Abstract
Objectives:
Examine the association between personality measures and perceived mental fatigability.
Methods:
We performed a cross-sectional analysis in N=1,670 men, age 84.3±4.1 years. Multivariable linear regression models were used to examine the covariate adjusted association between personality measures (conscientiousness, optimism, goal reengagement, goal disengagement) and perceived mental fatigability (measured with the validated 10-item Pittsburgh Fatigability Scale (PFS)).
Results:
One standard deviation lower conscientiousness (β=−0.91, p<0.0001) and optimism (β=−0.63, p<0.0001), and higher goal reengagement (β=0.51, p=0.01) scores were independently associated with higher PFS Mental scores adjusted for age, cognitive function, self-reported health status, depressive symptoms, sleep disturbance, physical activity, and goal disengagement.
Discussion:
Greater conscientiousness, optimism, and goal reengagement were linked with less mental fatigability in older men. Personality traits may potentially contribute to early risk assessment for fatigability in later life. Future work should be longitudinal in nature and include personality assessments to confirm the temporality of the relationships observed.
Keywords: fatigue, conscientiousness, optimism, goal adjustment
Introduction
Perceived mental fatigability, a person’s susceptibility to fatigue (i.e., tiredness, lack of energy) related to mental activities that engage cognitive function, is a prevalent condition amongst older adults and associated with functional decline, several health conditions, physical and cognitive functioning, and brain health (Baran, Zhang, Anderson, McDermott, & Lin, 2020; Burke et al., 2018; Carlozzi, Boileau, Murphy, Braley, & Kratz, 2019; Salerno et al., 2019; Simonsick et al., 2018; Wasson et al., 2019). Our previous work revealed that perceived mental fatigability was moderately to highly correlated with perceived physical fatigability (r=0.73 in Long Life Family Study cohort), but functions independently as a construct (Renner et al., 2021). For example, a small pilot study (Wasson et al., 2019) found that higher perceived mental fatigability, but not perceived physical fatigability, was associated with gray matter volume in the posterior cingulate and amygdala brain regions. Further, another study (Carlozzi et al., 2019) demonstrated the convergent and discriminant validity of perceived mental and physical fatigability measures, highlighting that perceived mental fatigability was more strongly associated with Generalized Anxiety Disorder 7-item scale scores (GAD-7), while perceived physical fatigability was more strongly related to Patient Report Outcomes Measurement Information Systems (PROMIS) fatigue motivational impact and physical function measures.
Specific personality attributes may pre-dispose individuals to greater perceived mental fatigability, given well-documented associations between personality and physical and cognitive health. The most commonly studied personality measures are the “Big Five” personality measures (McCrae & Costa Jr., 2008), including conscientiousness, or the tendency to be organized, thorough, rule-abiding, and self-disciplined (De Vries & Van Heck, 2002; Smagula et al., 2016). Across multiple longitudinal studies and aging cohorts, low conscientiousness has been found to be associated with poor scores on both self-report and performance-based measures of physical activity, self-rated health status, behavioral risk factors for diabetes, depressive symptoms, higher frailty, worsening sleep quality over time and increased risk for dementia or cognitive impairment (Aschwanden et al., 2021; Duberstein et al., 2003; Hakulinen et al., 2015; Kekäläinen, Terracciano, Sipilä, & Kokko, 2020; Sanatkar et al., 2020; Stephan, Bayard, Sutin, Krizan, & Terracciano, 2018; Stephan, Sutin, Canada, & Terracciano, 2017).
Optimism, or the general expectation that good things will happen, is another well studied personality measure consistently associated with downstream health outcomes. Similar to conscientiousness, low optimism has been linked to poor global sleep quality scores and depression (De Vries & Van Heck, 2002; Klein, Kotov, & Bufferd, 2011; Martin, Friedman, & Schwartz, 2007; Smagula et al., 2016). Additionally, in the Women’s Health Initiative measurement of cardiovascular disease, optimists had a decreased risk of coronary heart disease and mortality compared to pessimists (Tindle et al., 2009).
While less studied, goal adjustment attributes have also been linked to physical and mental health outcomes in community-dwelling older samples (Barlow, Wrosch, & McGrath, 2020; Rasmussen, Scheier, & Greenhouse, 2009; Smagula et al., 2016) and in longitudinal studies of treatment-seeking cancer patients (Zhu et al., 2015). Goal disengagement, or the ability to abandon unattainable goals, is protective against the accumulation of non-fulfillment of personal goals that may cause loss of motivation, time, and resources, while goal reengagement increases one’s ability to pursue new, more obtainable objectives (Wrosch, Scheier, Miller, Schulz, & Carver, 2003).
The observed relationship between personality and downstream health outcomes highlights the same complex psychosocial mechanism by which personality may be related to perceived mental fatigability. Personality attributes predict types of coping mechanisms, adherence to medical regimes, likelihood of physical and mental health outcomes, social support networks, and other health-behavior related attributes influencing health across the lifespan (Craig, Tran, Wijesuriya, & Boord, 2006; Hooker & McAdams, 2003; Srivastava & Das, 2013). Furthermore, both goal engagement attributes are highly related to an individual’s general self-efficacy, which may influence their health behaviors and outcomes across life, including fatigue (Barlow et al., 2020). The proposed conceptual framework for these analyses outlines the complex pathways to explain the association between personality, other potential influential factors, and perceived mental fatigability (Figure 1). Sociodemographic variables such as age and race are likely to act as confounding variables because they are consistent factors that have large influences on our environment and experiences that explain our personalities and our ability to perceive mental fatigability. Other variables, such as behavior- and health-based factors have the potential to act as mediators between personality, which is relatively stable over time, and perceived mental fatigability.
Figure 1.
