Abstract
The discovery of mesoderm inducing signals helped usher in the era of molecular developmental biology, and today the mechanisms of mesoderm induction and patterning are still intensely studied. Mesoderm induction begins during gastrulation, but recent evidence in vertebrates shows that this process continues after gastrulation in a group of posteriorly localized cells called neuromesodermal progenitors (NMPs). NMPs reside within the post-gastrulation embryonic structure called the tailbud, where they make a lineage decision between ectoderm (spinal cord) and mesoderm. The majority of NMP-derived mesoderm generates somites, but also contributes to lateral mesoderm fates such as endothelium. The discovery of NMPs provides a new paradigm in which to study vertebrate mesoderm induction. This review will discuss mechanisms of mesoderm induction within NMPs, and how they have informed our understanding of mesoderm induction more broadly within vertebrates as well as animal species outside of the vertebrate lineage. Special focus will be given to the signaling networks underlying NMP-derived mesoderm induction and patterning, as well as emerging work on the significance of partial epithelial to mesenchymal states in coordinating cell fate and morphogenesis.
Keywords: Neuromesodermal progenitors, mesoderm induction, mesoderm patterning, FGF, BMP, Wnt, Brachyury, epithelial to mesenchymal transition, EMT
1. Introduction
The vertebrate body axis forms in an anterior to posterior progression, with the head forming first (anterior) and the rest of the body plan forming sequentially away from the head [1]. A longstanding hypothesis, that this progressive mode of development relies on plastic multipotent progenitors at the posterior end of the embryo, was firmly supported in 2009 from retrospective clonal analysis in the mouse embryo [2]. This work showed that single cells in the mouse tailbud contribute daughter cells to both ectoderm (spinal cord) and mesoderm, and thus continue to make a germ layer decision after the end of gastrulation. These cells were given the name neuromesodermal progenitors (NMPs) based on their propensity to contribute to both neural and mesodermal lineages (Figure 1). Later work showed that cells in the tailbud of zebrafish embryos also have the potential to join the spinal cord or mesoderm, and that this decision is made continuously during axis elongation based on local canonical Wnt signaling cues [3]. High Wnt signaling induces mesoderm, while low Wnt signaling promotes spinal cord formation. The transition from NMP to mesoderm involves a developmental checkpoint that ensures neural specific genetic programs are repressed before cells can exit into mesodermal territories [4]. This checkpoint occurs during a partial epithelial to mesenchymal transition (EMT) as cells transition from epithelial NMPs to mesenchymal mesoderm [4]. More recent lineage tracing in chick embryos showed single NMPs contributing to both neural and mesodermal lineages [5]. The tailbud NMPs are identified as cells expressing both the neural associated transcription factor Sox2 and the mesoderm promoting transcription factor Brachyury [6], also referred to as Tbxt or T. In zebrafish there are two partially redundant brachyury genes tbxta and tbxtb, also referred to as ntla and ntlb, with tbxta playing the predominant role during development (based on loss of function phenotypes) [7]. This review will simply refer to “brachyury” to represent the combined function of the two zebrafish genes. Tailbud cells co-expressing Sox2 and Brachyury have been identified in mice, chick, zebrafish, and humans [3, 8–10], and thus NMPs appear to be a common feature of vertebrate embryonic development. NMPs have also recently been proposed to exist in invertebrate chordates [11].
Figure 1 – Neuromesodermal progenitors generate spinal cord and mesoderm after gastrulation.
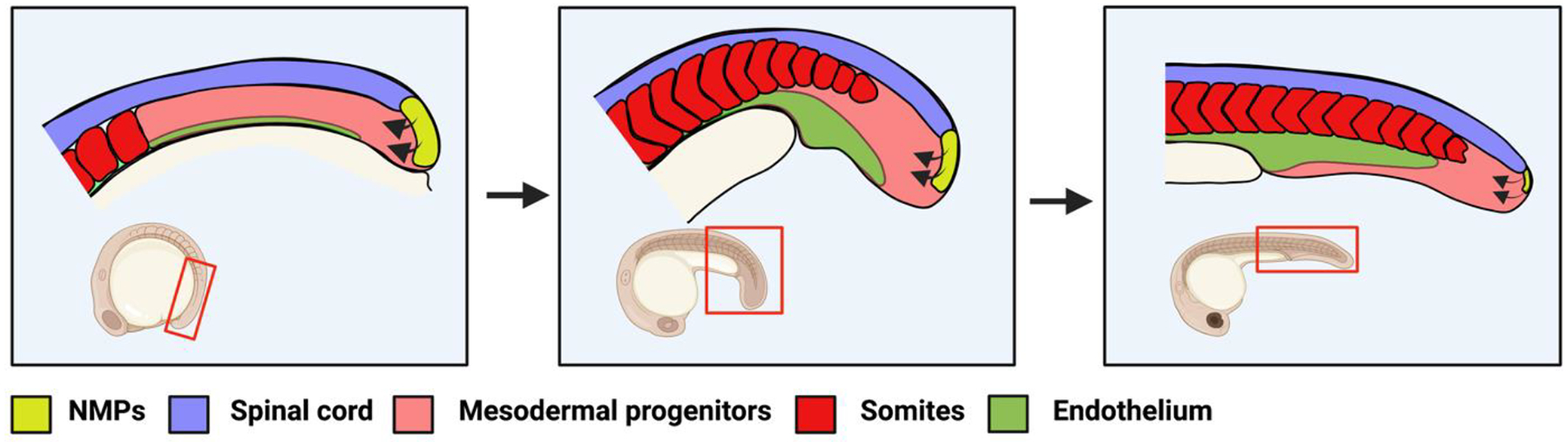
Vertebrate embryos (here is shown a zebrafish embryo) contain NMPs (yellow) in the tailbud which generate spinal cord (purple) and mesodermal progenitors (pink). The mesoderm will differentiate into somites (red) and vascular endothelium (green). The process of mesoderm induction from NMPs occurs continuously (arrows) over the course of axial extension until the NMPs are depleted. The red boxed region of the inset embryo represents the area depicted in the schematics.
The discovery of NMPs altered our understanding of lineage relationships within and between what are traditionally considered the three primary germ layers formed during gastrulation. While the spinal cord, brain, and epidermis are all considered to be tissues derived from the ectodermal germ layer, the normal biology of NMPs indicates that cells within the spinal cord are more closely related from a cell lineage perspective to paraxial mesoderm than they are to cells in the brain and epidermis [2]. This subsequently caused a broad shift in our understanding of neural induction and patterning. Instead of a model where all neural tissue is initially induced and then subsequently patterned into anterior (brain) and posterior (spinal cord) character, we now understand that there are separate cellular origins of brain and spinal cord [12–14]. Likewise, our view of mesodermal lineage relationships has changed, with certain anterior and ventral mesodermal types induced during gastrulation during primary germ layer segregation, while posterior mesoderm is generated from NMPs that share a common lineage with the spinal cord [2, 3]. The fact that mesoderm is continuously induced from NMPs during post-gastrulation axis extension provides a new context in which to study mechanisms of vertebrate mesoderm induction. This review will discuss aspects of NMP mesoderm induction that have revealed core conserved features of mesoderm induction in general, as well as mechanisms that appear to be NMP specific. Our consideration of mesoderm induction in this new context will further our understanding of the mechanisms of vertebrate body plan development, and how the mesodermal germ layer evolved within the animal lineage.
2. Signaling pathway usage during NMP and NMP-derived mesoderm induction
The mechanisms of vertebrate mesoderm induction have historically focused on gastrula stages of development and have revealed several signaling pathways critical for mesoderm induction during this period. These include the Nodal, Bone Morphogenetic Protein (BMP), Fibroblast Growth Factor (FGF), and canonical Wnt pathways [15]. These pathways play variable roles in the induction of NMPs and the subsequent induction and patterning of NMP-derived mesoderm (Table 1). The Nodal and FGF signaling pathways exhibit the most difference between their gastrula and post-gastrula stage roles in mesoderm induction and will serve as the basis of discussion in this section. The function of canonical Wnt and BMP signaling during NMP mesoderm induction and patterning will be discussed later (see sections 3 and 4 for Wnt and 5 for BMP).
