Abstract
We examined the effects of the COVID-19 pandemic on the screening, diagnosis and treatment of breast cancer in Hungary based on administrative data until June 2021, covering three pandemic waves. After correcting for trend and seasonality, the number of mammography examinations decreased by 68% in 2020q2, was around its usual level in 2020q3 and was reduced by 20–35% throughout 2020q4-2021q2. The reduction was caused by a combination of supply-side (temporary suspensions of screening) and demand-side (lower screening participation during the pandemic waves) factors. The number of new breast cancer diagnoses and mastectomy surgeries responded with a lag, and were below their usual level by 15-30% in all quarters between 2020q2 and 2021q2, apart from 2020q4, when there was no significant difference. Using a regression discontinuity framework, we found that the partial mastectomy rate (indicative of early diagnosis) dropped more substantially in 2020q2 in the 61–65 years old age group that was just below the age cut-off of organized screening than in the 66–70 years old age group, and this difference was partially offset in 2021q1. We suggest that policymakers need to motivate the target population (by providing both information and incentives) to catch up on missed screenings.
Keywords: Breast cancer incidence, Breast cancer screening, COVID-19, Partial mastectomy, Regression discontinuity
1. Introduction
Timely detection, diagnosis and treatment have important consequences on the outcomes of cancer patients. The impact of even a short-term delay (e.g., four-week in cancer treatment) can have a significant impact on mortality [12]. However, the COVID-19 pandemic has disrupted the spectrum of cancer care. Interventions to allocate more healthcare staff and resources to address the pandemic and to minimize the risk of SARS-CoV-2 transmission have led to suspending cancer screening of asymptomatic individuals at least temporarily, diagnostic interventions were delayed [22] and the management of cancer patients was hurdled by several direct and indirect effects [21].
Indeed, population-based quantitative studies indicate that the number of new cancer diagnoses substantially decreased in the first months of the pandemic and (partly) recovered afterwards. However, both the decrease and the subsequent recovery were heterogeneous across cancer types, age groups and gender [4,9,11]. Arguably, the drop in diagnoses was to some extent due to the limited access to cancer screening tests. For instance, it was shown that in the Dutch colorectal cancer screening programme a quarter of the individuals due for screening had not received invitation, and the participation rate was significantly lower than expected [17]. Similarly, in Germany, approximately one in ten individuals postponed cancer screening during the pandemic [10].
As far as breast cancer is concerned, population-based national screening programmes with mammography have been widely implemented across the world (and especially within the European Union) before the pandemic [7]. These services were paused or operated with reduced capacity after the outbreak of COVID-19 [8]. For instance, a temporary suspension in the Dutch breast cancer screening programme led to a lower number of cancer diagnoses in mid-2020 compared to historical trends, and this difference was much higher among the age groups eligible for screening (50–74 years) [5]. Also, the incidence of screen-detected cases decreased gradually, even reaching close to zero in certain weeks, while non-screen-detected cases decreased to a lesser extent. Consequently, the number of lower stage tumours decreased more than higher-stage ones in the Netherlands [6].
Hungary, the country this paper focuses on, is a European Union member state with approximately 9.7 million inhabitants. It has a single-payer public healthcare system, where the vast majority of outpatient and inpatient services do not formally require co-payments (although informal payments were common before a recently legislated ban on them, and there is a growing private healthcare sector as well). The country has a long history of population-level breast cancer screening, having established the national programme in 2002 for women aged 45–65 years with a two-year screening interval [1]. Before the pandemic, the examination rate in the target population was between 45 and 60% depending on the age group [2].
As Supplementary Fig. A1 shows, after a relatively mild first wave, Hungary was hit particularly hard – even in international comparison – by the second (2020q4) and the third (2021q1–2021q2) waves of the pandemic, with a death toll of 30,000 people, or 0.3% of the population, until June 2021 [26]. Meanwhile, a number of measures were introduced in the healthcare sector to contain the spread of the disease and to allocate healthcare resources to the treatment of COVID-19 patients (see [23], for a review). In particular, organized screening was suspended twice: first during the first wave for 2.5 months between 15 March 2020 and 1 June 2020 (with a gradual resumption) [13,14], and second for a much shorter period between 9 April and 29 April 2021 (during the third wave of the pandemic) [15,19]. The regulation specifically stated that the suspension of screening – and in fact any other suspension in outpatient or inpatient care – did not apply to diagnostic procedures and the treatment of cancer patients.
