Abstract
This is a review of preoperative cognitive assessment and other healthcare gaps in the care of older adults at risk for Alzheimer’s disease and related dementias (ADRD) who have elected surgery with anesthesia. It summarizes concerns regarding ADRD perioperative healthcare, perioperative cognitive, and neuronal domains of vulnerability. It also offers a plan for phased preoperative cognitive screening and perioperative cognitive intervention opportunities. An argument is made for why medical professionals in the perioperative setting need fundamental training in cognitive-behavioral principles, an understanding of neurodegenerative diseases of aging, and an appreciation of the immediate and long-term medical risks for such patients undergoing anesthesia. The author’s goal is to encourage readers to consider perioperative cognitive medicine as a new frontier for generating evidence-based care approaches for at-risk older adults with neurodegenerative disorders who require procedures with anesthesia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-021-01180-w.
Keywords: Anesthesia, Cognitive disorders, Evidence-based medicine, Delivery of health, Delirium, Neuropsychology
The World Health Organization predicts that by 2050, 2 billion people will be 60 and older, up from 1 billion in 2020 [1]. The number of persons aged 80 years or older is expected to triple and reach 426 million by 2050, with 21% of adults being 85 years and over [2]. Accompanying this change in population age will be increased rates of noncommunicable disorders impacting brain function, including cerebrovascular disease (small and large vessel), neurodegenerative disorders such as Parkinson’s disease, dementias such as Alzheimer’s disease (AD), and depression. For example, AD is currently diagnosed in at least 1 out of 9 people (11.3%) age 65 and older in the total US population [3]. Between 2020 and 2025, every state in the USA is expected to experience an increase of at least 6.7% in the number of individuals with AD. Certain states in the West and Southeast are projected to experience even more significant increases in dementia; Florida is projected to increase 24% from 2020 to 2025 (from 580,000 to 720,000 individuals age 65 and older with AD) [3].
ADRD and Perioperative Healthcare Concerns
Increases in population percentage of AD and Related Dementias (ADRD) will impact the function of states’ health care systems, including perioperative care. Currently, low- and high-risk surgical procedures are performed annually on more than half a million patients aged 65 and older [4]. At least 20–35% of older patients undergoing surgery have signs of a mild to major neurocognitive disorder [5–7]. Given the expected rate increase of neurodegenerative disorders in the populous, perioperative healthcare systems will face more significant numbers of individuals with early to late-stage AD [8] and other neurodegenerative diseases needing procedures with anesthesia due to severe health-related conditions (e.g., cardiac) or requesting surgeries for quality of life improvement (e.g., joint replacement) [4].
Individuals with memory or cognitive disorders who elect surgery with anesthesia present challenges to providers concerned about decision-making capacity, initial procedural planning, and discharge complications. Researchers report how older individuals classified with major neurocognitive disorder had poor pre-colonoscopy bowel preparation and higher no-show rates for procedures [9], increased use of emergency and rehabilitation services [10], and prolonged hospitalization and complications [11–15]. Preoperative cognitive-behavioral profiles predict postoperative clinical complications, hospital cost, discharge complications, and lost services [16–18]. Failure to identify preoperative cognitive vulnerabilities, therefore, decreases medical care efficiency.
Failure to identify preoperative cognitive vulnerability or the presence of neurodegenerative disease also places patients at risk. Older individuals with reduced cognitive functions are at greater risk for postoperative neurocognitive difficulties and mortality. This is most comprehensively explained in a well-written review by Oresanya, Lyons, and Finlayson (2014), summarizing preoperative clinical features associated with mortality and delirium. Of the studies reviewed from 2000 to 2013, authors concluded that the presence of a clinical diagnosis of dementia or impairment on general cognitive screeners was independently associated with perioperative mortality and a 2- to 17-fold increased risk for postoperative delirium (of note, postoperative delirium experience is a significant risk factor to later cognitive decline [19–21] and greater mortality ([22], see [23] for a review)). Neurodegenerative disorders without dementia, such as Parkinson’s disease, are also vulnerable [24, 25]. Retrospective studies reviewing hospital or national database systems indicate that a diagnosis of PD is associated with higher rates of postoperative delirium, cognitive decline [26], prolonged hospitalization, increased risk of recurrent dislocation within their first postoperative year, and poor long-term prognosis relative to non-PD patients who underwent the same procedures [27]. Other independent older adult risk factors for neurocognitive complications include fewer years of educational attainment, depression [28–30], frailty [31, 32], and presurgical evidence of a non-symptomatic stroke and presurgical neuroimaging markers of atrophy [33–35]. Overall, preoperative indicators of peripheral and central nervous system integrity correlate strongly with older adults’ postoperative surgical success.
Vulnerable Cognitive/Neuronal Systems
Investigations using neuropsychology metrics identify vulnerability in systems of processing speed, working memory, inhibitory function, and episodic memory. Individuals with lower preoperative working memory and episodic learning/memory scores have higher rates of delirium after cardiac and non-cardiac surgeries (e.g., [16, 36]). Rigorous prospective neuropsychology studies assessing type and severity of postoperative cognitive decline for cognitively well older adults after orthopedic surgery report lower post-surgery scores on measures of working memory, inhibitory, and episodic memory relative to non-surgery peers [35, 37]. Authors suggest surgeons can expect 15% of their older adult non-cardiac surgery patients to experience at least mild acute postoperative memory disturbances (1 standard deviation decline from baseline), with approximately 11% of all patients experiencing executive (inhibition/working memory) complications alone or in combination with memory problems [37]. High-risk older individuals undergoing aortic valve replacement reports have lower scores on working memory/inhibition tests at 4 to 6 weeks, relative to older adults with similar cardiovascular disease who did not have surgery [34]. Although postoperative cognitive dysfunction abates by one-year post-surgery for most cognitively well older adults [34, 38–40], the neuropsychology domain findings implicate specific brain systems vulnerable to perioperative stressors.
Prospective perioperative neuroimaging studies provide corresponding evidence. For cognitively well older adults electing orthopedic surgery, the severity of preoperative white matter disease predicts greater pre- to postoperative working memory and inhibitory change [35]. In what is known as the signature of AD-related cortical atrophy [41], thinner structural cortical thickness measurements predict postoperative delirium for individuals without dementia, with predictive power highest in the superior frontal gyrus [41]. Thinner entorhinal cortices, greater volume of white matter disease, and larger ventricles also explain intraindividual variability on two-channel derived EEG measures for older adults electing orthopedic surgery with general anesthesia [42], acute measurements in microstructural intra-extracellular free water [43], and pre- to postoperative functional network changes [44].