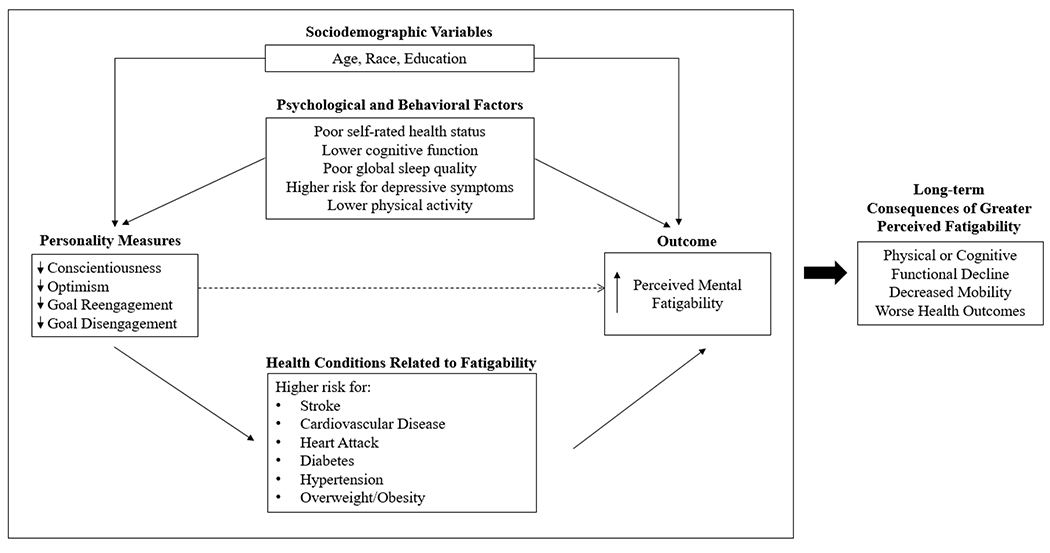
Conceptual model outlining the relationship between personality measures, covariates of interest, perceived mental fatigability, and long-term health outcomes
Moreover, personality attributes are observable earlier in the lifespan than perceived fatigability (Debast et al., 2014; Hampson & Friedman, 2008). Variation in perceived mental fatigability may be less detectable or reliable in younger groups, given traditional indices (e.g., Pittsburgh Fatigability Scale) have been validated only in older samples (Glynn et al., 2015). Thus, establishing personality correlates of perceived mental fatigability may be useful for targeting future research that seeks to identify factors at earlier ages (e.g., midlife) which signal future risk of fatigability in later life. These individuals may be prime candidates for early interventions to mitigate mental fatigue.
Personality measures have been shown to be weakly to moderately correlated with global measures of fatigue (Barlow et al., 2020; De Vries & Van Heck, 2002; Zhu et al., 2015). Measuring perceived mental fatigability in place of a traditional global fatigue assessment has emerged to be a more sensitive measure of the degree that fatigue limits one mentally in the context of physical function outcomes and serves as a prognostic marker of phenotypic aging (Renner et al., 2021; Schrack, Simonsick, & Glynn, 2020; Simonsick et al., 2018). To our knowledge, no studies have examined whether personality measures are related to the presence and severity of perceived mental fatigability.
Thus, the aim of this study was to examine the association between personality measures and perceived mental fatigability in older men. We hypothesized that lower scores on four personality domains (i.e., conscientiousness, optimism, goal reengagement and goal disengagement) would be associated with greater perceived mental fatigability, with lower conscientiousness and optimism scores having the strongest associations based on prior work in this same cohort (Smagula et al., 2016).
Methods
Study Population and Design
This study sample is from The Osteoporotic Fractures in Men Study (MrOS), a multicenter prospective longitudinal cohort study of community dwelling, ambulatory men aged 65 years and older (Blank et al., 2005). A total of 5,994 men were enrolled at baseline (2000 to 2002) from San Diego, CA; Palo Alto, CA; Pittsburgh, PA; Birmingham, AL; and Minneapolis, MN, with 10.6% of the overall sample being minority men. Additional recruitment information (Blank et al., 2005) and baseline characteristics (Orwoll et al., 2005) are reported elsewhere. The current analysis is a cross-sectional analysis of measures available from individuals who were willing and able to complete Visit 4 of MrOS (May 2014 through May 2016). All variables in the current analyses were collected at Visit 4 with the exception of date of birth, race and education, which were ascertained at the baseline assessment. The study protocol was approved by the institutional review boards at all participating centers.
Outcome Measurement - Perceived Mental Fatigability
Perceived mental fatigability was measured with the validated, 10-item, self-administered Pittsburgh Fatigability Scale (PFS) (Glynn et al., 2015; Renner et al., 2021). Participants rated the mental fatigue they would expect or imagine they would feel for activities across a range of intensities on a 0 (“no fatigue”) to 5 (“extreme fatigue”) scale; items were summed with PFS Mental scores ranging from 0 to 50 with higher scores denoting greater perceived mental fatigability (Glynn et al., 2015). Greater perceived mental fatigability was classified as PFS Mental scores ≥13 (Simonsick et al., 2018; Wasson et al., 2019). For individuals missing 1-3 responses, scores were imputed based on the mean value of the participant’s valid responses and accounted for intensity levels of the different activities as well as differences in fatigue level reported by those who had and had not done each activity (Cooper et al., 2019).
Independent Variables - Personality Measures
Conscientiousness, optimism, goal disengagement, and goal reengagement measures were assessed using 38 items measured on a scale ranging from 1 (“strongly disagree”) to 5 (“strongly agree”). Items pertaining to conscientiousness were based on the International Personality Item Pool (IPIP) scale (Goldberg, 1999; Scheier, Carver, & Bridges, 1994), while items on optimism were based on the Life Orientation Test-Revised (LOT-R). Goal disengagement and goal reengagement were both measured with the validated Goal Adjustment Scale (Wrosch et al., 2003). Summary scores were created for each of the four personality measures (conscientiousness 5-24, optimism 0-24, goal disengagement 4-20, and goal reengagement 6-30) by summing the responses to trait-specific questions after reverse-coding the appropriate items to a consistent scale.
