Abstract
Strain DCL14, which is able to grow on limonene as a sole source of carbon and energy, was isolated from a freshwater sediment sample. This organism was identified as a strain of Rhodococcus erythropolis by chemotaxonomic and genetic studies. R. erythropolis DCL14 also assimilated the terpenes limonene-1,2-epoxide, limonene-1,2-diol, carveol, carvone, and (−)-menthol, while perillyl alcohol was not utilized as a carbon and energy source. Induction tests with cells grown on limonene revealed that the oxygen consumption rates with limonene-1,2-epoxide, limonene-1,2-diol, 1-hydroxy-2-oxolimonene, and carveol were high. Limonene-induced cells of R. erythropolis DCL14 contained the following four novel enzymatic activities involved in the limonene degradation pathway of this microorganism: a flavin adenine dinucleotide- and NADH-dependent limonene 1,2-monooxygenase activity, a cofactor-independent limonene-1,2-epoxide hydrolase activity, a dichlorophenolindophenol-dependent limonene-1,2-diol dehydrogenase activity, and an NADPH-dependent 1-hydroxy-2-oxolimonene 1,2-monooxygenase activity. Product accumulation studies showed that (1S,2S,4R)-limonene-1,2-diol, (1S,4R)-1-hydroxy-2-oxolimonene, and (3R)-3-isopropenyl-6-oxoheptanoate were intermediates in the (4R)-limonene degradation pathway. The opposite enantiomers [(1R,2R,4S)-limonene-1,2-diol, (1R,4S)-1-hydroxy-2-oxolimonene, and (3S)-3-isopropenyl-6-oxoheptanoate] were found in the (4S)-limonene degradation pathway, while accumulation of (1R,2S,4S)-limonene-1,2-diol from (4S)-limonene was also observed. These results show that R. erythropolis DCL14 metabolizes both enantiomers of limonene via a novel degradation pathway that starts with epoxidation at the 1,2 double bond forming limonene-1,2-epoxide. This epoxide is subsequently converted to limonene-1,2-diol, 1-hydroxy-2-oxolimonene, and 7-hydroxy-4-isopropenyl-7-methyl-2-oxo-oxepanone. This lactone spontaneously rearranges to form 3-isopropenyl-6-oxoheptanoate. In the presence of coenzyme A and ATP this acid is converted further, and this finding, together with the high levels of isocitrate lyase activity in extracts of limonene-grown cells, suggests that further degradation takes place via the β-oxidation pathway.
Terpenes are the largest class of plant secondary metabolites (25). These compounds are hydrocarbons built from isoprene (C5) units and are classified based on the number of units linked. Monoterpenes are branched-chain C10 hydrocarbons formed from two isoprene units; they are widely distributed in nature, and more than 400 different naturally occurring monoterpenes have been identified (15). The amount of volatile monoterpenes emitted from trees is estimated to be 127 × 1014 g of carbon/year (23). Remarkably, little is known about the microbial metabolism of monoterpenes. In particular, information regarding the enzymes involved in monoterpene degradation pathways is scarce (44–46). The enzymes which have been studied most extensively are the enzymes involved in the (+)- and (−)-camphor degradation pathways of Pseudomonas putida ATCC 17453 (24, 44).
Limonene (4-isopropenyl-1-methylcyclohexene), a monocyclic monoterpene, is the most widespread terpene in the world and is formed by more than 300 plants (10). (4R)-(+)-Limonene is the most widespread form. (4R)-Limonene is the major constituent of citrus peel essential oils, in which it is usually found at concentrations between 90 and 96% (36). However, several plants form a mixture of both enantiomers, while others produce only (4S)-(−)-limonene (10).
There have been many reports concerning the biotransformation of limonene with a view towards potential production of more valuable natural flavor compounds (1, 8, 11, 12, 16, 18, 20, 29, 30, 33–35, 38, 42, 43, 51, 52). On the basis of “paper biochemistry” data, five different microbial biotransformation pathways for limonene have been proposed (Fig. 1). In most of the biotransformation studies performed previously, the researchers used microorganisms which do not grow on limonene as a sole source of carbon and energy, and many of the strains appeared to have more than one biotransformation pathway (8, 16). However, limonene is a relatively unstable compound, and some of the products identified in culture media are also the major autooxidation products of limonene (2). Since in many instances only small quantities of the products were produced, it is not known if the products formed were the result of biological activity.
FIG. 1.
Microbial biotransformation pathways for limonene. Route a is from references 8, 11, 12, 16, 18, 35, 38, and 52; route b is from references 1, 8, 16, 20, 34, 35, and 52; route c is from references 8, 16, 29, and 35; route d is from references 11, 12, 30, 33, 42, and 43; and route e is from references 29, 35, 51, and 52. The numbers in the limonene molecule refer to the carbon atom numbering of limonene.
So far, the degradation pathway for limonene has been determined by biochemical studies for only one microorganism, P. putida PL (17). In this microorganism limonene degradation is initiated by hydroxylation of limonene at the C-7 methyl group by a membrane-bound oxygenase, which results in the formation of perillyl alcohol (Fig. 1, route a). Perillyl alcohol is subsequently converted to perillyl aldehyde and perillic acid. Perillic acid is then oxidized in a coenzyme A (CoA)- and ATP-dependent reaction sequence analogous to the fatty acid β-oxidation reaction sequence; this results in the formation of 3-isopropenylpimelyl-CoA (17, 44). Two enzymes of this degradation pathway, perillyl alcohol dehydrogenase and perillyl aldehyde dehydrogenase, have been partially purified and characterized (4–6). The same degradation pathway is probably present in all other previously described microorganisms that are able to grow on limonene as a sole source of carbon and energy (11, 12, 18, 39, 43).
Previously, we isolated 56 bacteria that are able to grow on limonene as a sole source of carbon and energy (47). One of these strains, strain DCL14, neither grows on nor oxidizes perillyl alcohol, suggesting that this organism has a novel degradation pathway for limonene. In this report we discuss the enzymatic activities and intermediates involved in the degradation pathways for both (4R)-limonene and (4S)-limonene in strain DCL14.
MATERIALS AND METHODS
Strains.
Rhodococcus erythropolis DCL14 was isolated from an enrichment culture containing a 10-g sediment sample from a ditch in Reeuwijk, The Netherlands, diluted in 30 ml of mineral salts medium (pH 7.0) containing 1 mM (−)-dihydrocarveol (mixture of three stereoisomers) as the carbon and energy source in a 130-ml serum flask closed with a butyl rubber stopper. After this culture was incubated for 2 weeks on a shaker at 30°C and after two transfers into fresh medium, samples of the enrichment cultures were plated onto agar plates containing mineral salts medium. These plates were incubated in a desiccator in which (4R)-limonene was supplied in the gas phase. Colonies that developed were isolated and checked for purity by plating them onto yeast extract-glucose agar plates. Strain DCL14 was one of 10 strains isolated in this way. P. putida PpG1 (= ATCC 17453) was obtained from the American Type Culture Collection (Rockville, Md.).
Identification of strain DCL14.
The diamino acid content of the cell wall, the fatty acid profile, and the mycolic acid content of strain DCL14 were determined by the National Collection of Industrial and Marine Bacteria (Aberdeen, Scotland). The complete 16S rRNA gene sequence was determined by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany).
Growth conditions.
R. erythropolis DCL14 was subcultured once a month and was grown at 28°C on yeast extract-glucose agar plates (5 g of yeast extract per liter, 2 g of glucose per liter, 15 g of agar per liter) for 2 days, after which the plates were stored at 4°C. The growth substrate range of R. erythropolis DCL14 was determined by cultivating the strain in 25 ml of mineral salts medium (pH 7.0) (26) containing 1 mM substrate in 130-ml serum flasks. The flasks were incubated at 28°C. Cultures were grown on succinate in 5-liter Erlenmeyer flasks containing 1 liter of mineral salts medium supplemented with 2 g of disodium-succinate per liter. The flasks were incubated at 28°C on a horizontal shaker oscillating at 1 Hz with an amplitude of 10 cm.
Cells were grown on (4R)- or (4S)-limonene in a fed-batch fermentor as described previously (49). Cells were harvested by centrifugation (4°C, 10 min, 16,000 × g) and washed with 50 mM potassium phosphate buffer (pH 7.0). The pellet was resuspended in 7 ml of this buffer and stored at −20°C until it was used.
Respiration experiments.
Substrate-dependent oxygen uptake experiments were performed as described previously (26) by determining the difference in oxygen uptake rates of whole cells before substrate was added (endogenous oxygen uptake rate) and after substrate (final concentration, 0.1 mM) was added.
