported previously [16]. Briefly, eligible patients were aged 20 years or older with a performance status of 0–1, histologically confirmed oesophageal squamous cell carcinoma, and adequate primary organ function. The ESCC stages included cT1-4a N0-3 M0 and/or M1LYM metastases (confined to the supraclavicular lymph nodes), based on the TMN classification of the Union for International Cancer Control, seventh edition [17]. All patients provided written informed consent for trial participation. The study protocol was approved by the Institutional Review Board in each of six participating hospitals, before patient enrolment. The study was conducted in accordance with the Helsinki Declaration precepts, and it was registered with the University Hospital Medical Information Network Clinical Trials Registry (identification number: UMIN 000015788).
Study treatment
The study design was an open-label, randomised Phase II trial. Eligible patients were randomly assigned to either two or three courses of DCF. Each DCF course included: docetaxel 70 mg/m2 (1-h intravenous infusion) plus cisplatin 70 mg/m2 (1-h intravenous infusion) on day 1, followed by 5-fluorouracil (5-FU) 700 mg/m2 (continuous intravenous infusion) for 5 days. Courses were administered every 3 weeks [18–20]. Random assignments were stratified, according to the institution, cT stage, and cN stage, with a least-squares method.
To address adverse effects, the dose(s) of the probable causal agent(s) was adjusted in subsequent cycles, as follows [7, 11, 16, 21, 22]: for grade 4 leukopenia or neutropenia, febrile neutropenia, or grade 3 thrombocytopenia: all chemo-agent doses were reduced by 20%; for grade 3 stomatitis or diarrhoea: the 5-FU and docetaxel doses were reduced by 20%; for grade 2 nephrotoxicity: the cisplatin dose was reduced by 20%. A second cycle was administered unless progression or unacceptable toxicity had occurred.
Surgery was scheduled for 3–6 weeks after the start date of the last chemotherapy cycle. Patients underwent a subtotal oesophagectomy with either a two- or three-field lymphadenectomy with curative intent, via a right thoracotomy or thoracoscopic approach [20, 23, 24]. A transhiatal oesophagectomy was not acceptable. Regional lymphadenectomies included the mediastinal, perigastric, and celiac nodes. Distant lymphadenectomies included the cervical nodes.
Outcomes
The primary endpoint was 2-year progression-free survival (PFS). Secondary endpoints were OS, the pN0 rate, the number of pathological lymph nodes (pN), the pattern of disease recurrence, the R0 resection rate, the clinical/histopathological NAC response rate, postoperative complications, and safety. Disease recurrence was defined as locoregional (i.e., in the oesophageal bed, anastomotic, or regional lymph nodes) or distant (i.e., in non-regional lymph nodes, except for supraclavicular lymph nodes, or in distant organs). The histopathological tumour response was evaluated according to the histological criteria of the Japanese Society for Oesophageal Disease. Briefly, evaluations were classified into five categories, according to the proportion of tumour affected by degeneration or necrosis [8, 25–27]. Patients were followed up every 3 months, during the first 2 years after the date of random assignment; every 6 months for the next 3 years; then annually [28, 29].