Demographic variables included age, race (White, African American, Asian, Hispanic, other), and education (high school or less, some college/college graduate, or some/all graduate school), which were ascertained by self-reported questionnaire at baseline (Visit 1). Psychological and behavioral variables included self-rated health status (excellent/good, or poor/very poor/bad), and global cognitive function using the 100-point Teng Modified Mini-Mental State Exam (Teng & Chui, 1987). Global sleep quality was measured with the validated Pittsburgh Sleep Quality Index; a cut-point of score >5 indicated significant sleep disturbance (Buysse et al., 1991). The Geriatric Depression Scale was used to measure depression symptoms on a 0 to 15 scale; a score ≥6 indicates the presence of depression symptoms (Almeida & Almeida, 1999). The Physical Activity Scale for the Elderly (PASE) was used to assess physical activity (Washburn, Smith, Jette, & Janney, 1993). Health-related variables included body mass index (BMI, kg/m2) and self-reported history of hypertension, congestive heart failure, heart attack, diabetes, or stroke (Orwoll et al., 2005).
Statistical Analyses
Of 2,424 men that participated in MrOS Visit 4, a total of 2,206 completed the PFS and an additional 12 had imputed scores. Of these 2,218 participants, 82% (n=1812) completed the personality questionnaire. An additional 142 participants were excluded for missing data for the covariates of interest including Teng Modified Mini-Mental score (n=141) and Geriatric Depression Scale score (n=1). The final analytical sample consisted of N=1,670 participants with complete data.
Descriptive characteristics, including frequency and chi-squared tests for categorical variables, or mean ± standard deviation and t-tests or nonparametric alternatives for continuous variables, were reported overall and by higher and lower perceived mental fatigability. Covariates were divided into three groups: demographics, psychological/behavioral, and health-related factors. We reported Spearman correlation coefficients between all continuous independent variables.
Separate multivariable linear regression models were constructed to evaluate the association between each personality trait and continuous PFS Mental scores. Each model began with one personality trait of interest. Age was included in all models as it is known to be correlated with perceived mental fatigability (Wasson et al., 2019). Covariates were entered into the four models for each personality trait one group at a time: first demographic characteristics, then psychological/behavioral factors, and last health conditions. Only variables that were significant at α=.05 were retained in the model when the next covariate group was added. A final model was constructed that included all four personality measures plus the significant covariates. However, in a post-hoc analysis, we also constructed an additional model that included all variables, regardless of statistical significance. For all models, each personality variable was standardized such that mean (standard deviation) was 0 (1). The distribution of each of the standardized personality measures were assessed with Shapiro-Wilk test of normality. While personality measures were not statistically normal (p<0.0001), visual inspection of histograms demonstrated that the distribution of each measure approached normal. Absence of multicollinearity between independent variables was assessed using variance inflation factors ≤3. Interactions between significant variables were evaluated to determine if the effect of personality measures on PFS Mental scores depended on any third variable in our models. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Data Accessibility
Data, analytic methods and study materials are available to other researchers using the publicly available online study website link: https://mrosonline.ucsf.edu. Analytic code for this paper can be obtained from the corresponding author.
Results
In the analytical sample (N=1670), the mean age was 84.3 years (standard deviation=4.1), 90.6% of participants were white, and 82.3% had more than a high school education. The mean PFS Mental score was 7.69 (SD=8.27). Men with more perceived mental fatigability (PFS Mental scores ≥13) were significantly older, had poorer self-rated health, worse cognitive function scores, worse global sleep quality, higher prevalence of significant depressive symptoms, lower physical activity, and greater prevalence of diabetes compared to men with less perceived mental fatigability (PFS Mental scores <13) (Table 1). Men with more mental fatigue had lower mean conscientiousness and optimism scores (Table 1). Goal reengagement and goal disengagement scores were similar by perceived mental fatigability status. Lower conscientiousness (ρ=−0.19, p<.0001), optimism (ρ=−0.20, p<.0001) and goal reengagement (ρ=−0.05, p=.03) were associated with higher PFS Mental scores, but not goal disengagement (ρ=0.02, p=.41) (Table 2). As neither goal reengagement nor goal disengagement significantly differed by perceived mental fatigability status, these variables were not included as primary individual factors of interest in this paper, but findings from those regression analyses were included as supplementary information (Supplemental Tables 1 and 2).
Table 1.