Preparation of cell extract.
Aliquots (7 ml) of a frozen cell suspension were thawed and disrupted by sonication (20 min; 30% duty cycle; output control, 2.3) with a Branson model 250 Sonifier. To determine cytochrome P-450-dependent limonene monooxygenase activity, cells were broken with a Retsch model MM 2000 bead mill in the presence of 100 mM KCl and 5 mM dithiothreitol. An equal volume of glass pearls (diameter, 0.5 to 0.75 mm) was added to the cell suspension, and the cells were shaken for 15 min at 1,580 rpm. Cell debris was removed by centrifugation at 20,000 × g for 20 min. The supernatant was used as the cell extract. The protein content was determined by the method of Bradford (9) by using bovine serum albumin as the standard. The crude extracts were dialyzed against 500 volumes of 25 mM potassium phosphate buffer (pH 7.0) for 16 h at 4°C.
Separation of proteins by anion-exchange chromatography.
Cell extract (15 ml, 300 mg of protein) was applied to a DEAE-Sepharose CL-6B (Pharmacia) column (2.5 by 31 cm) equilibrated with 25 mM potassium phosphate buffer (pH 7.0) at 4°C. The column was washed with 100 ml of the same buffer (flow rate, 0.75 ml/min; collected fraction volume, 7.5 ml), and then the proteins were eluted with a linear 0 to 1 M NaCl gradient in the same buffer (total volume, 1 liter).
Enzyme assays.
All assays were performed at 30°C with freshly prepared cell extracts. The specific activities that were determined spectrophotometrically were calculated by using the linear part of the reaction, and the activity values were determined by using at least two separate measurements. The reactions were started by adding the substrate, and the rates were corrected for endogenous activity. Specific activities were expressed in nanomoles per minute per milligram of protein.
Limonene 1,2-monooxygenase activity was determined by monitoring limonene degradation by gas chromatography (GC). The reaction mixtures (total volume, 2.0 ml) contained 50 mM potassium phosphate buffer (pH 7.0), 10 mM NADH, 5 μM flavin adenine dinucleotide (FAD), 1 mM limonene, and dialyzed extract in 15-ml vials fitted with Teflon Mininert valves (Supelco Inc.), which prevented evaporation of limonene. Each reaction was started by adding 1 μl of a limonene-acetone (1:2) mixture, and the vials were placed in a shaking water bath (300 rpm, 30°C). At different times vials were removed from the water bath, and the reactions were terminated by adding 1 ml of ethyl acetate. The vials were vigorously shaken to quantitatively extract the terpenes. The ethyl acetate layer was pipetted into a microcentrifuge tube and centrifuged (3 min, 13,000 × g) in order to separate the two layers. Then 1 μl of the ethyl acetate layer was analyzed by GC. The presence of cytochrome P-450-dependent monooxygenase activity was determined by recording the CO difference spectra in the presence of the substrate and sodium dithionite (21, 32). To confirm the assay procedure used for this labile enzyme, the cytochrome P-450-dependent camphor monooxygenase activity in P. putida PpG1 (3) was determined as a blank.
Limonene-1,2-epoxide hydrolase activity was determined by monitoring limonene-1,2-epoxide degradation by chiral GC as described previously (49). dichlorophenolindophenol (DCPIP)-dependent limonene-1,2-diol dehydrogenase activity was assayed spectrophotometrically by monitoring the reduction of DCPIP at 600 nm in a reaction mixture containing 50 mM citrate buffer (pH 6.0), 0.075 mM DCPIP, 1 mM limonene-1,2-diol, and cell extract. The millimolar extinction coefficient for DCPIP (pH 6.0) is 14.24 cm−1 mM−1. NAD+-dependent limonene-1,2-diol dehydrogenase activity was determined spectrophotometrically by monitoring the reduction of NADH at 340 nm in a mixture containing 50 mM glycine-NaOH buffer (pH 10.5), 1 mM NAD+, 1 mM limonene-1,2-diol, and cell extract. The extinction coefficient for NAD+ is 6.23 cm−1 mM−1. 1-Hydroxy-2-oxolimonene 1,2-monooxygenase activity was measured spectrophotometrically by monitoring the oxidation of NADPH in a reaction mixture containing 50 mM Tris-HCl (pH 8.0), 0.3 mM NADPH, 1 mM 1-hydroxy-2-oxolimonene, and cell extract. 3-Isopropenyl-6-oxoheptanoyl-CoA synthetase activity was assayed by measuring 3-isopropenyl-6-oxoheptanoyl hydroxamate formation after the reaction was carried out in the presence of hydroxylamine (31). The reaction mixture contained 0.7 M hydroxylamine, 0.1 M Tris-HCl (pH 7.2), 20 mM MgCl2, 1 mM 3-isopropenyl-6-oxoheptanoate, 15 mM ATP, 0.2 mM CoA, and cell extract. After 5, 10, and 15 min, 300-μl samples were removed and put in microcentrifuge tubes containing 300 μl of 12% trichloroacetic acid and 300 μl of 3 N HCl. Just before the samples were centrifuged, 300 μl of 5% FeCl3 · 6H2O was added to each microcentrifuge tube. The vials were centrifuged (3 min, 13,000 × g), and the absorbance at 540 nm of the supernatant was determined. The extinction coefficient for 3-isopropenyl-6-oxoheptanoyl hydroxamate was estimated to be 0.6 cm−1 mM−1 based on the extinction coefficient for succinyl hydroxamate (0.484 cm−1 mM−1) (22) and the 25% higher extinction coefficients obtained for several hydroxymates of monocarboxylic acids (31). Lactone hydrolase activity was determined by monitoring degradation of ɛ-caprolactone (a commercially available substrate analogue) by GC in 2.0-ml mixtures containing 50 mM potassium phosphate (pH 7.0), 5 mM ɛ-caprolactone, and cell extract in 15-ml vials fitted with Teflon Mininert valves (Supelco Inc.). The vials were placed in a water bath, and at different times vials were removed from the water bath and the reactions were terminated by adding 1 ml ethyl acetate as described above for the limonene 1,2-monooxygenase assay. One microliter of the ethyl acetate layer was analyzed by GC. Isocitrate lyase activity was determined as described previously (48). l-(S)-Malate dehydrogenase activity was measured spectrophotometrically by monitoring the reduction of NAD+ in a reaction mixture containing 50 mM glycine-NaOH (pH 10.5), 1 mM NAD+, 1 mM (S)-malate, and cell extract.
Product accumulation studies.
Reaction mixtures (2 ml) similar to those described above were prepared in 15-ml vials fitted with Teflon Mininert valves. At different times vials were removed from the water bath, and the reactions were terminated by adding 1 ml ethyl acetate. In the experiments in which 3-isopropenyl-6-oxoheptanoate was used, 20 μl of a 2 N H2SO4 solution was also added to extract the acid in the organic phase. The vials were vigorously shaken to quantitatively extract the terpenes. The ethyl acetate layer was pipetted into a microcentrifuge tube and centrifuged (3 min, 13,000 × g) to separate the two layers. Then 1 μl of the ethyl acetate layer was analyzed by GC. For incubation mixtures containing 3-isopropenyl-6-oxoheptanoate, the organic phase was pipetted from the aqueous phase within 30 min and was analyzed immediately in order to prevent acid-catalyzed formation of lactones from 3-isopropenyl-6-oxoheptanoate (41, 54).
In the product identification studies, the biotransformations were done in the same way except that the scale was 10 times larger. At the end of each reaction, the liquid was extracted three times with 0.5 volume of ethyl acetate. The organic layers were combined, dried over MgSO4, and evaporated to dryness with a rotary evaporator under reduced pressure. The stereoisomers were identified by using a combination of chiral GC, nonchiral GC, GC-mass spectrometry (MS), specific optical rotation determination, 1H nuclear magnetic resonance (NMR), and 13C NMR (Table 1). The data obtained were compared with data obtained with authentic samples and/or previously published data.
TABLE 1.
