Abstract
Abstract
Purpose
To examine the prevalence of rapid discontinuation of chronic, high-dose opioid analgesic treatment, and identify associated patient, clinician, and community factors.
Methods
Using 2017–2018 retail pharmacy claims data from IQVIA, we identified chronic, high-dose opioid analgesic treatment episodes discontinued during these years and determined the percent of episodes meeting criteria for rapid discontinuation. We used multivariable logistic regression to estimate the probability of rapid discontinuation, conditional on having a discontinued chronic, high-dose opioid treatment episode, as a function of patient, provider, and county characteristics.
Results
We identified 810,120 new, chronic, high-dose opioid treatment episodes discontinued in 2017 or 2018, of which 72.0% (n=583,415) were rapidly discontinued. Rapid discontinuation was significantly more likely among Medicare (aOR 1.14, 95% CI 1.12 to 1.15) and Medicaid enrollees (aOR 1.03, 95% CI 1.02 to 1.05) compared to the commercially insured; in counties with higher fatal overdose rates (aOR 1.03, 95% CI 1.01 to 1.04) compared to counties with the lowest fatal overdose rates; and in counties with a higher percentage of non-white residents (aOR 1.21 for counties in the highest quartile relative to the lowest, 95% CI 1.19 to 1.24). Likelihood of rapid discontinuation also varied by prescriber specialty.
Conclusions
Most chronic, high-dose opioid treatment episodes that ended in 2017 or 2018 were discontinued more rapidly than recommended by clinical guidelines, raising concerns about adverse patient outcomes. Our findings highlight the need to understand what drives discontinuation and to inform safer opioid tapering and discontinuation practices.
KEY WORDS: opioid analgesic, state policy, prescribing, pain, drug safety
INTRODUCTION
Concerns about opioid overprescribing have prompted efforts to limit prescription opioid use. Over the past decade, opioid prescriptions have declined markedly1–3, from a peak of 81.3 per 100 persons in 2012 to 51.4 per 100 in 20184. The rate of initial opioid prescriptions and number of providers initiating opioids in opioid-naive patients have also decreased5.
Efforts to decrease opioid prescribing have largely focused on clinically questionable prescribing6 where alternative pain therapies would be equally efficacious; the amount prescribed exceed the amount necessary; or individuals could have chronic high-dose opioids tapered safely. Clinical guidelines and policy initiatives, such as the Centers for Disease Control’s (CDC) 2016 Guidelines, have often focused on high-dose prescribing7,8, such as discouraging daily doses above 90 morphine milligram equivalents (MME) due to associated risks9–13. Multiple states have introduced policies restricting high-dose prescribing9,14.
Prompted by increased attention to high-dose opioid prescribing, many clinicians are revisiting their prescribing practices15. With patient consent, thoughtful and clinically appropriate opioid tapering and eventual discontinuation can reduce patients’ risk of overdose and developing OUD and may improve functioning and quality of life16–18. However, rapid tapering and discontinuation have been associated with increased risk of adverse events such as opioid-related emergency department visits and hospitalizations19.
Multiple organizations and experts have released guidelines for tapering opioids among individuals on long-term opioid therapy20–22. To minimize withdrawal symptoms while maintaining pain control23, guidelines commonly recommend a taper of no more than a 10% weekly reduction in daily dose for most chronic high-dose opioid patients, excluding those at elevated risk for overdose.
Concerns have emerged that such guidance has elicited unintended responses6. Many providers appear unwilling to accept new chronic opioid patients24,25, and some clinicians caring for patients on long-term, high-dose opioids are tapering (i.e., reducing the opioid dosage) or discontinuing (i.e., stopping opioids altogether) chronic opioids faster than the guidelines recommend26. Approximately one-quarter of commercially insured and Medicare Advantage chronic opioid patients whose opioids were tapered experienced a tapering rate faster than the recommended 10% per week15. Tapering was more rapid among commercially insured individuals; however, rates of rapid discontinuation were not examined.