Baseline characteristics overall and by Pittsburgh Fatigability Scale (PFS) perceived mental fatigability status: MrOS (N=1,670)
| PFS Mental Fatigability Status | ||||
|---|---|---|---|---|
|
| ||||
|
Characteristics |
All N=1670 (mean±SD or n [%]) |
More Fatigability (≥13) n=394 (mean±SD or n [%]) |
Less Fatigability (<13) n=1276 (mean±SD or n [%]) |
P-value |
| Personality Measures a | ||||
| Conscientiousness (Scale: 5-50) | 38.1± 5.5 | 36.7± 5.7 | 38.5 ± 5.4 | <.0001 |
| Optimism (Scale: 5-30) | 22.5 ± 3.2 | 21.8 ± 3.3 | 22.8 ± 3.2 | <.0001 |
| Goal Reengagement (Scale: 6-30) | 21.2 ± 3.5 | 21.1 ± 3.4 | 21.3 ± 3.5 | .54 |
| Goal Disengagement (Scale: 4-20) | 11.4 ± 2.6 | 11.5 ± 2.7 | 11.4 ± 2.6 | .74 |
|
| ||||
| Demographics | ||||
| Age (years) | 84.3 ± 4.1 | 85.0 ± 4.5 | 84.1 ± 4.0 | <.0001 |
| Race | .43 | |||
| White | 90.6 [1513] | 90.4 [356] | 90.7 [1157] | |
| Black | 2.6 [44] | 3.1 [12] | 2.5 [32] | |
| Asian | 3.2 [53] | 2.0 [8] | 3.5 [45] | |
| Hispanic | 2.2 [37] | 2.8 [11] | 2.0 [26] | |
| Other | 1.4 [23] | 1.8 [7] | 1.3 [16] | |
| Education | .97 | |||
| High School or less | 17.7 [295] | 17.3 [68] | 17.8 [227] | |
| Some/all college | 38.2 [638] | 38.3 [151] | 38.2 [487] | |
| Some/all Graduate School | 44.1 [737] | 44.4 [175] | 44.0 [562] | |
|
| ||||
| Psychological and Behavioral Factors | ||||
| Self-rated health status | <.0001 | |||
| Good/Excellent | 89.5 [1495] | 81.7 [322] | 91.9 [1173] | |
| Poor/Very Poor/Fair | 10.5 [175] | 18.3 [72] | 8.1 [103] | |
| Cognitive functionb (3MS Scale: 0-100) | 92.3 ± 7.0 | 91.2 ± 8.1 | 92. 6 ± 6.6 | .002 |
| Global sleep qualityc | 59.7 [997] | 45.2 [178] | 64.2 [819] | <.0001 |
| No sleep disturbance (≤5) | 40.3 [673] | 54.8 [216] | 35.8 [457] | |
| Sleep disturbance (>5) | ||||
| Depression symptomsd | <.0001 | |||
| No depression (<6) | 94.0 [1570] | 85.0 [335] | 96.8 [1235] | |
| Depression (≥6) | 6.0 [100] | 15.0 [59] | 3.2 [41] | |
| Physical activitye | 116.7± 65.4 | 94.0 ± 63.1 | 123.7 ± 64.5 | <.0001 |
|
| ||||
| Health-related Conditions | ||||
| Hypertension | 51.4 [858] | 54.3 [214] | 50.5 [644] | .18 |
| Congestive Heart Failure | 8.6 [143] | 10.7 [42] | 7.9 [101] | .09 |
| Diabetes | 15.3 [255] | 18.5 [73] | 14.3 [182] | .04 |
| Heart Attack | 13.4 [223] | 13.7 [54] | 13.2 [169] | .81 |
| Stroke | 4.9 [81] | 5.3 [21] | 4.7 [60] | .61 |
| Body mass index (kg/m2) | 26.9 ± 3.7 | 27.1 ± 3.8 | 26.8 ± 3.7 | .17 |
Note. SD=Standard Deviation
Higher score = greater presentation of positive personality attribute
Cognitive function was measured with the Teng Mini Mental Scale (0-100); higher score = better cognitive functioning
Measured using the Pittsburgh Sleep Quality Index (PSQI)
Measured with The Geriatric Depression Scale
Physical activity was measured with Physical Activity Scale for the Elderly (PASE) score; higher score = more physically active
Table 2.
Spearman Correlations between Pittsburgh Fatigability Scale (PFS) Mental scores and continuous covariate measures: MrOS (N=1,670)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PFS Mental scores | |||||||||||
| 2. Conscientiousness | −0.19** | ||||||||||
| 3. Optimism | −0.20** | 0.30** | |||||||||
| 4. Goal Reengagement | −0.05* | 0.19** | 0.30** | ||||||||
| 5. Goal Disengagement | 0.02 | −0.10** | −0.07* | 0.02 | |||||||
| 6. Age | 0.13** | −0.05* | −0.11** | −0.09* | −0.00 | ||||||
| 7. Cognitive Functiona | −0.09* | 0.17** | 0.21** | 0.10** | −0.04 | −0.23** | |||||
| 8. Global Sleep Qualityb | 0.23** | −0.12** | −0.18** | −0.10** | 0.00 | 0.04 | −0.01 | ||||
| 9. Depressive Symptomsc | 0.31** | −0.21** | −0.36** | −0.27** | 0.01 | 0.19** | −0.13** | 0.31** | |||
| 10. Physical Activityd | −0.19** | 0.12** | 0.11** | 0.11** | −0.07* | −0.24** | 0.14** | −0.10** | −0.30** | ||
| 11. Body mass index | 0.03 | −0.07* | −0.01 | 0.01 | 0.04 | −0.10** | −0.02 | 0.06* | 0.06 | −0.10** |
Cognitive function was measured with the Teng Mini Mental Scale (0-100)
Measured using the Pittsburgh Sleep Quality Index (PSQI)
Measured with the Geriatric Depression Scale
Physical Activity Scale for the Elderly (PASE) score
p≤.01
p≤.0001
Other than age, no demographic characteristics impacted the association between lower conscientiousness and higher PFS Mental scores (Table 3, Model 2). The psychological and behavioral covariates added in Model 3, namely good/excellent health status, Teng Mini Mental cognitive function score, significant depressive symptoms, poor sleep quality, and lower physical activity attenuated the association between lower conscientiousness and higher PFS Mental scores by 35% (0.52 points) (Table 3, Model 3); the addition of BMI and health conditions did not substantively attenuate the association between conscientiousness and PFS Mental scores (Table 3, Model 4). The relationship between lower optimism and higher PFS mental scores was similar to that of the conscientiousness models (Table 4). In Model 4, each one standard deviation lower optimism score (about 3 points) was associated with 0.72 point higher PFS Mental score, independent of covariates.
Table 3.