Identification of intermediates in the limonene degradation pathway of R. erythropolis DCL14 grown on various substrates
| Compound | Analytical technique | Dataa |
|---|---|---|
| (1S,2S,4R)-Limonene-1,2-diol | MS | m/z 170 [M+] (1), 152 (19), 137 (13), 123 (7), 108 (30), 93 (24), 82 (25), 71 (100), 58 (31), 43 (92) |
| 1H NMR | 1.23 (s, 3H), 1.70 (s, 3H), 1.4-2.0 (m, 5H), 2.1-2.3 (m, 1H), 3.61 (t, J = 2.9 Hz, 1Heq), 4.70 (s, 2H) | |
| 13C NMR | 21.1 (q), 26.2 (t), 26.5 (q), 33.7 (t), 34.0 (t), 37.5 (d), 71.4 (s), 73.9 (d), 109.0 (t), 149.3 (s) | |
| [α]D20 | +41.8° (c = 1.0, acetone) | |
| Chiral GC | rt = 16.25 (140°C), rt = 38.42 (120°C) | |
| (1R,2R,4S)-Limonene-1,2-diolb | [α]D20 | −38.5° (c = 1.2, acetone) |
| Chiral GC | rt = 16.11 (140°C), rt = 38.06 (120°C) | |
| (1R,2S,4S)-Limonene-1,2-diol | MS | m/z 170 [M+] (1), 152 (15), 137 (14), 126 (12), 108 (48), 93 (32), 81 (30), 71 (100), 58 (23), 43 (56) |
| Chiral GC | rt = 13.3 (140°C) | |
| (1S,4R)-1-Hydroxy-2-oxolimonene | MS | m/z 168 (M+)c |
| 1H NMR | 1.33 (s, 3H), 1.69 (s, 3H), 1.6-2.0 (m, 4H), 2.5-2.9 (m, 3H), 3.40 (s, 1H, OH), 4.66 (s, 1H), 4.82 (s, 1H) | |
| 13C NMR | 21.6 (q), 25.1 (q), 25.2 (t), 37.0 (t), 41.4 (t), 43.9 (d), 75.7 (s), 112.1 (t), 146.0 (s), 213.6 (s) | |
| [α]D20 | +39.3° (c = 0.29, methanol) | |
| Chiral GC | rt = 9.66 (140°C), rt = 19.45 (120°C) | |
| (1R,4S)-1-Hydroxy-2-oxolimonened | [α]D20 | −42.2° (c = 0.28, methanol) |
| Chiral GC | rt = 9.77 (140°C), rt = 19.76 (120°C) | |
| (3R)-3-Isopropenyl-6-oxoheptanoate | MS | m/z 166 [M+ - H2O]e |
| 1H NMR | 1.62 (s, 3H), 2.10 (s, 3H), 1.5-1.9 (m, 3H), 2.3-2.7 (m, 4H), 4.74 (m, 1H), 4.78 (m, 1H) | |
| 13C NMR | 18.4 (q), 26.3 (t), 30.0 (q), 38.8 (t), 41.2 (t), 42.8 (d), 113.2 (t), 145.1 (s), 177.9 (s), 208.7 (s) | |
| [α]D20 | −8.0° (c = 0.81, methanol) | |
| Chiral GC | rt = 46.08 (140°C) | |
| (3S)-3-Isopropenyl-6-oxoheptanoatef | [α]D20 | +3.5° (c = 0.78, methanol) |
| Chiral GC | rt = 46.37 (140°C) |
For MS spectra the major fragmentation peaks and relative intensities are indicated in brackets. For 1H NMR and 13C NMR spectra the chemical shifts are reported in δ (parts per million); tetramethylsilane was used as the internal standard.
The MS, 1H NMR, and 13C NMR data are the same as the data for (1S,2S,4R)-limonene-1,2-diol.
The mass spectrum is shown in Fig. 4A.
The MS, 1H NMR, and 13C NMR data are the same as the data for (1S,4R)-1-hydroxy-2-oxolimonene.
The mass spectrum is shown in Fig. 4B.
The MS, 1H NMR, and 13C NMR data are the same as the data for (3R)-3-isopropenyl-6-oxoheptanoate.
Analytical methods.
All terpenes were analyzed by chiral GC by using fused silica cyclodextrin capillary columns (type α-DEX 120; length, 30 m; inside diameter, 0.25 mm; film thickness, 0.25 μm; Supelco, Zwijndrecht, The Netherlands). GC was performed with a Chrompack CP9000 GC equipped with a flame ionization detector by using N2 as the carrier gas. The detector and injector temperatures were 250 and 200°C, respectively, and the split ratio was 1:50. The stereoisomers of limonene, limonene-1,2-epoxide, limonene-1,2-diol, 1-hydroxy-2-oxolimonene, and 3-isopropenyl-6-oxoheptanoate were analyzed isocratically at oven temperatures of 80, 100, 140, 140, and 180°C, respectively.
GC-MS analyses were carried out with a model HP5970B quadrupole MS coupled to a model HP6890 GC equipped with a fused silica capillary column (nonchiral GC; type HP-5MS; length, 30 m; inside diameter, 0.25 mm; film thickness, 0.25 μm). The carrier gas was He at a flow rate of 1.0 ml min−1. The injector temperature was 220°C, and the temperature was programmed to increase from 70 to 175°C at a rate of 7°C min−1. The injection volume was 1 μl, and the split ratio was 1:50. Electron impact MS data were obtained at 70 eV.
1H NMR and 13C NMR spectra were recorded with a Bruker model AC-E 200 spectrometer at 200 and 50 MHz, respectively. Optical rotations were measured with a Perkin-Elmer model 241 polarimeter.
Chemicals.
(4R)-Limonene and (4R)- and (4S)-limonene-1,2-epoxides were purchased from Acros, and ɛ-caprolactone was purchased from Aldrich. (4S)-Limonene and (+)-cis-(1R,2S,4R)- and (+)-trans-(1S,2R,4R)-limonene-1,2-epoxides were obtained from Fluka. (+)-Limonene-1,2-diol [an 85:15 mixture of the (1S,2S,4R) and (1R,2R,4R) isomers] was prepared from (4R)-limonene-1,2-epoxide by acid hydrolysis of the epoxide (40). Ten milliliters of (4R)-limonene-1,2-epoxide, 150 ml of demineralized water, and 0.5 ml of 2 N H2SO4 were incubated for 16 h at 37°C. The reaction mixture was extracted three times with 0.5 volume of ethyl acetate. The organic layers were combined, dried over MgSO4, and evaporated to dryness with a rotary evaporator under reduced pressure. (−)-Limonene-1,2-diol [an 85:15 mixture of the (1R,2R,4S) and (1S,2S,4S) isomers] was prepared from (4S)-limonene-1,2-epoxide as described above for (+)-limonene-1,2-diol. Optically pure (1S,2S,4R)- and (1R,2R,4S)-limonene-1,2-diols were prepared with cell extracts of R. erythropolis DCL14; 200 μl of (4R)- or (4S)-limonene-1,2-epoxide, 50 ml of 50 mM potassium phosphate buffer (pH 7.0), and 2 ml of cell extract (36 mg of protein) were incubated for 16 h at 30°C. The reaction mixture was worked up as described above. (1S,4R)- and (1R,4S)-1-hydroxy-2-oxolimonenes were prepared from (+)- and (−)-limonene-1,2-diols, respectively, by using a slightly modified method described by Kido et al. (28). To 1.68 g (10 mmol) of (+)-limonene-1,2-diol in 15 ml of CH2Cl2, 4.68 g (12.4 mmol) of pyridinium dichromate was added. After the reaction mixture was stirred at 25°C overnight, it was filtered over a short pad of Hyflo. The filtrate was concentrated under reduced pressure, and the resulting diastereomeric mixture was purified by flash chromatography with a 9:1 petroleum ether (bp, 40 to 60°C)-ethyl acetate mixture in order to obtain optically pure (1S,4R)-1-hydroxy-2-oxolimonene (250 mg). (1R,4S)-1-Hydroxy-2-oxolimonene (450 mg) was prepared in the same way from (−)-limonene-1,2-diol. 3-Isopropenyl-6-oxoheptanoate was synthesized from 1-hydroxy-2-oxolimonene under acidic conditions in the presence of an equimolar concentration of periodate (16). Twenty-five milligrams of 1-hydroxy-2-oxolimonene, 15 ml of 5 mM NaIO4, and 75 μl of 2 N H2SO4 were incubated for 1.5 h at 30°C. The acid concentration and incubation time were critical because of further conversion of the 3-isopropenyl-6-oxoheptanoate to the corresponding lactones (41, 54). The reaction mixture was worked up as described above. All of the other chemicals used were of the highest purity commercially available.
Nucleotide sequence accession number.
The 16 rRNA sequence of the organism used in this study has been deposited in the EMBL data bank under accession no. AJ131637.
RESULTS
Identification of strain DCL14.