Functional brain MRI studies show perioperative change to the cingulate cortex, specifically the posterior cingulate cortex (PCC). The PCC plays a prominent role in pain and episodic memory retrieval, topographic and topokinetic memory (orientation of the body in space), and working memory information transfer. Functional changes occur in the PCC from pre to 6 weeks post-cardiac surgery [45]. Reduced functional connectivity involving anterior and posterior cingulate cortex nodes also occur within 48 h after total knee arthroplasty with general anesthesia [44]. These changes are most noticeable for individuals meeting research criteria for mild cognitive impairment, and the reduction is marked within the PCC and angular gyri [46]. Anesthesia research implicates the PCC during sedation [47, 48], and functional MRI delirium investigations identify abnormal interactions between PCC and the dorsolateral prefrontal cortex [48]—a region necessary for working memory [49]. Cholinergic input to the cingulate cortex and frontal–temporal-parietal cortices is dependent on the Basal Nucleus of Meynert (BNM), an essential nucleus for cholinergic transmission. The cholinergic projections provide the chemical shift between synchronous versus dyssynchronous states of cortical activation mediating consciousness [48] and response to anesthesia [50].
Cognitive and neuronal systems implicated in perioperative risk profiles overlap with those observed in common dementias and neurodegenerative disorders (e.g., AD, small vessel vascular dementia, and PD with or without dementia) as well as major depression [51] and chronic pain [52]. For example, the number of cells within the BNM decreases with normal aging. It is accentuated for individuals with prodromal neurodegenerative disorders presenting signs of executive and declarative memory dysfunction (e.g., AD [53, 54] and PD [55]). We can now surmise why preoperative clinical diagnosis of dementia or memory/executive dysfunction has a 2- to 17-fold increased risk for delirium. Preexisting vulnerability places the brain at risk for symptom onset or disease acceleration after perioperative stressors—i.e., brain reserve and the threshold theory [56].
Biomarker Considerations
The perioperative environment is essentially a “stress test” involving at minimum disrupted sleep, activity changes, exposure to new medications, anesthesia, and acute pain. One or more of these “stressors” may contribute to neurodegeneration and increased vulnerability to tau and amyloid-beta accumulation. Although some associations remain controversial [57], studies show how sleep disruption [58, 59] and physical activity levels [60] contribute to increased generation and aggregation of amyloid β in cognitively well older adults. Rodent studies link general anesthesia-induced apoptosis to increased generation and aggregation of amyloid β [61–63] and increased tau hyperphosphorylation [64]. Through the mediation of glucocorticoids, stress responses may stimulate these actions [65–67]. Individual susceptibility combined with stressors within the perioperative experience may result in delirium.
Due to the heterogeneity of dementia and the familial and sporadic neurodegenerative disorders developing in the older adult population [68], it appears too simplistic to rely upon biofluid biomarkers alone for perioperative risk detection. Apolipoprotein E (ApoE) allele 4, the most robust genetic risk marker for late-onset AD, does not predict delirium [69–73] and has mixed findings for predicting postoperative cognitive dysfunction [74–76]. It only indirectly modifies the relationship of delirium when postoperative protein markers of biological stress are considered [73]. Further, associations between the levels of cerebrospinal fluid amyloid and tau biomarkers with disease severity or rate of AD progression remains inconsistent [77–79]. Factors such as age, sex, comorbidities, medications, lifestyle factors, and genetic variation also impact the clinical interpretation of these biomarkers [80]. Future researchers will need to integrate biomarker metrics with patient demographic, clinical, and psychosocial characteristics to improve risk detection and perioperative intervention approaches.
The Translational Call to Action
International and national anesthesiology societies and some surgery position papers are actively recommending providers regularly address perioperative brain health for older adults [81–85]. Although in 2013, the United States Preventive Services Task Force (USPSTF) refrained from recommending routine screening for cognitive impairment (citing stress-related to misdiagnosis and the absence of efficacious treatment to mitigate cognitive decline, thereby reducing the potential benefits associated with early detection of dementia) [86], the new 2019 American College of Surgeons Geriatric Surgery Verification (ACS GSV) Program [87] strongly recommends preoperative and postoperative cognitive and functional status screening for older adults electing surgery. Differentiating delirium from dementia has been particularly challenging in a postoperative setting if the patient has no caregiver or has no medical history and no dementia diagnosis or cognitive screening recorded [88]. Completed preoperative cognitive screening documents should be attached to medical records for postoperative multidisciplinary care teams and primary care physician reviews. Based on the ACS GSV recommendations and the plethora of growing data showing the importance of preoperative cognition on outcomes, perioperative medical teams are now faced with the dilemma of which measure(s) to choose, how to use them in the clinical setting appropriately, and how to adjust individual patient care.
Colleagues in the American Society of Anesthesiology (ASA) and the International Anesthesia Research Society (IARS) are now collaborating with neuropsychologists and geriatricians to address perioperative “brain health” status [89]. Anesthesiologists have pushed the concept of a “perioperative surgical home” for patient care [90–92], emphasizing the triple aims of improving health, improving health care delivery, and reducing the cost of care. Anesthesiologists and surgeons are not trained in brain-behavioral approaches; However, professionals with an understanding of brain-behavioral profiles (e.g., neuropsychologists, geriatricians, and behavioral neurologists) are needed to bridge the gap within the perioperative setting to address brain-anesthesia interactions [5, 93–95].