In this paper, based on nationwide administrative data, we investigate the impact of COVID-19 on patient pathways from screening and early diagnosis to the treatment of breast cancer in Hungary. We examine the heterogeneous effect of COVID-19 on breast cancer care by age group. In particular, we exploit the age cut-off of organized screening at 65 years to analyse the effect of screening disruption as a quasi-experiment.
2. Materials and methods
2.1. Patient pathway in breast cancer
A simplified patient pathway in breast cancer can be described as follows ([18]; NHS [20]). After a patient obtains an abnormal screening mammogram or presents with symptoms in the healthcare system, further diagnostic procedures are performed that rule out or prove the presence of malignancy and determine the stage of the disease. Apart from stage IV cancer (that has already spread beyond the breast and nearby lymph nodes), surgery is the mainstay of treatment. Two main types are (1) breast conserving surgery or partial mastectomy (when only the cancer is removed with some normal tissue around it, but not the whole breast) and (2) total mastectomy, i.e. the removal of the whole breast. (Although the terminology is somewhat ambiguous, we followed the Hungarian DRG system by referring to the two groups of surgeries as “partial” and “total” mastectomy throughout the paper.) An earlier stage is associated with a greater probability of partial mastectomy as opposed to total mastectomy, although this relationship is not deterministic because of patient choice and (in some cases) clinical judgement. Screen-detected cancer is more often treated with partial mastectomy than symptomatic cancer [24].
2.2. Data
We used administrative data for outpatient and inpatient services that were collected by the National Health Insurance Fund Administration (NHIFA), the single payer of the Hungarian healthcare system, and were obtained via the NHIFA or the Pulvita system of the National Healthcare Service Centre (ÁEEK). The following variables were available on the disaggregated level of the age of the patient:
-
-
the number of patients undergoing mammography examination, defined according to the ICPM (International Classification of Procedures in Medicine) code of the outpatient event [monthly data between 2015m1 and 2021m6];
-
-
the number of newly diagnosed breast cancer patients, defined as those who were hospitalised with C50 (breast cancer) main diagnosis code but had not been hospitalised with such a code within the previous five years [monthly data between 2017m1 and 2021m6];
-
-
the number of patients undergoing total mastectomy and partial mastectomy, defined by the Hungarian DRG (diagnosis related group) code of such inpatient events [quarterly data between 2015q1 and 2021q2].
We note that screening and diagnostic mammography cannot be distinguished meaningfully in the administrative database due to the coding practices of the mammography centres. However, the combined examination rate roughly corresponds to the screening participation rate in the 45–65 years old population because the vast majority of mammography procedures are performed for screening and not for diagnostic purposes in this age group. To justify this assumption, we reviewed the administrative data before the analysis and found that in the 45–65 years age group between 2017 and 2019, the number of mammography procedures was about 100 times the number of new breast cancer cases, and only about 3% of the diagnostic ICD codes attached to the mammography procedures were cancer diagnoses. In contrast, for women older than 65 years, the latter number was 16%, hence the mammography examination rate is less indicative for the screening participation rate in the older population.
Per capita values were calculated by a division with the (calendar year specific) size of the female population (100,000 capita) of the corresponding ages.
2.3. Statistical analysis
After presenting descriptive time trends and age-specific distributions of mammography, breast cancer diagnosis and mastectomy rates, we carried out three types of statistical analysis. See Appendix 1 for the formal description of the models.
First, we estimated how the logarithm of aggregate per capita variables (and specifically of the per capita variables in the 45–65 years and the 66+ years age groups) deviated from their historical time trend and seasonality during each quarter of the pandemic. To do this, we used conventional time series regressions with linear trend, calendar quarter dummies and separate dummies for each quarter between 2020q1 an d 2021q2. Similar models with trend, seasonality and monthly indicator variables between 2020m1 and 2021m6 were estimated on the variables available on the monthly frequency.
Second, since the age cut-off of invitation to breast cancer screening is 65 years, we estimated the above types of models specifically on the 61–65 and 66–70 years age groups and calculated their quarter-specific parameters during the pandemic. Then we compared these parameters in a difference-in-differences setting, i.e. by applying interaction terms of age group with the explanatory variables in the quarterly panel data set of two age groups. This way, we could test whether the time effects during the pandemic differ in the two groups. For a robustness check, we also estimated similar models on the levels of the per capita variables (instead of the logarithms).
Third, to examine whether the structural change in the parameters really occurs at 65 years, we fitted the time series models separately on each year of age between 61 and 70 years and then plotted the quarter-specific parameter estimates during the pandemic.
Throughout the calculations, we used the Stata software (version 16).