Linear regression model examining the association between conscientiousness and Pittsburgh Fatigability Scale (PFS) Mental scores: MrOS (N=1670)
| Characteristics | Model 1 β coefficient (SE) |
Model 2 β coefficient (SE) |
Model 3 β coefficient (SE) |
Model 4 β coefficient (SE) |
|---|---|---|---|---|
| Personality Measure | ||||
| Conscientiousness | −1.49 (0.20)* | −1.50 (0.20)* | −0.98 (0.19)* | −0.86 (0.20)* |
|
| ||||
| Demographics | ||||
| Age | 0.28 (0.05)* | 0.29 (0.05)* | 0.17 (0.05)* | 0.18 (0.05)* |
| Race (ref=White) | ||||
| Black | 0.22 (1.23) | |||
| Asian | −0.87 (1.13) | |||
| Hispanic | 1.31 (1.34) | |||
| Other | 1.78 (1.70) | |||
| Education (ref= ≤high school) | ||||
| Some/all college | 0.41 (0.57) | |||
| Some/all graduate school | 0.30 (0.56) | |||
|
| ||||
| Psychological and Behavioral Factors | ||||
| Cognitive Function a | −0.06 (0.03)* | −0.06 (0.03)* | ||
| Good/Excellent Health Status | −2.30 (0.65)* | −2.16 (0.66)* | ||
| Depressive Symptoms b | 4.97 (0.76)* | 5.05 (0.84)* | ||
| Sleep Disturbance c | 2.08 (0.39)* | 2.06 (0.39)* | ||
| Physical Activity d | −0.02 (0.00)* | −0.02 (0.00)* | ||
|
| ||||
| Health-related Conditions | ||||
| Hypertension | 0.15 (0.39) | |||
| Heart Failure | 0.52 (0.70) | |||
| Stroke | 0.62 (0.88) | |||
| Heart Attack | −0.54 (0.57) | |||
| Diabetes | −0.69 (0.54) | |||
| Body Mass Index, kg/m2 | 0.02 (0.05) | |||
Note. SE=Standard Error
Cognitive function was measured with Teng Mini Mental Scale (0-100); higher score = better cognitive functioning
Significant depressive symptoms are indicated with a score ≥ 6 on the Geriatric Depression Scale
Sleep disturbance is indicated with a score >5 on the Pittsburgh Sleep Quality Index (PSQI)
Physical activity was measured with Physical Activity Scale for the Elderly (PASE) score; higher score = more physically active
p≤.05
Table 4.
Linear regression model examining the association between optimism and Pittsburgh Fatigability Scale (PFS) Mental scores: MrOS (N=1,670)
| Model 1 β coefficient (SE) |
Model 2 β coefficient (SE) |
Model 3 β coefficient (SE) |
Model 4 β coefficient (SE) |
|
|---|---|---|---|---|
| Personality Measure | ||||
| Optimism | −1.46 (0.20)* | −1.53 (0.20)* | −0.71 (0.20)* | −0.72 (0.20)* |
|
| ||||
| Demographics | ||||
| Age | 0.26 (0.05)* | 0.27 (0.05)* | 0.16 (0.05)* | 0.17 (0.05)* |
| Race (ref=White) | −0.18 (1.23) | |||
| Black | −1.30 (1.13) | |||
| Asian | 1.55 (1.34) | |||
| Hispanic | 1.48 (1.70) | |||
| Other | ||||
| Education (ref= ≤high school) | ||||
| Some/all college | 0.87 (0.57) | |||
| Some/all graduate school | 0.85 (0.57) | |||
|
| ||||
| Psychological and Behavioral Factors | ||||
| Cognitive Functiona | −0.07 (0.03)* | −0.07 (0.02)* | ||
| Good/Excellent Health Status | −2.16 (0.66)* | −1.97 (0.67)* | ||
| Depressive Symptomsb | 4.83 (0.86)* | 4.90 (0.86)* | ||
| Sleep Disturbancec | 2.04 (0.39)* | 2.01 (0.39)* | ||
| Physical Activityd | −0.02 (0.00)* | −0.02 (0.00)* | ||
|
| ||||
| Health-related Conditions | ||||
| Hypertension | 0.10 (0.34) | |||
| Heart Failure | 0.58 (0.70) | |||
| Stroke | 0.62 (0.88) | |||
| Heart Attack | −0.50 (0.57) | |||
| Diabetes | 0.83 (0.54) | |||
| Body Mass Index, kg/m2 | 0.03 (0.05) | |||
Note. SE=Standard Error
Cognitive function was measured with Teng Mini Mental Scale (0-100); higher score = better cognitive functioning
Significant depressive symptoms are indicated with a score ≥ 6 on the Geriatric Depression Scale
Sleep disturbance is indicated with a score >5 on the Pittsburgh Sleep Quality Index (PSQI)
Physical activity was measured with Physical Activity Scale for the Elderly (PASE) score; higher score = more physically active
p≤.05
In the final model that included all four personality measures, lower conscientiousness (β=−0.93, 95% CI: (−1.31, −0.52), p<.0001) and optimism scores (β=−0.63, 95% CI: (−1.05, −0.21), p=.003), and higher goal reengagement scores (β=0.51, 95% CI: (0.12, 0.90), p=.010), were independently associated with higher PFS Mental scores (Figure 2 and Supplemental Table 3). This final model was adjusted for age, self-rated health status, cognitive function, clinical depression, global sleep quality, and physical activity. No significant interactions were found between any of the four personality measures and covariates in this final model, and there was no collinearity amongst the variables (variance inflation factors ≤3). In the post-hoc analysis where all covariates were retained in the regression models regardless of statistical significance, the effect estimates and significance level for each of the personality measures remained unchanged (Supplemental Table 4).
Figure 2.
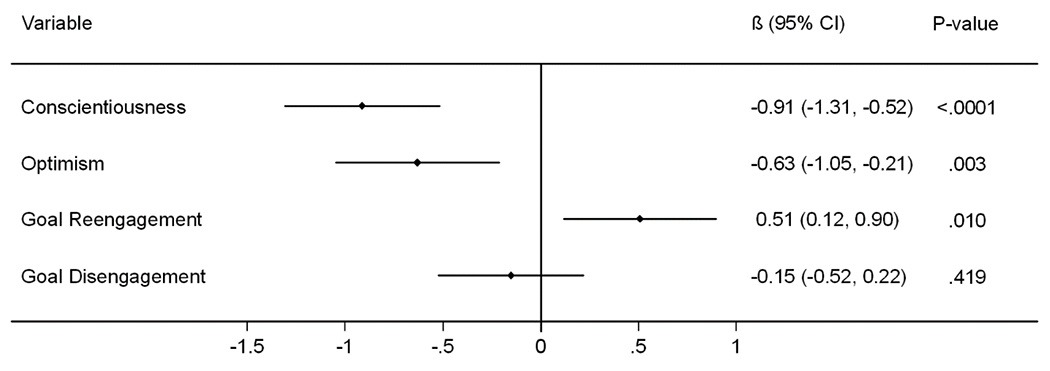
Relationship between personality measures and higher Pittsburgh Fatigability Scale Mental scores: MrOS (N=1,670)
All personality measures were standardized in scale such that mean=0 and standard deviation=1. Beta coefficients for each of the personality measures shown above are adjusted for all statistically significant (p<.05) covariates: age, cognitive function, self-reported health status, depressive symptoms, sleep disturbance, and physical activity, as well as each of the other personality measures.