Strain DCL14 was isolated from a sediment sample from a ditch by using (−)-dihydrocarveol as the sole source of carbon and energy. This organism produces whitish to pink, slightly slimy colonies on glucose-yeast extract agar. The cells form an elementary branched mycelium, which fragments into short rod-shaped and coccoid elements. It is catalase and gram positive. Temperatures greater than 30°C inhibit growth completely. The cell wall of strain DCL14 contains meso-diaminopimelic acid as the only cell wall diamino acid and also contains mycolic acids. The major fatty acids are straight-chain saturated and unsaturated acids, as well as a branched-chain acid having the methyl group on carbon 10 (10-methyloctadecanoic acid or tuberculostearic acid). A comparison of the fatty acid profile with the database at the National Collection of Industrial and Marine Bacteria resulted in low levels of similarity to Nocardia and Rhodococcus spp. More than 95% of the 16S rRNA gene nucleotide sequence of strain DCL14 has been determined. This sequence is identical to the sequence of the type strain of R. erythropolis (NCIMB 11148). Based on these chemotaxonomic and genetic data, strain DCL14 was identified as strain of R. erythropolis.
Growth characteristics.
R. erythropolis DCL14 utilizes (4R)-(+)- and (4S)-(−)-limonene, (4R)- and (4S)-limonene-1,2-epoxide, (4R)- and (4S)-limonene-1,2-diol, (4S)- and (4R)-carveol, (4S)- and (4R)-carvone, (4R)- and (4S)-dihydrocarveol, (4R)-dihydrocarvone, (−)-menthone, (−)-menthol, linalool, geraniol, isobutyrate, butyrate, propionate, acetate, lactate, succinate, ethanol, gluconate, and d-glucose as sole sources of carbon and energy for growth. (4R)- and (4S)-perillyl alcohol, (4R)- and (4S)-α-terpineol, α-terpinene, γ-terpinene, cyclohexane, cyclohexene, (±)-camphor, (+)-menthol, (+)-menthone, (1R,5R)- and (1S,5S)-α-pinene, (1S,5S)-β-pinene, α-pinene oxide, β-pinene oxide, isoprene, acetone, (R)-mandelate, and citraconate are not utilized.
Whole-cell oxidation studies.
Respiration experiments in which suspensions of (4R)- and (4S)-limonene- and succinate-grown washed cells of R. erythropolis DCL14 were used were performed to obtain information about the limonene degradation pathway in this strain (Table 2). (4R)- or (4S)-limonene-grown cells of R. erythropolis DCL14 readily oxidized limonene-1,2-epoxide, limonene-1,2-diol, 1-hydroxy-2-oxolimonene, and (4S)-carveol. Perillyl alcohol, isopulegol, and piperitone, intermediates of three other limonene biotransformation pathways (Fig. 1), were not oxidized. Succinate-grown cells did not significantly oxidize any of the monoterpenes tested (Table 2).
TABLE 2.
Rates of oxygen uptake by washed-cell suspensions of R. erythropolis DCL14 incubated with various substrates
| Substrate | Rate of oxygen uptake (nmol of O2 · min−1 · mg of protein−1) by cells grown on:a
|
||
|---|---|---|---|
| (4R)-Limonene | (4S)-Limonene | Succinate | |
| (4R)-Limonene | 270 | 240 | <5 |
| (4S)-Limonene | 310 | 215 | <5 |
| (4R)-Limonene-1,2-epoxide | 430 | 220 | <5 |
| (4S)-Limonene-1,2-epoxide | 345 | 255 | <5 |
| (1S,2S,4R)-Limonene-1,2-diol | 380 | 245 | 15 |
| (1R,2R,4S)-Limonene-1,2-diol | 370 | 220 | <5 |
| (1S,4R)-1-Hydroxy-2-oxolimonene | 210 | 185 | <5 |
| (1R,4S)-1-Hydroxy-2-oxolimonene | 190 | 160 | <5 |
| (R)-Perillyl alcohol | 25 | 20 | <5 |
| (S)-Perillyl alcohol | 30 | 15 | <5 |
| (S)-Carveol | 300 | 225 | 7 |
| (R)-Carveol | 45 | 65 | <5 |
| (±)-Isopulegol | 20 | 25 | <5 |
| Piperitone | <5 | <5 | <5 |
| (R)-α-Terpineol | 15 | 15 | <5 |
| (S)-α-Terpineol | 10 | 10 | <5 |
| Succinate | <5 | <5 | 160 |
The rates of oxygen uptake were corrected for endogenous oxygen uptake.
Limonene 1,2-monooxygenase activity.
An FAD- and NADH-dependent (4R)- and (4S)-limonene 1,2-monooxygenase activity was detected in cell extracts. In the absence of oxygen, no (4R)- or (4S)-limonene conversion was observed. The limonene-converting activity was greater when dialyzed extracts were used due to the high NADH oxidase activity in crude cell extracts. When NADH was replaced by NADPH or in the absence of FAD, the limonene degradation rate was fivefold lower. The limonene monooxygenase activity was present in the 100,000-×-g supernatant. No cytochrome P-450-dependent limonene monooxygenase activity was detected in cell extracts, as determined by using CO difference spectrum measurements.
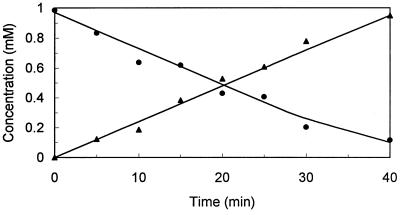
During the conversion of (4R)-limonene by dialyzed cell extracts, stoichiometric accumulation of a product was observed (Fig. 2). The MS, 1H NMR, and 13C NMR spectra and the specific optical rotation of this compound (Table 1) were identical to the spectra and specific optical rotation of authentic (1S,2S,4R)-limonene-1,2-diol and previously reported data for (1S,2S,4R)-limonene-1,2-diol (1, 16, 34, 40, 52). The retention time after chiral GC was identical to that of authentic (1S,2S,4R)-limonene-1,2-diol.
FIG. 2.
Accumulation of (1S,2S,4R)-limonene-1,2-diol from (4R)-limonene by dialyzed cell extracts of (4R)-limonene-grown cells of R. erythropolis DCL14. Each reaction mixture (30°C) contained dialyzed cell extract (1.75 mg of protein/ml), 50 mM potassium phosphate buffer (pH 7.0), 10 mM NADH, 0.005 mM FAD, and 1 mM (4R)-limonene. Symbols: ●, (4R)-limonene; ▴, (1S,2S,4R)-limonene-1,2-diol.
Stoichiometric accumulation of a product was also observed during the conversion of (4S)-limonene (data not shown). The MS, 1H-NMR, and 13C-NMR spectra, specific optical rotation, and retention time after chiral GC of this product were identical to the spectra, specific optical rotation, and retention time of authentic (1R,2R,4S)-limonene-1,2-diol (Table 1) and agreed with previously reported 13C NMR data for this compound (1).
No accumulation of limonene-1,2-epoxide was observed. Attempts to obtain this epoxide by separating the limonene-1,2-epoxide hydrolase activity from the limonene 1,2-monooxygenase activity by gel filtration or anion-exchange chromatography were unsuccessful due to the instability of limonene 1,2-monooxygenase and the fact that the limonene-1,2-epoxide hydrolase activity in cell extracts was 50-fold greater than the limonene 1,2-monooxygenase activity (Table 3).
TABLE 3.
Specific activities of enzymes involved in limonene degradation in cell extracts of R. erythropolis DCL14 grown on various carbon sources
| Enzyme | Sp act (nmol · min−1 · mg of protein−1) of cells grown on:
|
||
|---|---|---|---|
| (4R)-Limonene | (4S)-Limonene | Succinate | |
| (4R)-Limonene 1,2-monooxygenase | 17 | 14 | <0.5 |
| (4S)-Limonene 1,2-monooxygenase | 10 | 13 | <0.5 |
| (4R)-Limonene-1,2-epoxide hydrolasea | 2,900 | 2,200 | 0.7 |
| (4S)-Limonene-1,2-epoxide hydrolasea | 800 | 650 | 0.6 |
| (1S,2S,4R)-Limonene-1,2-diol dehydrogenase | |||
| DCPIP dependent | 40 | 33 | <1 |
| NAD+ dependent | 23 | 27 | 15 |
| (1R,2R,4S)-Limonene-1,2-diol dehydrogenase | |||
| DCPIP dependent | 25 | 20 | <1 |
| NAD+ dependent | 6.0 | 2.8 | 0.5 |
| (1S,4R)-1-Hydroxy-2-oxolimonene 1,2-monooxygenase | 100 | 120 | 1.0 |
| (1R,4S)-1-Hydroxy-2-oxolimonene 1,2-monooxygenase | 140 | 160 | 1.3 |
| (3R)-3-Isopropenyl-6-oxoheptanoyl-CoA synthetase | 4.6 | 3.1 | 4.7 |
| (3S)-3-Isopropenyl-6-oxoheptanoyl-CoA synthetase | 9.3 | 6.9 | 4.4 |
| Lactone hydrolaseb | 5,800 | 2,600 | 18 |
| Isocitrate lyase | 27 | 21 | <1 |
| (S)-Malate dehydrogenase | 11 | 8.5 | 19 |
Activity with the (1R,2S) stereoisomer.