3. Results
3.1. Aggregate time series results
According to the descriptive time series of Supplementary Fig. A2, before the COVID-19 pandemic, the quarterly mammography examination rate was on average 2470, breast cancer incidence was 37.4, total mastectomy rate was 13.2 and partial mastectomy rate was 20.2 per 100,000 female inhabitants, without substantial pre-pandemic trends in either series, and all series decreased significantly from 2020q2 onwards.
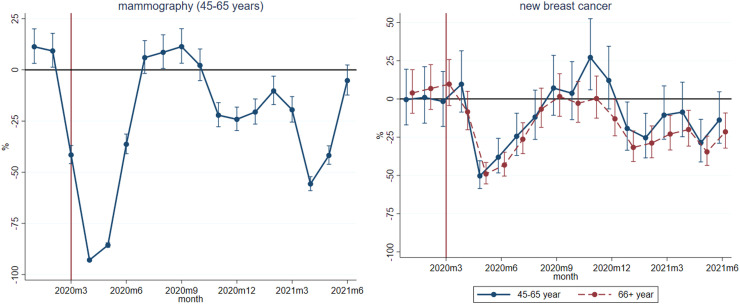
Table 1 displays the regression-estimated quarterly deviations of the series from their trend and usual seasonality during the pandemic, and Fig. 1 shows the monthly deviations of the two variables that were available on the monthly frequency. In Fig. 1, deviation of new breast cancer cases is displayed separately for the two age groups defined by the presence or absence of organized screening (45–65 and 66+ years). Fig. 1 and Table 1 show mammography examination data specifically for the 45–65 years old population because the mammography examination rate reflects on the screening participation rate only in this age group.
Table 1.
Quarterly deviation of breast cancer variables from their trend and seasonality during the COVID-19 pandemic
| Quarter-specific effects in percentages |
||||||
|---|---|---|---|---|---|---|
| Per capita dependent variable | 2020q1 | 2020q2 | 2020q3 | 2020q4 | 2021q1 | 2021q2 |
| Mammography | -9.1** | -67.5*** | 6.4 | -17.6*** | -22.7*** | -34.9*** |
| (3.5) | (1.2) | (4.0) | (3.1) | (3.2) | (2.7) | |
| Mammography (45-65 years old) | -8.0* | -71.3*** | 10.5** | -13.2*** | -17.1*** | -33.6*** |
| (4.0) | (1.3) | (4.6) | (3.8) | (3.8) | (3.1) | |
| New breast cancer | 3.1 | -27.0*** | -12.3*** | 5.5 | -21.6*** | -17.4*** |
| (3.3) | (2.3) | (2.8) | (3.4) | (2.9) | (3.1) | |
| Mastectomy | 2.6 | -19.2*** | -14.4*** | 4.8 | -16.2*** | -15.2*** |
| (3.9) | (3.1) | (3.3) | (4.0) | (3.4) | (3.4) | |
| Total mastectomy | -1.2 | -19.2*** | -18.9*** | -3.2 | -22.1*** | -19.1*** |
| (5.0) | (4.1) | (4.1) | (4.9) | (4.2) | (4.4) | |
| Partial mastectomy | 5.1 | -19.2*** | -11.3*** | 10.0** | -12.5*** | -12.7*** |
| (4.2) | (3.2) | (3.5) | (4.4) | (3.7) | (3.7) | |
*** p<0.01, ** p<0.05, * p<0.1. Percentage effects: . Standard errors in parentheses (transformed with the delta method from the logarithmic models). Quarterly data between 2017q1 and 2021q2 for new breast cancer and 2015q1-2021q2 for the other variables. Number of quarters: 18 and 26. Controls: linear trend and seasonal dummies.
Fig. 1.
Monthly deviation of the mammography examination rate and breast cancer incidence from their trend and seasonality during the COVID-19 pandemic by age group. Percentage effects: . With 95% confidence intervals (transformed from the logarithmic models). Based on monthly data between 2015q1 and 2021q2 for mammography and 2017q1-2021q2 for new breast cancer. Number of months: 54 and 78. Controls: linear trend and seasonal dummies.
According to the left panel of Fig. 1, the mammography examination rate among the 45-65 years old women decreased to close to zero in April – May 2020, then gradually increased in June 2020 and the service operated slightly above its usual level in much of the summer and early autumn. It decreased again during the subsequent waves of the pandemic (between November 2020 and March 2021), collapsed in April – May 2021 and reached again the historical average only in the summer of 2021. While the sharp reductions in the springs of 2020 and 2021 were due to the two suspensions of mammography screening, the decreases in between were mainly caused by more nuanced supply- and demand-side factors such as a decreased willingness of the population to take part in screening. According to the quarterly parameter estimates of Table 1, the number of mammography examinations in the 45-65 years old age group decreased by 71% in 2020q2, was above its usual level by 11% in 2020q3 and was again reduced by 15-35% throughout 2020q4-2021q2.