Discussion
To our knowledge, this study is the first to report, as hypothesized that conscientiousness, optimism, and goal reengagement were independently association with perceived mental fatigability after adjustment for potential demographic, psychological and behavioral, and health-related conditions confounders. Prior studies have found independent associations between personality, global fatigue and health outcomes (De Vries & Van Heck, 2002; Smagula et al., 2016). Ours is the first study to suggest that personality may act on fatigue, in part, through an individual’s psychological predisposition to fatigue (i.e., perceived mental fatigability) (Glynn et al., 2015; Renner et al., 2021).
The magnitude of associations between personality factors and perceived mental fatigability were relatively large compared to other included covariates. SD differences in conscientiousness and optimism were associated with −0.90 SD (~0.77 point) and −0.70 SD (~0.60 point) differences in PFS Mental scores, respectively. In contrast, age and cognitive function were only associated with 0.18 SD (~0.15 point) and −0.06 SD (~0.05 point) differences in PFS Mental score in the final model. Thus, personality factors appear to contribute meaningfully to differences in perceived mental fatigability relative to and after adjusting for established predictors.
Personality attributes may impact perceived fatigability through influencing self-appraisals of an individual’s ability to meet task demands given their current health status. For example, optimists are more likely to attribute negative events to external factors beyond their control and view negative events as temporary (Gillham, Shatté, Reivich, & Seligman, 2001). Men with higher optimism may have a more positive outlook on their ability to meet task demands relative to less optimistic men of similar health, resulting in lower perceived mental fatigability despite similar functional capacity. This psychological pathway may be independent of depressive symptoms and self-perceived health, which also were significantly associated with perceived mental fatigability in adjusted analyses.
While our goal was to measure the direct relationship between personality and perceived mental fatigability, we recognize that there are also a number of indirect pathways that may function with many of the covariates in our analyses as mediators through a health behavioral pathway. For example, conscientiousness has been associated with positive health behaviors (e.g., preventive health visits) that lead to better maintenance of physical and cognitive health over time (Reiss, Eccles, & Nielsen, 2014), potentially also resulting in lower perceived fatigability. While we adjusted for specific health conditions (e.g., hypertension) and behaviors (e.g., smoking, physical activity) in the current analysis, there may be unobserved health factors that were not included. Additionally, controlling for mediating factors may have weakened the overall effect size of our estimates by effectively blocking these indirect pathways. Future studies should consider mediation analysis to better understand the full relationship. Future work should also consider additional health factors that were outside the scope of the current analysis. Of particular interest is the relationship between domain-specific (vs. global) cognitive functioning (e.g., processing speed, executive functioning) and perceived mental fatigability, which may partly account for the association between these personality factors and perceived mental fatigability, and which is currently being researched in our group.
Our findings also provide initial evidence for the contribution of personality measures to early risk assessment of perceived mental fatigability. While personality attributes may change over time due to normative development and specific life events (Chopik, Kim, Schwaba, Krämer, & Smith, 2020; Debast et al., 2014) these changes are measurable in general samples and have been well-studied throughout the lifespan. In contrast, perceived mental fatigability typically only has significant variation in populations at more immediate risk of physical and cognitive declines, including older and patient populations. For example, the PFS was only validated in samples age ≥60 years (Glynn et al., 2015; Renner et al., 2021). Thus, since variation in personality is observable earlier in life, it would be useful to know whether certain personality subgroups are at higher risk of later-life fatigability to inform early interventions to mitigate fatigue. To this end, future longitudinal research can better establish a temporal relationship between personality and perceived fatigability and further determine whether personality changes earlier in life predict future levels of perceived mental fatigability.
A limitation of this work is that the MrOS sample is restricted to primarily white men, most of whom have higher educational attainment and lower prevalence (24%) of greater perceived mental fatigability than other comparable cohorts (Cohen et al., 2021; Simonsick et al., 2018). We also excluded individuals without complete data (more likely to be Black or Asian, older, lower cognitive function scores, and reported worse health, all p<0.05). However, our conservative approach likely biased our results toward the null hypothesis and controlling for the suite of covariates in these analyses allows for the assumption that the data in our sample was missing at random. Additionally, previous studies on personality and downstream health effects have found that the relationships are not significantly moderated by sex or race, suggesting that it is possible that findings from our somewhat limited sample may still generalize to women and more diverse populations (Hakulinen et al., 2015; Stephan et al., 2018). Nonetheless, future analyses should aim to test the relationship between personality and perceived mental fatigability in samples that include women and people of color. An additional limitation of this analysis is that only individuals with complete data at Visit 4 of the MrOS study, which took place 14 to 16 years after the baseline enrollment, were included. Therefore, our analysis likely included individuals who were healthier, and therefore potentially higher on conscientiousness and optimism and lower on perceived mental fatigability than the cohort as a whole, subjecting this study to selection bias. Selection bias of healthier individuals would most likely reduce the range of variance of lower scores of conscientiousness and optimism, moving the significance of our findings toward the null. As the generalizability of our findings are limited, additional work may be needed in extremely frail, low functioning, or institutionalized or clinical populations.
Temporality of the association between personality, potential cofounders, and perceived mental fatigability could also not be assessed due to the cross-sectional nature of this work. Important strengths are the large sample size of community-dwelling older men that allowed us to assess the relationship between personality and fatigability with greater precision and in a non-clinical context. Furthermore, the PFS is easier to administer than performance-based mental fatigability tests, making it a useful tool for many research studies and clinical settings (Glynn et al., 2015; Renner et al., 2021).