Table 1 –
Mesoderm inducing signaling pathways and their role in NMPs.
| Signaling Pathway | Role in gastrula stage mesoderm induction and patterning | Role in NMP-derived mesoderm induction | Role in NMP-derived mesoderm patterning |
|---|---|---|---|
| Nodal | Essential for mesoderm (and endoderm) induction and promotes dorsal/medial patterning [15, 16]. | Not essential as loss of function during NMP-derived mesoderm induction has no impact on mesoderm formation [17]. | No role currently identified. |
| FGF | Essential for induction of posterior mesoderm and patterning into dorsal/medial fates [15, 18]. | Essential for EMT completion during NMP-derived mesoderm induction. Loss of FGF signaling in mesoderm fated NMPs causes them to be trapped in the partial EMT state and prevents mesodermal differentiation [19]. | Essential for patterning NMP-derived mesoderm into the paraxial mesoderm fate [20]. |
| Canonical Wnt | Essential for mesoderm induction and patterning, with maternal signaling promoting dorsal/medial fates and zygotic signaling promoting ventral/lateral fates [15, 21]. | Essential for both the maintenance of NMPs and the induction of NMP-derived mesoderm. This pathway is required for EMT initiation during NMP-derived mesoderm induction [3, 10, 22]. | Essential for patterning NMP-derived mesoderm into the paraxial mesoderm fate [3]. |
| BMP | Plays a role in mesoderm induction and patterning. Best known for pattering mesoderm into ventral/lateral fates [15, 23]. | No direct role currently identified. | Essential for patterning NMP-derived mesoderm into the lateral mesoderm fate [20, 24]. |
2.1. Nodal signaling
Nodal signaling plays a key conserved role during both gastrula-stage mesoderm and endoderm induction in vertebrate embryos [15, 16]. Genetic analysis has indicated in both zebrafish and mouse that Nodal signaling plays a critical role during mesoderm and endoderm induction [15, 16]. However, this role appears to be restricted specifically to anterior mesoderm. A loss of function mutation in the single mouse Nodal gene disrupts primitive streak formation, but in 25% of embryos there are posteriorly localized mesodermal cells, indicating that Nodal signaling is not absolutely required for all mesoderm to form [25]. Likewise, loss of function of the two early acting Nodal genes in zebrafish, or of the essential co-receptor tdgf1 (also referred to as one-eyed pinhead or crypto) results in an absence of anterior mesoderm, but posterior mesoderm, including somitic tissue, is formed [26–28]. Posterior somites are generated from NMPs, indicating that Nodal signaling is not required for NMP or NMP-derived mesoderm induction. Supporting this, timed inactivation of Nodal signaling in zebrafish using small molecule inhibitors showed that once gastrulation begins, inhibition of Nodal signaling does not impact mesoderm induction in any region of the embryo [17].
2.2. FGF signaling
FGF signaling was first identified as being important for mesoderm induction based on its ability to induce mesoderm in frog embryos when over-expressed [29]. Later work showed that inhibition of FGF signaling in frogs and zebrafish causes a specific loss of posterior mesoderm, with anterior mesoderm forming [30–32]. Subsequent work in a number of other vertebrate model systems revealed a conserved role of FGF signaling in inducing posterior mesoderm [18]. In addition to vertebrate mesoderm induction, FGF signaling also plays key roles in mesoderm migration and patterning [18, 33]. As development progresses, the role of FGF signaling in mesoderm induction changes over time. During gastrula stages, FGF induces posterior mesoderm at least in part through transcriptional activation of brachyury. In zebrafish and Xenopus, loss of FGF signaling results in a loss of brachyury expression, and brachyury is in turn itself required for posterior mesoderm formation [32, 34–36]. In zebrafish, inhibition of FGF signaling after gastrulation also disrupts mesoderm formation, as NMP-derived cells fail to transition into committed mesodermal progenitors. However, the mechanism is distinct, as loss of FGF signaling after gastrulation results in an expansion of brachyury expression as opposed to a loss [19]. In this context, FGF signaling activates the expression of transcription factors msgn1 and tbx16, which in turn are required to repress NMP markers brachyury and sox2 [19, 37–39]. In the absence of FGF signaling, prospective NMP-derived mesoderm cells become trapped in the partial EMT state and remain in the tailbud, unable to exit and commit to mesodermal differentiation (discussed in further detail in section 4) [19]. In the chick model, FGF signaling was shown to play an additional role in cells once they join the paraxial mesoderm. Here, FGF signaling promotes the random motility of paraxial mesoderm cells which is essential for the proper axial extension of the embryo [40]. In zebrafish, convergence and extension of the paraxial mesoderm is important for axial elongation and is also associated with non-directional rearrangement of cells [41].
Given that much of the posterior mesoderm in vertebrate embryos is derived from NMPs, the data suggests that FGF signaling induces NMPs during gastrulation, and then FGF is required within NMPs to induce mesoderm through regulation of transcription factors that promote EMT and mesoderm differentiation. In addition to gastrula stage FGF signaling being required for posterior mesoderm formation, it is also required for posterior neural (spinal cord) formation. This activity includes activation of soxB1 transcription factor expression and is independent of the neural inducing activity of BMP signaling inhibition [14, 42–45]. This activity adds further support for a role of FGF signaling in inducing the NMP population during gastrulation. Furthermore, protocols for the in vitro derivation of NMPs from pluripotent stem cells require the addition of FGF [6, 10, 46, 47].
2.3. Integrating new views of neural and mesoderm induction during development
The discovery of NMPs created a shift in our understanding of nervous system development by showing that spinal cord cells originate from a population of cells that is unique from those that give rise to the brain. This is opposed to a historical model of neural development called the activation and transformation model, which posits that all neural tissue is first induced (activation) with an anterior brain character and then patterned (transformed) into posterior tissues of the hindbrain and spinal cord by morphogen gradients [48]. Our current updated understanding is that the cells that generate the brain are induced first through local inhibition of BMP signaling, whereas the spinal cord forms from induction by FGF signaling, independent of BMP inhibition [12, 14, 42]. The cells that form spinal cord also come from a distinct source (the NMPs) compared to cells that will generate the brain, and do not pass through an anterior neural intermediate before becoming spinal cord [13]. It is not clear whether all, or just part of the spinal cord is generated from NMPs in all vertebrates. For instance, fate mapping in zebrafish indicates that portions of the spinal cord exhibit clonal restriction to that fate without contributions to paraxial mesoderm [49]. However, Wnt signaling manipulations suggest that these spinal cord cells come from a neuromesodermal competent population, despite not realizing both fates during development [3]. The role of Nodal and FGF signaling in mesoderm induction point towards a similar division within the mesodermal germ layer with respect to distinct signaling mechanisms and cellular origins of anterior vs. posterior tissues. In the mesoderm, Nodal signaling induces anterior tissues, while FGF signaling is required for generating posterior mesoderm. Most of the posterior mesoderm also comes from a distinct cellular origin (NMPs) compared to the anterior tissue. Thus, the generation of neural and mesodermal tissues in vertebrate embryos appear to occur through distinct anterior and posterior development modules. However, these modules interact with each other based on positive feedback between FGF and Nodal signaling during early development [50].
3. The role of Brachyury (T) during mesoderm induction
NMPs are defined as cells that co-express the transcription factors sox2 and brachyury [3]. Brachyury, which is the founding member of the T-box transcription factor family, has long been associated with mesoderm development since the Brachyury mouse mutant was first described in 1927 [51]. Mice heterozygous for loss of Brachyury function have short tails, hence the name Brachyury which means “short tail” in Greek. Homozygous mutant loss of function results in embryonic lethality and a loss of posterior mesoderm, with only the anterior-most 8–12 somites forming of the approximately 60 that normally form [52, 53]. Mutant embryos also lack axial mesoderm that will form the notochord. The essential role of Brachyury in notochord formation is conserved within the chordates [54–60]. Additionally, work in many different animal species have revealed that Brachyury plays an essential role in posterior mesoderm induction in many of them, including all vertebrates that have been examined [61].
Investigating the role that Brachyury plays during continuous mesoderm production from NMPs has revealed molecular insights into its function as a mesoderm inducing factor. While Brachyury is an essential positive transcriptional regulator required for mesoderm induction from NMPs at the whole embryo level, its function is not absolutely required at the cell autonomous level. Mosaic analysis of Brachyury function in both zebrafish and mouse has revealed that even in the complete absence of Brachyury function, NMPs can still contribute to posterior somites when surrounded by wild-type cells [7, 62, 63]. This indicates that an essential function of Brachyury is the transcriptional regulation of genes that function in a cell non-autonomous fashion, such that wild-type cells that surround cells lacking Brachyury function can rescue the mutant phenotype. In zebrafish, critical direct targets of Brachyury are the canonical Wnt ligands wnt3a and wnt8a (Figure 2A) [7, 64]. Wnt signaling in turn activates brachyury expression, which creates an autoregulatory loop to sustain posterior Wnt signaling during the course of axis extension [7]. The posteriorly localized Wnt signal is necessary for both NMP maintenance and for mesoderm induction from the NMPs (as further discussed in section 4) [3, 10, 22]. After the discovery that zebrafish Brachyury directly activates Wnt ligands, it was subsequently discovered that this is also true in mouse, sea urchin, and sea anemone [65–67]. Additionally, a Brachyury/canonical Wnt signaling autoregulatory loop that was found to be critical for NMP derived mesoderm formation has also been observed in a diverse set of animals, including mouse, sea urchin, acorn worm, and sea anemone, revealing the deep evolutionary ancestry of this regulatory relationship (Figure 2B) [22, 65–70]. Thus, a central evolutionarily conserved role of Brachyury (predating the Cambrian explosion over 500 million years ago) during mesoderm induction is the maintenance of canonical Wnt signaling [61].
Figure 2 – Conserved direct Brachyury targets involved in NMP maintenance and NMP-derived mesoderm induction.