As shown in Table 1, the number of new breast cancer diagnoses and mastectomies were below their usual levels by 20–30% in 2020q2, by 12–15% in 2020q3 and by 15–25% in 2021q1-2021q2, while there was no significant difference in 2020q4. On the aggregate level, partial mastectomies decreased by a slightly lower rate than total mastectomies.
The monthly time series of breast cancer incidence on the right panel of Fig. 1 shows sharp reductions in May 2020 and 2021, a gradual increase in 2020q3 and significantly higher than average values at the end of 2020 in the 45–65 years old age group. This suggests that changes in breast cancer detection lagged behind changes in mammography examination rates by 1, 2 months. Also, after a collapse of similar magnitude at the beginning of the pandemic, breast cancer incidence of 45–65 years old women – compared to that of women older than 65 years – recovered more strongly at the end of 2020 and decreased by a smaller rate in the first half of 2021.
3.2. Analysis using the age cut-off in screening
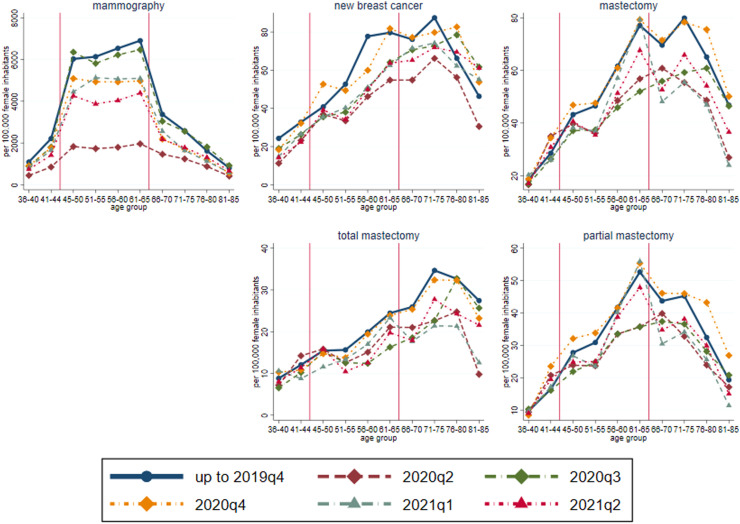
The above aggregate and broad age group specific analyses hide more nuanced age patterns. Indeed, according to Supplementary Fig. A3, before the pandemic, there was a strong discontinuity in the quarterly number of mammography examinations around age 65 (a decrease from 7000 [at age 65] to 3300 [at age 67] per 100,000 female inhabitants of the given age). This implied a fall in breast cancer incidence and partial mastectomy prevalence (for the latter, a decrease from 52 to 44 per 100,000 female inhabitants) – but not in total mastectomy prevalence. Consistent with a delay in the diagnosis just after the upper age limit of organized screening, breast cancer incidence and the mastectomy rate were lower among the 66-69 years old and reached the values of 65 years again at around 70 years, but with a substantially larger prevalence of total mastectomy and a lower prevalence of partial mastectomy. Above 75 years of age, breast cancer incidence and the mastectomy rate decreased gradually.
These patterns were influenced significantly by the pandemic. Fig. 2 shows the values for the last five quarters (2020q2-2021q2) by five-year age group, along with the historical values. For all age groups, mammography became rare in 2020q2 (and the usual jump around 65 years disappeared), the number returned afterwards to the historical level in 2020q3 and decreased again in 2020q4-2021q2. Meanwhile, the drop of mastectomy (especially of partial mastectomy) rate at age 65 completely disappeared in 2020q2-2020q3 but appeared again in 2020q4 and further strengthened in 2021q1-2021q2. Apart from 2020q4, mastectomy rates in the older age groups (above 65 years) were markedly lower than usual. Meanwhile, there was no substantial drop in breast cancer incidence and the mastectomy rate among women younger than 50 years.
Fig. 2.