Few studies have examined rapid opioid discontinuation. One examined discontinuation among Medicaid enrollees in a single state between 2013 and 2017. Of almost 700 individuals with a daily MME greater than 120 for more than 90 days, 86% went from their first prescription for less than 120 MME per day (defined as the beginning of the discontinuation) to no opioid prescriptions within 3 weeks 19. A more recent study of Medicare Part D beneficiaries on opioids for more than a year examined if the average daily MME in the last month of treatment was more than 50% of the average daily MME of a baseline period of 7–12 months before discontinuation, finding this occurred in more than 70% of beneficiaries. 27 Finally, in another study of Medicare beneficiaries and commercially insured individuals, 28 researchers found that less than 25% of individuals on long-term opioids had an average daily MME in the last 30 days more than 10% lower than the daily MME in the preceding 30 days. However, none of these studies have examined how type of insurance or community context may influence the prevalence of rapid discontinuation, nor to what extent it may vary by prescriber specialty. Prior studies have identified differences in opioid prescribing patterns by county urbanicity29,30, physician supply31, county racial/ethnic composition31, and drug mortality rates32; similar factors may be associated with opioid discontinuation.
We used claims data from over 500,000 chronic high-dose opioid episodes initiated during 2017 and 2018 to examine prevalence of rapid discontinuation and identify associated patient demographics, clinician, and community factors.
METHODS
Data
To examine rapid discontinuation for new, high-dose opioid treatment episodes, we used de-identified pharmacy claims from IQVIA Real World Data–Longitudinal Prescriptions33. These data, which capture about 90% of all prescriptions filled at retail pharmacies in all 50 states and the District of Columbia, contain information on the prescription, payer, patient demographics, and the prescribing provider’s specialty and location. The study was deemed exempt by the corresponding author’s Institutional Review Board.
Sample
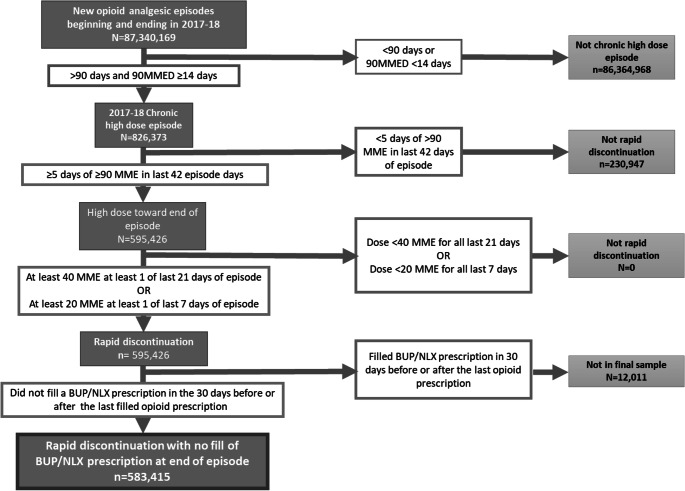
We identified new, chronic, high-dose opioid treatment episodes started during 2017 or 2018. We defined new episodes as beginning with the first opioid analgesic prescription following a period of at least 90 days in which no opioid analgesic prescriptions were filled. We identified opioid analgesic episodes that were both chronic (had at least a 90-day supply of opioid analgesic from the first observed filled prescription), and high-dose (had an MME daily dose exceeding 90 on more than 14 days at any time during the episode). We excluded individuals who filled a buprenorphine/naloxone prescription in the 30 days before or after the last filled opioid prescription, as such individuals may have had opioids rapidly discontinued as a result of being diagnosed with opioid use disorder. We identified those new, chronic, high-dose opioid analgesic treatment episodes that were discontinued during 2017 or 2018, defined as at least a 30-day period with no opioid prescription fills following the date when the days’ supply of any prior opioid prescription had run out (Figure 1). In situations in which there was overlapping days supply from multiple opioid prescriptions being filled, if prescriptions were filled the same day they were considered to be taken concurrently, and if the prescriptions were not filled the same day there were considered to be taken sequentially. Individuals could have more than one long-term opioid episode.
Figure 1.
Identification of new chronic high-dose opioid analgesic episodes and episodes with rapid discontinuation.
