Abstract
Background
Platelet rich fibrin (PRF) has shown great potential in osteogenesis; however, some studies still question utilizing it as a grafting material. Thus, the aim of this review is to evaluate the effect of PRF when used in socket and ridge preservation procedures.
Methods
Electronic searches through MEDLINE, EMBASE, and Cochrane, Science Citation Index Expanded databases and manual searches of unpublished data, academic theses, and journals were conducted up until July 2021. The outcomes were to assess the ability of PRF as a graft material to preserve bone width, height, and density after tooth extraction.
Results
Twelve studies were included in the review, using PRF showed significant results in all three outcomes when compared to no grafting at all, however when compared to other commonly used grafting materials it showed a lesser effect. On the other hand, most studies included reported mixing PRF with a graft material showed the best result. The meta-analysis also revealed the significant results in using PRF on the three outcomes.
Conclusion
The meta-analysis of the studies included proved the beneficial effect of PRF in socket preservation surgeries alone or in combination with other graft materials, but further individual multi-centre randomized controlled studies with appropriate sample size are still needed to further confirm our findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-021-00428-y.
Keywords: Fibrin, Regeneration, Socket preservation, Bone graft, Platelet rich fibrin
Introduction
Dental extraction initiates a cascade of biological events that eventually alter a previously intact alveolar ridge morphology, leaving behind an inadequate implant-bearing site. But with the surge of implant dentistry research, more focus has been raised on maintaining future implant-bearing structures. According to the American Academy of Periodontology Glossary of Periodontal Terms, ridge preservation is a surgical procedure aimed at preventing ridge collapse and preserving ridge dimension after dental extraction, typically done for purposes of implant site development. This could be achieved using bone grafts or bone substitute with or without membranes or use of biological mediators [1]. To facilitate the sockets healing after extraction, several attempted had been proposed. These include platelet rich fibrin (PRF) which is the most studied type of platelet concentrates when it comes to accelerating wound healing especially for soft tissue migration. Platelet rich fibrin (PRF) can reduce inflammation of the periodontal tissues, preserve alveolar site and alveolar bone defect repair, thereby enhancing bone regeneration. L-PRF promotes fast neo-angiogenesis and stimulates bone regeneration by releasing growth factors and by providing good cloth stability.
It is easily manufactured and has bioactive elements not requiring anticoagulants in its preparation [2]. Clinically, it has exhibited good handling properties in being resistant to traction and elasticity and the ability to be sutured easily [3]. It can either be applied with or without combining it with other substitute materials [2].
PRF contains growth factors which has been found to enhance the regeneration of bone [2]. It was proven to enhance osteoblasts proliferation and the expression of collagen type I alpha1 (COL1A), runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), osteocalcin (OCN) and GAPDH genes which play a major role in bone formation [4]. PRF can either be applied as a single filling material or in combination with other bone graft materials [5].
When in it comes to the PRF potential in osteogenesis, many authors believe that PRF alone can enhance bone formation, however it has been proven to have limited osteogenic capability after comparing it with commonly used osteogenic materials [6]. In two clinical studies, PRF was used alone to form bone in a sinus lift and in an intrabody defect augmentation procedures and bone formation was seen radiologically and histologically was seen in both studies after a six month follow up visit [7, 8].
The aim of the present systematic review is to evaluate the effect of PRF when used in socket / ridge preservation procedures in terms of clinical and histological outcomes measured.
Focused question
Does PRF have a positive effect on socket preservation procedures in term of bone quality and preservation of socket wall dimensions?
Material and methods
Protocol
The protocol for this systematic review was registered on PROSPERO under registration number: CRD42021261939 on 27th of July 2021 and it followed the recommendations of the PRISMA guidelines and statements checklist for reporting a systematic review [9].
Selection of studies and eligibility criteria
Clinical trials, case–control, cross-sectional and cohort studies were eligible for inclusion with no date or language restriction. The inclusion criteria were based on the focused question and PICO strategy [10] As follows:
- Population
Systemically healthy humans with at least one socket preservation procedure.
- Intervention
augmenting extracted sockets using PRF alone or in combination with other biomaterials
- Comparison
other socket preservation materials or none.
Outcomes
- Primary:
Reduction in width, Reduction in height and change in bone density.
- Secondary:
Pain assessment, epithelial migration, soft tissue healing and maturation, bone to implant contact and bone microarchitecture.