Quarterly values of breast cancer variables by five-year age group (average up to 2019q4 and quarterly in 2020q2-2021q2)
Various mechanisms may explain these age patterns during the pandemic. For instance, younger patients are relatively more likely to be diagnosed with fast-growing or higher-grade breast cancer [25], potentially leading to a quicker diagnosis and hence a smaller decrease in cancer incidence and surgery. At the other end of the age spectrum, older patients may have been more likely to avoid care due to their fear from COVID-19 (on the demand side) and may have been more likely to be sorted in the healthcare system (on the supply side). Hence, to show the direct effect of lower screening activity on cancer incidence and treatment we compared the cohorts just below and just above 65 years. In this comparison it is realistic to assume that the patterns of various breast cancer types are similar and patients are treated similarly in the healthcare system in case of illness.
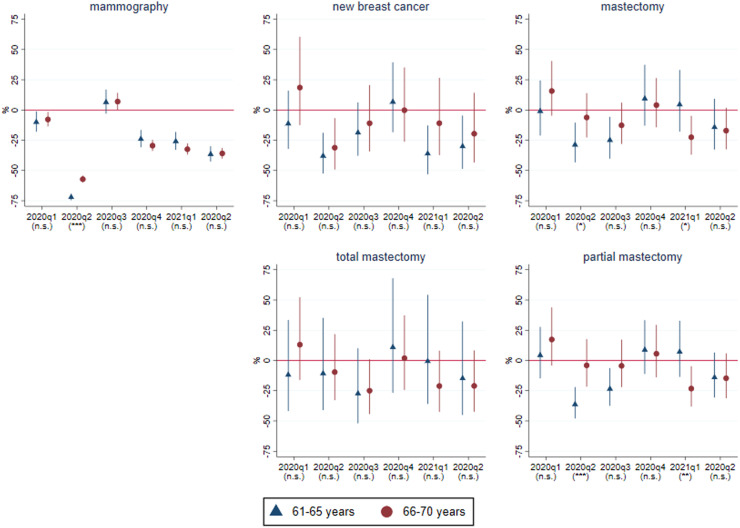
Based on the models estimated for the 61–65 and 66–70 years old age groups, Fig. 3 displays that the number of partial mastectomies decreased by 36% in 2020q2 and by 24% in 2020q3 among the 61-65 years old, while it remained essentially unchanged in these quarters among the 66–70 years old. According to the significance marks of Fig. 3 the difference between the two groups was statistically significant in 2020q2. (The detailed parameter estimates of the difference-in-differences models are presented in Appendix 1 Table A1). Meanwhile, the number of total mastectomies was roughly at its historical average for both age groups. This indicates a reduction in the diagnosis of early-stage breast cancer as a result of the disruption of mammography screening at the beginning of the pandemic. Although standard errors are much larger, a more detailed, age-specific analysis suggests that the discontinuity in partial mastectomies in 2020q2 occurred exactly between ages 65 and 66 years, in line with the observed patterns of mammography procedures (see Appendix 1 Fig. A4).
Fig. 3.
Effects of the COVID-19 pandemic on breast cancer variables for the 61-65 and 66-70 years old age groups, results from difference-in-differences models. Percentage effects: . With 95% confidence intervals (transformed from the logarithmic models). The statistical significance of the difference of the parameters of the two age groups (i.e. of the interaction terms in the difference-in-differences models) is indicated in parentheses: *** p<0.01, ** p<0.05, * p<0.1, n.s. not significant. Quarterly data between 2015q1 and 2021q2 (and, for new breast cancer cases, 2017q1-2021q2). Number of quarters: 18 and 26. Number of age groups: 2. Controls: linear trend and seasonal dummies interacted with age group. Parameter estimates are also shown in Appendix 1 Table A1.
The large drop of mammography procedures was partly counterbalanced in 2020q3, which may explain, with a lag, why we find statistically significantly more partial mastectomies in 2021q1 in the 61–65 than in the 66–70 years old age group (see the 2021q1 parameters in the corresponding panel of Fig. 3).
Finally, we note that the above results (a larger drop in partial mastectomies in 2020q2 in the 61–65 years old vs. the 66–70 years old age group, and opposite developments in 2021q1) also hold according to models estimated on the levels of the variables instead of the logarithms (Appendix 1 Table A2).
4. Discussion
Our results indicate several ways in which the pandemic affected the patient pathway of breast cancer in Hungary. First, the time series of new diagnoses of breast cancer and of mastectomies (both partial and total) demonstrate such a sizeable drop from established stable levels as cannot have been caused by anything else than COVID-19. The size of the drop and – if comparable – the overall pattern (i.e. a return to roughly the baseline level in 2020q4) are broadly in line with studies for other countries [4,6] and also with the published annual data of the National Cancer Register for Hungary for 2020 (reported by [3]). However, adding to those, we could extend the analysis to the first half of 2021 as well, when we observed further reductions probably related to the subsequent waves of the pandemic. Another strength of our paper is that, after presenting descriptive graphs, we considered trends and seasonality using relatively long historical series. All in all, the time series results suggest that the social cost of the epidemic should not only be measured in deaths and suffering caused directly by the virus, but it also had a significant indirect effect as well in terms of missed diagnoses.