Measures
Guidelines commonly recommend gradually decreasing the amount of opioids prescribed by 10% per week from the prior dose as measured in MME daily doses. This benchmark has been used in prior studies of tapering15. However, many individuals whose opioids are discontinued have periods of increases as well as decreases in the prescribed MME daily dose between their peak MME daily dose and discontinuation. This variation complicates definitions of rapid discontinuation that require consistent reductions in the average MME daily dose exceeding 10% per week from the prior dose. To define rapid discontinuation more flexibly, we identified new, chronic, high-dose opioid analgesic treatment episodes in which (1) the individual received at least 90 MME daily for at least 5 days during the last 6 weeks of the episode, and then had either (2) at least 40 MME prescribed for at least 1 day during the final 21 days of the episode, or had (3) at least 20 MME prescribed for at least 1 day during the last 7 days of the episode (Figure 1). All episodes meeting these criteria will have had a weekly decrease in opioid MME of greater than 10%.
We categorized prescribers according to credentials and specialty: adult primary care physicians (including internal medicine and family practice); advanced practice providers (nurse practitioners and physician’s assistants); pain and anesthesia physicians; oncologists; surgeons; and other physician specialties. Episodes in which the patient filled opioid prescriptions from 2 or more prescribers were attributed to the prescriber who wrote the last filled opioid prescription during the episode.
We categorized patients by gender and age group (18–25, 26–35, 36–45, 46–55, 56–65, and over 65 years). We determined the primary payment source for the opioid episode (Medicaid; Medicare; commercial insurance; cash pay; and other insurance, including Tricare and prescription discount cards) by the payment source used for the majority of the days of opioid supply during the episode. We used the 5-digit Federal Information Processing Standards (FIPS) code of the episode prescriber to determine the county in which the episode occurred. We categorized county urbanicity based on Rural-Urban Continuum Codes (RUCC) from the Area Health Resources Files (AHRF), with counties classified as “metropolitan” (RUCC 1, 2, or 3), “rural adjacent” (RUCC 4, 6, or 8), or “rural remote” (RUCC 5, 7, or 9)34. We calculated county fatal overdose rates as the per-capita rate of overdose deaths due to any drug in 2017, using data from the CDC35, and assigned counties to terciles based on their fatal overdose rate. We categorized a county as a primary care health professional shortage area (HPSA) if any part of the county was designated as a primary care HPSA in the AHRF36. We excluded individuals for whom county-level variables were unavailable.
Analytic Approach
We conducted analyses at the episode level. We first calculated the number and unadjusted percentages of discontinued new, chronic, high-dose opioid treatment episodes during our observation period overall and by patient, provider, and county characteristics. We then calculated the number and percentage of rapidly discontinued episodes overall and by patient demographic, provider, and county characteristics. Finally, we used multivariable logistic regression to examine the probability of rapid discontinuation, conditional on having a discontinued chronic high-dose opioid treatment episode, as a function of patient demographic, prescriber, and county characteristics. Our models adjusted for time-invariant state characteristics using state fixed effects; we clustered standard errors at the patient level to account for correlation of errors within individuals who had multiple treatment episodes. As patients of oncologists may be receiving opioids at end-of-life, and opioid prescription fills may have stopped as a result of patient death, we conducted a sensitivity analysis excluding opioid episodes in which an oncologist was the prescriber. The results (available from the authors) were not meaningfully different than the primary results. We report adjusted odds ratios and 95% confidence intervals.
RESULTS
We identified 810,120 new, chronic, high-dose opioid treatment episodes ending between January 1, 2017, and December 31, 2018. Slightly over half involved women (50.6%; n=410,040) and individuals over the age of 55 (56.1%; n=455,060) (Table 1). Adult primary care physicians were the primary prescriber in about one-third of discontinued episodes (34.5%; n=279,269); in about a quarter of episodes, the primary prescriber was a pain specialist (25.7%; n=208,382); in another 20% of episodes, the primary prescriber was an advanced practice provider (20.8%; n=168,725). Medicare (37.0%; n=299,652) and commercial insurance (28.0%; n=226,451) were each responsible for paying for more than a quarter of discontinued episodes. Rapid discontinuation occurred in 72.0% (n=583,415) of high-dose chronic opioid treatment episodes.
Table 1.