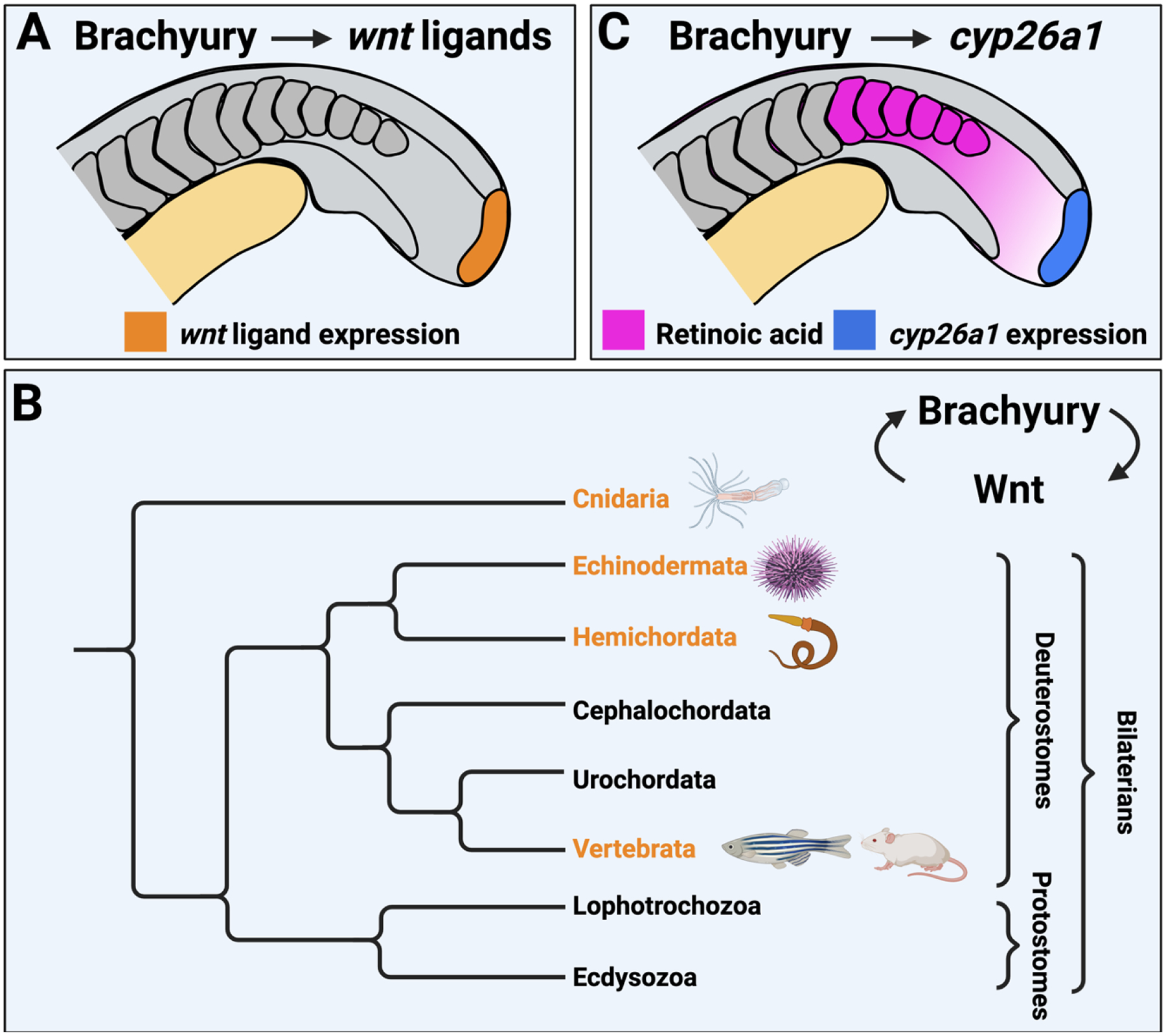
(A) Brachyury directly activates the expression of canonical Wnt ligands in the NMP region (orange). Wnt signaling in turn activates brachyury expression. (B) A simplified animal phylogeny illustrating the groups in which there is experimental evidence for a Brachyury/Wnt autoregulatory loop (orange). The specific animals in which this has been observed are shown as icons next to the phylogenetic tree. (C) Brachyury target gene cyp26a1 is expressed in the NMPs (blue). Cyp26a1 degrades retinoic acid to protect NMPs from the nearby posterior somite retinoic acid source (magenta).
In addition to the activation of canonical Wnt ligands, zebrafish Brachyury also directly activates the expression of the retinoic acid metabolizing enzyme cyp26a1 [62], and this positive regulation by Brachyury is conserved in mouse (Figure 2C) [63, 71]. During mouse gastrulation, retinoic acid signaling plays an important role in inducing the NMP population [72], a function that exhibits species specific differences between mouse and zebrafish [73]. However, in both species, as well as in chick, low levels of retinoic acid signaling is required for post-gastrulation NMP maintenance [8, 62, 74, 75]. Retinoic acid is normally produced in the most recently formed somites of vertebrate embryos, in cells that express the enzyme aldh1a2, which catalyzes the formation of retinoic acid from retinaldehyde [76]. The most recently formed somites are in close proximity to the tailbud and the NMP population. Retinoic acid is a potent inhibitor of brachyury expression, which in turn causes a loss of Wnt ligand expression [62]. Thus, in order for NMPs to be sustained in the undifferentiated state, they must be protected from the neighboring retinoic acid source (Figure 2C). Indeed, loss of Cyp26a1 function in zebrafish and mouse causes a failure to sustain NMPs and results in posterior truncations and a loss of the posterior-most somites [62, 74, 75]. In zebrafish, cyp26a1 mutant cells transplanted into wild-type host embryos are able to contribute to the posterior-most somites of the host embryos, which would normally be missing in whole embryos cyp26a1 mutants [62]. This result suggests that neighboring wild-type cells that express Cyp26a1 can act as a retinoic acid sink and degrade enough retinoic acid to protect transplanted cells lacking Cyp26a1. This non-autonomous role of Cyp26a1 is also supported by other work in zebrafish [77]. Thus Brachyury, by direct activation of canonical Wnt ligands and cyp26a1, creates a molecular niche that supports the maintenance of NMPs and their subsequent induction into mesoderm [62].
There is much still to be learned about the role of Brachyury in both NMP maintenance and differentiation into mesoderm. Many other direct transcriptional target genes regulated by Brachyury that have been identified, suggesting that Brachyury is also regulating NMPs and mesoderm induction in other ways. A report using mice indicated that Brachyury plays a direct role in the neural/mesodermal fate decision by antagonizing the function of the Sox2 transcription factor, which promotes neural fate in NMPs [71]. However more recent work has suggested that Brachyury does not play such a role, as mosaic analysis in mouse embryos of Brachyury mutant cells shows there is not an increased propensity for these cells to become neural instead of mesoderm [63]. Some of the Brachyury mutant cells in mosaic embryos are able to contribute to posterior somites (as mentioned above), but many tend to stay in the tailbud in the region of the NMPs, suggesting a role in promoting exit of NMPs into the mesodermal compartment [63]. Recent work using quail embryos also showed that the NMPs with a higher ratio of Brachyury compared to Sox2 exhibit increased motility and exit into paraxial mesoderm [78]. Whether this activity is related to the regulation of Wnt and/or retinoic acid signaling in the mouse remains to be seen.
Some of the other Brachyury direct targets are required for the proper segmentation of the NMP-derived paraxial mesoderm into somites, thereby coordinating the generation of new mesoderm with the continuous segmentation process that occurs in vertebrate axial elongation [79, 80]. The Brachyury/Wnt autoregulatory loop also intersects with Hox gene regulation. Hox genes are transcription factors that activate genes important for specifying cell fate at specific axial levels of the body [81]. Wnt signaling activates Hox gene expression through regulation of the Caudal homeobox (Cdx) transcription factors, which in turn activate Hox gene expression [61]. The regulation of Hox genes by Wnt signaling adds an additional layer of coordination to the induction of NMP-derived mesoderm and the acquisition of axial identity. This topic was extensively covered in a recent review [6], however, even more recent work has shown that the brachyury (tbxta) promoter in zebrafish is directly activated by a posterior Hox gene, which helps drive brachyury expression in the NMP region [82]. Thus, there appears to be a positive feedback loop between the Brachyury/Wnt loop and Hox genes.
As mentioned above, Brachyury is also required for the formation of the notochord in chordate embryos. Both the notochord and floor-plate are generated from midline progenitors that reside within the tailbud, and which have NMP-like characteristics including co-expression of brachyury and sox2, as well as the ability to continuously generate both mesoderm (notochord) and neural tissue (floor plate) [83]. However, unlike the cell non-autonomous role of Brachyury in NMP-derived mesoderm, Brachyury is required in a cell-autonomous fashion for notochord development [84]. Mosaic analysis of brachyury mutant cells in both zebrafish and mouse indicates that they are completely unable to join the notochord, and in the case of zebrafish instead join the floor plate [84, 85]. The distinct activity of Brachyury between these two populations of cells is likely due at least in part to differences in cofactors present. Brachyury binds to the BMP effector Smad1, which can cause differential target gene regulation compared to Brachyury not bound to Smad1 [86]. The NMPs are a region of high BMP activity, while the midline and notochord progenitors have low BMP activity, and thus an absence of activated Smad1 [20, 87]. In Xenopus embryos, the absence of Smad1 binding to Brachyury promotes activation of notochord marker goosecoid expression [86]. The difference in Brachyury function within NMPs and midline progenitors may also be the result of differential regulation of signaling pathways in these populations. FGF signaling plays an essential role in promoting notochord fate, which is conserved across chordates [30–32, 88–90]. Brachyury functions in an autoregulatory loop with FGF signaling [35]. Brachyury can induce FGF ligand expression, and FGF signaling is in turn required for brachyury expression [35]. Loss of Brachyury functional analysis in zebrafish revealed that the regulation of FGF signaling activation by Brachyury appears restricted to the axial mesoderm [7], which may play a role in the differential activity of Brachyury revealed by mosaic analysis.