Search strategy
A comprehensive three-step search approach was established to identify studies for this systematic review with no restrictions to language. Electronic searches through MEDLINE (via PubMed), EMBASE, and Cochrane, Science Citation Index Expanded databases and manual searches of unpublished data, academic theses, and journals were conducted up until July 2021. Furthermore, reference lists and trial records were examined, and regulatory agency websites and manufacturers were enquired. This search and subsequent review took a period of three months. The online database search was performed using the following search strategy prepared for MEDLINE: (autograft) OR (allograft) OR (alloplasty) OR (xenograft) OR (bone graft) OR (membrane) OR (collaplug) OR (gelfoam) OR (CTG) OR (FGG) OR (autogenous tissue) AND ((PRF) OR (Platelet rich fibrin)) AND ((socket preservation) OR (ridge preservation)) AND ((pain) OR (bleeding) OR (epithelial migration) OR (epithelial) OR (epithelium) OR (healing) OR (healing process) OR (Tissue vascularity) OR (blood flow) OR (Implant Stability quotient) OR (ISQ) OR (bone mineralization) OR (bone) OR (proliferation) OR (soft tissue proliferation)).
Selection of included studies
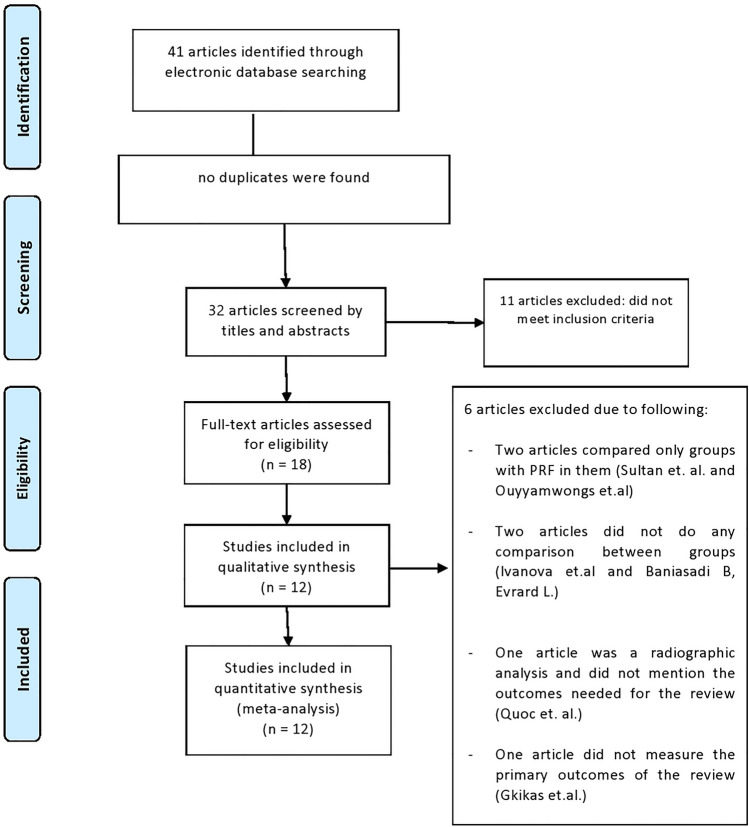
The search process was made by two independent reviewers (R.N.J. and Y.F.R.), the titles were screened first followed by the abstracts, then the full texts of the papers were thoroughly reviewed to be included. Any dissimilarity between the two reviewers were fixed through discussion until consensus was reached. Cohen’s Kappa score was used to assess the inter-reviewer agreement between the two reviewers for the selection process [11]. The causes for excluding studies after fully reviewing them were documented and presented in the figure (Fig. 1). Studies that met the review’s inclusion requirements underwent data extraction and synthesis.
Fig. 1.
PRISMA flow diagram
Data extraction
A form that was prepared beforehand was used to extract the following data from the included studies: the name of the author(s); the place and year of the study publication; type of funding and its sources; any conflict of interest that was mentioned; the design of the study; the sample size; the duration of follow-up; the source of the study population, their selection methods, and demographic data; the study intervention definition and their measurements method; the controls; the study outcomes and their concluded results; and lastly any biases.
Data synthesis
The data were organized into evidence tables according to PRISMA guidelines [9] and a descriptive summary was created to determine the study’s characteristics, quality, and results. Descriptive statistical analysis according to the mean values was used to evaluate the outcomes in Supplementary table 1.