Individual, Prescriber, and County Characteristics of Chronic High-Dose Opioid Treatment Episodes
| N | % | |
|---|---|---|
| Total | 810,120 | |
| Gender | ||
| Female | 410,040 | 50.6 |
| Male | 400,080 | 49.4 |
| Age cohort | ||
| 18–25 | 7,331 | 0.9 |
| 26–35 | 54,032 | 6.7 |
| 36–45 | 110,218 | 13.6 |
| 46–55 | 184,479 | 22.8 |
| 56–65 | 239,640 | 29.6 |
| >65 | 214,420 | 26.5 |
| Prescriber specialty | ||
| PCP adult | 279,269 | 34.5 |
| Pain | 208,382 | 25.7 |
| Advanced practice providers | 168,725 | 20.8 |
| Surgeon | 66,203 | 8.2 |
| Oncology | 50,664 | 6.3 |
| Other | 36,877 | 4.6 |
| Payer | ||
| Commercial | 226,451 | 28.0 |
| Medicare | 299,652 | 37.0 |
| Medicaid | 119,998 | 14.8 |
| Cash | 41,346 | 5.1 |
| Other | 122,673 | 15.1 |
| County rural-urban status (RUCC) | ||
| Metropolitan | 719,100 | 88.8 |
| Rural adjacent | 59,088 | 7.3 |
| Rural remote | 31,932 | 3.9 |
| Fatal overdose per capita | ||
| Low | 247,987 | 30.6 |
| Mid | 274,837 | 33.9 |
| High | 287,296 | 35.5 |
| Primary care health professional shortage area | ||
| None | 52,047 | 6.4 |
| Partial or whole county | 758,073 | 93.6 |
| Rapid Discontinuation | ||
| Yes | 583,415 | 72.0 |
| No | 226,705 | 28.0 |
Our multivariable logistic regression analysis found that the likelihood of rapid discontinuation was significantly associated with patient demographic characteristics. It was significantly more likely for men than for women (aOR 1.21, 95% CI 1.20–1.22). In Table 2, Models in column 3 estimate the likelihood of rapid discontinuation conditional on an opioid treatment episode being discontinued, adjusting for age, fatal overdose per capita, gender, payer, proportion of non-White county residents, provider specialty, RUCC category, HPSA, and state, clustering by subject.
Table 2.
Individual, Prescriber, and County Characteristics Associated with Rapid Discontinuation of Chronic High-Dose Opioid Treatment Episodes
| (1) | (2) | (3) | |
|---|---|---|---|
| N | Unadjusted rate of rapid discontinuation | Adjusted odds ratio of likelihood of rapid discontinuation (95% CI) | |
| Total | 583,415 | 72.0 | – |
| Gender | |||
| Female | 286,667 | 69.9 | Ref |
| Male | 296,748 | 74.2 | 1.21 (1.20, 1.22) |
| Age cohort | |||
| 18–25 | 5,034 | 68.7 | 0.86 (0.81, 0.90) |
| 26–35 | 39,336 | 72.8 | 1.00 (0.98, 1.03) |
| 36–45 | 79,276 | 71.9 | 0.99 (0.98, 1.01) |
| 46–55 | 132,466 | 71.8 | Ref |
| 56–65 | 173,034 | 72.2 | 1.01 (0.999, 1.03) |
| >65 | 154,269 | 71.9 | 0.96 (0.95, 0.98) |
| Provider specialty | |||
| PCP adult | 208,008 | 74.5 | Ref |
| Pain | 152,922 | 73.4 | 0.93 (0.92, 0.94) |
| Advanced practice providers | 116,878 | 69.3 | 0.78 (0.77, 0.79) |
| Surgeon | 38,579 | 58.3 | 0.48 (0.47, 0.49) |
| Oncology | 41,273 | 81.5 | 1.54 (1.50, 1.57) |
| Other | 25,755 | 69.8 | 0.76 (0.74, 0.78) |
| Payer | |||
| Commercial | 157,569 | 69.6 | Ref |
| Medicare | 217,182 | 72.5 | 1.14 (1.12, 1.15) |
| Medicaid | 85,035 | 70.9 | 1.03 (1.02, 1.05) |
| Cash | 34,773 | 84.1 | 2.19 (2.13, 2.25) |
| Other | 88,856 | 72.4 | 1.14 (1.13, 1.16) |
| County rural-urban status (RUCC) | |||
| Metropolitan | 518,680 | 72.1 | Ref |
| Rural adjacent | 42,307 | 71.6 | 1.01 (0.99, 1.03) |
| Rural remote | 22,428 | 70.2 | 0.98 (0.96, 1.01) |
| Fatal overdose per capita | |||
| Low | 175,539 | 70.8 | Ref |
| Mid | 199,500 | 72.6 | 1.02 (1.00, 1.03) |
| High | 208,376 | 72.5 | 1.03 (1.01, 1.04) |
| Primary care health professional shortage area | |||
| No | 37,557 | 72.2 | Ref |
| Yes | 545,858 | 72.0 | 0.98 (0.96, 1.00) |
| % of non-White county residents (quartiles) | |||
| 1 (lowest % of non-White county residents) | 138,221 | 70.7 | Ref |
| 2 | 145,966 | 71.5 | 1.05 (1.03, 1.06) |