In conclusion, the strength of the relationship between personality, particularly lower conscientiousness and optimism, and higher PFS Mental scores warrants further investigation into how personality attributes may help clinicians design more targeted and effective interventions to reduce perceived mental fatigability, and consequently lower the risk of several adverse aging-related health outcomes. Lastly, future work should be longitudinal in nature and include personality assessments to confirm the temporality of the relationships observed in these analyses.
Supplementary Material
ACKNOWEDGMENTS
Preliminary findings were presented at the 2020 Gerontological Society of American Annual Scientific Meeting, Virtual, November 2020.
FUNDING
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. Additionally, the Claude D. Pepper Older Americans Independence Center, Research Registry and Developmental Pilot Grant (NIH P30 AG024827), and the Intramural Research Program, National Institute on Aging supported N.W.G. to develop the Pittsburgh Fatigability Scale. The Epidemiology of Aging training grant at the University of Pittsburgh (NIA T32 AG000181) supported T.G and K.D.M.
Footnotes
Conflict of Interest: None to declare.
REFERENCES
- Almeida OP, & Almeida SA (1999). Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. International Journal of Geriatric Psychiatry, 14(10), 858–865. [DOI] [PubMed] [Google Scholar]
- Aschwanden D, Strickhouser JE, Luchetti M, Stephan Y, Sutin AR, & Terracciano A (2021). Is personality associated with dementia risk? A meta-analytic investigation. Ageing Research Reviews, 67, 101269. 10.1016/j.arr.2021.101269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran TM, Zhang Z, Anderson AJ, McDermott K, & Lin F (2020). Brain structural connectomes indicate shared neural circuitry involved in subjective experience of cognitive and physical fatigue in older adults. Brain imaging and behavior, 14(6), 2488–2499. 10.1007/s11682-019-00201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow MA, Wrosch C, & McGrath JJ (2020). Goal adjustment capacities and quality of life: A meta-analytic review. Journal of Personality, 88(2), 307–323. 10.1111/jopy.12492 [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, & Delay RR (2005). Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemporary clinical trials, 26(5), 557–568. 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Burke SE, Babu Henry Samuel I, Zhao Q, Cagle J, Cohen RA, Kluger B, & Ding M (2018). Task-Based Cognitive Fatigability for Older Adults and Validation of Mental Fatigability Subscore of Pittsburgh Fatigability Scale. Frontiers in aging neuroscience, 10, 327. 10.3389/fnagi.2018.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, & Kupfer DJ (1991). Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep, 14(4), 331–338. 10.1093/sleep/14.4.331 [DOI] [PubMed] [Google Scholar]
- Carlozzi NE, Boileau NR, Murphy SL, Braley TJ, & Kratz AL (2019). Validation of the Pittsburgh Fatigability Scale in a mixed sample of adults with and without chronic conditions. Journal of Health Psychology, 1359105319877448. 10.1177/1359105319877448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopik WJ, Kim ES, Schwaba T, Krämer MD, & Smith J (2020). Changes in optimism and pessimism in response to life events: Evidence from three large panel studies. Journal of research in personality, 88. 10.1016/j.jrp.2020.103985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RW, Meinhardt AJ, Gmelin T, Qiao YS, Moored KD, Katz RD, LLFS Research Group. (2021). Prevalence and severity of perceived mental fatigability in older adults: The Long Life Family Study. Journal of the American Geriatrics Society. 10.1111/jgs.17075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Popham M, Santanasto AJ, Hardy R, Glynn NW, & Kuh D (2019). Are BMI and inflammatory markers independently associated with physical fatigability in old age? International Journal of Obesity, 43(4), 832–841. 10.1038/s41366-018-0087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A, Tran Y, Wijesuriya N, & Boord P (2006). A controlled investigation into the psychological determinants of fatigue. Biological Psychology, 72(1), 78–87. 10.1016/j.biopsycho.2005.07.005 [DOI] [PubMed] [Google Scholar]
- De Vries J, & Van Heck GL (2002). Fatigue: relationships with basic personality and temperament dimensions. Personality and Individual Differences, 33(8), 1311–1324. 10.1016/S0191-8869(02)00015-6 [DOI] [Google Scholar]
- Debast I, van Alphen S. P. J. (Bas), Rossi G, Tummers JHA, Bolwerk N, Derksen JJL, & Rosowsky E (2014). Personality Traits and Personality Disorders in Late Middle and Old Age: Do They Remain Stable? A Literature Review. Clinical gerontologist, 37(3), 253–271. 10.1080/07317115.2014.885917 [DOI] [Google Scholar]
- Duberstein PR, Sörensen S, Lyness JM, King DA, Conwell Y, Seidlitz L, & Caine ED (2003). Personality is associated with perceived health and functional status in older primary care patients. Psychology and aging, 18(1), 25–37. 10.1037/0882-7974.18.1.25 [DOI] [PubMed] [Google Scholar]
- Gillham JE, Shatté AJ, Reivich KJ, & Seligman MEP (2001). Optimism, pessimism, and explanatory style. In Chang EC (ed.), Optimism & pessimism: Implications for theory, research, and practice. (pp. 53–75). Washington: American Psychological Association. 10.1037/10385-003 [DOI] [Google Scholar]
- Glynn NW, Santanasto AJ, Simonsick EM, Boudreau RM, Beach SR, Schulz R, & Newman AB (2015). The Pittsburgh Fatigability scale for older adults: development and validation. Journal of the American Geriatrics Society, 63(1), 130–135. 10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR (1999). A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. Personality psychology in Europe, 7(1), 7–28. [Google Scholar]
- Hakulinen C, Elovainio M, Pulkki-Råback L, Virtanen M, Kivimäki M, & Jokela M (2015). Personality and depressive symptoms: individual participant meta-analysis of 10 cohort studies. Depression and Anxiety, 32(7), 461–470. 10.1002/da.22376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson SE, & Friedman HS (2008). Personality and health: A lifespan perspective. In John OP, Robins RW, & L. Pervin A (eds.), Handbook of personality: Theory and research (pp. 770–794). The Guilford Press. [Google Scholar]