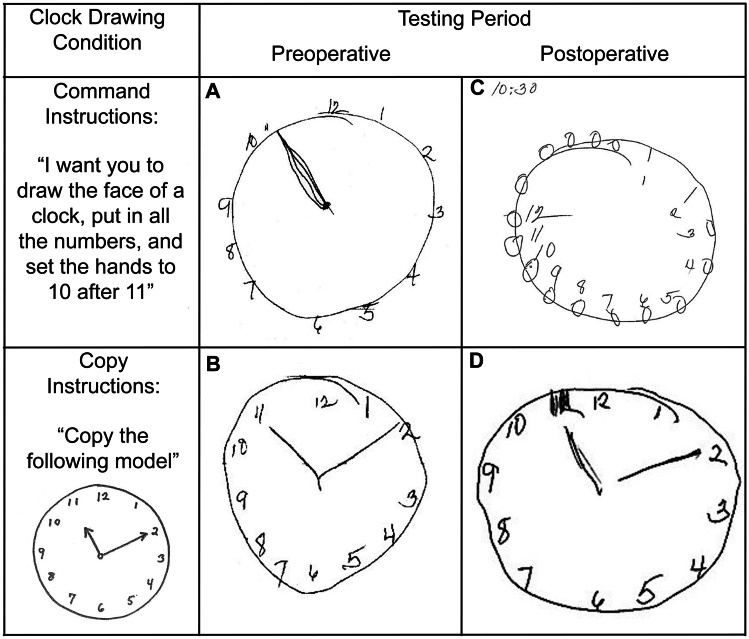
In 2019, the Society for Perioperative Assessment and Quality Improvement (SPAQI) convened experts in neuropsychology, geriatric medicine, and anesthesiology to review the literature and compile a comprehensive list of cognitive screening tools used within primary care and preoperative settings. The team identified seven cognitive screenings previously used in preoperative settings with administration time ranging from 1 to 10 min [94]. These tests included clock drawing (to command and copy conditions) [96], the Mini-Cog© [97], mini-mental state exam [98], months backward [99], short-blessed test, and the short orientation memory concentration test [100, 101], telephone interview for cognitive status [102], and time and change [103]. Of these, tests with some element of clock drawing are the most reported in the perioperative literature [5, 6, 11, 17]. This is likely due to its long history as a rapid cognitive screener (particularly for dementias [96, 104]), a wide variety of scoring options, and the stimulus value a patients’ drawing provides for rapid recognition of cognitive impairment (Fig. 1). However, the test is associated with interrater reliability difficulties [105, 106] unless error analyses are used.
Fig. 1.
Clock drawing test to command and copy conditions completed preoperatively (7 days before surgery) and postoperatively (42 days postoperatively) in a 76-year-old individual. Preoperative drawings show frontal compromise on the command condition (A, incorrect hand placement, gap in number spacing) and subtle signs in the copy condition (B, hands touching numbers, hands equal in length, gap in number spacing). Postoperative drawings show frontal impairment in command condition (C, increased perseverations, intrusions, incorrect number placement, missing hands) with subtle signs in the copy condition (D, hands of equal length, number gaps, self-correction of hand and number)
The Future of Digital Cognitive Capture Tools
Researchers have begun to explore the value of digital capture tools for data gathering field [107] to assist with the cognitive screener time constraints and interrater scoring difficulties. For example, research teams have employed digital technology to assess preoperative nuances during the visuoconstruction of clock drawing for adults aged 65 or older electing surgery with anesthesia [17, 107, 108]. Using a set of normative data from cognitively healthy adults [109] and cluster classification [110, 111], teams show how specific timed metrics and graphomotor features within the command and copy clock condition provide insight into neurodegenerative disorders [111, 112]. Applying this digital technology research in the perioperative setting reveals subtle behaviors predictive of postoperative outcome, including length of stay and hospital cost after certain surgeries [17]. Key elements of the clock drawing change after surgical procedures [108]. Relative to the command condition, the copy condition appears most beneficial for predicting outcome [5, 17], supporting previous assertions that command and copy conditions provide specific information about brain-behavioral profiles and as a unit are diagnostically meaningful [106, 113]. The field faces challenges with rapid digital test applications for disease detection in perioperative settings. We know little regarding clock drawing profiles in patients from diverse backgrounds such as non-Native English speaker status, individuals with few to years of formal education, and reading difficulties [94]. These issues require careful consideration before large-scale digital technology application.
A Multidisciplinary Approach
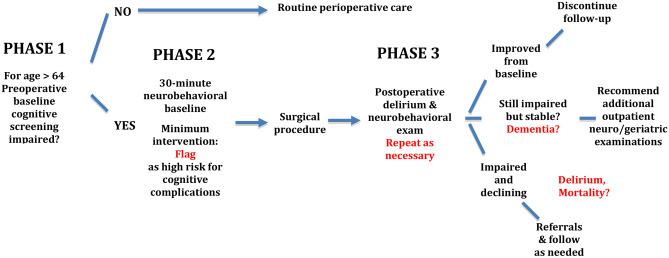
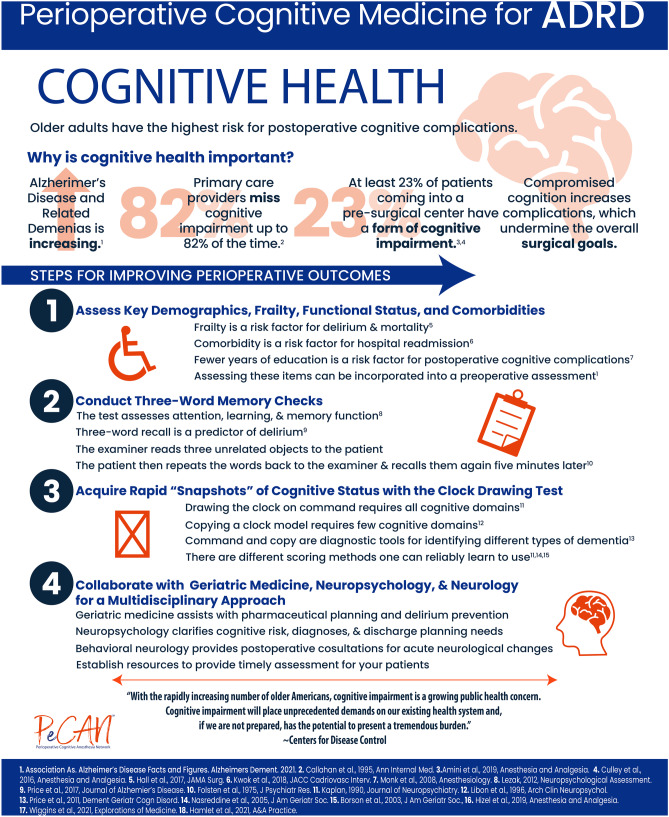
Suppose clinical screening with a brief test such as the Mini-Cog© field [11] or command/copy clock drawing field [5] can be incorporated into a preoperative anesthesia clinic. In that case, the next question involves what to do with the acquired information. Some sites have developed staged assessment programs. Duke University refers patients to geriatricians for optimization (e.g., Perioperative Optimization of Senior Health, POSH) [18] to reduce negative postoperative outcomes [114]. The University of Florida has embedded neuropsychology practitioners into a preoperative anesthesia clinic to provide immediate neurobehavioral differential diagnosis, perioperative recommendations, and post-operative referrals (i.e., Perioperative Cognitive Anesthesia Network, PeCAN; [115, 116]; Figs. 2 and 3).