Quality and assessment of biases:
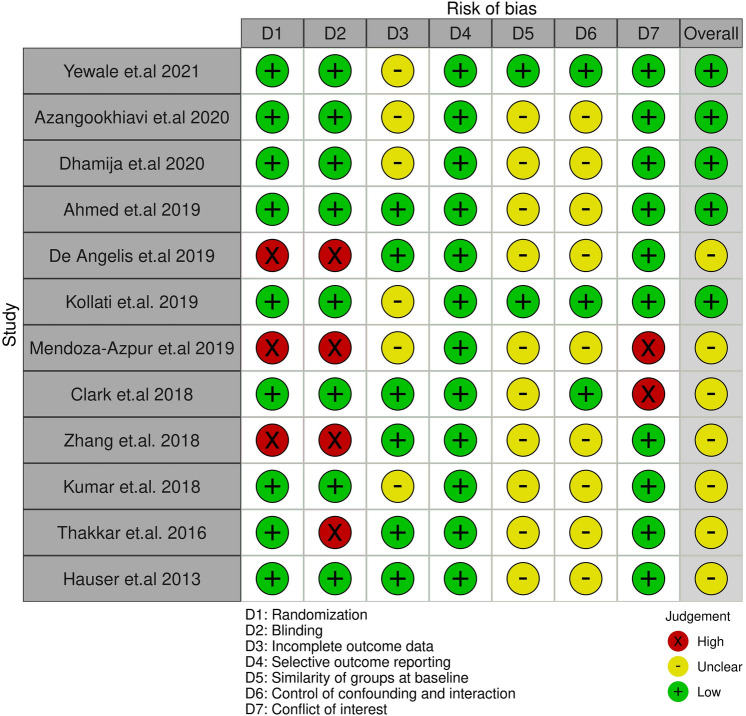
The guidelines by PRISMA were used to evaluate the quality of the included studies and were recorded into tables [9], while focusing on the following points: (1) the study participants randomization method, and it can be: (I) adequate, when random-number tables, a tossed coin, or shuffled cards were used; (II) inadequate, when other methods were used, such as alternate assignment, hospital number, or odd/even date of birth; and (III) unclear, when the randomization method was not reported or explained. (2) Allocation concealment (i.e., how the sequence of randomization was hidden from the examiners): (I) adequate, when examiners were kept ignorant of the randomization method (e.g., by means of central randomization or opaque envelopes); (II) inadequate, when other methods were utilized, such as alternative assignment or file number; and (III) unclear, when the method used was not stated or described. (3) The examiners blindness regarding the treatment procedures used in the study was also assessed. (4) The follow-up completion was based on the following question: Was the number of study participants at the beginning of the trial and at completion of the follow-up period stated? Other assessments included the explanations and reasons for dropouts. Studies were excluded if they did not report the completion of follow-up. (5) The similarity between groups at baseline. (6) Assessment of any analysis performed to control for confounding factors that can impact the final outcomes (Table 1). Furthermore, based on the criteria defined in the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0, the risk-of-biases were given grades as low, high, or unclear for each domain [12]. We also utilized Robvis R package software to create summary “traffic light” plots for our findings as shown in Fig. 2 [13].
Table 1.
Risk of bias assessment
| Randomization | Blinding | Incomplete outcome data | Selective outcome reporting | Similarity of groups at baseline | Control of confounding and interaction | Conflict of interest | References |
|---|---|---|---|---|---|---|---|
| Yes (adequate) | Yes |
Yes, Type of tooth extracted was not mentioned |
No | Yes | Yes (adequate) | No | Yewale et al. [14] |
| Yes (adequate) | Yes |
Yes, Type of tooth extracted was not mentioned |
No | No | No (inadequate) | No | Azangookhiavi et al. [14] |
| Yes (adequate) | Yes |
Yes, Type of tooth extracted was not mentioned |
No | No | No (inadequate) | No | Dhamija et al. [16] |
| Yes (adequate) | Yes | No | No | No | No (inadequate) | No | Ahmed et al. [17] |
| No (inadequate) | No | No | No | No | No (inadequate) | No | De Angelis et al. [23] |
| Yes (adequate) | Yes |
Yes, Type of tooth extracted was not mentioned |
No | No | Yes (adequate) | No | Kollati et al. [18] |
| No (inadequate) | No |
Yes, Type of tooth extracted was not mentioned |
No | No | No (inadequate) |
Yes, The material was given by a company for free |
Mendoza-Azpur et al. [24] |
| Yes (adequate) | Yes | No | No | No | Yes (adequate) |
Yes, the study was funded by a dental company |
Clark et al. [19] |
| No (inadequate) | No | No | No | No | No (inadequate) | No | Zhang et al. [25] |
| Yes (adequate) | Yes |
Yes, Type of tooth extracted was not mentioned |
No | No | No (inadequate) | No | Kumar et al. [20] |
| Yes (adequate) | No | No | No | No | No (inadequate) | No | Thakkar et al. [22] |
| Yes (adequate) | Yes | No | No | No | No (inadequate) | No | Hauser et al. [21] |
Fig. 2.