4. Insights into the epithelial to mesenchymal transition that generates mesoderm
Epithelial to mesenchymal transition (EMT) describes the cellular state change that occurs in tightly adhering epithelial cells as they lose their adhesions and become migratory and invasive mesenchymal cells [91, 92]. The term EMT was coined by Elizabeth Hay, who first described the process after observing mesoderm formation in the chick embryo [93]. Since then, EMT has been recognized to be a critical event during mesoderm induction across animal species, where cells undergo EMT to internalize and form the mesodermal germ layer [91, 94]. Here I will discuss the process of EMT in NMP-derived mesoderm induction and how it has informed our understanding of the molecular regulation of this process, as well as the biological importance of intermediate transitional states between full epithelial and mesenchymal characteristics.
4.1. NMPs undergo a two-step EMT during mesoderm induction
Several lines of evidence suggest that NMPs are an epithelial cell type, and subsequently undergo EMT as they form mesoderm [6]. Much of our understanding of the NMP to mesoderm EMT comes from zebrafish, based on their amenability for pairing live imaging with genetic manipulations. Live-imaging of zebrafish tailbud cell movements revealed that cells within the region corresponding to the location of NMPs have collective epithelial-like migration and then transition to rapid individual cell migration as they transition to mesoderm, consistent with these cells being in a mesenchymal state [95]. Live-imaging of zebrafish mesoderm formation during gastrulation and in the tailbud at post-gastrulation stages showed that the EMT process occurs in a two-step fashion [19, 96, 97]. In the first step, cells transition from epithelium to mesenchyme that migrates in a non-directional fashion. In the second step the cells migrating in a non-directional manner switch to directional migration as measured by individual cell tracking, which allows them to join the mesodermal cell population [96]. The cells in the transitional, or partial EMT state, are more adhesive than the fully mesenchymal mesoderm formed after the completion of the second step [97]. The T-box transcription factor Tbx16 is essential for the completion of the second EMT step in zebrafish [96, 98]. In the absence of Tbx16 function, cells complete the first EMT step but are unable to complete the second, and thus remain trapped in the partial EMT state indefinitely. This causes the cells to remain in the tailbud until the end of axis extension, unable to join the mesoderm [99, 100]. Mouse embryos with a loss of function in the related transcription factor TBX6 have a similar phenotype, where embryos have enlarged tailbuds due to cells being unable to leave and join the mesoderm [101]. However, unlike in zebrafish tbx16 mutants, a portion of cells in the mouse Tbx6 mutants exit the tailbud to join the region normally occupied by the paraxial mesoderm, but instead give rise to ectopic spinal cords [101]. The nature of this phenotype, and the difference with zebrafish, is discussed in section 4.2.
The cellular transition and molecular regulation of the two-step EMT during NMP-derived mesoderm induction was further characterized in zebrafish embryos. The initiation of the 1st EMT step is regulated by canonical Wnt signaling, with Wnt signaling inducing an apical constriction and delamination of cells from the NMP epithelium, a process which, with respect to cell morphology and Wnt signaling dependence, looks remarkably like the mesodermal EMT during mouse gastrulation (Figure 3) [19, 102]. In the absence of Wnt signaling NMPs downregulate expression of the direct Wnt target gene brachyury and do not leave the NMP epithelium. These low Wnt cells will eventually contribute to the spinal cord [3, 7, 19]. Once cells enter the partial EMT state, FGF signaling is required to promote the 2nd EMT step, causing them to obtain directional migration out of the tailbud to join the mesodermal population. The activity of FGF signaling is due at least in part to its positive regulation of tbx16 and msgn1 expression, which promote exit from the tailbud in both zebrafish and mouse (Tbx6 in the case of mouse) [37, 38, 100, 101, 103]. The FGF and Wnt signaling pathways activate each other in the zebrafish tailbud, which may help ensure the continuous and balanced allocation of cells to the mesoderm by positive reinforcement between the 1st and 2nd EMT steps [104]. In the mouse, a partial EMT occurs in the epiblast, and the cells that enter the partial EMT state are incorporated into the tailbud as NMPs. This process is dependent on the activities of the Tgf-beta signaling pathway and the SNAI1 transcription factor [105]. Thus, partial EMT appears to be a common aspect of NMP development, although with species specific differences in molecular regulation and timing.
Figure 3 – Insights into the mesodermal epithelial to mesenchymal transition.
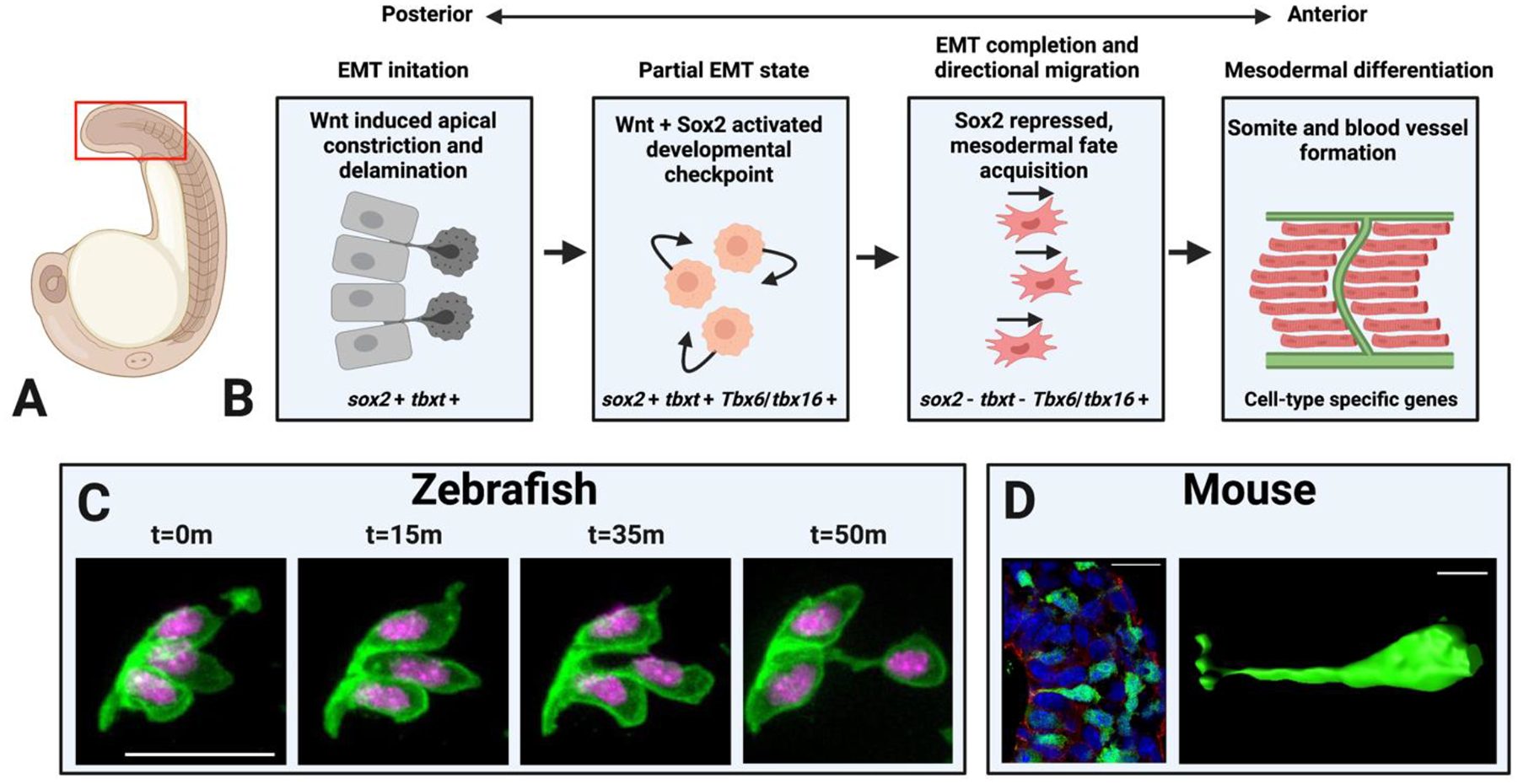
(A) A zebrafish embryo depicting (red box) the region of interest shown in (B). (B) The stages of the NMP-derived mesodermal EMT are depicted as schematics with events occurring beginning in the posterior wall of the tailbud (left) and progressing towards the anterior (right). The marker genes expressed at each step are indicated under each schematic. (C) Micrographs from a time-lapse of an individual cell undergoing EMT during zebrafish NMP-derived mesoderm induction, adapted from [16] with permission. Scale bar = 20μm. (D) Micrograph (left) and surface rendering (right) of an individual cell undergoing EMT during gastrula stage mesoderm induction in a mouse embryo, adapted from [94] with permission. Scale bar in left image = 25μm, in right image = 10μm). Both the mouse and zebrafish cells exhibit apical constriction and eventual delamination from the epithelium.