Fig. 2.
A staged model of perioperative care for adults age 65 or older electing major surgery with anesthesia and entering a preoperative anesthesia clinic. Phase one and two are currently active at the University of Florida, Gainesville, Florida. Phase three involves geriatric medicine provider assessment using baseline evaluation from phase two as a preoperative reference
Fig. 3.
Information graphic summarizing statistics and rationale for a perioperative cognitive medicine program for ADRD with cognitive screening stages
The PeCAN clinic, for example, works in stages. Phase one involves medical staff acquiring information on a patient’s years of education years, frailty [117], clock production to command and copy [96, 118], and 3-word recall [119]. If signs of compromise are noted per screening guidelines, providers immediately refer the patient to an on-site neuropsychologist who conducts an interview and neurobehavioral status exam [120]. The evaluation concludes with immediate patient feedback with recommendations and a routed to the surgical team and primary care providers. The review does not recommend surgical cancellation but rather highlights risks and recommendations for discharge planning [95, 115, 116]. The POSH and PeCAN program provide opportunities for tailored individual care with the perioperative medical team (e.g., [114, 115]).
These preoperative cognitive clinical services also provide crucial venues for cross-pollination between disciplines for education and research purposes. Multidisciplinary team members engage in clinical science problem solving by addressing preoperative, intraoperative, and postoperative cognitive care dilemmas. This focused preoperative clinical setting can also assist with ADRD perioperative research investigation recruitment (see [121] for a review of traditional recruitment challenges).
Summary
Anticipated ADRD rates will challenge our healthcare system. Providers will observe increased rates of delirium and postoperative cognitive complications (accelerated cognitive change) in our older adult population within the hospital and after discharge. This is mainly because individuals with ADRD are not sufficiently identified preoperatively, and care systems lack evidence-based perioperative medical approaches for ADRD. Consequently, preoperative identification of ADRD is critically essential for enhancing recovery from surgery in every facet.
Healthcare systems currently have limited fundamental staff with training in cognitive-behavioral principles, neurodegenerative disease profiles, delirium, and medication interactions. To date, the brain is not routinely considered a vital organ to preoperative assessment. However, it is the organ directly targeted by general anesthesia, essential for sympathetic and parasympathetic responses, and experiences traumatic transformation with neurodegenerative disorders. Anesthesiologists are leading the call for older adult brain health awareness. The American Board of Anesthesiology’s exam includes a few questions on geriatric anesthesia/aging relative to fifteen or more for pediatrics (https://theaba.org). Geriatric clinical care also appears to be dwindling for older adults. For the past 5 years, geriatric medicine has had one of the lowest match rates for fellowship specialty training [122]. There are no evidence-based care methods addressing anesthetic agents or doses for individuals with ADRD. Hospitals are not routinely integrating behavioral strategies for reducing the risk of cognitive complications such as delirium [123]. Further, delirium training is minimal within most hospitals [124], and delirium assessments are not routinely performed [125].
We can improve healthcare services and quality by expanding the perioperative clinical team to include neuropsychologists and geriatricians well-versed in cognitive-behavioral profiles and intervention planning. Multidisciplinary high-risk surgery committees may improve perioperative decision-making, particularly for high-risk ADRD surgical patients [126]. This goal is consistent with The United Nations General Assembly who declared 2021–2030 the Decade of Healthy Ageing [127] and the Anesthesia Brain Health Initiative. Identifying ADRD preoperatively is critically important if we are going to enhance recovery from surgery. This could be the most significant benefit of this new frontier in medicine.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
I sincerely thank the reviewers for their comments and Shawna Amini, MPH, and Christina Hendricks, B.S, for the Figure 3 infographic. Thank you to Laura Anderson for her time editing this manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
Support was provided through K07 AG066813 and a Paul Satz Term Professorship. Perioperative and neurodegenerative research and career research training were provided through R01 NS082386, K23 NS060660, NIA R01 AG055337, NSF 13–543, and R01 NR014810.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Aging and Health. 2021. https://www.who.int/en/news-room/fact-sheets/detail/ageing-and-health2021.
- 2.Vincent GK, Velkoff VA. The next four decades. The older population in the United States: 2010 to 2050. In: Bureau USC, editor. Washington, DC. 2010.