The traffic plot reveals the risk of bias in each domain and the overall risk of bias for the included studies based on CONSORT
Quantitative analysis
The Meta-analysis of this systematic review analysis was carried out using MedCalc for windows version 15.0 (MedCalc Software, Ostend, Belgium. The Meta-analysis was carried out for three quantitative (continuous) variables: Reduction in width, Reduction in height and change in bone density. Descriptive statistics (mean and standard deviation) were used to describe these variables. As the outcome variables quantitative, the Standardized mean difference (SMD) was used a summary pooled statistic with the cut-off value of pooled effect of 0.2 = small, 0.5 = medium & 0.8 and above as large effect, as recommended by Cohen. The pooled estimates of SMD were obtained by using both the fixed effect and random effect models. The statistical significance of SMD was done by using Student’s t-test. The heterogeneity in the pooled data was observed by using Cochran’s Q (weighted sum of squares on standardized scale) and I2 was used, which indicates percentage of total variation across the studies included in this analysis. A cut-off values of I2 > 50% was used to rule out the higher levels of unexplained variability in the effect sizes. The significance of publication bias was assessed by using Egger’s test and Begg’s test. A p-value of ≤ 0.05 and 95% confidence intervals were used to report the Statistical significance and precision of estimates. Forest plots were used to show graphically the results (pooled effect using both fixed and random effect models) of studies included in the meta-analysis. Also Funnel plots were used to identify the publication bias of studies used in this quantitative systematic review.
Results
Reviewers’ agreement and kappa score
Electronic searches yielded 41 articles, nine articles were excluded after reviewing their titles, and 14 articles were further excluded after reviewing their abstracts. Five articles were further excluded after reviewing the full manuscript, and the reasons for exclusion are listed in (Fig. 1). Cohen's kappa score was used to measure the agreement level between the reviewers, it was 1 after analysing the titles, 0.9 after analysing the abstracts, and after analysing the full article the Kappa score was also 0.9 which indicated an almost perfect agreement [11].
Study design and patients features
12 studies after carefully going through their content were included in the present review and their data were summarised in Supplementary table 1. The studies publication time were between the year 2013 and 2021 which were conducted in different countries that include: Switzerland, China, Ireland, Italy, USA, Peru, India, and Iran. Nine studies were randomized and out of those nine, eight were blinded [13, 15–21] and one was not [22] and the other three were both not randomized and not blinded [23–25]. Two studies had some conflicts of interest as they were funded by dental companies [19, 24]. The total number of participants in all included studies were 215 male and 257 female individuals. In terms of age distribution, all participants ranged from 18 to 80 years old.
Pre-surgical preparation
Preparation before surgeries was variant among included studies. One study mentioned their protocol before starting the surgical procedures, which is having all subjects undergone periodontal procedures to establish adequate oral hygiene conditions [23]. While other had mentioned prescribing a pre-surgical rinse of chlorhexidine gluconate 0.12% mouthwash for 2 min and extraoral scrub with 5% Betadine solution as an asepsis protocol [20]. Ahmed et al. had described using a standard precaution for asepsis that were carried out for all patients without specifying anything in particular [17]. Furthermore, Clark et al. administered all the patients with 600 mg ibuprofen and had the rinse with chlorhexidine gluconate 0.12% mouthwash at the surgical appointment [19]. Yewale et al. had each participant receiving a session of oral prophylaxis and polishing with a rubber cup and low abrasive paste and emphasised proper oral hygiene and in case the adjacent tooth at site of interest was decayed they had it restored prior to surgery [14]. Furthermore, Dhamija et al. emphasised that all their sample were subjected to routine blood investigations and were given a prophylactic regimen that included a 2 g of amoxicillin 1 h preoperatively [16]. Finally, the six remaining studies did not mention any specific pre-surgical preparations. [15, 18, 21, 22, 24, 25].