Activity with ɛ-caprolactone as the substrate.
Limonene-1,2-epoxide hydrolase activity.
Cell extracts of (4R)- or (4S)-limonene-grown cells of R. erythropolis DCL14 contained a high level of limonene-1,2-epoxide hydrolase activity (Table 3). Dialysis of the extract did not affect this activity.
Both stereoisomers of (4R)-limonene-1,2-epoxide were stoichiometrically converted to optically pure (1S,2S,4R)-limonene-1,2-diol (data not shown). This is the same product as the product formed from (4R)-limonene (Fig. 2). In contrast, both stereoisomers of (4S)-limonene-1,2-epoxide were converted to optically pure (1R,2R,4S)-limonene-1,2-diol, the same limonene-1,2-diol stereoisomer as the stereoisomer formed from (4S)-limonene.
Limonene-1,2-diol dehydrogenase activity.
Both NAD+- and DCPIP-dependent (1S,2S,4R)- and (1R,2R,4S)-limonene-1,2-diol dehydrogenase activities were present in cell extracts of R. erythropolis DCL14 (Table 3). Only the DCPIP-dependent limonene-1,2-diol dehydrogenase activity, which was maximal at pH 6.0, was specifically induced after growth on limonene (Table 3), suggesting that this enzyme is the main enzyme involved in in vivo limonene degradation.
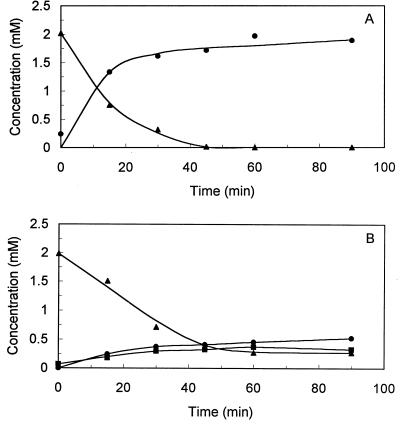
Formation of a product was observed during DCPIP-dependent conversion of (1S,2S,4R)-limonene-1,2-diol (Fig. 3A). GC-MS analysis revealed that this product had a molecular mass of 168, and 1H NMR and 13C NMR spectroscopy identified the compound as 1-hydroxy-2-oxolimonene (Fig. 4A and Table 1). The 1H NMR spectrum agreed with the spectrum previously reported for this compound (28). The retention time of the product after chiral GC and the specific optical rotation were identical to the retention time and specific optical rotation of authentic (1S,4R)-1-hydroxy-2-oxolimonene.
FIG. 3.
DCPIP-dependent conversion of (1S,2S,4R)-limonene-1,2-diol (A) and (1R,2R,4S)-limonene-1,2-diol (B) by cell extracts of R. erythropolis DCL14. Each reaction mixture (30°C) contained cell extract (4.5 mg of protein/ml), 50 mM citrate buffer (pH 6.0), 3 mM DCPIP, and 2 mM optically pure limonene-1,2-diol. Symbols: ▴, limonene-1,2-diol; ●, 1-hydroxy-2-oxolimonene [the (1S,4R) and (1R,4S) stereoisomers in panels A and B, respectively]; ■, (1R,2S,4S)-limonene-1,2-diol.
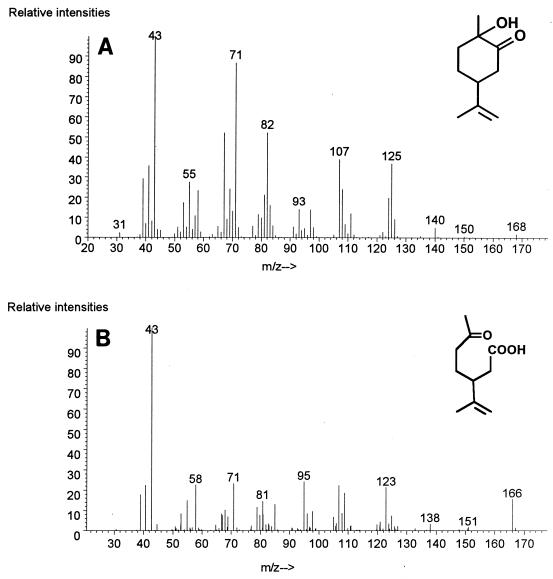
FIG. 4.
Mass spectra of metabolites in the limonene degradation pathway of R. erythropolis DCL14 obtained by GC-MS. (A) 1-Hydroxy-2-oxolimonene. (B) 3-Isopropenyl-6-oxoheptanoic acid.
When cell extract was incubated with (1R,2R,4S)-limonene-1,2-diol and DCPIP, two products were formed (Fig. 3B). The MS, 1H-NMR, and 13C-NMR spectra, the specific optical rotation, and the retention time after chiral GC of the first product were identical to data for authentic (1R,4S)-1-hydroxy-2-oxolimonene. The second product had a molecular mass of 170 and exhibited a fragmentation pattern which was almost identical to that of (1S,2S,4R)- and (1R,2R,4S)-limonene diols (Table 1). Based on the retention time and mass spectral data, we concluded that a second stereoisomer of limonene-1,2-diol was formed during conversion of (1R,2R,4S)-limonene-1,2-diol by cell extracts. In dialyzed extracts, the highest isomerization rate was observed in the presence of DCPIP and NADH. In the absence of a cofactor, no isomeric limonene-1,2-diol was formed, while a much lower conversion rate was observed with either DCPIP or NADH alone. Also, isomeric limonene-1,2-diol was formed when dialyzed extract was incubated with (1R,4S)-1-hydroxy-2-oxolimonene and NADH. We concluded, therefore, that isomerization of (1R,2R,4S)-limonene-1,2-diol was the result of the following two enzymatic activities: a DCPIP-dependent limonene-1,2-diol dehydrogenase activity and an NADH-dependent 1-hydroxy-2-oxolimonene reductase activity (Fig. 5). We also concluded that the isomeric limonene-1,2-diol was the (1R,2S,4S) stereoisomer.
FIG. 5.
(+)-(4R)- and (−)-(4S)-limonene degradation pathways in R. erythropolis DCL14. a, limonene 1,2-monooxygenase; b, limonene-1,2-epoxide hydrolase; c, DCPIP-dependent limonene-1,2-diol dehydrogenase; d, 1-hydroxy-2-oxolimonene 1,2-monooxygenase; e, spontaneous reaction; f, 3-isopropenyl-6-oxoheptanoyl-CoA synthetase; g, NAD+-dependent limonene-1,2-diol dehydrogenase.
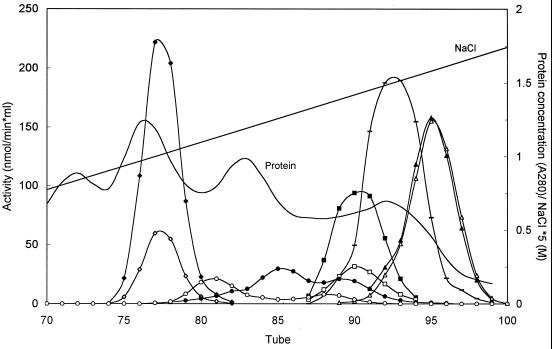
After the proteins present in cell extracts of limonene-grown strain DCL14 were separated by anion-exchange chromatography, we observed that the NAD+-dependent limonene-1,2-diol dehydrogenase activity was due to at least three different proteins (Fig. 6). Remarkably, in contrast to the DCPIP-dependent limonene-1,2-diol dehydrogenase, at least two of the NAD+-dependent limonene-1,2-diol dehydrogenases seemed to be stereospecific; one enzyme that eluted mainly in tube 81 converted (1R,2R,4S)-limonene-1,2-diol, while the enzyme that eluted mainly in tube 85 was active mainly with the (1S,2S,4R)-limonene-1,2-diol enantiomer (Fig. 6).
FIG. 6.