Second, our results also show that, before the pandemic, there used to be a marked cut-off in mammography rates around the age of 65 (the upper age limit for organized screening), which resulted in more partial mastectomies in the case of women just under 65 years compared to those who were slightly older and thus no more invited to be screened within the organized screening programme. We also found that the drop in mammography rates at the outbreak of the pandemic was much steeper for the age group that was or would have been subject to organized screening than the neighbouring age group that was not. Even more ominously, the frequency of partial mastectomies (indicative of early diagnosis and promising a better prognosis) dropped much more from pre-pandemic levels in 2020q2-2020q3 for the below-65 age group than for the above-65 age group – albeit the difference was partly counterbalanced in 2021q1. This finding provides strong indirect evidence of the effectiveness of organized breast cancer screening in the Hungarian setting. Although substantial evidence is already available on the impact of breast cancer screening on cancer-specific mortality, these results mainly come from well-organized programmes outside Eastern Europe [27]. Therefore, findings such as ours are important in countries like Hungary, where the impact of screening has not yet been evaluated systematically.
Third, our results on the monthly level (lagged decrease of cancer incidence after the decrease in mammography rates during suspension periods and pandemic waves) suggest that the screening channel behind the reduction in cancer diagnoses and treatment acted through a combination of supply-side factors (such as the suspension of or limited access to screening) and demand-side mechanisms. The latter can have included reasons as diverse as a drop in readiness to be screened for breast cancer due to the fear of contracting COVID-19 during or on the way to the outpatient encounter, worse health service expected because of COVID-19 or a higher opportunity cost of leaving home because of secondary effects of the pandemic like increased childcare duties due to school closures, just to name a few. Although the second and the third waves of the pandemic resulted in significantly more COVID-19 cases and deaths, breast cancer screening declined the most during the first wave. This suggests that, after the shock of the first wave, the supply and the demand side of screening adjusted somewhat to the pandemic. Besides sending out invitation that had been withheld during the suspension period, there were no specific efforts at the health policy level to increase screening activities. This also implies that the effects might not be fully homogenous over time and we should be careful in generalizing the short-term observations to the complete course of the pandemic.
Fourth, we applied a regression discontinuity framework in the analysis (similarly as in [16], for the USA for an earlier period) by comparing age groups just below and just above the upper age limit of organized screening. Our findings show that this approach provides better estimates on the effect of lower screening activity during the pandemic than an aggregate analysis or a comparison of wider age groups (e.g. 45-65 vs. 66+ years) would do because many other age-related demand- and supply-side mechanisms may have operated in the healthcare system. For instance, while the aggregate total mastectomy rate declined more than the partial mastectomy rate (Table 1), their relative changes were heterogeneous across age groups (Fig. 2), and in fact we found the opposite (i.e. a larger decline of partial than of total mastectomies) in the age group of our main interest (61–65 years) (Fig. 3). Finally, we note that applying a regression discontinuity design is valid in our setting because – apart from the screening age cut-off – there is no other policy discontinuity at 65 years of age in Hungary. For instance, the effective retirement age of women is well below 65 years, so there is no jump in their labour market participation around that age.
The analysis comes with some caveats. First, our data are so recent that a key health outcome variable of cancer care and prevention, mortality rates in the cohorts under scrutiny cannot yet be meaningfully examined. To investigate this outcome without waiting too long, a modelling approach could be applied, relying on up-to-date analyses such as ours as inputs [8]. Second, and relatedly, we could only use partial mastectomy as an indicator of earlier diagnosis of breast cancer and thus a predictor of better outcome. Surely, this is an imperfect indicator since diagnostic parameters such as stage or tumour size and symptom related factors are also important to define early diagnosis. Third, we could not examine the longer-term evolution of the variables of interest. It may well be the case that breast cancer incidence and the total mastectomy rate will increase in the future with a lag as the previously undetected (and on average more advanced stage) cancer cases get diagnosed. Fourth, we should proceed with great caution when interpreting our results in terms of causality because we do not have direct evidence on the relative role of the supply- and demand-side factors discussed above. Finally, instead of using individual-level data that would be ideal for this setting, we could base our examination only on cohort-level aggregate variables with all their limitations.