- Hooker K, & McAdams DP (2003). Personality reconsidered: a new agenda for aging research. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 58(6), P296–304. 10.1093/geronb/58.6.p296 [DOI] [PubMed] [Google Scholar]
- Kekäläinen T, Terracciano A, Sipilä S, & Kokko K (2020). Personality traits and physical functioning: a cross-sectional multimethod facet-level analysis. European review of aging and physical activity : official journal of the European Group for Research into Elderly and Physical Activity, 17(1), 20. 10.1186/s11556-020-00251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Kotov R, & Bufferd SJ (2011). Personality and depression: explanatory models and review of the evidence. Annual Review of Clinical Psychology, 7, 269–295. 10.1146/annurev-clinpsy-032210-104540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LR, Friedman HS, & Schwartz JE (2007). Personality and mortality risk across the life span: the importance of conscientiousness as a biopsychosocial attribute. Health Psychology, 26(4), 428–436. 10.1037/0278-6133.26.4.428 [DOI] [PubMed] [Google Scholar]
- McCrae RR, & Costa PT Jr. (2008). The five-factor theory of personality. In John OP, Robins RW, & Pervin LA (eds.), Handbook of personality: Theory and research (pp. 159–181). The Guilford Press. [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, … Stone K (2005). Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials, 26(5), 569–585. 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- Rasmussen HN, Scheier MF, & Greenhouse JB (2009). Optimism and physical health: a meta-analytic review. Annals of Behavioral Medicine, 37(3), 239–256. 10.1007/s12160-009-9111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Eccles JS, & Nielsen L (2014). Conscientiousness and public health: synthesizing current research to promote healthy aging. Developmental Psychology, 50(5), 1303–1314. 10.1037/a0036473 [DOI] [PubMed] [Google Scholar]
- Renner SW, Bear TM, Brown PJ, Andersen SL, Cosentino S, Gmelin T, … Glynn NW (2021). Validation of perceived mental fatigability using the pittsburgh fatigability scale. Journal of the American Geriatrics Society. 10.1111/jgs.17017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno EA, Wanigatunga AA, An Y, Urbanek JK, Simonsick EM, Ferrucci L, … Schrack JA (2019). Longitudinal Association Between Perceived Fatigability and Cognitive Function in Older Adults: Results from the Baltimore Longitudinal Study of Aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 10.1093/gerona/glz287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanatkar S, Baldwin P, Clarke J, Fletcher S, Gunn J, Wilhelm K, … Proudfoot J (2020). The influence of personality on trajectories of distress, health and functioning in mild-to-moderately depressed adults with type 2 diabetes. Psychology, health & medicine, 25(3), 296–308. 10.1080/13548506.2019.1668567 [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, & Bridges MW (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology, 67(6), 1063–1078. 10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- Schrack JA, Simonsick EM, & Glynn NW (2020). Fatigability: A prognostic indicator of phenotypic aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(9), e63–e66. 10.1093/gerona/glaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Schrack JA, Santanasto AJ, Studenski SA, Ferrucci L, & Glynn NW (2018). Pittsburgh Fatigability Scale: One-Page Predictor of Mobility Decline in Mobility-Intact Older Adults. Journal of the American Geriatrics Society, 66(11), 2092–2096. 10.1111/jgs.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Faulkner K, Scheier MF, Tindle HA, Cauley JA, & Osteoporotic Fractures in Men (MrOS) Study Group. (2016). Testing the Independence of Multiple Personality Factors in Relation to Health Among Community-Dwelling Older Men. Journal of Aging and Health, 28(4), 571–586. 10.1177/0898264315597649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K, & Das RC (2013). Personality pathways of successful ageing. Industrial psychiatry journal, 22(1), 1–3. 10.4103/0972-6748.123584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Bayard S, Sutin AR, Krizan Z, & Terracciano A (2018). Personality and Sleep Quality: Evidence From Four Prospective Studies. Health Psychology, 37(3), 271–281. 10.1037/hea0000577.supp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Canada B, & Terracciano A (2017). Personality and frailty: evidence from four samples. Journal of research in personality, 66, 46–53. 10.1016/j.jrp.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng EL, & Chui HC (1987). Modified Mini Mental State Examination (3MS). Canadian Journal of Psychiatry, 41(2), 114–121. [PubMed] [Google Scholar]
- Tindle HA, Chang Y-F, Kuller LH, Manson JE, Robinson JG, Rosal MC, … Matthews KA (2009). Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women’s Health Initiative. Circulation, 120(8), 656–662. 10.1161/CIRCULATIONAHA.108.827642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, & Janney CA (1993). The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of Clinical Epidemiology, 46(2), 153–162. 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- Wasson E, Rosso AL, Santanasto AJ, Rosano C, Butters MA, Rejeski WJ, … LIFE Study Group. (2019). Neural correlates of perceived physical and mental fatigability in older adults: A pilot study. Experimental Gerontology, 115, 139–147. 10.1016/j.exger.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrosch C, Scheier MF, Miller GE, Schulz R, & Carver CS (2003). Adaptive self-regulation of unattainable goals: goal disengagement, goal reengagement, and subjective well-being. Personality and Social Psychology Bulletin, 29(12), 1494–1508. 10.1177/0146167203256921 [DOI] [PubMed] [Google Scholar]
- Zhu L, Ranchor AV, van der Lee M, Garssen B, Sanderman R, & Schroevers MJ (2015). The role of goal adjustment in symptoms of depression, anxiety and fatigue in cancer patients receiving psychosocial care: a longitudinal study. Psychology & health, 30(3), 268–283. 10.1080/08870446.2014.969263 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, analytic methods and study materials are available to other researchers using the publicly available online study website link: https://mrosonline.ucsf.edu. Analytic code for this paper can be obtained from the corresponding author.