4.2. The partial EMT transitional state acts as a developmental checkpoint
Work from the cancer field has indicated that cells in the partial or intermediate EMT state have unique properties, such as increased stemness, invasiveness, and drug resistance, making the study of partial EMT states in cancer progression particularly important [106]. However, much less is known about the biological properties of cells in the partial EMT state in normal developmental processes, and whether there is significance to maintenance of metastable partial EMT states. As mentioned previously, zebrafish cells fated to become NMP-derived mesoderm that lack tbx16 function become trapped in the partial EMT state, a phenotype similar to mouse Tbx6 mutants. Both zebrafish Tbx16 and mouse TBX6 play an important role in transcriptional repression of the NMP marker sox2 [107, 108]. It was recently shown in zebrafish that ectopically maintaining sox2 in mesoderm fated NMPs is sufficient to phenocopy the Tbx16 loss of function phenotype, and eliminating Sox2 function in tbx16 mutants allows NMP fated mesoderm to exit the tailbud and differentiate into mesoderm [4]. The activity of Sox2 in preventing NMP fated mesoderm from exiting the tailbud and holding them in a metastable partial EMT state was found to be dependent upon interactions with the mesoderm inducing canonical Wnt signal. Inhibiting Wnt signaling in Sox2 gain of function or Tbx16 loss of function mesoderm fated cells (which maintain sox2 expression) allows them to exit the tailbud, however they differentiate into ectopic spinal cord rather than mesoderm [4]. Thus, the partial EMT state, which is promoted by a unique interaction of Sox2 and Wnt signaling, acts as a developmental checkpoint to ensure mesoderm fated cells that express sox2 do not exit the tailbud, making certain these cells adopt the appropriate mesodermal fate instead of neural fate (Figure 3) [4]. Recent work using quail embryos also showed that higher levels of Sox2 relative to Brachyury limits NMP cell migration and prevents incorporation into mesoderm [78].
In zebrafish, lowering Wnt signaling in Tbx16 loss of function cells causes them to differentiate into ectopic spinal cord [4]. This phenotype is similar to Tbx6 mutant mouse embryos, which form ectopic spinal cords [101], suggesting that differences in Wnt signaling levels between the two species may account for the normal differences in phenotypes. Based on the phenotypes, the differences imply that zebrafish have a higher relative level of Wnt signaling in the NMP region than mouse, and lowering the level in tbx16 loss of function phenocopies the mouse Tbx6 mutant. Wnt signal activation was previously shown to accelerate the tailbud exit and differentiation of NMP-fated mesoderm [3, 108]. Since zebrafish development is extremely rapid relative to mouse, which facilitates their need to swim and evade predators early in development, the hypothesized higher levels of Wnt signaling may play a role in part to promote rapid mesoderm induction and differentiation.
5. Mesoderm patterning by BMP and FGF signaling
Newly induced NMP-derived mesoderm primarily gives rise to paraxial mesoderm, which forms the somites. Although there are distinct non-NMP sources of lateral mesoderm [9, 109], a minority of NMP-derived mesoderm adopts lateral mesodermal fates [2, 3, 24]. The primary lateral fates identified thus far are vascular endothelium that will form posterior blood vessels and nephric mesenchyme [3, 20, 24]. This medial-lateral (also referred to as dorsal-ventral) mesodermal patterning is much simpler than what occurs during gastrulation-stage mesodermal patterning, where there are many more types of mesoderm being specified at the same time that mesodermal cells are undergoing very broad cell movements [15]. The simplified patterning event in NMP-derived mesoderm provides an opportunity to better understand mechanisms of patterning, without the complicated pleiotropies caused by gastrula-stage manipulations.
The first indication that NMP-derived mesoderm is continuously patterned by local signaling cues after it is induced came from experiments manipulating canonical Wnt signaling [3]. The role of Wnt signaling in patterning gastrula-stage mesoderm is well studied and is critical for promoting dorsal/medial cell fates such as axial and paraxial mesoderm [110]. During NMP-derived mesoderm patterning, this role appears to be conserved. After mesoderm is induced, Wnt signaling is required for paraxial mesoderm fate, and in its absence cells adopt the lateral endothelial fate [3]. Wnt signaling directly activates the expression of key paraxial mesoderm genes msgn1 and tbx16 (in zebrafish) and the patterning role of Wnt signaling in NMP-derived mesoderm is likely due at least in part to the regulation of these genes [108, 111–114]. The knowledge that NMP-derived mesoderm is patterned into multiple cell fates set the stage to examine other signaling pathways known to pattern mesoderm, and to use the relative simplicity of the system to tease out the molecular mechanism of patterning downstream of signal activation.
The BMP and FGF signaling pathways play key roles in mesodermal patterning, in addition to their roles in mesoderm induction. BMP induces ventral/lateral mesoderm, whereas FGF induces medial/dorsal fates [18, 23]. However, the downstream targets of these pathways that mediate the patterning during gastrulation were unknown. Like their gastrulation stage roles, BMP and FGF signaling also pattern NMP-derived mesoderm, with BMP inducing the lateral/ventral endothelial and nephric mesenchyme fates, and FGF inducing the medial/dorsal paraxial mesoderm fate [20, 24]. With respect to the paraxial/endothelial fate decision, the absence of BMP signaling causes endothelial fated cells to become paraxial mesoderm, and form ectopic somites at the ventral midline where axial blood vessels would normally form. On the other hand, activation of BMP induces endothelial fate in the paraxial mesoderm fated cells, and after signaling activation a large functional network of blood vessels form where somites normally occur (Figure 4A). Loss of FGF signaling gives the same patterning phenotype as gain of BMP signaling (Figure 4A) [20]. Although FGF signaling functions to repress BMP signaling during gastrulation [115, 116], the FGF loss of function phenotype in NMP derived mesodermal patterning is independent of any direct repressive role on BMP signaling. Instead, BMP and FGF signaling interact at the level of bHLH transcription factor activity, with the medial FGF patterning the result of FGF induced msgn1, myf5, and myod [20]. BMP signaling inhibits their activity by activation of Id proteins (id1, id3), which are HLH proteins that bind to and inhibit the activity of bHLH transcription factors [20]. This mechanism mediated by FGF and BMP signaling also functions during gastrula stage mesoderm patterning in zebrafish and in mouse NMP-derived mesoderm (Figure 4B) [20].
Figure 4 – Patterning of mesoderm by modulation of bHLH transcription factor activity.
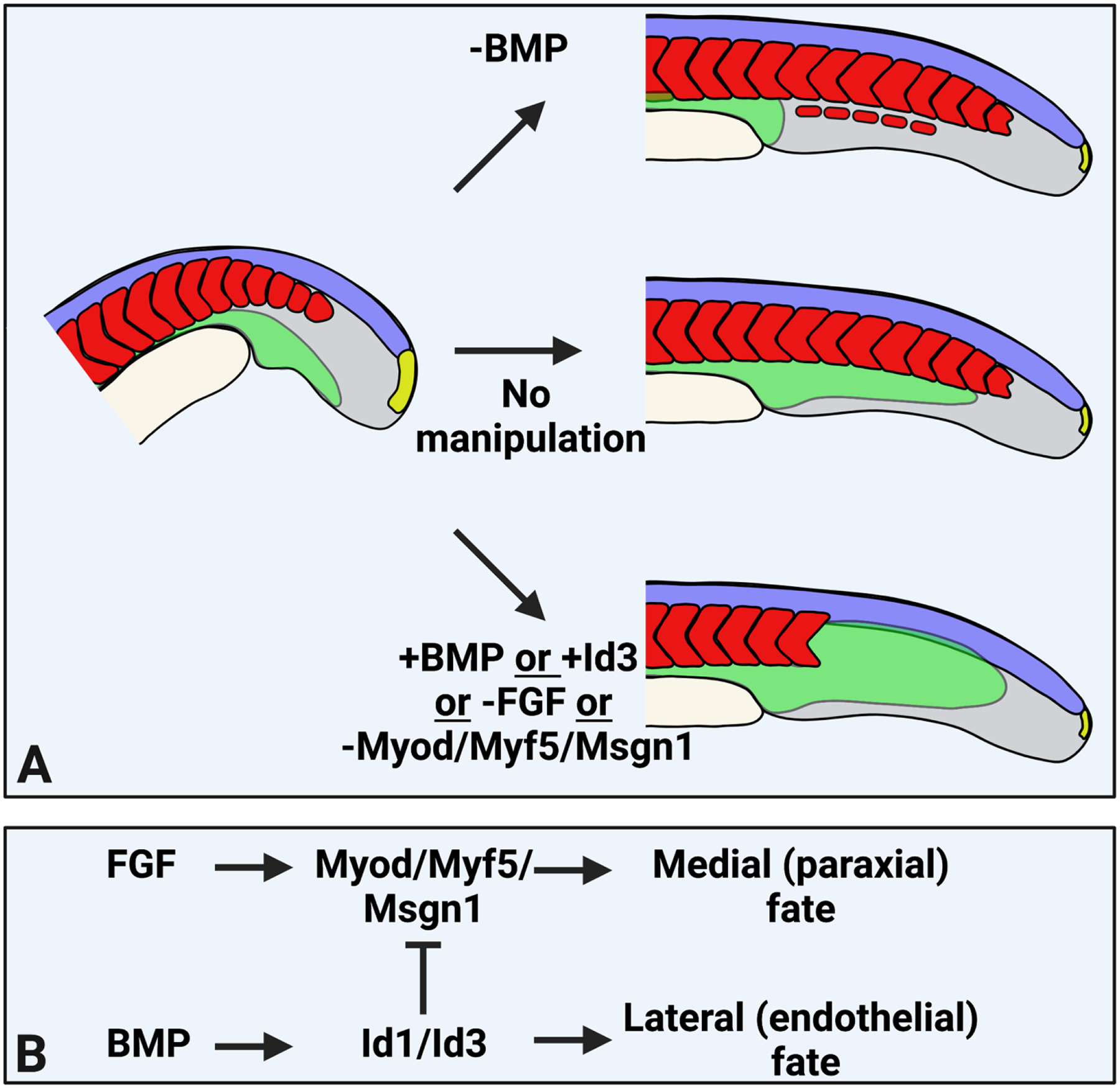
(A) Experimental manipulations that change the patterning of NMP-derived mesoderm in vivo, resulting in loss of endothelium and formation of ectopic somite tissue (top), or loss of somites and expansion of endothelium (bottom). (B) A model depicting the molecular mechanism of patterning downstream of BMP and FGF signaling. FGF signaling activates bHLH transcription factor expression, while BMP signaling activates the Id genes which inhibit bHLH proteins. This mechanism has been shown in zebrafish and in cultured mouse NMP-derived mesoderm.