PRF protocol
Seven studies had shown to use the same PRF preparation protocol as they followed Dohan’s protocol [26], in which they collected a blood sample without anticoagulant in 10-mL tubes which are immediately centrifuged at 3000 rpm for 10 min. [15, 17, 18, 20, 22, 24, 25].
On the other hand, some differences had been shown among other studies. Two studies followed Choukroun’s new protocol [27] which composed of collecting 10 ml of venous blood without anticoagulants and centrifuging it for 1,300 rpm for 8 min [14, 19].
De Angelis et al. reported that they collected a total of 9 ml of blood without anticoagulants and the was centrifuged using a sterile glass-coated plastic tube and each L-PRF clot was removed from the tube and separated from the red element phase and were squeezed between a sterile glass plate and a metal box to obtain L-PRF membranes [23]. While Hauser et al. mentioned that they collected 8 ml of venous blood without anticoagulants or bovine thrombin and were centrifuged at 2700 rpm for 12 min [21]. Finally, Dhamija et al. reported that they only collected 5 ml of venous blood without anticoagulant and were centrifuged at 3000 revolutions per minute (rpm) for 10 min. [16].
Surgical technique
Five studies included teeth were extracted with minimal trauma using a surgical periotome, then the tooth was removed from the socket using extraction forceps and the socket was debrided using a surgical curette and irrigated with saline [15, 16, 18, 20, 22].
Yelwale et al. reported that extraction was carried out atraumatically, by using a #15 blade to make an intrasulcular incision to elevate the marginal gingiva and adjacent interdental papilla and the flap was then reflected by using a periosteal elevator to expose the crestal bone, then a surgical curette was used to debride the extraction socket [14].
In another included study, a full-thickness flap was raised, and the tooth extraction was performed by using a periotome and dental forceps [24]. In two other studies non-traumatic tooth extraction was performed without elevating a flap but no clear specification for tools used and then the socket was curetted then irrigated with saline [19, 25].
Furthermore, one study described performing a simple extraction by using a scalpel to cut the periodontal ligaments and the extraction was performed using dental elevators, and forceps and after that curettage was made [21].
Finally, the two last studies unclearly described their surgical protocol, and no clear information was elaborated about the method of extraction [17, 23].
Medications prescribed and post-operative management
In terms of antibiotic use, four studies had prescribed Amoxicillin 500 mg three times daily for 5 to 7 days after surgery [14–16, 18]. Furthermore, Mendoza-Azpur et al. reported the use of amoxicillin 750 mg three times daily for 7 days after surgery [24]. While De Angelis et al. prescribed 1 g of amoxicillin every 12 h for 6 days for their participants after procedure [23]. Finally, Kumar et al. reported the use of antibiotics, but no clear information was given [20].
Regarding use of analgesics, six studies had described the use of Ibuprofen, However the dosage and frequency of use was variant. As dosage ranged between 400–600 mg. While frequency of use ranged between twice, three, four and six times per day [14, 15, 18, 24]. A seventh study reported the use of over-the-counter non-steroidal anti-inflammatory drugs in addition to narcotics when needed [19]. While another, noted the use of paracetamol but did the dose was mentioned [21]. Finally, analgesics was given in three studies, but the type was not specified [16, 17, 20].
Outcomes measured
Primary outcomes
Reduction in width (RW)
Yewale et al. reported that significant less RW was shown in test group (1.69 ± 1.28 mm) when compared to control (0.596 ± 1.59 mm) (p ≤ 0.05) [14]. This was also shown by Ahmed et al., RW in both test groups which consisted of PRF alone (0.47 ± 0.36 mm) and PRF + collagen (0.16 ± 0.35 mm) was significantly less than control group (1.71 ± 0.49 mm) (p ≤ 0.001) [17]. In addition, Kollati et al. also reported that the test group revealed significantly less RW (1.47 ± 0.21 mm) compared to the control (2.75 ± 0.22 mm) (p ≤ 0.05) [18]. Similarly, Clark et al., reported that RW in both test groups which composed of PRF (1.8 ± 1.8 mm) and PRF + FDBA (1.7 ± 1.2 mm) was significantly less in comparison to both control group; FDBA (1.5 ± 1.2 mm) and no graft (1.02 ± 0.21 mm) (p ≤ 0.05) [19]. Furthermore, Thakkar et al. also showed that the test group had significant less RW (0.75 ± 0.51 mm) than the control (1.36 ± 0.53 mm) (p ≤ 0.001) [22]. Finally, this significance was also shown by Hauser et al. as less RW was shown in the test group (0.06 ± 0.28 mm) when compared to the control (0.43 ± 0.21 mm) (p ≤ 0.05) [21].