| 3 | 146,524 | 72.0 | 1.09 (1.07, 1.11) |
| 4 (greatest % of non-White county residents) | 152,704 | 73.8 | 1.21 (1.19, 1.24) |
Models in column 3 estimate the likelihood of rapid discontinuation conditional on an opioid treatment episode being discontinued, adjusting for age, fatal overdose per capita, gender, payer, proportion of non-White county residents, provider specialty, RUCC category, HPSA, and state, clustering by subject
Rapid discontinuation was least likely among young adults ages 18 to 25 (aOR 0.86, 95% CI 0.81–0.90). It was statistically significantly but only slightly less likely among adults over the age of 65 (aOR 0.96, 95% CI 0.95–0.98) compared to the reference group of adults age 46 to 55. Comparing payers, relative to episodes among commercially insured individuals, rapid discontinuation was most likely for cash pay episodes (aOR 2.19, 95% CI 2.13–2.25), followed by episodes among Medicare beneficiaries (aOR 1.14, 95% CI 1.12–1.15) and episodes with an unspecified primary payer (i.e., “other;” aOR 1.14, 95% CI 1.13–1.16). Rapid discontinuation was significantly, but only slightly, more common among Medicaid than commercial enrollees (aOR 1.03, 95% CI 1.02–1.05),
Likelihood of rapid discontinuation also varied significantly based on the prescriber’s specialty. Compared to adult PCPs, rapid discontinuation was least likely among surgeons (aOR 0.48, 95% CI 0.47–0.49), and also less likely in episodes where an advanced practice provider (aOR 0.78, 95% CI 0.77–0.79) or other physician specialty (aOR 0.76, 95% CI 0.74–0.78) was the prescriber. It was significantly, though only slightly, less likely among episodes where a pain specialist (aOR 0.93, 95% CI 0.92–0.94) was the prescriber. Rapid discontinuation was more likely among episodes where the prescribers were oncologists (aOR 1.54, 95% CI 1.50–1.57) relative to adult PCPs.
The likelihood of rapid discontinuation was associated with characteristics of the primary prescriber’s county. Compared to counties with the lowest percentage of non-White residents, episodes in counties with more non-White residents had higher rates of rapid discontinuation. Compared to counties with the lowest fatal overdose rates, episodes in counties with high fatal overdose rates had slightly higher rates of rapid discontinuation (aOR 1.03, 95% CI 1.01–1.04), while counties containing primary care health professional shortage areas had slightly lower rates of rapid discontinuation (aOR 0.98, 95% CI 0.96–1.00) than counties without such shortage areas. There was no significant difference in the likelihood of rapid discontinuation by county rurality/urbanicity.
DISCUSSION
Using data on approximately 90% of prescriptions filled at retail pharmacies across the USA, we identified more than half a million new episodes of chronic, high-dose opioid analgesic treatment that were discontinued in 2017 or 2018. In more than 70% of those episodes, discontinuation occurred more rapidly than specified in clinical guidelines. Rapid discontinuation may be clinically appropriate in certain circumstance—for example, among patients with a high risk of experiencing adverse events from continuing to receive high-dose opioids9. For most patients, however, the risks of rapid discontinuation outweigh its potential benefits by putting the patient at increased risk for opioid withdrawal and potentially other adverse events, including opioid-related hospital and emergency department visits, and overdose or self-harm19. Efforts to limit overuse of opioid analgesics by tapering or discontinuing high-dose opioid therapy where appropriate must minimize risk of patient discomfort and negative consequences26.