Statistical analysis
A power calculation for the present study indicated that 164 patients were required to detect increases in the 2-year PFS of 55% in the two-course group and 70% in the three-course group, with 80% power to show a significant difference between groups, and a 10% type I error. Assuming an approximate drop-out rate of 10%, we planned to enroll a total of 180 patients. Continuous variables are expressed as the median and range. Nonparametric variables were compared between groups with the Mann–Whitney U test. Categorical data are expressed as frequencies (percentages). These were compared between groups with the Fisher exact test or the Pearson χ2 test. The level of significance was set at P = 0.05 for all tests. The 2-year PFS and OS were calculated, starting from the date of random assignment, estimated with the Kaplan–Meier method, and compared with the log-rank test on an intent-to-treat basis. To assess the effects of contributing factors, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
Patient characteristics
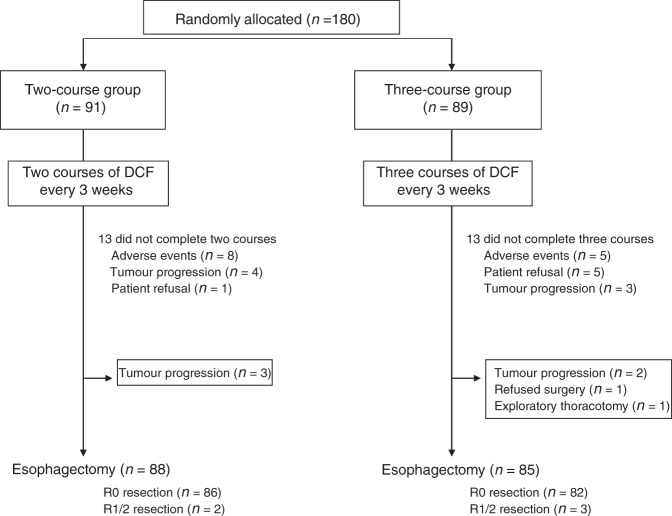
We randomly assigned 180 patients from six institutions to either two (N = 91) or three (N = 89) courses of DCF, between July 2014 and December 2018. The number of patients enrolled by each hospital ranged from 4 to 73, with a mean of 30. The patients were predominantly males (85%), with a median age of 67 years (range 37–79). The majority of tumours were located within the middle thorax (43.3%), and most were staged as cT3 (68.9%). Of the 180 patients, 143 (79.4%) had clinically-positive lymph node metastases. The treatment groups were well-balanced for baseline characteristics, including age, sex, performance status, tumour location, histological differentiation, and staging, including cT, cN, cM and cStage (Table 1). In the two-course group, 78 (85.7%) patients completed both courses of preoperative DCF. The reasons for not completing both courses of chemotherapy were: severe adverse effects (N = 8), progressive disease (N = 4), and patient refusal (N = 1). In the three-course group, 76 (85.4%) patients completed all three courses of chemotherapy. In this group, the reasons for not completing the planned NAC courses were: severe adverse effects (N = 5), patient refusal (N = 5), and progressive disease (N = 3). After preoperative chemotherapy, 88 (96.7%) patients in the two-course group and 85 (95.5%) patients in the three-course group underwent oesophagectomies. The reasons for not undergoing surgery in the two-course group were unresectable (progressive) disease (N = 3). The reasons for not undergoing surgery in the three-course group were progressive disease (N = 2), patient refusal of treatment (N = 1), and exploratory thoracotomy, due to an unresectable tumour (n = 1, Fig. 1).
Table 1.
Clinicopathological characteristics of patients with oesophageal squamous cell carcinoma that underwent 2 vs. 3 courses of neoadjuvant chemotherapy (NAC).
| Characteristic | Category | 2 NAC courses (N = 91) | 3 NAC courses (N = 89) | P value |
|---|---|---|---|---|
| Age (years) | Median | 67 | 67 | 1.000 |
| Range | 37–79 | 44–79 | ||
| Sex | Male | 78 (85.7%) | 75 (84.3%) | 0.786 |
| Female | 13 (14.3%) | 14 (15.7%) | ||
| Performance status (ECOG) | 0 | 82 (90.1%) | 81 (91.0%) | 0.836 |
| 1 | 9 (9.9%) | 8 (9.0%) | ||
| Location | Upper | 16 (17.6%) | 13 (14.6%) | 0.587 |
| Middle/lower | 75 (82.4%) | 76 (85.4%) | ||
| Histological differentiation (squamous cell carcinoma) | G1a | 11 (12.1%) | 6 (6.7%) | 0.673 |
| G2b | 13 (14.3%) | 13 (14.6%) | ||
| G3c | 4 (4.4%) | 4 (4.5%) | ||
| GX (unknown) | 63 (69.2%) | 66 (74.2%) | ||
| cT stage | 1 | 6 (6.6%) | 4 (4.5%) | 0.779 |
| 2 | 22 (24.2%) | 24 (27.0%) | ||
| 3 | 63 (69.2%) | 61 (68.5%) | ||
| cN stage | 0 | 18 (19.8%) | 19 (21.4%) | 0.965 |
| 1 | 55 (60.4%) | 53 (59.6%) | ||
| 2 | 18 (19.8%) | 17 (19.1%) | ||
| 3 | 0 (0%) | 0 (0%) | ||
| cM stage | 0 | 83 (91.2%) | 80 (89.9%) | 0.762 |
| 1 | 8 (8.8%) | 9 (10.1%) | ||
| cStage | I | 8 (8.8%) | 5 (5.6%) | 0.543 |
| II | 24 (26.4%) | 31 (34.8%) | ||
| III | 51 (56.0%) | 44 (49.4%) | ||
| IV | 8 (8.8%) | 9 (9.4%) | ||
| Total relative dose intensity (%) | 1.79 (1–2) | 2.57 (1–3) | <0.0001 |
ECOG the Eastern Cooperative Oncology Group.
aWell-differentiated, bmoderately differentiated, cpoorly-differentiated squamous cell carcinoma.