An early response to either FGF loss of function or BMP gain of function in NMP-derived mesoderm is the expanded expression of the endothelial inducing gene etv2 [20]. Etv2 is both necessary and sufficient for inducing endothelial fate [117–122], and recent single cell sequencing of mutant zebrafish etv2 embryos revealed that in the absence of Etv2 function presumptive endothelial cells become somite derived skeletal muscle [123]. Gain of Etv2 function can also transfate cells in the somite into endothelial cells [118]. Etv2 labels both endothelial and hematopoietic progenitors in mouse and zebrafish. In the mouse, loss of ETV2 function results in the complete loss of blood and vessels [119]. In zebrafish, loss of Etv2 function results in the loss of vessels and primitive myeloid cells. Further research is needed to determine whether hematopoietic lineages are also derived in part from NMPs, and if BMP and FGF signaling play a similar patterning role.
7. Conclusions and future directions
The study of NMP-derived mesoderm induction is in its infancy, and while it so far has revealed critical insights into mesoderm induction, there are still many unanswered questions. Key molecular aspects of molecular pathways involved in NMP-derived mesoderm induction are yet to be described, such as the direct molecular targets of the canonical Wnt pathway that induce apical constriction in NMPs and the initiation of the EMT process. There are other basic questions that remain, such as why NMPs exhibit enrichment in the G2 phase of the cell cycle [124–126], and whether chemo-attractants or repellents play a role in directed migration out of the tailbud into the mesodermal territory, among many others. Furthermore, differences exist between the growth dynamics and lineage contribution of NMPs between vertebrate species, and further work is needed to determine how these differences may impact body plan variations [127, 128].
Additional important evolutionary questions remain as well. It is not clear to what extent partial EMT states observed in mouse and zebrafish NMPs occur during mesoderm formation in other animals. Recent evidence shows that in the diploblastic cnidarian Nematostella vectensis, the endomesodermal germ layer is established through cells entering into a partial EMT state, without becoming fully mesenchymal [129]. However, these cells can be artificially induced to become fully mesenchymal causing individual cell delamination [129]. Additionally, the mesodermal patterning mechanism involving regulation of bHLH transcription factor activity first observed in NMP-derived mesoderm may also have ancient origins, as bHLH transcription factors of the type found in vertebrate mesoderm as well as their HLH inhibitors are found in the earliest branching metazoans [130]. Finally, evidence is building that NMP-like cells or the regulatory circuits that govern their development exist outside of the vertebrate lineage [11, 66], and it will be fascinating to see how broadly they are represented across the animal kingdom.
Acknowledgements
I thank David Matus for critical reading of the manuscript and lab members for helpful discussions. The lab’s work on NMPs is supported by the NSF (IOS 1452928) and NIH NIGMS (R01GM124282).
References
- [1].Kimelman D, Martin BL. Anterior-posterior patterning in early development: three strategies. Wiley Interdiscip Rev Dev Biol 1(2) (2012) 253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas J-F. Redefining the Progression of Lineage Segregations during Mammalian Embryogenesis by Clonal Analysis. Developmental cell 17(3) (2009) 365–376. [DOI] [PubMed] [Google Scholar]
- [3].Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev Cell 22(1) (2012) 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kinney BA, Al Anber A, Row RH, Tseng YJ, Weidmann MD, Knaut H, Martin BL. Sox2 and Canonical Wnt Signaling Interact to Activate a Developmental Checkpoint Coordinating Morphogenesis with Mesoderm Fate Acquisition. Cell Rep 33(4) (2020) 108311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guillot C, Djeffal Y, Michaut A, Rabe B, Pourquie O. Dynamics of primitive streak regression controls the fate of neuromesodermal progenitors in the chicken embryo. Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wymeersch FJ, Wilson V, Tsakiridis A. Understanding axial progenitor biology in vivo and in vitro. Development 148(4) (2021). [DOI] [PubMed] [Google Scholar]
- [7].Martin BL, Kimelman D. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev Cell 15(1) (2008) 121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Olivera-Martinez I, Harada H, Halley PA, Storey KG. Loss of FGF-Dependent Mesoderm Identity and Rise of Endogenous Retinoid Signalling Determine Cessation of Body Axis Elongation. Plos Biology 10(10) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wymeersch FJ, Huang Y, Blin G, Cambray N, Wilkie R, Wong FC, Wilson V. Position-dependent plasticity of distinct progenitor types in the primitive streak. Elife 5 (2016) e10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsakiridis A, Huang Y, Blin G, Skylaki S, Wymeersch F, Osorno R, Economou C, Karagianni E, Zhao S, Lowell S, Wilson V. Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development 141(6) (2014) 1209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hudson C, Yasuo H. Neuromesodermal Lineage Contribution to CNS Development in Invertebrate and Vertebrate Chordates. Genes (Basel) 12(4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Al Anber A, Martin BL. Transformation of a neural activation and patterning model. EMBO Rep 20(8) (2019) e48060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Metzis V, Steinhauser S, Pakanavicius E, Gouti M, Stamataki D, Ivanovitch K, Watson T, Rayon T, Mousavy Gharavy SN, Lovell-Badge R, Luscombe NM, Briscoe J. Nervous System Regionalization Entails Axial Allocation before Neural Differentiation. Cell 175(4) (2018) 1105–1118 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Polevoy H, Gutkovich YE, Michaelov A, Volovik Y, Elkouby YM, Frank D. New roles for Wnt and BMP signaling in neural anteroposterior patterning. EMBO Rep 20(6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet 7(5) (2006) 360–72. [DOI] [PubMed] [Google Scholar]
- [16].Schier AF. Nodal morphogens. Cold Spring Harb Perspect Biol 1(5) (2009) a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hagos EG, Dougan ST. Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev Biol 7 (2007) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development 137(22) (2010) 3731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goto H, Kimmey SC, Row RH, Matus DQ, Martin BL. FGF and canonical Wnt signaling cooperate to induce paraxial mesoderm from tailbud neuromesodermal progenitors through regulation of a two-step epithelial to mesenchymal transition. Development 144(8) (2017) 1412–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Row RH, Pegg A, Kinney BA, Farr GH 3rd, Maves L, Lowell S, Wilson V, Martin BL. BMP and FGF signaling interact to pattern mesoderm by controlling basic helix-loop-helix transcription factor activity. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kimelman D, Griffin KJ. Vertebrate mesendoderm induction and patterning. Curr Opin Genet Dev 10(4) (2000) 350–6. [DOI] [PubMed] [Google Scholar]
- [22].Garriock RJ, Chalamalasetty RB, Kennedy MW, Canizales LC, Lewandoski M, Yamaguchi TP. Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development 142(9) (2015) 1628–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tuazon FB, Mullins MC. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin Cell Dev Biol 42 (2015) 118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hayashi S, Suzuki H, Takemoto T. The nephric mesenchyme lineage of intermediate mesoderm is derived from Tbx6-expressing derivatives of neuro-mesodermal progenitors via BMP-dependent Osr1 function. Dev Biol 478 (2021) 155–162. [DOI] [PubMed] [Google Scholar]
- [25].Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120(7) (1994) 1919–28. [DOI] [PubMed] [Google Scholar]
- [26].Harvey SA, Tumpel S, Dubrulle J, Schier AF, Smith JC. no tail integrates two modes of mesoderm induction. Development 137(7) (2010) 1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395(6698) (1998) 181–5. [DOI] [PubMed] [Google Scholar]
- [28].Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97(1) (1999) 121–32. [DOI] [PubMed] [Google Scholar]
- [29].Kimelman D, Abraham JA, Haaparanta T, Palisi TM, Kirschner MW. The presence of fibroblast growth factor in the frog egg: its role as a natural mesoderm inducer. Science 242(4881) (1988) 1053–6. [DOI] [PubMed] [Google Scholar]
- [30].Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66(2) (1991) 257–70. [DOI] [PubMed] [Google Scholar]
- [31].Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development 118(2) (1993) 477–87. [DOI] [PubMed] [Google Scholar]
- [32].Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development 121(9) (1995) 2983–94. [DOI] [PubMed] [Google Scholar]
- [33].Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell 1(1) (2001) 37–49. [DOI] [PubMed] [Google Scholar]
- [34].Casey ES, O’Reilly MAJ, Conlon FL, Smith JC. The T-box transcription factor Brachyury regulates expression of eFGF through binding to a non-palindromic response element. Development 125(19) (1998) 3887–3894. [DOI] [PubMed] [Google Scholar]
- [35].Schulte-Merker S, Smith JC. Mesoderm formation in response to Brachyury requires FGF signalling. Curr Biol 5(1) (1995) 62–7. [DOI] [PubMed] [Google Scholar]