Separation of enzymes involved in limonene degradation present in cell extracts of (4R)-limonene-grown R. erythropolis DCL14 by anion-exchange chromatography. The activities with the (4R) stereoisomers (solid symbols) and the activities with the (4S) stereoisomers (open symbols) were determined. Symbols: ◊ and ⧫, limonene-1,2-epoxide hydrolase (1:100); □ and ■, DCPIP-dependent limonene-1,2-diol dehydrogenase; ○ and ●, NAD+-dependent limonene-1,2-diol dehydrogenase; ▵ and ▴, 1-hydroxy-2-oxolimonene 1,2-monooxygenase (1:2); —, lactone hydrolase (1:50). A280, absorbance at 280 nm.
The same limonene-1,2-diol degradation and product accumulation profiles were observed with dialyzed extracts when NAD+ was the cofactor as when DCPIP was the cofactor (Fig. 3); when (1S,2S,4R)-limonene-1,2-diol was the substrate, quantitative formation of (1S,4R)-1-hydroxy-2-oxolimonene occurred, while when (1R,2R,4S)-limonene-1,2-diol was the substrate, both (1R,4S)-1-hydroxy-2-oxolimonene and (1R,2S,4S)-limonene-1,2-diol were formed (data not shown).
1-Hydroxy-2-oxolimonene 1,2-monooxygenase activity.
An inducible 1-hydroxy-2-oxolimonene 1,2-monooxygenase activity was present in R. erythropolis DCL14 (Table 3). This Baeyer-Villiger type of monooxygenase was NADPH dependent and exhibited optimal activity at pH 8.0. In the absence of oxygen, conversion of 1-hydroxy-2-oxolimonene did not occur. When NADH was the cofactor, degradation of (1S,4R)- and (1R,4S)-1-hydroxy-2-oxolimonenes did occur; however, this resulted in the formation of limonene-1,2-diol, suggesting that the reverse of the limonene-1,2-diol dehydrogenase reaction was occurring. No dehydrogenase type of ring-opening activity, analogous to the cleavage of (methyl)acetoin by the acetoin dehydrogenase complex, was detected in cell extracts (37).
During NADPH-dependent conversion of (1S,4R)-1-hydroxy-2-oxolimonene by cell extracts, a product accumulated (Fig. 7). This product was identified by MS and by 1H NMR and 13C NMR spectroscopy as 3-isopropenyl-6-oxoheptanoate (Table 1 and Fig. 4B). The 1H NMR data agreed with partial 1H NMR data reported previously for this compound (54). The retention time of this product after chiral GC and its specific optical rotation agreed with the retention time and specific optical rotation of authentic (3R)-3-isopropenyl-6-oxoheptanoate, and the specific optical rotation was similar to the specific optical rotation previously reported for this compound (41).
FIG. 7.
Conversion of (1S,4R)-1-hydroxy-2-oxolimonene into (3R)-isopropenyl-6-oxoheptanoate by cell extracts of (4R)-limonene-grown cells of R. erythropolis DCL14. Each reaction mixture (30°C) contained cell extract (0.82 mg of protein/ml), 50 mM Tris-HCl buffer (pH 8.0), 3 mM NADPH, and 2 mM (1S,4R)-1-hydroxy-2-oxolimonene. Symbols: ▴, (1S,4R)-1-hydroxy-2-oxolimonene; ●, (3R)-3-isopropenyl-6-oxoheptanoate.
During conversion of (1R,4S)-1-hydroxy-2-oxolimonene one product accumulated (data not shown). The GC-MS fragmentation pattern, the 1H NMR and 13C NMR spectra, the specific optical rotation, and the retention time after chiral GC identified this product as (3S)-3-isopropenyl-6-oxoheptanoate (Table 1).
3-Isopropenyl-6-oxoheptanoate degradation.
Cell extracts of limonene-grown cells of R. erythropolis DCL14 exhibited 3-isopropenyl-6-oxoheptanoyl-CoA synthetase activity (Table 3). In dialyzed extracts, degradation of 3-isopropenyl-6-oxoheptanoate occurred only after CoA, ATP, and MgCl2 were added (data not shown). Elevated isocitrate lyase activity was also present in cell extracts of limonene-grown cells (Table 3).
DISCUSSION
In this report we describe the degradation of (4R)- and (4S)-limonene in R. erythropolis DCL14. Growth experiments revealed that this strain grew on a broad range of monoterpenes. However, perillyl alcohol, an intermediate in the only limonene degradation pathway described so far (Fig. 1) (17), neither supported growth nor was oxidized very well by limonene-grown cells of R. erythropolis DCL14 (47). As terpenes are very lipophilic, they can diffuse freely across the cell membrane. Consequently, microorganisms that degrade limonene via a certain pathway should also be able to oxidize the (neutral) intermediates of this pathway. The preliminary results (47) suggested that strain DCL14 contains a novel degradation pathway for limonene.
The proposed degradation pathway for (4R)- and (4S)-limonene in R. erythropolis DCL14 are shown in Fig. 5. These pathways are supported by (i) the substrate utilization pattern of the microorganism, (ii) the results of oxygen uptake experiments performed with induced and uninduced cells (Table 2), (iii) the presence of the (inducible) required enzymatic activities (Table 3), and (iv) the results of the product accumulation and identification studies (Fig. 2, 3, and 7 and Table 1). The pathways for the enantiomers of limonene are analogous, but the stereochemical configurations of the intermediates of the (4R)-limonene degradation pathway and the stereochemical configurations of the intermediates of the (4S)-limonene degradation pathway are opposite (Fig. 5).
The degradation pathway for limonene in R. erythropolis DCL14 starts with attack at the 1,2 double bond by an FAD- and NADH-dependent monooxygenase. This limonene 1,2-monooxygenase activity has not been described previously, and it resembles two previously described epoxide-forming monooxygenase activities, α-pinene 1,2-monooxygenase activity (13) and styrene monooxygenase activity (27), with respect to cofactor dependence. We have not been able to obtain limonene-1,2-epoxide accumulation with either (4R)- or (4S)-limonene, but both limonene and limonene-1,2-epoxide were converted to the same stereoisomer of limonene-1,2-diol (Fig. 2). However, we were not able to establish if the limonene-1,2-epoxide formed by limonene 1,2-monooxygenase is an optically pure compound or a mixture of two stereoisomers.
A very active and inducible limonene-1,2-epoxide hydrolase, which catalyzed the hydrolysis of limonene-1,2-epoxide to limonene-1,2-diol (Fig. 5, reaction b), was present in cell extracts of R. erythropolis DCL14. We recently purified, characterized, cloned, and sequenced the gene coding for this novel enzyme, which belongs to a novel class of epoxide hydrolases (7, 49).
Remarkably, the only reaction product of the (4R) stereoisomers of limonene and limonene-1,2-epoxide was optically pure diaxial (1S,2S,4R)-limonene-1,2-diol, whereas (4S)-limonene and limonene-1,2-epoxide yielded only (1R,2R,4S)-diaxial limonene-1,2-diol. Although the enzyme mechanism for this type of reaction is difficult to envisage, the product formed is consistent with the Fürst-Plattner rule for chemical acid- or base-catalyzed hydrolysis of substituted cyclohexene epoxides, which states that these epoxides invariably open to give diaxial products (40).
Several alcohol dehydrogenases that convert limonene-1,2-diol into 1-hydroxy-2-oxolimonene were detected in cell extracts of R. erythropolis DCL14 (Fig. 6). Only the DCPIP-dependent activity was specifically induced by growth on limonene (Table 3). We recently purified, characterized, and cloned the gene encoding this novel DCPIP-dependent alcohol dehydrogenase (50). This enzyme is a nicotinoprotein belonging to the short-chain dehydrogenase-reductase superfamily. The NAD+-dependent limonene-1,2-diol dehydrogenase activity was much less inducible by growth in the presence of limonene (Table 3), suggesting that this activity is catalyzed by nonspecific alcohol dehydrogenases with broad substrate specificities unrelated to limonene metabolism. When (1R,2R,4S)-limonene-1,2-diol was the substrate, isomerization of this compound into (1R,2S,4S)-limonene-1,2-diol was observed (Fig. 3B), which was due to the different stereospecificities of some of the limonene-1,2-diol-oxidizing alcohol dehydrogenases present in R. erythropolis DCL14.
The 1-hydroxy-2-oxolimonene formed by the limonene-1,2-diol dehydrogenases was subsequently converted by an NADPH-dependent monooxygenase. This lactone-forming monooxygenase is a novel enzymatic activity. The product of this enzymatic reaction was 3-isopropenyl-6-oxoheptanoate (Fig. 4B and 7) and not a lactone, which is the expected product for this Baeyer-Villiger type of monooxygenase. However, lactones with the oxygen between a hydroxy group and a keto group are unstable (14, 44) and spontaneously rearrange to form the corresponding oxo acids. This type of spontaneous rearrangement was observed previously in the cyclohexane-1,2-diol degradation pathway of Nocardia globerula CL1 and an Acinetobacter sp. (14, 19).