Fig. 1. CONSORT diagram shows patient allocations, treatment and outcomes.
DCF docetaxel, cisplatin, and 5-fluorouracil.
Histopathological tumour response and pathological stage
Table 2 summarises the pathological stages and histological tumour responses in each group. The median numbers of dissected lymph nodes were 61 (range 21–148) in the two-course group and 60.5 (range 25–140) in the three-course group (P = 0.878). The median number of lymph node metastases was 1 in both the two- (range 0–22) and three-course (range 0–12) groups (P = 0.2144) and, there was no difference in the distribution of pN stages between the two groups (P = 0.5840). Although, overall, the numbers of patients in each stage were similar between the groups, the incidences of both the pathological M1 stage and the pStage IV, due to subclavian lymph node metastasis, tended to be lower in the two-course group than in the three-course group (3.4 vs 10.6%, P = 0.0581, for both stages). The R0 resection rate was 97.7% in the two-course group and 96.5% in the three-course group (P = 0.621). Although the three-course group showed a relatively higher pathological complete response (CR) rate for the primary tumour than the two-course group, the two groups showed similar histological responses to NAC (P = 0.898). Other parameters did not differ between the two groups, including the rates for the pT categories and the rates of lymphatic and venous invasion.
Table 2.
Pathological stages and histological tumour responses in patients with oesophageal cancer that underwent 2 vs. 3 courses of neoadjuvant chemotherapy (NAC).
| Characteristic | Category | 2 NAC courses (N = 88) | 3 NAC courses (N = 85) | P value |
|---|---|---|---|---|
| pT stage | 0 | 8 (9.1%) | 14 (16.5%) | 0.333 |
| Tis | 0 (0%) | 1 (1.2%) | ||
| 1 | 25 (28.4%) | 19 (22.3%) | ||
| 2 | 14 (15.9%) | 18 (21.2%) | ||
| 3 | 40 (45.5%) | 34 (40.0%) | ||
| 4 | 1 (1.1%) | 0 (0%) | ||
| Number of pNs | 1 (0–22) | 1 (0–12) | 0.214 | |
| pN stage | 0 | 39 (44.3%) | 30 (35.3%) | 0.584 |
| 1 | 32 (36.4%) | 35 (41.2%) | ||
| 2 | 13 (14.8%) | 16 (18.8%) | ||
| 3 | 4 (4.5%) | 4 (4.7%) | ||
| pM stage | 0 | 85 (96.6%) | 76 (89.4%) | 0.058 |
| 1 | 3 (3.4%) | 9 (10.6%) | ||
| pStage | 0 | 5 (5.7%) | 11 (12.9%) | 0.077 |
| I | 19 (21.6%) | 12 (14.1%) | ||
| II | 33 (37.5%) | 24 (28.2%) | ||
| III | 28 (31.8%) | 29 (34.2%) | ||
| IV | 3 (3.4%) | 9 (10.6%) | ||
| Lymphatic invasion | Absent | 60 (68.2%) | 53 (62.4%) | 0.550 |
| Present | 27 (30.7%) | 29 (33.0%) | ||
| Unknown | 1 (1.1%) | 3 (3.6%) | ||
| Venous invasion | Absent | 67 (76.1%) | 63 (74.1%) | 0.930 |
| Present | 20 (22.7%) | 19 (22.4%) | ||
| Unknown | 2 (2.2%) | 3 (3.5%) | ||
| Residual tumour | R0 | 86 (97.7%) | 82 (96.5%) | 0.621 |
| R1/2 | 2 (2.3%) | 3 (3.5%) | ||
| Histopathological tumour response | Grade 0 | 3 (3.4%) | 2 (2.4%) | 0.898 |