- 3.Association As. Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2021;17. [DOI] [PubMed]
- 4.Williams SN, Wolford ML, Bercovitz A. Hospitalization for total knee replacement among inpatients aged 45 and over: 2000–2010. In: Services USDoHaH, editor. Centers for Disease Control and Prevention: NCHS Data Brief. 2015. [PubMed]
- 5.Amini S, Crowley S, Hizel L, Arias F, Libon DJ, Tighe P, et al. Feasibility and rationale for incorporating frailty and cognitive screening protocols in a preoperative anesthesia clinic. Anesth Analg. 2019;129(3):830–838. doi: 10.1213/ANE.0000000000004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culley DJ, Flaherty D, Reddy S, Fahey MC, Rudolph J, Huang CC, et al. Preoperative cognitive stratification of older elective surgical patients: a cross-sectional study. Anesth Analg. 2016;123(1):186–192. doi: 10.1213/ANE.0000000000001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silbert BS, Scott DA, Evered LA, Lewis MS, Maruff PT. Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg. 2007;104(5):1023–8, tables of contents. [DOI] [PubMed]
- 8.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 9.Arias F, Riverso M, Levy SA, Armstrong R, Estores DS, Tighe P, et al. Pilot study: neurocognitive disorders and colonoscopy in older adults. Anesth Analg. 2019;129(3):e89–e93. doi: 10.1213/ANE.0000000000004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Gelder J, Lucke JA, de Groot B, Fogteloo AJ, Anten S, Heringhaus C, et al. Predictors and outcomes of revisits in older adults discharged from the emergency department. J Am Geriatr Soc. 2018;66(4):735–741. doi: 10.1111/jgs.15301. [DOI] [PubMed] [Google Scholar]
- 11.Culley DJ, Flaherty D, Fahey MC, Rudolph JL, Javedan H, Huang CC, et al. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology. 2017;127(5):765–774. doi: 10.1097/ALN.0000000000001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 13.Fick DM, Kolanowski AM, Waller JL, Inouye SK. Delirium superimposed on dementia in a community-dwelling managed care population: a 3-year retrospective study of occurrence, costs, and utilization. J Gerontol A Biol Sci Med Sci. 2005;60(6):748–753. doi: 10.1093/gerona/60.6.748. [DOI] [PubMed] [Google Scholar]
- 14.Morandi A, Davis D, Bellelli G, Arora RC, Caplan GA, Kamholz B, et al. The diagnosis of delirium superimposed on dementia: an emerging challenge. Journal of the American Medical Directors Association. 2016. [DOI] [PMC free article] [PubMed]
- 15.Morandi A, Davis D, Fick DM, Turco R, Boustani M, Lucchi E, et al. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. J Am Med Dir Assoc. 2014;15(5):349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price CC, Garvan C, Hizel LP, Lopez MG, Billings FTt. Delayed recall and working memory mmse domains predict delirium following cardiac surgery. J Alzheimers Dis. 2017;59(3):1027–35. [DOI] [PMC free article] [PubMed]
- 17.Wiggins ME, Dion C, Formanski E, Davoudi A, Amini S, Heilman KM, et al. Proof of concept: digital clock drawing behaviors prior to transcatheter aortic valve replacement may predict length of hospital stay and cost of care. Explor Med. 2021;2:110–121. doi: 10.37349/emed.2021.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zietlow KE, Wong S, McDonald SR, Colon-Emeric C, Cassas C, Lagoo-Deenadayalan S, et al. Perioperative Optimization of Senior Health (POSH): a descriptive analysis of cancelled surgery. World J Surg. 2021;45(1):109–115. doi: 10.1007/s00268-020-05772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evered L, Scott DA, Silbert B. Cognitive decline associated with anesthesia and surgery in the elderly: does this contribute to dementia prevalence? Curr Opin Psychiatry. 2017;30(3):220–226. doi: 10.1097/YCO.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg TE, Chen C, Wang Y, Jung E, Swanson A, Ing C, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77(11):1373–1381. doi: 10.1001/jamaneurol.2020.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racine AM, Touroutoglou A, Abrantes T, Wong B, Fong TG, Cavallari M, et al. Older patients with Alzheimer's disease-related cortical atrophy who develop post-operative delirium may be at increased risk of long-term cognitive decline after surgery. J Alzheimers Dis. 2020;75(1):187–199. doi: 10.3233/JAD-190380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottesman RF, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338–344. doi: 10.1002/ana.21899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Aminoff MJ, Christine CW, Friedman JH, Chou KL, Lyons KE, Pahwa R, Bloem BR, Parashos SA, Price CC, Malaty IA, Iansek R, Bodis-Wollner I, Suchowersky O, Oertel WH, Zamudio J, Oberdorf J, Schmidt P, Okun MS. National Parkinson Foundation Working Group on Hospitalization in Parkinson's Disease. Management of the hospitalized patient with Parkinson's disease: current state of the field and need for guidelines. Parkinsonism Relat Disord. 2011 Mar;17(3):139–45. 10.1016/j.parkreldis.2010.11.009. Epub 2010 Dec 14. PMID: 21159538; PMCID: PMC3070297. [DOI] [PMC free article] [PubMed]
- 25.Enemark M, Midttun M, Winge K. Evaluating outcomes for older patients with Parkinson's disease or dementia with Lewy bodies who have been hospitalised for hip fracture surgery: potential impact of drug administration. Drugs Aging. 2017;34(5):387–392. doi: 10.1007/s40266-017-0454-x. [DOI] [PubMed] [Google Scholar]
- 26.Newman JM, Sodhi N, Dalton SE, Khlopas A, Newman RP, Higuera CA, et al. Does Parkinson disease increase the risk of perioperative complications after total hip arthroplasty? A nationwide database study J Arthroplasty. 2018;33(7S):S162–S166. doi: 10.1016/j.arth.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Jamsen E, Puolakka T, Peltola M, Eskelinen A, Lehto MU. Surgical outcomes of primary hip and knee replacements in patients with Parkinson's disease: a nationwide registry-based case-controlled study. Bone Joint J. 2014;96-B(4):486–91. [DOI] [PubMed]
- 28.Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2009;110(4):788–95. [DOI] [PMC free article] [PubMed]
- 29.Price CC, Pereira DB, Andre R, Garvan CW, Nguyen P, Herman M, et al. Prospective pilot investigation: presurgical depressive symptom severity and anesthesia response in women undergoing surgery for gynecologic mass removal. 2015;22(4):521–529. doi: 10.1007/s12529-014-9451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PJ, Attix DK, Weldon BC, Greene NH, Monk TG. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology. 2009;110(4):781–787. doi: 10.1097/ALN.0b013e31819b5bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin HS, Peel NM, Hubbard RE. Baseline Vulnerability and Inpatient Frailty Status in Relation to Adverse Outcomes in a Surgical Cohort. J Frailty Aging. 2016;5(3):180–182. [PubMed] [Google Scholar]