- [36].Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67(1) (1991) 79–87. [DOI] [PubMed] [Google Scholar]
- [37].Fior R, Maxwell AA, Ma TP, Vezzaro A, Moens CB, Amacher SL, Lewis J, Saude L. The differentiation and movement of presomitic mesoderm progenitor cells are controlled by Mesogenin 1. Development 139(24) (2012) 4656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yabe T, Takada S. Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites. Dev Biol 370(2) (2012) 213–22. [DOI] [PubMed] [Google Scholar]
- [39].Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 129(14) (2002) 3311–23. [DOI] [PubMed] [Google Scholar]
- [40].Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 466(7303) (2010) 248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thomson L, Muresan L, Steventon B. The zebrafish presomitic mesoderm elongates through compaction-extension. Cells Dev (2021) 203748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rentzsch F, Bakkers J, Kramer C, Hammerschmidt M. Fgf signaling induces posterior neuroectoderm independently of Bmp signaling inhibition. Dev Dyn 231(4) (2004) 750–7. [DOI] [PubMed] [Google Scholar]
- [43].Rogers CD, Archer TC, Cunningham DD, Grammer TC, Casey EM. Sox3 expression is maintained by FGF signaling and restricted to the neural plate by Vent proteins in the Xenopus embryo. Dev Biol 313(1) (2008) 307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature 406(6791) (2000) 74–8. [DOI] [PubMed] [Google Scholar]
- [45].Wills AE, Choi VM, Bennett MJ, Khokha MK, Harland RM. BMP antagonists and FGF signaling contribute to different domains of the neural plate in Xenopus. Dev Biol 337(2) (2010) 335–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol 12(8) (2014) e1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turner DA, Hayward PC, Baillie-Johnson P, Rue P, Broome R, Faunes F, Martinez Arias A. Wnt/beta-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development 141(22) (2014) 4243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nieuwkoop PD, Nigtevecht GV. Neural Activation and Transformation in Explants of Competent Ectoderm under the Influence of Fragments of Anterior Notochord in Urodeles. Embryol Exp Morph 2 (1954) 175–193. [Google Scholar]
- [49].Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development 108(4) (1990) 581–94. [DOI] [PubMed] [Google Scholar]
- [50].Mathieu J, Griffin K, Herbomel P, Dickmeis T, Strahle U, Kimelman D, Rosa FM, Peyrieras N. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development 131(3) (2004) 629–41. [DOI] [PubMed] [Google Scholar]
- [51].Dobrovolskaia-Zavadskaia N. Regarding the spontaneous mortification of the tail of a new-born mouse and the existence of a hereditary characteristic (factor). Comptes Rendus Des Seances De La Societe De Biologie Et De Ses Filiales 97 (1927) 114–116. [Google Scholar]
- [52].Chesley P. Development of the short-tailed mutant in the house mouse. Journal of Experimental Zoology 70(3) (1935) 429–459. [Google Scholar]
- [53].Gluecksohn-Schoenheimer S. The development of normal and homozygous Brachy (T/T) mouse embryos in the extraembryonic coelom of the chick. Proc Natl Acad Sci U S A 30 (1944) 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yasuo H, Satoh N. Conservation of the developmental role of Brachyury in notochord formation in a urochordate, the ascidian Balocynthia roretzi. Dev Biol 200(2) (1998) 158–70. [DOI] [PubMed] [Google Scholar]
- [55].Takahashi H, Hotta K, Erives A, Di Gregorio A, Zeller RW, Levine M, Satoh N. Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev 13(12) (1999) 1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yasuo H, Satoh N. Function of vertebrate T gene. Nature 364(6438) (1993) 582–3. [DOI] [PubMed] [Google Scholar]
- [57].Satou Y, Imai KS, Satoh N. Action of morpholinos in Ciona embryos. Genesis 30(3) (2001) 103–6. [DOI] [PubMed] [Google Scholar]
- [58].Di Gregorio A, Harland RM, Levine M, Casey ES. Tail morphogenesis in the ascidian, Ciona intestinalis, requires cooperation between notochord and muscle. Dev Biol 244(2) (2002) 385–95. [DOI] [PubMed] [Google Scholar]
- [59].Yamada L, Shoguchi E, Wada S, Kobayashi K, Mochizuki Y, Satou Y, Satoh N. Morpholino-based gene knockdown screen of novel genes with developmental function in Ciona intestinalis. Development 130(26) (2003) 6485–95. [DOI] [PubMed] [Google Scholar]
- [60].Chiba S, Jiang D, Satoh N, Smith WC. Brachyury null mutant-induced defects in juvenile ascidian endodermal organs. Development 136(1) (2009) 35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Martin BL, Kimelman D. Wnt signaling and the evolution of embryonic posterior development. Curr Biol 19(5) (2009) R215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Martin BL, Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev 24(24) (2010) 2778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Guibentif C, Griffiths JA, Imaz-Rosshandler I, Ghazanfar S, Nichols J, Wilson V, Gottgens B, Marioni JC. Diverse Routes toward Early Somites in the Mouse Embryo. Dev Cell 56(1) (2021) 141–153 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Morley RH, Lachani K, Keefe D, Gilchrist MJ, Flicek P, Smith JC, Wardle FC. A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc Natl Acad Sci U S A 106(10) (2009) 3829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Amin S, Neijts R, Simmini S, van Rooijen C, Tan SC, Kester L, van Oudenaarden A, Creyghton MP, Deschamps J. Cdx and T Brachyury Co-activate Growth Signaling in the Embryonic Axial Progenitor Niche. Cell Rep 17(12) (2016) 3165–3177. [DOI] [PubMed] [Google Scholar]
- [66].Schwaiger M, Andrikou C, Dnyansagar R, Murguia PF, Paganos P, Voronov D, Zimmermann B, Lebedeva T, Schmidt H, Genikhovich G, Benvenuto G, Arnone M, Technau U. An ancestral Wnt-Brachyury feedback loop and recruitment of mesoderm-determining target genes revealed by comparative Brachyury target screens. PREPRINT (Version 2) available at Research Square: 10.21203/rs.3.rs-753399/v1 (2021). [DOI] [PubMed] [Google Scholar]
- [67].Evans AL, Faial T, Gilchrist MJ, Down T, Vallier L, Pedersen RA, Wardle FC, Smith JC. Genomic targets of Brachyury (T) in differentiating mouse embryonic stem cells. PLoS One 7(3) (2012) e33346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yamaguchi TP, Takada S, Yoshikawa Y, Wu NY, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes & Development 13(24) (1999) 3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Servetnick MD, Steinworth B, Babonis LS, Simmons D, Salinas-Saavedra M, Martindale MQ. Cas9-mediated excision of Nematostella brachyury disrupts endoderm development, pharynx formation and oral-aboral patterning. Development 144(16) (2017) 2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fritzenwanker JH, Uhlinger KR, Gerhart J, Silva E, Lowe CJ. Untangling posterior growth and segmentation by analyzing mechanisms of axis elongation in hemichordates. Proc Natl Acad Sci U S A 116(17) (2019) 8403–8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Koch F, Scholze M, Wittler L, Schifferl D, Sudheer S, Grote P, Timmermann B, Macura K, Herrmann BG. Antagonistic Activities of Sox2 and Brachyury Control the Fate Choice of Neuro-Mesodermal Progenitors. Dev Cell 42(5) (2017) 514–526 e7. [DOI] [PubMed] [Google Scholar]
- [72].Gouti M, Delile J, Stamataki D, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. A Gene Regulatory Network Balances Neural and Mesoderm Specification during Vertebrate Trunk Development. Dev Cell 41(3) (2017) 243–261 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Berenguer M, Lancman JJ, Cunningham TJ, Dong PDS, Duester G. Mouse but not zebrafish requires retinoic acid for control of neuromesodermal progenitors and body axis extension. Dev Biol 441(1) (2018) 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes & Development 15(2) (2001) 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Emoto Y, Wada H, Okamoto H, Kudo A, Imai Y. Retinoic acid-metabolizing enzyme Cyp26a1 is essential tor determining territories of hindbrain and spinal cord in zebrafish. Developmental Biology 278(2) (2005) 415–427. [DOI] [PubMed] [Google Scholar]
- [76].Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Reviews Molecular Cell Biology 16(2) (2015) 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rydeen A, Voisin N, D’Aniello E, Ravisankar P, Devignes CS, Waxman JS. Excessive feedback of Cyp26a1 promotes. cell non-autonomous loss of retinoic acid signaling. Developmental Biology 405(1) (2015) 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Romanos M, Allio G, Roussigne M, Combres L, Escalas N, Soula C, Medevielle F, Steventon B, Trescases A, Benazeraf B. Cell-to-cell heterogeneity in Sox2 and Bra expression guides progenitor motility and destiny. Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Martin BL. Factors that coordinate mesoderm specification from neuromesodermal progenitors with segmentation during vertebrate axial extension. Semin Cell Dev Biol 49 (2016) 59–67. [DOI] [PubMed] [Google Scholar]
- [80].Martin B, Progenitor Cells in Vertebrate Segmentation, in: Chipman AD (Ed.), Cellular Processes in Segmentation, CRC Press, Boca Raton, 2020. [Google Scholar]
- [81].Mallo M. Reassessing the Role of Hox Genes during Vertebrate Development and Evolution. Trends in genetics : TIG 34(3) (2018) 209–217. [DOI] [PubMed] [Google Scholar]