| Grade 1a | 27 (30.7%) | 31 (36.4%) | ||
| Grade 1b | 23 (26.1%) | 18 (21.2%) | ||
| Grade 2 | 27 (30.7%) | 21 (24.7%) | ||
| Grade 3 | 8 (9.1%) | 13 (15.3%) |
Survival analysis
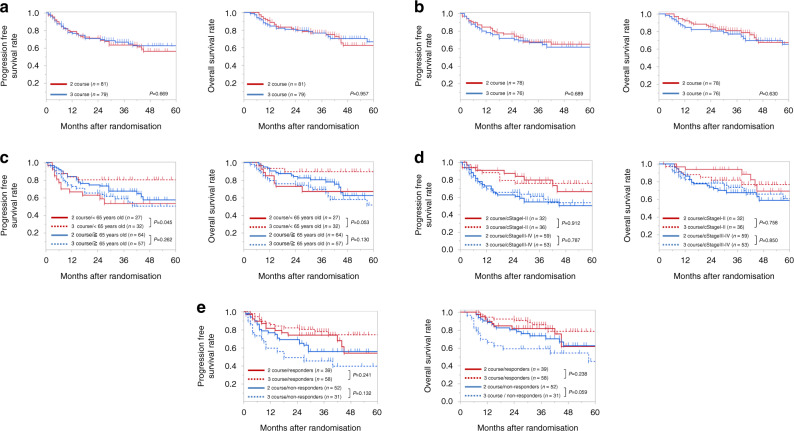
The ITT analysis showed 2-year PFS rates of 71.4% in the two-course group and 71.1% in the three-course group (P = 0.6688; Fig. 2a). In addition, 2-year OS rates were similar between the two-course (81.1%) and three-course groups (79.9%, P = 0.9567; Fig. 2a). Moreover, the per protocol analysis showed that the two-course (n = 78) and three-course (n = 76) groups had similar 2-year PFS rates (76.9 and 71.8%, respectively; P = 0.6888) and similar OS rates (85.8% and 80.7%, respectively; P = 0.6297; Fig. 2b).
Fig. 2. Kaplan–Meier survival curves show progression-free and overall survival.
(Left) Progression-free survival and (right) overall survival results are shown from (a) an intention-to-treat analysis and (b) a per protocol analysis of the two-course (red lines) and three-course (blue lines) treatment groups. c–e Kaplan–Meier survival curves show (left) progression-free and (right) overall survival in groups stratified according to the two-course (solid lines) and three-course (dotted lines) treatment groups and by the (c) age group (<65 y = red; ≥65 y = blue, (d) cStage (Stages I–II = red; Stages III–IV = blue), and (e) overall clinical response to neoadjuvant chemotherapy (responders = red; non-responders = blue).
We next performed a subgroup analysis of patients under or over age 65 years. We found that, among younger patients, two courses of DCF led to significantly shorter 2-year PFS and OS compared to three courses of DCF (2-year PFS: 63.0 vs. 80.8%, P = 0.0448, 2-year OS: 73.0 vs. 90.0%, P = 0.0526). In contrast, among older patients, the 2-year PFS (P = 0.262) and OS (P = 0.130) rates were similar between the two treatment groups (Fig. 2c).
We also performed a subgroup analysis of patients with cStage I–II or cStage III–IV tumours. We found that, in both subgroups, the 2-year PFS and OS rates were similar between the two- and three-course treatment groups (cStage I–II, PFS: 87.4 vs. 79.2%, P = 0.912, OS: 93.8 vs. 85.1%, P = 0.758; cStage III–IV, PFS: 62.7 vs. 65.8%, P = 0.787, OS: 74.1 vs. 78.5%, P = 0.850; Fig. 2d).
Finally, we performed a subgroup analysis of patients that did or did not show a clinical response to the overall NAC courses (responders and non-responders, respectively). Importantly, in the clinical non-responder group, the 2-year PFS and OS rates tended to be higher with two courses of DCF, compared to three courses of DCF (2-year PFS: 69.2 vs. 49.2%, P = 0.132, 2-year OS: 80.4 vs. 59.2%, P = 0.059). In contrast, in the clinical responder group, the 2-year PFS and OS rates were similar between the two treatment groups (2-year PFS: 74.3 vs. 82.5%, P = 0.241, 2-year OS: 82.0 vs. 90.7%, P = 0.238; Fig. 2e).