5. Conclusion
Our study has important policy implications related to the patient pathways in breast cancer. First, the decreased observed incidence suggests that many patients remained undiagnosed during the pandemic, and the lower rates of partial mastectomies imply that some of the patients could have been diagnosed at an earlier stage with screening. In order to avoid the long-term consequences of these developments, policymakers need to make further efforts to reach out to and motivate the target population to catch up on missed screenings by providing both information and incentives. Second, as a policy implication beyond the pandemic, we showed that the age cut-off of breast cancer screening significantly influenced partial mastectomy rates. This indirectly provides evidence for the effectiveness of screening and further research is warranted to validate it with other long-term outcomes such as survival. Specifically, we recommend that policymakers should use these types of evidence for motivating the target population to attend breast cancer screening as it can provide a solid basis for screening related communication activities and raising the awareness among non-attendees.
Funding
Elek was supported by the János Bolyai research scholarship and by the Lendület research programme of the Hungarian Academy of Sciences. Elek, Mayer and Váradi were supported by OTKA (National Research, Development and Innovation Fund) research grant no. 134573.
Declaration of Competing Interest
None declared.
Acknowledgment
The authors would like to thank the useful comments made by the members of the COVID-19 Working Group of the Hungarian Health Economics Association.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.healthpol.2022.05.013.
Appendix 2. Details of the estimated statistical models
First, we estimated time series regressions of the form
| (1) |
where denotes time (quarter), is the per capita variable, is the j-th calendar quarter (the first quarter being the baseline) and ) are the quarters of the pandemic. The parameters of interest are , which show the quarter-specific deviation from the usual trend and seasonality during the pandemic. Finally, is the error term. The models were estimated with ordinary least squares (OLS), which is an appropriate estimator here because – as it turned out during the estimation – the error terms were not autocorrelated in either model. Similar models with trend, seasonality and monthly indicator variables between 2020m1 and 2021m6 were estimated on the variables that were available on the monthly frequency.
Second, we estimated models specifically on the 61-65 and 66-70 years age groups and then compared their parameters. Formally, we estimated the difference-in-differences specification
| (2) |
where denotes the age group (61-65 years or 66-70 years) and denotes quarter. Here, and are the quarter-specific deviations during the pandemic in the two age groups, respectively, and we evaluated the statistical significance of . This formulation is equivalent to the more usual difference-in-differences setup that uses the interaction terms of age group with each variable (trend, seasonality, pandemic dummies).
As a robustness check we also fitted the same difference-in-differences models on levels (instead of logarithms).
Third, we fitted the time series models separately on each year of age between 61 and 70 years.
Appendix 1. Additional descriptive figures and tables
References
- 1.Boncz I., Döbrőssy L., Péntek Z., et al. The attendance of the third (2006–2007) screening round of the Hungarian organized breast cancer screening programme (in Hungarian) Hungarian Oncology. 2013;57:140–146. https://huon.hu/2013/57/3/0140/0140a.pdf [Google Scholar]
- 2.Boncz I., Döbrőssy L., Péntek Z., et al. Attendance of the fourth (2008–2009) screening round of the Hungarian organized, nationwide breast cancer screening programme (in Hungarian) Orv Hetil. 2013;154(50):1975–1983. doi: 10.1556/oh.2013.29744. [DOI] [PubMed] [Google Scholar]
- 3.Central Statistical Office . 2021. Newly diagnosed malignant neoplasms.https://www.ksh.hu/stadat_files/ege/en/ege0025.html (Downloaded on 26-11-2021) [Google Scholar]
- 4.Coma E., Guiriguet C., Mora N., et al. Impact of the COVID-19 pandemic and related control measures on cancer diagnosis in Catalonia: a time-series analysis of primary care electronic health records covering about five million people. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinmohamed A.G., Cellamare M., Visser O., et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol Oncol. 2020;13:147. doi: 10.1186/s13045-020-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eijkelboom A.H., de Munck L., Lobbeset M. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Commission . 2017. Cancer Screening in the European Union (2017) Report on the implementation of the Council Recommendation on cancer screening.https://ec.europa.eu/health/sites/default/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf (Downloaded on 26-11-2021) [Google Scholar]