Prior research has found faster rates of opioid tapering among commercially insured adults compared to Medicare Advantage enrollees15; we find a higher likelihood of rapid discontinuation for opioid analgesic episodes paid for by Medicare or Medicaid compared to commercial insurance. Medicare and many state Medicaid agencies may have implemented policies and utilization management techniques to limit chronic high-dose opioid prescribing37–40, and clinicians may be responding with more aggressive efforts to wean publicly insured individuals off chronic opioids. A number of commercial insurers have also discouraged high-dose opioid prescribing41; however, such efforts might be less uniform across payers, resulting in inconsistent effects on clinician behavior when examined at the national level.
Prior studies have documented substantial variation by prescriber specialty,42 consistent with our finding of substantial variation in the likelihood of rapid discontinuation by prescriber specialty. Differences in clinical populations across specialties likely explain much of this variation. For example, the higher likelihood of rapid discontinuation among oncologists may result from the common use of opioids to manage patient pain43, with no subsequent filled prescriptions after the patient dies, consistent with prior studies finding cancer patients had higher rates of abrupt discontinuation with no evidence of tapering.28 Further research is needed to better understand varying opioid discontinuation patterns across specialties; such information could inform efforts to support clinicians of different specialties in treating chronic pain safely and effectively.
We did not find significant differences by county rural/urban status; however, rapid discontinuation was more likely among residents of counties with higher percentages of racial/ethnic minority residents and in counties with higher fatal overdose rates. Clinicians may be more concerned about the risks of opioids in communities with higher fatal overdose rates; however, rapid discontinuation may actually increase the risk of adverse events such as overdoses19. Multiple studies have documented substantially lower rates of opioid prescribing among individuals from racial/ethnic minority groups44–48, but we are unaware of studies examining differences in rapid discontinuation associated with the racial/ethnic composition of a county. Further work is needed to recognize whether these patterns are observed across individuals from different racial/ethnic groups and to understand the implications for racial/ethnic disparities in the quality of pain care.
Our findings must be considered in the context of study limitations. We have pharmacy claims but no information on an individual’s medical encounters or clinical status. Thus, we cannot identify situations in which rapid discontinuation of opioids is clinically indicated, such as being diagnosed with opioid use disorder, or when it was the result of an individual’s death or an interruption in ambulatory prescribing due to a prolonged hospitalization, during which patients would receive medication from the hospital rather than from a retail pharmacy. We also have no information about external factors such as loss of insurance or closing of a clinician’s practice that could contribute to rapid discontinuation. We see only filled prescriptions, and therefore have no information regarding written prescriptions that went unfilled or prescriptions filled at a pharmacy not included in the IQVIA data. We also have no information regarding any instructions the prescriber communicated to the patient regarding taking the medication that was not reflected in the prescription signature, nor do we have information about opioids that a patient obtained without a prescription. We identify counties using information on the prescriber, not the patient, and individuals may obtain prescriptions from a clinician in a county in which they do not reside. Finally, we have no information on a patient’s clinical outcomes after opioid discontinuation. Research is needed to explore how rapid discontinuation affects clinical outcomes and to determine how patients’ characteristics and their local environment might affect this relationship.
Despite these limitations, this study provides the most comprehensive national estimates to date of the prevalence of rapid discontinuation of opioid analgesics, using data on the vast majority of retail prescriptions across multiple payer types. The significant differences in the likelihood of rapid discontinuation by payer, provider specialty, and county characteristics that we observed can guide further research to identify factors that contribute to rapid discontinuation of opioid analgesics and to determine the appropriate clinical and policy responses.
Acknowledgements
The authors thank Mary Vaiana, Alexandra Pelz, and Hilary Peterson for their feedback and editorial assistance on the earlier versions of the manuscript.
Funding
This research was supported by grants from the National Institutes of Health (R01DA045055-02 and P50DA046351). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest. This article was conceived and drafted when Dr. Sherry was employed at the RAND Corporation, and the findings and views in this article do not necessarily reflect the official views or policy of her current employer, the U.S. Department of Health and Human Services, or the U.S. Government.