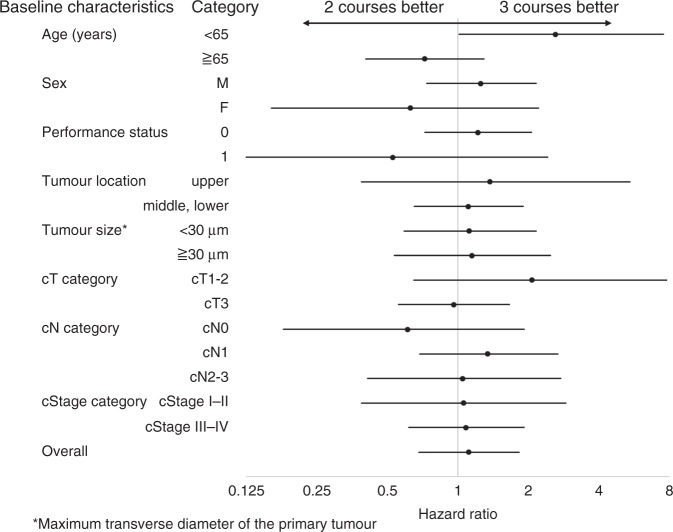
Figure 3 summarises the results of subgroup analyses of all baseline characteristics in all 180 patients with ESCC. We found that the PFS was not significantly different when two or three courses of DCF were administered to subgroups divided by sex, performance status, tumour location, tumour size, cT stage, cN stage or cStage. As mentioned above, only age showed a significant effect on the PFS achieved with two- vs. three DCF courses. In the subgroup of patients under 65 years old, three courses provided a survival benefit over two courses of DCF (HR = 2.612, 95% CI = 1.012–7.517, P = 0.0471). In contrast, no survival benefit was identified in patients over 65 years old (HR = 1.384, 95% CI = 0.777-2.467, P = 0.268).
Fig. 3. Forest plot shows hazard ratios (dots) and 95% confidence intervals (lines) for factors that could potentially affect progression-free survival.
The analysis included 180 patients with oesophageal cancer; the factors are baseline characteristics.
Finally, we examined the pattern of disease recurrence. We found that the two groups showed similar rates of overall disease recurrence (29.1 vs. 26.8%, P = 0.493; Table 3). Moreover, the patterns of recurrence were similar between the two groups. Thus, similar proportions of patients in the two groups developed recurrences in locoregional and distant sites (Table 3).
Table 3.
Patterns of disease recurrence in patients with oesophageal cancer that achieved R0 resection after 2 vs. 3 courses of neoadjuvant chemotherapy (NAC).
| Recurrence site | 2 NAC courses (N = 86) | 3 NAC courses (N = 82) | P value |
|---|---|---|---|
| Overall number of recurrences | 25 (29.1%) | 20 (26.8%) | 0.493 |
| Locoregional | 9 (34.6%) | 7 (35.0%) | 0.978 |
| Distant | 16 (61.5%) | 13 (65.0%) | 0.809 |
| Lymph node | 6 (23.1%) | 5 (25.0%) | 0.880 |
| Liver | 4 (15.4%) | 1 (5.0%) | 0.243 |
| Lung | 4 (15.4%) | 5 (25.0%) | 0.417 |
| Bone | 4 (15.4%) | 3 (15.0%) | 0.971 |
| Brain | 0 (%) | 1 (5.0%) | 0.193 |
| Pleura | 2 (7.7%) | 0 (0%) | 0.125 |
| Others | 1 (3.9%) | 2 (10.0%) | 0.403 |
Discussion
In the present multi-institutional, randomised, Phase II trial, we demonstrated that two and three courses of DCF provided similar R0 resection rates and histological responses to NAC. The 2-year PFS and OS rates were also comparable between the two groups, except among patients under 65 years old. In younger patients, the three-course treatment was associated with a significantly higher PFS compared to the two-course treatment. However, among patients that showed no clinical response to NAC, survival tended to be worse in the with three courses than with two courses of DCF. To the best of our knowledge, the present study was the first randomised trial to compare different numbers of NAC cycles for treating patients with ESCC. Our findings suggested that two courses of DCF showed potential as an optional NAC protocol for patients with locally advanced ESCC.