Despite the spontaneous rearrangement of 7-hydroxy-4-isopropenyl-7-methyl-2-oxo-oxepanone, a very high level of an inducible lactone hydrolase activity was detected in cell extracts of R. erythropolis DCL14 (Table 3). This enzymatic activity is not required in the proposed limonene degradation pathway of this strain, as the lactone formed rearranges spontaneously (Fig. 5). Moreover, if this enzyme were involved in the limonene degradation pathway, a product with a molecular weight of 200, not a product with a molecular weight of 184, would be formed (Fig. 4B and Table 1). Actually, in the cyclohexane-1,2-diol-degrading Acinetobacter sp., no lactone hydrolase activity was present, suggesting that lactone hydrolase activity is not necessary for degradation of cyclohexane-1,2-diol derivatives (14). However, the lactone hydrolase present in R. erythropolis DCL14 might be essential for complete mineralization of other monoterpenes used as carbon and energy sources by this strain, such as dihydrocarvone and menthol. These monoterpenes do require lactone hydrolase activity for complete mineralization (44, 53). Apparently, limonene acts as a fortuitous inducer for other monoterpene degradation pathways present in R. erythropolis DCL14.
3-Isopropenyl-6-oxoheptanoate degradation occurs only in the presence of Mg2+, CoA, and ATP, and this, together with the high isocitrate lyase activity in extracts of limonene-grown cells, suggests that further metabolism of this acyclic catabolic intermediate takes place via the β-oxidation pathway (Fig. 5).
Separation of the enzymatic activities involved in limonene degradation by anion-exchange chromatography showed that the limonene-1,2-epoxide hydrolase, DCPIP-dependent limonene-1,2-diol dehydrogenase, and 1-hydroxy-2-oxolimonene 1,2-monooxygenase activities, which convert the (4R)- and (4S) intermediates of the (4R)- and (4S)-limonene degradation pathways, were present in the same fractions (Fig. 6). This suggested that one enzyme converted both enantiomers. Recently, we purified limonene-1,2-epoxide hydrolase and DCPIP-dependent limonene-1,2-diol dehydrogenase and found that these enzymes did indeed convert all four stereoisomers of limonene-1,2-epoxide and both (1S,2S,4R)- and (1R,2R,4S)-limonene-1,2-diols, respectively, although the reaction rates were different (49, 50).
This is the first time that it has been unequivocally established that microorganisms mineralize limonene by using a degradation pathway initiated by attack of the 1,2 double bond of limonene (Fig. 5). Previously, limonene-1,2-diol was reported to be the major limonene biotransformation product in yeast and fungi (1, 8, 20, 34, 35, 52), and it was also described as a minor bacterial biotransformation product of limonene (16). In the instances in which the stereochemical configuration was established, the limonene-1,2-diol formed from (4R)- or (4S)-limonene had the same stereochemical configuration as the intermediate in the (4R)- and (4S)-limonene degradation pathway of R. erythropolis DCL14 (1, 20, 35), while in other reports only the trans nature of this diol was established (16, 34, 52). Only P. putida PL also accumulated 1-hydroxy-2-oxolimonene as a biotransformation product (16), but this strain, which grows on limonene as a sole source of carbon and energy, degrades limonene via the perillyl alcohol route (17). Only one of the fungi and yeasts examined, Cladosporium sp. strain T7, was able to grow on limonene (34). This strain accumulated high concentrations (1.5 g/liter) of limonene-1,2-diol in the culture fluid when it was grown on limonene (34). The degradation pathway for limonene in this strain was not determined, and as generally only dead-end metabolites, formed due the broad substrate specificity of some enzymes, accumulate in growth media (44), the data indicate that this fungus uses another pathway for limonene degradation.
In conclusion, R. erythropolis DCL14 degrades both (4R)- and (4S)-limonene via a novel degradation pathway that involves the following four novel enzymatic activities; limonene 1,2-monooxygenase activity, limonene-1,2-epoxide hydrolase activity, limonene-1,2-diol dehydrogenase activity, and 1-hydroxy-2-oxo-limonene 1,2-monooxygenase activity.
ACKNOWLEDGMENTS
This work was supported by grant BIO4-CT95-0049 from the European Community.
Martie S. van Dyk (University of the Free State, Bloemfontein, South Africa) is acknowledged for sending us a sample of piperitone. We thank Martin de Wit for technical assistance.
REFERENCES
- 1.Abraham W-R, Stumpf B, Kieslich K. Microbial transformation of terpenoids with 1-p-menthene skeleton. Appl Microbiol Biotechnol. 1986;24:24–30. [Google Scholar]
- 2.Anandaraman S, Reineccius G A. Stability of encapsulate orange peel oil. Food Technol. 1986;40:88–93. [Google Scholar]
- 3.Aramaki H, Fujita M, Sagara Y, Amemura A, Horiuchi T. Heterologous expression of the cytochrome P450cam hydroxylase operon and the repressor gene of Pseudomonas putida in Escherichia coli. FEMS Microbiol Lett. 1994;123:49–54. doi: 10.1111/j.1574-6968.1994.tb07200.x. [DOI] [PubMed] [Google Scholar]
- 4.Ballal N R, Bhattacharyya P K, Rangachari P N. Perillyl alcohol dehydrogenase from a soil pseudomonad. Biochem Biophys Res Commun. 1966;23:473–478. doi: 10.1016/0006-291x(66)90752-2. [DOI] [PubMed] [Google Scholar]
- 5.Ballal N R, Bhattacharyya P K, Rangachari P N. Perillyl aldehyde dehydrogenase from a soil pseudomonad. Biochem Biophys Res Commun. 1967;29:275–280. doi: 10.1016/0006-291x(67)90448-2. [DOI] [PubMed] [Google Scholar]
- 6.Ballal N R, Bhattacharyya P K, Rangachari P N. Microbiological transformations of terpenes. Part XIV. Purification & properties of perillyl alcohol dehydrogenase. Indian J Biochem. 1968;5:1–6. [PubMed] [Google Scholar]
- 7.Barbirato F, Verdoes J C, de Bont J A M, van der Werf M J. The Rhodococcus erythropolis DCL14 limonene-1,2-epoxide hydrolase gene encodes an enzyme belonging to a novel class of epoxide hydrolases. FEBS Lett. 1998;438:293–296. doi: 10.1016/s0014-5793(98)01322-2. [DOI] [PubMed] [Google Scholar]
- 8.Bowen E R. Potential by-products from microbial transformation of d-limonene. Florida State Horticultural Society; 1975. pp. 304–308. [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Burdock G A. Fenaroli’s handbook of flavour ingredients. 3rd ed. Boca Raton, Fla: CRC Press; 1995. p. 107. [Google Scholar]
- 11.Cadwallader K R, Braddock R J, Parish M E, Higgins D P. Bioconversion of (+)-limonene by Pseudomonas gladioli. J Food Sci. 1989;54:1241–1245. [Google Scholar]
- 12.Chang H C, Oriel P. Bioproduction of perillyl alcohol and related monoterpenes by isolates of Bacillus stearothermophilus. J Food Sci. 1994;59:660–662. , 686. [Google Scholar]
- 13.Colocousi A, Saqib K M, Leak D J. Mutants of Pseudomonas fluorescens NCIMB 11671 defective in the catabolism of α-pinene. Appl Microbiol Biotechnol. 1996;45:822–830. [Google Scholar]
- 14.Davey J F, Trudgill P W. The metabolism of trans-cyclohexan-1,2-diol by an Acinetobacter species. Eur J Biochem. 1977;74:115–127. doi: 10.1111/j.1432-1033.1977.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 15.Devon T K, Scott A I. Handbook of naturally occurring compounds. II. Terpenes. New York, N.Y: Academic Press; 1972. The monoterpenes; pp. 3–54. [Google Scholar]
- 16.Dhavalikar R S, Bhattacharyya P K. Microbiological transformations of terpenes. Part VIII. Fermentation of limonene by a soil pseudomonad. Indian J Biochem. 1966;3:144–157. [PubMed] [Google Scholar]
- 17.Dhavalikar R S, Rangachari P N, Bhattacharyya P K. Microbiological transformations of terpenes. Part IX. Pathways of degradation of limonene in a soil pseudomonad. Indian J Biochem. 1966;3:158–164. [PubMed] [Google Scholar]