- [82].Ye Z, Braden CR, Wills A, Kimelman D. Identification of in vivo Hox13-binding sites reveals an essential locus controlling zebrafish brachyury expression. Development 148(11) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Row RH, Tsotras SR, Goto H, Martin BL. The zebrafish tailbud contains two independent populations of midline progenitor cells that maintain long-term germ layer plasticity and differentiate in response to local signaling cues. Development 143(2) (2016) 244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75(1) (1993) 99–111. [PubMed] [Google Scholar]
- [85].Wilson V, Manson L, Skarnes WC, Beddington RS. The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development 121(3) (1995) 877–86. [DOI] [PubMed] [Google Scholar]
- [86].Messenger NJ, Kabitschke C, Andrews R, Grimmer D, Nunez Miguel R, Blundell TL, Smith JC, Wardle FC. Functional specificity of the Xenopus T-domain protein Brachyury is conferred by its ability to interact with Smad1. Dev Cell 8(4) (2005) 599–610. [DOI] [PubMed] [Google Scholar]
- [87].Esterberg R, Delalande JM, Fritz A. Tailbud-derived Bmp4 drives proliferation and inhibits maturation of zebrafish chordamesoderm. Development 135(23) (2008) 3891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Imai KS, Satoh N, Satou Y. Early embryonic expression of FGF4/6/9 gene and its role in the induction of mesenchyme and notochord in Ciona savignyi embryos. Development 129(7) (2002) 1729–38. [DOI] [PubMed] [Google Scholar]
- [89].Kim GJ, Nishida H. Role of the FGF and MEK signaling pathway in the ascidian embryo. Dev Growth Differ 43(5) (2001) 521–33. [DOI] [PubMed] [Google Scholar]
- [90].Shimauchi Y, Murakami SD, Satoh N. FGF signals are involved in the differentiation of notochord cells and mesenchyme cells of the ascidian Halocynthia roretzi. Development 128(14) (2001) 2711–21. [DOI] [PubMed] [Google Scholar]
- [91].Sheng G. Defining epithelial-mesenchymal transitions in animal development. Development 148(8) (2021). [DOI] [PubMed] [Google Scholar]
- [92].Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, Garcia de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RJY, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massague J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G, Association EMTI. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 21(6) (2020) 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 154(1) (1995) 8–20. [DOI] [PubMed] [Google Scholar]
- [94].Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ 50(9) (2008) 755–66. [DOI] [PubMed] [Google Scholar]
- [95].Lawton AK, Nandi A, Stulberg MJ, Dray N, Sneddon MW, Pontius W, Emonet T, Holley SA. Regulated tissue fluidity steers zebrafish body elongation. Development 140(3) (2013) 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Manning AJ, Kimelman D. Tbx16 and Msgn1 are required to establish directional cell migration of zebrafish mesodermal progenitors. Dev Biol (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Row RH, Maitre JL, Martin BL, Stockinger P, Heisenberg CP, Kimelman D. Completion of the epithelial to mesenchymal transition in zebrafish mesoderm requires Spadetail. Developmental Biology 354(1) (2011) 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Griffin KJP, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development 125(17) (1998) 3379–3388. [DOI] [PubMed] [Google Scholar]
- [99].Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature 348(6303) (1990) 728–30. [DOI] [PubMed] [Google Scholar]
- [100].Kimmel CB, Kane DA, Walker C, Warga RM, Rothman MB. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature 337(6205) (1989) 358–62. [DOI] [PubMed] [Google Scholar]
- [101].Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391(6668) (1998) 695–697. [DOI] [PubMed] [Google Scholar]
- [102].Ramkumar N, Omelchenko T, Silva-Gagliardi NF, McGlade CJ, Wijnholds J, Anderson KV. Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nat Cell Biol 18(12) (2016) 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yoon JK, Wold B. The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes Dev 14(24) (2000) 3204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Stulberg MJ, Lin A, Zhao H, Holley SA. Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud. Dev Biol 369(2) (2012) 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Dias A, Lozovska A, Wymeersch FJ, Novoa A, Binagui-Casas A, Sobral D, Martins GG, Wilson V, Mallo M. A Tgfbr1/Snai1-dependent developmental module at the core of vertebrate axial elongation. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol 30(10) (2020) 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Takemoto T, Uchikawa M, Yoshida M, Bell DM, Lovell-Badge R, Papaioannou VE, Kondoh H. Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470(7334) (2011) 394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bouldin CM, Manning AJ, Peng YH, Farr GH, Hung KL, Dong A, Kimelman D. Wnt signaling and tbx16 form a bistable switch to commit bipotential progenitors to mesoderm. Development 142(14) (2015) 2499–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Prummel KD, Hess C, Nieuwenhuize S, Parker HJ, Rogers KW, Kozmikova I, Racioppi C, Brombacher EC, Czarkwiani A, Knapp D, Burger S, Chiavacci E, Shah G, Burger A, Huisken J, Yun MH, Christiaen L, Kozmik Z, Muller P, Bronner M, Krumlauf R, Mosimann C. A conserved regulatory program initiates lateral plate mesoderm emergence across chordates. Nat Commun 10(1) (2019) 3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol 5(1) (2013) a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chalamalasetty RB, Dunty WC Jr., Biris KK, Ajima R, Iacovino M, Beisaw A, Feigenbaum L, Chapman DL, Yoon JK, Kyba M, Yamaguchi TP. The Wnt3a/beta-catenin target gene Mesogenin1 controls the segmentation clock by activating a Notch signalling program. Nature Communications 2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chalamalasetty RB, Garriock RJ, Dunty WC Jr., Kennedy MW, Jailwala P, Si H, Yamaguchi TP. Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development 141(22) (2014) 4285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Szeto DP, Kimelman D. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development 131(15) (2004) 3751–60. [DOI] [PubMed] [Google Scholar]
- [114].Wittler L, Shin E.-h., Grote P, Kispert A, Beckers A, Gossler A, Werber M, Herrmann BG. Expression of Msgn1 in the presomitic mesoderm is controlled by synergism of WNT signalling and Tbx6. Embo Reports 8(8) (2007) 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Furthauer M, Van Celst J, Thisse C, Thisse B. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development 131(12) (2004) 2853–64. [DOI] [PubMed] [Google Scholar]
- [116].Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17(24) (2003) 3023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Salanga MC, Meadows SM, Myers CT, Krieg PA. ETS family protein ETV2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the Xenopus embryo. Dev Dyn 239(4) (2010) 1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Veldman MB, Zhao C, Gomez GA, Lindgren AG, Huang H, Yang H, Yao S, Martin BL, Kimelman D, Lin S. Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol 11(6) (2013) e1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2(5) (2008) 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rasmussen TL, Kweon J, Diekmann MA, Belema-Bedada F, Song Q, Bowlin K, Shi X, Ferdous A, Li T, Kyba M, Metzger JM, Koyano-Nakagawa N, Garry DJ. ER71 directs mesodermal fate decisions during embryogenesis. Development 138(21) (2011) 4801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol 4(1) (2006) e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H, Nishikawa S. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRalpha+ primitive mesoderm. Blood 118(26) (2011) 6975–86. [DOI] [PubMed] [Google Scholar]
- [123].Chestnut B, Casie Chetty S, Koenig AL, Sumanas S. Single-cell transcriptomic analysis identifies the conversion of zebrafish Etv2-deficient vascular progenitors into skeletal muscle. Nat Commun 11(1) (2020) 2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Adikes RC, Kohrman AQ, Martinez MAQ, Palmisano NJ, Smith JJ, Medwig-Kinney TN, Min M, Sallee MD, Ahmed OB, Kim N, Liu S, Morabito RD, Weeks N, Zhao Q, Zhang W, Feldman JL, Barkoulas M, Pani AM, Spencer SL, Martin BL, Matus DQ. Visualizing the metazoan proliferation-quiescence decision in vivo. Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bouldin CM, Snelson CD, Farr GH, Kimelman D. Restricted expression of cdc25a in the tailbud is essential for formation of the zebrafish posterior body. Genes & Development 28(4) (2014) 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Morabito RD, Adikes RC, Matus DQ, Martin BL. Cyclin-Dependent Kinase Sensor Transgenic Zebrafish Lines for Improved Cell Cycle State Visualization in Live Animals. Zebrafish (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Attardi A, Fulton T, Florescu M, Shah G, Muresan L, Lenz MO, Lancaster C, Huisken J, van Oudenaarden A, Steventon B. Neuromesodermal progenitors are a conserved source of spinal cord with divergent growth dynamics. Development 145(21) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Steventon B, Duarte F, Lagadec R, Mazan S, Nicolas JF, Hirsinger E. Species-specific contribution of volumetric growth and tissue convergence to posterior body elongation in vertebrates. Development 143(10) (2016) 1732–41. [DOI] [PubMed] [Google Scholar]
- [129].Salinas-Saavedra M, Rock AQ, Martindale MQ. Germ layer-specific regulation of cell polarity and adhesion gives insight into the evolution of mesoderm. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Fortunato SAV, Vervoort M, Adamski M, Adamska M. Conservation and divergence of bHLH genes in the calcisponge Sycon ciliatum. Evodevo 7 (2016) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]