In recent years, multimodal treatments have proven to be an appropriate therapeutic approach for locally advanced ESCC and two major neoadjuvant approaches, i.e., NAC and neoadjuvant chemoradiotherapy (NCRT) have been adopted. In the CROSS trial [4], patients with ESCC that received NCRT, followed by surgery, achieved greater survival benefit than patients that received surgery alone. The 2-year OS (in an ITT analysis) of patients with ESCC that received NCRT plus surgery was 73.1%, which was comparable to the 81.1% (two-course group) and 79.9% (three-course group) 2-year OS rates observed in the present study. More recently, adjuvant immunotherapy has become the new standard treatment for resected oesophageal/gastroesophageal junction cancer with residual pathological disease after NCRT [30]. Thus, adjuvant immunotherapy may improve survival in patients with ESCC that receive NCRT, followed by surgery. On the other hand, NAC plus surgery improved the 5-year overall survival (OS) by 6% and 13%, compared the OS rates achieved with surgery alone in the OEO2 [31] or MAGIC [13] studies, respectively. However, those trials predominantly included adenocarcinoma, rather than squamous cell carcinoma. To date, studies have provided limited evidence on the differences in survival rates between patients treated with NCRT and patients treated with NCT. However, a recent propensity score-matched study compared NAC to NCRT for patients with ESCC in China [32]. They reported no difference in survival between patients treated with NAC and patients treated with NCRT, although a trend showed more favourable survival rates with NCRT; this trend may have been related to the significantly higher pCR rates observed with NCRT, compared to NAC. In Japan, an ongoing randomised Phase III trial (JCOG1109) [15] is currently being conducted to compare three neoadjuvant treatments: a doublet (CF) or triplet (DCF) regimen for NAC treatment, versus NCRT (41.4 Gy irradiation with a CF regimen). In the near future, those results are expected to indicate the most appropriate preoperative treatment for ESCC.
Prior to starting this study, we hypothesised that patient survival rates would be higher in the three-course group than in the two-course group. Theoretically, NAC aims to eradicate micrometastases that exist outside the surgical field. Although it remains unclear how long treatment should be continued to achieve the optimal eradication, we had hypothesised that two courses of NAC would be too short to achieve complete eradication. Therefore, we expected the three-course treatment to improve survival, particularly in advanced cases. In support of this hypothesis, when we examined short-term outcomes in a previous study, we found that two- and three-course DCF regimens in the NAC setting were equally feasible, and an additional (i.e., 3rd) DCF course led to a better clinical response rate and a relatively higher pathological CR rate for the primary tumour [16].
Nevertheless, in this study, the overall pathological responses to NAC were similar between treatment groups, and both the survival rates and the disease recurrence patterns were comparable between the two groups. Moreover, none of the subgroups showed a tendency to better survival with the three-course, compared to the two-course treatment, except among patients under 65 years old. However, the result from this subgroup (under 65 years old) was based on a post-hoc analysis with a very small sample size (n = 59); therefore, we could not definitively conclude that a three-course DCF treatment would improve survival in patients with ESCC that were under 65 years old. Albeit, we found no significant differences in background or chemotherapy-related parameters between patients under and over 65 years old, including performance status, the dose reduction rate, the discontinuation rate, the relative dose intensity, NAC-related adverse events, and the tumour response to NAC (Supplemental Table 1).
We observed some drawbacks to administering a three-course DCF. First, five patients in the three-course group refused to receive the planned courses for NAC. A potential explanation might be that, particularly among patients that developed severe adverse effects, patients were more likely to be discouraged about undergoing a third, long-duration, NAC treatment. Thus, the three-course NAC approach might negatively influence patient motivation or compliance. Another issue was that, notably, among clinical non-responders to NAC, significantly worse survival rates were observed in the three-course group than in the two-course group. In fact, out of 76 patients that completed three courses of DCF, 12 (15.8%) showed progressive disease (PD) during the 3rd course. This rate was significantly higher than the 5.1% (4/75) PD rate observed during the 2nd course in the two-course group (P = 0.0254). Moreover, patients with PD during the 3rd DCF course were prone to developing distant metastases after surgery; they comprised 4 (80%) out of 5 total disease recurrences. In addition, they tended to show worse survival compared to patients with PD during 2nd course (2-year PFS: 30.0 vs. 75.0%, P = 0.489). This finding implied that patients not responding should avoid further DCF course and, to this end, an optimal timing or method of response evaluation using PET-CT etc, needs to be established. This might also be the case with other regimens for treating oesophageal adenocarcinoma, or even other cancer types beyond the Japanese population. Considering the extra cost and adverse effects associated with an additional (i.e., 3rd) DCF cycle and the comparable survival between the two groups, we concluded that two courses of DCF chemotherapy should be considered optimal for patients in the setting of NAC treatment for resectable advanced ESCC.