- 18.Dhere S G, Dhavlikar R S. Microbial transformations of terpenoids: limonene. Sci Cult. 1970;7:292. [Google Scholar]
- 19.Donoghue N A, Trudgill P W. The metabolism of cyclohexane-1,2-diols by Nocardia globerula CL1. Biochem Soc Trans. 1973;1:1287–1290. [Google Scholar]
- 20.Draczyńska-Łusiak B, Siewiński A. Enantioselectivity of the metabolism of some monoterpenic components of coniferous tree resin by Armillariella mellea (honey fungus) J Basic Microbiol. 1989;29:269–275. [Google Scholar]
- 21.Eltis L D, Karlson U, Timmis K N. Purification and characterization of cytochrome P450rr1 from Rhodococcus rhodochrous. Eur J Biochem. 1993;213:211–216. doi: 10.1111/j.1432-1033.1993.tb17750.x. [DOI] [PubMed] [Google Scholar]
- 22.Gibson J, Upper C D, Gunsalus I C. Succinyl coenzyme A synthetase from Escherichia coli. I. Purification and properties. J Biol Chem. 1967;242:2474–2477. [PubMed] [Google Scholar]
- 23.Guenther A, Hewitt C N, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Ledau M, McKay W A, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. A global model of natural volatile organic compound emissions. J Geophys Res. 1995;100:8873–8892. [Google Scholar]
- 24.Gunsalus I C, Marshall V P. Monoterpene dissimilation: chemical and genetic models. Crit Rev Microbiol. 1971;1:291–310. [Google Scholar]
- 25.Harborne J B. Recent advances in the ecological chemistry of plant terpenoids. In: Harborne J B, Tomas-Barberan F A, editors. Ecological chemistry and biochemistry of plant terpenoids. Oxford, United Kingdom: Clarendon Press; 1991. pp. 399–426. [Google Scholar]
- 26.Hartmans S, Smits J P, van der Werf M J, Volkering F, de Bont J A M. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl Environ Microbiol. 1989;55:2850–2855. doi: 10.1128/aem.55.11.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmans S, van der Werf M J, de Bont J A M. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol. 1990;56:1347–1351. doi: 10.1128/aem.56.5.1347-1351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kido F, Yamaji K, Sinha S C, Abiko T, Kato M. Carbocyclic construction by the [2,3]sigmatropic rearrangement of cyclic sulfonium ylides. A new entry for the stereoselective synthesis of substituted cyclohexanones. Tetrahedron. 1995;51:7697–7714. [Google Scholar]
- 29.Kieslich K, Abraham W R, Stumpf B, Thede B, Washausen P. Transformations of terpenoids. In: Brunke E-H, editor. Progress in essential oil research. Berlin, Germany: Walter de Gruyter; 1986. pp. 367–394. [Google Scholar]
- 30.Kraidman G, Mukherjee B B, Hill I D. Conversion of d-limonene into an optically active isomer of α-terpineol by a Cladosporium species. Bacteriol Proc. 1969;1969:63. [Google Scholar]
- 31.Lipmann F, Tuttle L C. A specific micromethod for the determination of acyl phosphates. J Biol Chem. 1945;159:21–28. [Google Scholar]
- 32.Liu W, Rosazza J P N. A soluble Bacillus cereus cytochrome P-450cin system catalyzes 1,4-cineole hydroxylations. Appl Environ Microbiol. 1993;59:3889–3893. doi: 10.1128/aem.59.11.3889-3893.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattison J E, McDowell L L, Baum R H. Cometabolism of selected monoterpenoids by fungi associated with monoterpenoid containing plants. Bacteriol Proc. 1971;1971:141. [Google Scholar]
- 34.Mukherjee B B, Kraidman G, Hill I D. Synthesis of glycols by microbial transformation of some monocyclic terpenes. Appl Microbiol. 1973;25:447–453. doi: 10.1128/am.25.3.447-453.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noma Y, Yamasaki S, Asakawa Y. Biotransformation of limonene and related compounds by Aspergillus cellulosae. Phytochemistry. 1992;31:2725–2727. [Google Scholar]
- 36.Nonino E A. Where is the citrus industry going? Perfumer Flavorist. 1997;22:53–58. [Google Scholar]
- 37.Opperman F B, Schmidt B, Steinbüchel A. Purification and characterization of acetoin:2,6-dichlorophenolindophenol oxidoreductase, dihydolipoamide dehydrogenase, and dihydrolipoamide acetyltransferase of the Pelobacter carbinolicus acetoin dehydrogenase enzyme system. J Bacteriol. 1991;173:757–767. doi: 10.1128/jb.173.2.757-767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rama Devi J, Bhattacharyya P K. Microbiological transformations of terpenes. Part XXII. Fermentation of geraniol, nerol & limonene by a soil pseudomonad, Pseudomonas incognita (Linalool strain) Indian J Biochem Biophys. 1977;14:288–291. [PubMed] [Google Scholar]
- 39.Rama Devi J, Bhattacharyya P K. Microbiological transformations of terpenes. Part XXIV. Pathways of degradation of linalool, geraniol, nerol & limonene by Pseudomonas incognita (Linalool strain) Indian J Biochem Biophys. 1977;14:359–363. [PubMed] [Google Scholar]
- 40.Royals E E, Leffingwell J C. Reactions of the limonene-1,2-oxides. I. The stereospecific reactions of the (+)-cis- and (+)-trans-limonene-1,2-oxides. J Org Chem. 1966;31:1937–1944. [Google Scholar]
- 41.Schmidt H. p-Menthen-(8.9)-diol-(1.2), ein autoxydationsproduct des limonenes. Berichte. 1949;82:11–16. [Google Scholar]
- 42.Tan Q, Day D F. Bioconversion of limonene to α-terpineol by immobilized Penicillium digitatum. Appl Microbiol Biotechnol. 1998;49:96–101. [Google Scholar]
- 43.Teunissen M J, de Bont J A M. Will terpenes be of any significance in future biotechnology? In: Étiévant P, Schreier P, editors. Bioflavour 95. Paris, France: INRA; 1995. pp. 329–337. [Google Scholar]
- 44.Trudgill P W. Terpenoid metabolism by Pseudomonas. In: Sokatch J R, editor. The bacteria, a treatise on structure and function. X. Orlando, Fla: Academic Press; 1986. pp. 483–525. [Google Scholar]
- 45.Trudgill P W. Microbial metabolism and transformation of selected monoterpenes. In: Ratledge C, editor. Biochemistry of microbial degradation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 33–61. [Google Scholar]
- 46.van der Werf M J, de Bont J A M, Leak D J. Opportunities in microbial biotransformation of monoterpenes. Adv Biochem Eng Biotechnol. 1997;55:147–177. [Google Scholar]
- 47.van der Werf M J, de Bont J A M. Screening for microorganisms converting limonene into carvone. Stud Org Chem. 1998;53:231–234. [Google Scholar]
- 48.van der Werf M J, Guettler M V, Jain M K, Zeikus J G. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch Microbiol. 1997;167:332–342. doi: 10.1007/s002030050452. [DOI] [PubMed] [Google Scholar]
- 49.van der Werf M J, Overkamp K, de Bont J A M. Limonene-1,2-epoxide hydrolase from Rhodococcus erythropolis DCL14 belongs to a novel class of epoxide hydrolases. J Bacteriol. 1998;180:5052–5057. doi: 10.1128/jb.180.19.5052-5057.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Werf, M. J., C. van der Ven, F. Barbirato, M. H. M. Eppink, J. A. M. de Bont, and W. J. van Berkel. Stereoselective carveol dehydrogenase from Rhodococcus erythropolis DCL14. A novel nicotinoprotein belonging to the short-chain dehydrogenase/reductase superfamily. Submitted for publication. [DOI] [PubMed]
- 51.van Dyk M S, van Rensburg E, Moleleki N. Hydroxylation of (+)limonene, (−)α-pinene and (−)β-pinene by a Hormonema sp. Biotechnol Lett. 1998;20:431–436. [Google Scholar]
- 52.van Rensburg E, Moleleki N, van der Walt J P, Botes P J, van Dyk M S. Biotransformation of (+)-limonene and (−)-piperitone by yeasts and yeast-like fungi. Biotechnol Lett. 1997;19:779–782. [Google Scholar]
- 53.Williams D R, Trudgill P W. Ring cleavage reactions in the metabolism of (−)-menthol and (−)-menthone by a Corynebacterium sp. Microbiology. 1994;140:611–616. [Google Scholar]
- 54.Wolinsky J, Chan D. Carbonyl-olefin reactions. The cyclization of 3-isopropenyl-6-oxoheptanoic acid. J Org Chem. 1966;31:2471–2474. [Google Scholar]