Footnotes
Prior Presentations None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guy GP, Jr, Zhang K, Bohm MK, et al. Vital Signs: Changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohnert ASB, Guy GP, Jr, Losby JL. Opioid prescribing in the United States before and after the Centers For Disease Control and Prevention's 2016 opioid guideline. Ann Intern Med. 2018;169(6):367–375. doi: 10.7326/M18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IQVIA. Medicine Use and Spending in the U.S.: A Review of 2017 and Outlook to 2022. 2018: https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-review-of-2017-outlook-to-2022. Accessed November 20, 2020.
- 4.Centers for Disease Control and Prevention. U.S. Opioid Prescribing Rate Maps. https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html. Accessed April 8, 2020.
- 5.Zhu W, Chernew ME, Sherry TB, Maestas N. Initial opioid prescriptions among U.S. commercially insured patients, 2012-2017. N Engl J Med. 2019;380(11):1043–1052. doi: 10.1056/NEJMsa1807069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285–2287. doi: 10.1056/NEJMp1904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heins SE, Castillo RC. The Impact of Morphine Equivalent Daily Dose Threshold Guidelines on Prescribed Dose in a Workers' Compensation Population. Med Care. 2020;58(3):241–247. doi: 10.1097/MLR.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heins SE, Frey KP, Alexander GC, Castillo RC. Reducing High-Dose Opioid Prescribing: State-Level Morphine Equivalent Daily Dose Policies, 2007-2017. Pain Med. 2020;21(2):308–316. doi: 10.1093/pm/pnz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose AJ, Bernson D, Chui KKH, et al. Potentially inappropriate opioid prescribing, overdose, and mortality in Massachusetts, 2011-2015. J Gen Intern Med. 2018;33(9):1512–1519. doi: 10.1007/s11606-018-4532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 13.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 14.Heins SE, Frey KP, Alexander GC, Castillo RC. Reducing High-Dose Opioid Prescribing: State-Level Morphine Equivalent Daily Dose Policies, 2007-2017. Pain Med. 2019. [DOI] [PMC free article] [PubMed]
- 15.Fenton JJ, Agnoli AL, Xing G, et al. Trends and rapidity of dose tapering among patients prescribed long-term opioid therapy, 2008-2017. JAMA Netw Open. 2019;2(11):e1916271. [DOI] [PMC free article] [PubMed]
- 16.Huffman KL, Sweis GW, Gase A, Scheman J, Covington EC. Opioid use 12 months following interdisciplinary pain rehabilitation with weaning. Pain Med. 2013;14(12):1908–1917. doi: 10.1111/pme.12201. [DOI] [PubMed] [Google Scholar]
- 17.Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: Comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140(1):177–189. doi: 10.1016/j.pain.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91(4):919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark TL, Parish W. Opioid medication discontinuation and risk of adverse opioid-related health care events. J Subst Abuse Treat. 2019;103:58–63. doi: 10.1016/j.jsat.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: Evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90(6):828–842. doi: 10.1016/j.mayocp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659–E666. doi: 10.1503/cmaj.170363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Atlanta: U.S. Department of Health and Human Services; 2012.
- 23.Centers for Disease Control and Prevention. Pocket Guide: Tapering Opioids for Chronic Pain. https://www.cdc.gov/drugoverdose/pdf/Clinical_Pocket_Guide_Tapering-a.pdf. Accessed August 27, 2020.
- 24.Lagisetty PA, Healy N, Garpestad C, Jannausch M, Tipirneni R, Bohnert ASB. Access to primary care clinics for patients with chronic pain receiving opioids. JAMA Netw Open. 2019;2(7):e196928. doi: 10.1001/jamanetworkopen.2019.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.North Carolina Medical Board. North Carolina Medical Board (NCMB) Licensee Survey Results. July 2018: https://www.ncmedboard.org/images/uploads/article_images/NCMBLicensee_Survey_July2018_-_Public.pdf. Accessed September 9, 2020.
- 26.U.S. Food and Drug Administration. FDA Identifies Harm Reported from Sudden Discontinuation of Opioid Pain Medicines and Requires Label Changes to Guide Prescribers on Gradual, Individualized Tapering. https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes. Published 2019. Accessed September 9, 2020.