- 8.Figueroa J.D., Gray E., Pashayanet N. The impact of the Covid-19 pandemic on breast cancer early detection and screening. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood E., Swanton C. Consequences of COVID-19 for cancer care — a CRUK perspective. Nat Rev Clin Oncol. 2021;18:3–4. doi: 10.1038/s41571-020-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajek A., De Bock F., Huebl L., et al. Determinants of postponed cancer screening during the COVID-19 pandemic: evidence from the Nationally Representative COVID-19 Snapshot Monitoring in Germany (COSMO) Risk Manag Healthc Policy. 2021;14:3003–3011. doi: 10.2147/RMHP.S297326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton A.C., Donnelly D.W., Loughrey M.B., et al. Inequalities in the decline and recovery of pathological cancer diagnoses during the first six months of the COVID-19 pandemic: a population-based study. Br J Cancer. 2021;125:798–805. doi: 10.1038/s41416-021-01472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna T.P., King W.D., Thibodeau S., et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020;371:m4087. doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hungarian Official Gazette . 2020. 10/2020. (III. 14.) Decree of the Ministry of Human Capacities. (On the amendment of certain ministerial decrees on health matters due to the declared emergency.)https://magyarkozlony.hu/dokumentumok/9421b74af54379d07ba8934a5fe3180c7f62d0be/megtekintes (in Hungarian) [Google Scholar]
- 14.Hungarian Official Gazette . 2020. 17/2020. (IV. 30.) Decree of the Ministry of Human Capacities. (On the amendment of the 18/1998 (VI. 3.) NM decree on epidemiological measures necessary for the prevention of communicable diseases and epidemics and of the 4/2000. (II. 25.) EüM decree on the activities of general practitioners, pediatricians and dentists.)https://magyarkozlony.hu/dokumentumok/9c64e7fe063b644848c7a154b073c481466b4362/megtekintes (in Hungarian) [Google Scholar]
- 15.index hu . 2021. Preventive oncological screening resumes on Thursday.https://index.hu/belfold/2021/04/28/egeszsegugy-jarvany-kasler-miklos-szurovizsgalat-onkologia/ (in Hungarian) (Downloaded on 26-11-2021) [Google Scholar]
- 16.Kadiyala S., Strumpf E. How effective is population-based cancer screening? Regression discontinuity estimates from the US guideline screening initiation ages. Forum Health Econ Policy. 2016;19:87–139. doi: 10.1515/fhep-2014-0014. [DOI] [PubMed] [Google Scholar]
- 17.Kortlever T.L., De Jonge L., Wisse P., et al. The national FIT-based colorectal cancer screening program in the Netherlands during the COVID-19 pandemic. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of National Resources . 2014. Guideline for the treatment of breast cancer. (in Hungarian) [Google Scholar]
- 19.National Public Health Centre . 2021. The targeted (organized) oncological public health screening programmes mean the screening of the healthy population (in Hungarian)https://www.nnk.gov.hu/index.php/kozlemenyek/1080-a-nepegeszsegugyi-celu-celzott-szervezett-onkologiai-szurovizsgalatok-az-egeszsegesnek-velt-lakossag-szureset-jelentik Press release. (Downloaded on 26-11-2021) [Google Scholar]
- 20.England NHS. 2018. Clinical guidelines for the management of breast cancer. West Midlands Expert Advisory Group for Breast Cancer.https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/02/guidelines-for-the-management-of-breast-cancer-v1.pdf (Downloaded on 26-11-2021) [Google Scholar]
- 21.Raymond E., Thieblemont C., Alran S., et al. Impact of the COVID-19 outbreak on the management of patients with cancer. Target Oncol. 2020;15:249–259. doi: 10.1007/s11523-020-00721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards M., Anderson M., Carter P., et al. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1:565–567. doi: 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagan A., Bryndova L., Kowalska-Bobko I., et al. A reversal of fortune: Comparison of health system responses to COVID-19 in the Visegrad group during the early phases of the pandemic. Health Policy. 2021 doi: 10.1016/j.healthpol.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starikov A., Askin G., Blackburn A., et al. Mode of detection matters: differences in screen-detected versus symptomatic breast cancers. Clin Imaging. 2021;80:11–15. doi: 10.1016/j.clinimag.2021.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Thomas G.A., Leonard R.C.F. How age affects the biology of breast cancer. Clin Oncol. 2009;21(2):81–85. doi: 10.1016/j.clon.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 26.WHO . 2021. Daily cases and deaths by date reported to WHO.https://covid19.who.int/WHO-COVID-19-global-data.csv (Downloaded on 26-11-2021) [Google Scholar]
- 27.Zielonke N., Gini A., Jansen E.E.L., et al. Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: a systematic review. Eur J Cancer. 2020;127:191–206. doi: 10.1016/j.ejca.2019.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.