This study had several limitations. This Phase II trial included a limited number of patients, therefore it was underpowered for drawing any definitive conclusions regarding the long-term outcome. Thus, our results should be confirmed in a larger, Phase III trial. In addition, the median follow-up time for surviving patients was 39 months; therefore, a follow-up study would be necessary to assess long-term outcomes for this trial. Finally, to determine the optimal NAC treatment for resectable ESCC, a larger Phase III randomised controlled trial should be performed to compare DCF regimens with CRT, another standard treatment currently administered for advanced ESCC.
In conclusion, this study was the first to demonstrate that two courses of DCF showed potential as an optional NAC treatment for locally advanced ESCC patients.
Supplementary information
Acknowledgements
The authors would like to thank the staff of all the centres that participated in the data collection process for this study.
Author contributions
TM, SU and TI contributed to the design and analysis of the results and the writing of the manuscript. MY, KT, KY, OS, KS, HM, MM, KF, AT, MH, YK and TS contributed to the implementation of the research and analysis of the results. MY, HE, YD and TY contributed to the design and implementation of the research.
Funding
This study was not supported by any funding.
Ethics approval and consent to participate
All patients provided written informed consent for trial participation. The study protocol was approved by the Institutional Review Board in each of six participating hospitals, before patient enrolment.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01726-5.
References
- 1.Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterological Surg. 2017;1:5–10. doi: 10.1002/ags3.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Hofstetter W, Swisher SG, Correa AM, Hess K, Putnam JB, Jr, Ajani JA, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:376–84. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl J Med. 2012;366:2074–84.. [DOI] [PubMed]
- 5.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 6.Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907) Ann Surg Oncol. 2012;19:68–74. doi: 10.1245/s10434-011-2049-9. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, et al. Multicenter randomized phase ii study of cisplatin and fluorouracil plus docetaxel (dcf) compared with cisplatin and fluorouracil plus adriamycin (acf) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (ogsg1003) Ann Oncol. 2017;28:116–20. doi: 10.1093/annonc/mdw439. [DOI] [PubMed] [Google Scholar]
- 8.Hagi T, Makino T, Yamasaki M, Yamashita K, Tanaka K, Saito T, et al. Pathological regression of lymph nodes better predicts long-term survival in esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery. Ann Surg. 2020; 10.1097/SLA.0000000000004238. [DOI] [PMC free article] [PubMed]
- 9.Makino T, Yamasaki M, Tanaka K, Masuike Y, Tatsumi M, Motoori M, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270:1090–5. doi: 10.1097/SLA.0000000000002808. [DOI] [PubMed] [Google Scholar]
- 10.Urakawa S, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2021;273:1141–9. doi: 10.1097/SLA.0000000000003445. [DOI] [PubMed] [Google Scholar]
- 11.Shiraishi O, Yamasaki M, Makino T, Motoori M, Miyata H, Shinkai M, et al. Feasibility of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil versus adriamycin, cisplatin, and 5-fluorouracil for resectable advanced esophageal cancer. Oncology. 2021;92:101–8. doi: 10.1159/000452765. [DOI] [PubMed] [Google Scholar]
- 12.Makino T, Yamasaki M, Miyazaki Y, Wada N, Takahashi T, Kurokawa Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (dcf) for t4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018; 10.1093/dote/dox130. [DOI] [PubMed]
- 13.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T, Morita S, Tanabe K, Nishikawa K, Ito Y, Matsui T, et al. Survival results of a randomised two-by-two factorial phase ii trial comparing neoadjuvant chemotherapy with two and four courses of s-1 plus cisplatin (sc) and paclitaxel plus cisplatin (pc) followed by d2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer. 2016;62:103–11. doi: 10.1016/j.ejca.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, et al. Three-arm phase iii trial comparing cisplatin plus 5-fu (cf) versus docetaxel, cisplatin plus 5-fu (dcf) versus radiotherapy with cf (cf-rt) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, next study) Jpn J Clin Oncol. 2013;43:752–5. doi: 10.1093/jjco/hyt061. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi O, Makino T, Yamasaki M, Tanaka K, Yamashita K, Ishida T, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: short-term outcomes of a multicenter randomized phase II trial. Esophagus. 2021; 10.1007/s10388-021-00831-3. [DOI] [PubMed]
- 17.Sobin LH GM, Wittekind C. TNM classification of malignant tumors. 7th edn. Oxford: Wiley-Blackwell; 2010.