- 27.Neprash HT, Gaye M, Barnett ML. Abrupt discontinuation of long-term opioid therapy among Medicare beneficiaries, 2012-2017. J Gen Intern Med. 2021. [DOI] [PMC free article] [PubMed]
- 28.Bao Y, Wen K, Johnson P, Witkin LR, Reid MC. Abrupt discontinuation of long-term opioid therapies among privately insured or medicare advantage adults, 2011-2017. Pain Med. 2020. [DOI] [PMC free article] [PubMed]
- 29.Prunuske JP, St Hill CA, Hager KD, et al. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: A population-based study using 2010 NAMCS data. BMC Health Serv Res. 2014;14:563. doi: 10.1186/s12913-014-0563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system - United States, 2014-2017. MMWR Morb Mortal Wkly Rep. 2019;68(2):25–30. doi: 10.15585/mmwr.mm6802a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13(10):988–996. doi: 10.1016/j.jpain.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monnat SM, Peters DJ, Berg MT, Hochstetler A. Using census data to understand county-level differences in overall drug mortality and opioid-related mortality by opioid type. Am J Public Health. 2019;109(8):1084–1091. doi: 10.2105/AJPH.2019.305136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.IQVIA: Real World Data and Insights. https://www.iqvia.com/solutions/real-world-evidence/real-world-data-and-insights. Accessed July 13, 2020.
- 34.National Center for Health Statistics. 2013 NCHS Urban–Rural Classification Scheme for Counties; Vol 2. Atlanta: Centers for Disease Control and Prevention; 2014.
- 35.Centers for Disease Control Prevention. Drug Overdose Deaths. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Published 2020. Accessed May 20, 2020.
- 36.U.S. Department of Health and Human Services Health Resources and Services Administration. Area Health Resources Files. 2006-2019. In.
- 37.FACTSHEET: Texas’ Oversight of Opioid Prescribing and Monitoring of Opioid Use. A-06-18-04000 Office of the Inspector General; 2019: https://oig.hhs.gov/oas/reports/region6/61804000_Factsheet.pdf. Accessed October 30, 2020.
- 38.Bui L. Oregon's Opioid Initiative. Oregon Health Authority. https://www.medicaid.gov/state-resource-center/innovation-accelerator-program/iap-downloads/reducing-substance-use-disorders/alt-pain-treatment-webinar.pdf. Published 2019. Accessed October 30, 2020.
- 39.Brandt K. New Part D Policies Address Opioid Epidemic. CMSgov. 2019. https://www.cms.gov/blog/new-part-d-policies-address-opioid-epidemic. Accessed October 30, 2020.
- 40.Traylor C. CMCS Informational Bulletin: Medicaid Strategies for Non-Opioid Pharmacologic and NonPharmacologic Chronic Pain Management. Centers for Medicare & Medicaid Services; 2019: https://www.medicaid.gov/federal-policy-guidance/downloads/cib022219.pdf. Accessed October 30, 2020.
- 41.Garcia MC, Dodek AB, Kowalski T, et al. Declines in opioid prescribing after a private insurer policy change - Massachusetts, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(41):1125–1131. doi: 10.15585/mmwr.mm6541a1. [DOI] [PubMed] [Google Scholar]
- 42.Guy GP, Jr, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153–e155. doi: 10.1016/j.amepre.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henson LA, Maddocks M, Evans C, Davidson M, Hicks S, Higginson IJ. Palliative care and the management of common distressing symptoms in advanced cancer: Pain, breathlessness, nausea and vomiting, and fatigue. J Clin Oncol. 2020;38(9):905–914. doi: 10.1200/JCO.19.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison JM, Lagisetty P, Sites BD, Guo C, Davis MA. Trends in prescription pain medication use by race/ethnicity among US adults with noncancer pain, 2000-2015. Am J Public Health. 2018;108(6):788–790. doi: 10.2105/AJPH.2018.304349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singhal A, Tien YY, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016;11(8):e0159224. doi: 10.1371/journal.pone.0159224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman J, Kim D, Schneberk T, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med. 2019;179(4):469–476. doi: 10.1001/jamainternmed.2018.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meghani SH, Byun E, Gallagher RM. Time to take stock: A meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2):150–174. doi: 10.1111/j.1526-4637.2011.01310.x. [DOI] [PubMed] [Google Scholar]
- 48.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]