- 32.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi: 10.1186/s12877-016-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto T, Yoshitake A, Baba T, Shibata Y, Sakata R, Uozumi H. Cerebral ischemic disorders and cerebral oxygen balance during cardiopulmonary bypass surgery: preoperative evaluation using magnetic resonance imaging and angiography. Anesth Analg. 1997;84(1):5–11. doi: 10.1213/00000539-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Giovannetti T, Price CC, Fanning M, Messe S, Ratcliffe SJ, Lyon A, et al. Cognition and cerebral infarction in older adults after surgical aortic valve replacement. Ann Thorac Surg. 2019;107(3):787–794. doi: 10.1016/j.athoracsur.2018.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, Heilman K, McDonagh DL, Libon DJ, Leonard C, Bowers D, Monk TG. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014 Mar;120(3):601–13. 10.1097/ALN.0000000000000080. PMID: 24534857; PMCID: PMC3930070. [DOI] [PMC free article] [PubMed]
- 36.Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology. 2009;110(4):788–795. doi: 10.1097/ALN.0b013e31819b5ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price CC, Garvan CW, Monk TG. Subtype and severity of cognitive impairment in older adults after non-cardiac surgery. Anesthesiology. 2008;108:8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–61. [DOI] [PubMed]
- 39.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 40.Selnes OA, Grega MA, Borowicz LM, Jr., Royall RM, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75(5):1377–84; discussion 84–6. [DOI] [PubMed]
- 41.Racine AM, Fong TG, Travison TG, Jones RN, Gou Y, Vasunilashorn SM, et al. Alzheimer's-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol Aging. 2017;59:55–63. doi: 10.1016/j.neurobiolaging.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernaiz Alonso C, Tanner JJ, Wiggins ME, Sinha P, Parvataneni HK, Ding M, et al. Proof of principle: Preoperative cognitive reserve and brain integrity predicts intra-individual variability in processed EEG (Bispectral Index Monitor) during general anesthesia. PLoS One. 2019;14(5):e0216209. [DOI] [PMC free article] [PubMed]
- 43.Tanner JJ, Amin M, Hardcastle C, Parvataneni H, Vaillancourt DE, Mareci TH, et al. Better brain and cognition prior to surgery is associated with elevated postoperative brain extracellular free-water in older adults. Front Aging Neurosci. 2019;11:117. doi: 10.3389/fnagi.2019.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Tanner J, Parvataneni H, Rice M, Horgas A, Ding M, et al. Impact of total knee arthroplasty with general anesthesia on brain networks: cognitive efficiency and ventricular volume predict functional connectivity decline in older adults. J Alzheimers Dis. 2018;62(1):319–333. doi: 10.3233/JAD-170496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browndyke JN, Berger M, Harshbarger TB, Smith PJ, White W, Bisanar TL, et al. Resting-state functional connectivity and cognition after major cardiac surgery in older adults without preoperative cognitive impairment: preliminary findings. J Am Geriatr Soc. 2017;65(1):e6–e12. doi: 10.1111/jgs.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardcastle C, Huang H, Crowley S, Tanner J, Hernaiz C, Rice M, et al. Mild cognitive impairment and decline in resting state functional connectivity after total knee arthroplasty with general anesthesia. J Alzheimers Dis. 2019;69(4):1003–1018. doi: 10.3233/JAD-180932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2(6):291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stamatakis EA, Adapa RM, Absalom AR, Menon DK. Changes in resting neural connectivity during propofol sedation. PLoS One. 2010;5(12):e14224. [DOI] [PMC free article] [PubMed]
- 49.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pratico C, Quattrone D, Lucanto T, Amato A, Penna O, Roscitano C, et al. Drugs of anesthesia acting on central cholinergic system may cause post-operative cognitive dysfunction and delirium. Med Hypotheses. 2005;65(5):972–982. doi: 10.1016/j.mehy.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/S0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 52.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17(8):485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 53.Baranov D, Bickler PE, Crosby GJ, Culley DJ, Eckenhoff MF, Eckenhoff RG, et al. Consensus statement: First International Workshop on Anesthetics and Alzheimer's disease. Anesth Analg. 2009;108(5):1627–1630. doi: 10.1213/ane.0b013e318199dc72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silbert B, Evered L, Scott DA, Maruff P. Anesthesiology must play a greater role in patients with Alzheimer's disease. Anesth Analg. 2011;112(5):1242–1245. doi: 10.1213/ANE.0b013e3182147f5b. [DOI] [PubMed] [Google Scholar]
- 55.Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221(2):564–573. doi: 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. doi: 10.1037/0894-4105.7.3.273. [DOI] [Google Scholar]
- 57.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Machulda M, Lowe VJ, et al. Effect of intellectual enrichment on AD biomarker trajectories: longitudinal imaging study. Neurology. 2016;86(12):1128–1135. doi: 10.1212/WNL.0000000000002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho DZ, St Louis EK, Boeve BF, Mielke MM, Przybelski SA, Knopman DS, et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 2017;32:236–243. doi: 10.1016/j.sleep.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carvalho DZ, St Louis EK, Knopman DS, Boeve BF, Lowe VJ, Roberts RO, et al. Association of excessive daytime sleepiness with longitudinal beta-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 2018;75(6):672–680. doi: 10.1001/jamaneurol.2018.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown BM, Sohrabi HR, Taddei K, Gardener SL, Rainey-Smith SR, Peiffer JJ, et al. Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer's disease. Alzheimers Dement. 2017;13(11):1197–1206. doi: 10.1016/j.jalz.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64(6):618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhen Y, Dong Y, Wu X, Xu Z, Lu Y, Zhang Y, et al. Nitrous oxide plus isoflurane induces apoptosis and increases beta-amyloid protein levels. Anesthesiology. 2009;111(4):741–752. doi: 10.1097/ALN.0b013e3181b27fd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66(5):620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Freche H, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116(4):779–787. doi: 10.1097/ALN.0b013e31824be8c7. [DOI] [PubMed] [Google Scholar]