- 18.Makino T, Yamasaki M, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, et al. Short- and long-term outcomes of larynx-preserving surgery for cervical esophageal cancer: analysis of 100 consecutive cases. Ann Surg Oncol. 2016;23:858–65. doi: 10.1245/s10434-016-5511-x. [DOI] [PubMed] [Google Scholar]
- 19.Makino T, Yamasaki M, Tanaka K, Tatsumi M, Takiguchi S, Hatazawa J, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in t4 esophageal cancer. Surgery. 2017;162:836–45. doi: 10.1016/j.surg.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Makino T, Yamasaki M, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, et al. Solitary lymph node recurrence of esophageal squamous cell carcinoma: surgical failure or systemic disease? Ann Surg Oncol. 2016;23:2087–93. doi: 10.1245/s10434-015-5086-y. [DOI] [PubMed] [Google Scholar]
- 21.Hagi T, Makino T, Yamasaki M, Tanaka K, Nishida N, Sakai D, et al. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26:4754–64. doi: 10.1245/s10434-019-07744-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, Makino T, Sato E, Noma T, Urakawa S, Takeoka T, et al. Tumor-infiltrating m2 macrophage in pretreatment biopsy sample predicts response to chemotherapy and survival in esophageal cancer. Cancer Sci. 2020;111:1103–12. doi: 10.1111/cas.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino T, Miyata H, Yamasaki M, Fujiwara Y, Takiguchi S, Nakajima K, et al. Utility of response evaluation to neo-adjuvant chemotherapy by (18)f-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148:908–18. doi: 10.1016/j.surg.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita K, Makino T, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, et al. Postoperative infectious complications are associated with adverse oncologic outcomes in esophageal cancer patients undergoing preoperative chemotherapy. Ann Surg Oncol. 2016;23:2106–14. doi: 10.1245/s10434-015-5045-7. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2020;271:875–84. doi: 10.1097/SLA.0000000000003129. [DOI] [PubMed] [Google Scholar]
- 26.Sugase T, Makino T, Yamasaki M, Tanaka K, Hashimoto T, Miyazaki Y, et al. Histological changes of superficial esophageal squamous cell carcinoma after preoperative chemotherapy. Esophagus. 2018; 10.1007/s10388-018-0626-8. [DOI] [PubMed]
- 27.Makino T, Yamasaki M, Miyata H, Yoshioka S, Takiguchi S, Fujiwara Y, et al. P53 mutation status predicts pathological response to chemoradiotherapy in locally advanced esophageal cancer. Ann Surg Oncol. 2010;17:804–11. doi: 10.1245/s10434-009-0786-9. [DOI] [PubMed] [Google Scholar]
- 28.Makino T, Yamasaki M, Takeno A, Shirakawa M, Miyata H, Takiguchi S, et al. Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 2009;101:1298–306. doi: 10.1038/sj.bjc.6605313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino T, Yamasaki M, Takemasa I, Takeno A, Nakamura Y, Miyata H, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2058–64. doi: 10.1245/s10434-009-0476-7. [DOI] [PubMed] [Google Scholar]
- 30.Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl J Med. 2021;384:1191–203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 31.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Cin Oncol. 2009;27:5062–7. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, Zhang C, Sun N, Xue L, Yang Z, Fang L, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the National Cancer Center in China. J Cancer Res Clin Oncol. 2021; 10.1007/s00432-021-03659-7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.