- 65.Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, et al. Stress acts cumulatively to precipitate Alzheimer's disease-like tau pathology and cognitive deficits. J Neurosci. 2011;31(21):7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26(35):9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arenaza-Urquijo EM, Przybelski SA, Machulda MM, Knopman DS, Lowe VJ, Mielke MM, et al. Better stress coping associated with lower tau in amyloid-positive cognitively unimpaired older adults. Neurology. 2020;94(15):e1571–e1579. doi: 10.1212/WNL.0000000000008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dorszewska J, Prendecki M, Oczkowska A, Dezor M, Kozubski W. Molecular basis of familial and sporadic Alzheimer's disease. Curr Alzheimer Res. 2016;13(9):952–963. doi: 10.2174/1567205013666160314150501. [DOI] [PubMed] [Google Scholar]
- 69.Abelha FJ, Fernandes V, Botelho M, Santos P, Santos A, Machado JC, et al. Apolipoprotein E e4 allele does not increase the risk of early postoperative delirium after major surgery. Journal of anesthesia. 2012. [DOI] [PubMed]
- 70.Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJ. APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry. 2007;22(7):688–694. doi: 10.1002/gps.1732. [DOI] [PubMed] [Google Scholar]
- 71.Alexander SA, Ren D, Gunn SR, Kochanek PM, Tate J, Ikonomovic M, et al. Interleukin 6 and apolipoprotein E as predictors of acute brain dysfunction and survival in critical care patients. Am J Crit Care. 2014;23(1):49–57. doi: 10.4037/ajcc2014578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bryson GL, Wyand A, Wozny D, Rees L, Taljaard M, Nathan H. A prospective cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E genotype following open aortic repair. Can J Anaesth. 2011;58(3):246–255. doi: 10.1007/s12630-010-9446-6. [DOI] [PubMed] [Google Scholar]
- 73.Vasunilashorn SM, Ngo LH, Inouye SK, Fong TG, Jones RN, Dillon ST, et al. Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: Importance of gene-protein interactions. Alzheimers Dement. 2020;16(3):572–580. doi: 10.1016/j.jalz.2019.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 75.Steed L, Kong R, Stygall J, Acharya J, Bolla M, Harrison MJ, et al. The role of apolipoprotein E in cognitive decline after cardiac operation. Ann Thorac Surg. 2001;71(3):823–826. doi: 10.1016/S0003-4975(00)02511-X. [DOI] [PubMed] [Google Scholar]
- 76.Tardiff BE, Newman MF, Saunders AM, Strittmatter WJ, Blumenthal JA, White WD, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64(3):715–20. [DOI] [PubMed]
- 77.Wattmo C, Blennow K, Hansson O. Cerebro-spinal fluid biomarker levels: phosphorylated tau (T) and total tau (N) as markers for rate of progression in Alzheimer's disease. BMC Neurol. 2020;20(1):10. doi: 10.1186/s12883-019-1591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blennow K, Zetterberg H. Biomarkers for Alzheimer's disease: current status and prospects for the future. J Intern Med. 2018;284(6):643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 79.Karikari TK, Emersic A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer's disease diagnosis. Alzheimers Dement. 2021;17(5):755–767. doi: 10.1002/alz.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teunissen CE, Verberk IMW, Thijssen EH, Vermunt L, Hansson O, Zetterberg H, et al. Blood-based biomarkers for Alzheimer's disease: towards clinical implementation. The Lancet Neurology. 2021. [DOI] [PubMed]
- 81.Berger M, Schenning KJ, Brown CHt, Deiner SG, Whittington RA, Eckenhoff RG, et al. Best practices for postoperative brain health: recommendations From the Fifth International Perioperative Neurotoxicity Working Group. Anesth Analg. 2018. [DOI] [PMC free article] [PubMed]
- 82.Berger M, Burke J, Eckenhoff R, Mathew J. Alzheimer's disease, anesthesia, and surgery: a clinically focused review. J Cardiothorac Vasc Anesth. 2014;28(6):1609–1623. doi: 10.1053/j.jvca.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crosby G, Culley DJ, Hyman BT. Preoperative cognitive assessment of the elderly surgical patient: a call for action. Anesthesiology. 2011;114(6):1265–1268. doi: 10.1097/ALN.0b013e31821b1bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hogue CW, Selnes OA, McKhann G. Should all patients undergoing cardiac surgery have preoperative psychometric testing: a brain stress test? Anesth Analg. 2007;104(5):1012–1014. doi: 10.1213/01.ane.0000263281.45718.e4. [DOI] [PubMed] [Google Scholar]
- 86.Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(9):601–12. [DOI] [PubMed]
- 87.Surgeons ACo. Optimal resources for geriatric surgery: 2019 standards. 633 N. Saint Clair St., Chicago, IL. 2019.
- 88.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, Browndyke JN, Wright CB, Culley DJ, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123(4):464–478. doi: 10.1016/j.bja.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shafer SL. Anesthesia & Analgesia's 2015 Collection on the perioperative surgical home. Anesth Analg. 2015;120(5):966–967. doi: 10.1213/ANE.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 91.Shafer SL, Donovan JF. Anesthesia & Analgesia's collection on the perioperative surgical home. Anesth Analg. 2014;118(5):893–895. doi: 10.1213/ANE.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 92.Alvis BD, Amsler RG, Leisy PJ, Feng X, Shotwell MS, Pandharipande PP, et al. Effects of an anesthesia perioperative surgical home for total knee and hip arthroplasty at a Veterans Affairs Hospital: a quality improvement before-and-after cohort study. Can J Anaesth. 2021;68(3):367–375. doi: 10.1007/s12630-020-01865-4. [DOI] [PubMed] [Google Scholar]
- 93.Arias F, Wiggins M, Urman RD, Armstrong R, Pfeifer K, Bader AM, et al. Rapid in-person cognitive screening in the preoperative setting: test considerations and recommendations from the Society for Perioperative Assessment and Quality Improvement (SPAQI). Perioper Care Oper Room Manag. 2020;19. [DOI] [PMC free article] [PubMed]
- 94.Arias F, Wiggins M, Urman RD, Armstrong R, Pfeifer K, Bader AM, et al. Rapid in-person cognitive screening in the preoperative setting: test considerations and recommendations from the Society for Perioperative Assessment and Quality Improvement (SPAQI). J Clin Anesth. 2020;62:109724. [DOI] [PMC free article] [PubMed]
- 95.Wiggins M, Arias F, Urman RD, Richman DC, Sweitzer BJ, Edwards AF, et al. Common neurodegenerative disorders in the perioperative setting: recommendations for screening from the Society for Perioperative Assessment and Quality Improvement (SPAQI). Perioper Care Oper Room Manag. 2020;20. [DOI] [PMC free article] [PubMed]
- 96.Libon DJ, Malamut BL, Swenson MR, Cloud BS. Further analysis of clock drawings among demented and non-demented subjects. Arch Clin Neuropsychol. 1996;11:193–211. doi: 10.1093/arclin/11.3.193. [DOI] [PubMed] [Google Scholar]
- 97.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 98.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed]
- 99.Lamar M, Price CC, Davis KL, Kaplan E, Libon DJ. Capacity to maintain mental set in dementia. Neuropsychologia. 2002;40(4):435–445. doi: 10.1016/S0028-3932(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 100.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 101.O'Sullivan D, O'Regan NA, Timmons S. Validity and reliability of the 6-item cognitive impairment test for screening cognitive impairment: a review. Dement Geriatr Cogn Disord. 2016;42(1–2):42–49. doi: 10.1159/000448241. [DOI] [PubMed] [Google Scholar]
- 102.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 1993;6(2):103. [Google Scholar]
- 103.Inouye SK, Robison JT, Froehlich TE, Richardson ED. The time and change test: a simple screening test for dementia. J Gerontol A Biol Sci Med Sci. 1998;53(4):M281–M286. doi: 10.1093/gerona/53A.4.M281. [DOI] [PubMed] [Google Scholar]
- 104.Libon DJ, Swenson RA, Barnoski EJ, Sands LP. Clock drawing as an assessment tool for dementia. Arch Clin Neuropsychol. 1993;8(5):405–415. doi: 10.1093/arclin/8.5.405. [DOI] [PubMed] [Google Scholar]
- 105.Frei BW, Woodward KT, Zhang MY, Amini S, Tighe P, Garvan CW, Giordano C, Price CC. Considerations for Clock Drawing Scoring Systems in Perioperative Anesthesia Settings. Anesth Analg. 2019 May;128(5):e61–e64. 10.1213/ANE.0000000000004105. PMID: 30896604; PMCID: PMC6545889. [DOI] [PMC free article] [PubMed]
- 106.Price CC, Cunningham H, Coronado N, Freedland A, Cosentino S, Penney DL, et al. Clock drawing in the Montreal Cognitive Assessment: recommendations for dementia assessment. Dement Geriatr Cogn Disord. 2011;31(3):179–187. doi: 10.1159/000324639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buckley RA, Atkins KJ, Fortunato E, Silbert B, Scott DA, Evered L. A novel digital clock drawing test as a screening tool for perioperative neurocognitive disorders: a feasibility study. Acta Anaesthesiol Scand. 2020. [DOI] [PubMed]
- 108.Hizel LP, Warner ED, Wiggins ME, Tanner JJ, Parvataneni H, Davis R, et al. Clock drawing performance slows for older adults after total knee replacement surgery. Anesth Analg. 2019;129(1):212–219. doi: 10.1213/ANE.0000000000003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davoudi A, Dion C, Formanski E, Frank BE, Amini S, Matusz EF, et al. Normative references for graphomotor and latency digital clock drawing metrics for adults age 55 and older: operationalizing the production of a normal appearing clock. J Alzheimers Dis. 2021;82(1):59–70. doi: 10.3233/JAD-201249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davoudi A, Dion C, Amini S, Libon DJ, Tighe PJ, Price CC, et al. Phenotyping cognitive impairment using graphomotor and latency features in digital clock drawing test. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:5657–5660. doi: 10.1109/EMBC44109.2020.9176469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davoudi A, Dion C, Amini S, Tighe PJ, Price CC, Libon DJ, et al. Classifying non-dementia and Alzheimer's disease/vascular dementia patients using kinematic, time-based, and visuospatial parameters: the digital clock drawing test. J Alzheimers Dis. 2021;82(1):47–57. doi: 10.3233/JAD-201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dion C, Frank BE, Crowley SJ, Hizel LP, Rodriguez K, Tanner JJ, et al. Parkinson's disease cognitive phenotypes show unique clock drawing features when measured with digital technology. J Parkinsons Dis. 2021;11(2):779–791. doi: 10.3233/JPD-202399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Libon DJ, Malamut BL, Swenson R, Sands LP, Cloud BS. Further analyses of clock drawings among demented and nondemented older subjects. Arch Clin Neuropsychol. 1996;11(3):193–205. doi: 10.1093/arclin/11.3.193. [DOI] [PubMed] [Google Scholar]
- 114.McDonald SR, Heflin MT, Whitson HE, Dalton TO, Lidsky ME, Liu P, et al. Association of integrated care coordination with postsurgical outcomes in high-risk older adults: the Perioperative Optimization of Senior Health (POSH) Initiative. JAMA Surg. 2018;153(5):454–462. doi: 10.1001/jamasurg.2017.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamlet KM, Pasternak E, Rabai F, Mufti M, Hernaiz Alonso C, Price CC. Perioperative multidisciplinary delirium prevention: a longitudinal case report. A A Pract. 2021;15(1):e01364. [DOI] [PMC free article] [PubMed]
- 116.Arias F, Bursian AC, Sappenfield JW, Price CE. Delirium history and preoperative mild neurocognitive disorder: An Opportunity for Multidisciplinary Patient-Centered Care. Am J Case Rep. 2018 Nov 6;19:1324-1328. 10.12659/AJCR.911437. PMID: 30397190; PMCID: PMC6232917. [DOI] [PMC free article] [PubMed]
- 117.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 118.Frei BW, Woodward KT, Zhang MY, Amini S, Tighe P, Garvan CW, et al. Considerations for clock drawing scoring systems in perioperative anesthesia settings. Anesth Analg. 2019;128(5):e61–e64. doi: 10.1213/ANE.0000000000004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amini S, Crowley S, Hizel L, Arias F, Libon DJ, Tighe P, et al. Feasibility and rationale for incorporating frailty and cognitive screening protocols in a preoperative anesthesia clinic. Anesth Analg. 2019. [DOI] [PMC free article] [PubMed]
- 120.Arias F, Riverso M, Levy SA, Armstrong R, Estores DS, Tighe P, et al. Pilot study: neurocognitive disorders and colonoscopy in older adults. Anesth Analg. 2019. [DOI] [PMC free article] [PubMed]
- 121.Price CC, Levy SA, Tanner J, Garvan C, Ward J, Akbar F, et al. Orthopedic surgery and post-operative cognitive decline in idiopathic Parkinson's disease: considerations from a pilot study. J Parkinsons Dis. 2015;5(4):893–905. doi: 10.3233/JPD-150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Program NRM. National resident matching program, results and data: specialties matching service 2021 appointment year. Washington, DC; 2021.
- 123.Hughes CG, Boncyk CS, Culley DJ, Fleisher LA, Leung JM, McDonagh DL, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention. Anesth Analg. 2020;130(6):1572–1590. doi: 10.1213/ANE.0000000000004641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solberg LM, Campbell CS, Jones K, Vaughn I, Suryadevara U, Fernandez C, et al. Training hospital inpatient nursing to know (THINK) delirium: A nursing educational program. Geriatr Nurs. 2021;42(1):16–20. doi: 10.1016/j.gerinurse.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 125.Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125(6):1229–1241. doi: 10.1097/ALN.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jones TS, Jones EL, Barnett CC, Jr, Moore JT, Wikiel KJ, Horney CP, et al. A multidisciplinary high-risk surgery committee may improve perioperative decision making for patients and physicians. J Palliat Med. 2021;24(12):1863–1866. doi: 10.1089/jpm.2021.0141. [DOI] [PubMed] [Google Scholar]
- 127.Lloyd-Sherlock P, Kalache A, Kirkwood T, McKee M, Prince M. WHO's proposal for a decade of healthy ageing. Lancet. 2019;394(10215):2152–2153. doi: 10.1016/S0140-6736(19)32522-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.