In the other hand, Differences in RW among study groups was not significant in remaining included studies. Zhang et al. also reported RW in test group was 1.05 ± 0.78 mm while control was 2.08 ± 1.67 mm (p > 0.05) [25]. Mendoza-Azpur et al. also reported the amount of RW in test (1.00 ± 0.14 mm) was less than control (1.02 ± 0.21 mm) with no significant difference (p > 0.05) [24]. Kumar et al. also reported similar RW between one of their test groups that consisted of PRF (3 ± 0.8 mm) and control (3 ± 0.8 mm), while it was less in their second test group consisting of PRF + POP (2.9 ± 0.8) (p > 0.05) [20].
In contrast, Azangookhiavi et al. reported the amount RW was more in the test group (1.1 ± 2.0 mm) when compared to the control group (0.5 ± 1.4 mm) but with no significant differences (p > 0.05) [14]. Similarly, Dhamija et al. reported RW in test (3.27 ± 0.2 mm) was more when than control (2.67 ± 0.4 mm) but with no significant difference (p > 0.05) [16]. De Angelis et al. also revealed that RW in one of the test groups which composed of PRF (2.80 ± 0.31 mm) was more than control (1.12 ± 0.28 mm) while the other test composing of PRF + Xenograft (1.05 ± 0.23 mm) but less but with no significant difference shown among comparing all groups (p > 0.05) [23].
Reduction in height (RH)
Yewale et al. reported the amount of RH was significantly less in the test group (1.48 ± 1.53 mm) when compared to the control (1.67 ± 1.610 mm) (p ≤ 0.05) [14]. This was also shown by Dhamija et al., the amount of RH was significant in the test (− 1.6 ± 0.5 mm) when compared to the control (− 1.87 ± 0.3 mm) (p ≤ 0.05) [16]. Ahmed et al. showed similar results in terms of RH, the amount in the test groups which consisted of PRF alone (0.17 ± 0.44 mm) and PRF + collagen (0.14 ± 0.38) was significantly better compared to control (2.12 ± 0.69 mm) (p < 0.001) [17]. De Angelis et al. also reported a significant difference between the test groups, the PRF only group (1.89 ± 0.55 mm) showed more RH than control (0.61 ± 0.42 mm) but the other test which combined PRF with a Xenograft (0.56 ± 0.34) showed the best result. (p ≤ 0.05) [23]. Kollati et al. Similarly, showed significant results in in RH, after comparing the test group (1.00 ± 0.34 mm) with the control (2.22 ± 0.42 mm) (p ≤ 0.05) [18].
Similar to RW, RH did not show any significant results in the remaining studies, the first group by Mendoza-Azpur et al. revealed that RH was better in test group (0.50 ± 0.24 mm) compared to control (0.56 ± 0.33 mm) (p > 0.05) [24]. Furthermore, Clark et al. reported that RH in both test groups which composed of PRF (1.8 ± 2.1 mm) and PRF + FDBA (1.0 ± 2.3) was less in comparison to both control groups; FDBA (2.2 ± 1.8 mm) and no graft (1.8 ± 1.3 mm) (p > 0.05) [19]. Zhang et al. Also reported similar results in terms of RH, the test group (1.60 ± 1.46 mm) was better compared to control (2.80 ± 1.81 mm) (p > 0.05) [25]. Kumar et al. also reported similar RH, it was shown to be better in both test groups that consisted of PRF (3 ± 0.64) and PRF + POP (2.8 ± 0.46 mm) while it was higher in the control (3.3 ± 0.61 mm) (p > 0.05) [20]. For the last group by Thakkar et al., they reported the amount of RH in the test group (1.08 ± 0.66 mm) was better than the control (1.36 ± 0.71 mm) (p > 0.05) [22]. In contrast, only one study by Azangookhiavi et al. reported that the amount of HR in the test group (0.1 ± 0.7 mm) was higher than the control was (0.0 ± 0.2 mm) (p > 0.05) [15]
Change in bone density (BD)
Only three studies showed significant results in terms of BD, and the first was by Ahmed et al. they reported a significant difference in both test groups which consisted of PRF alone (− 0.44 ± 1.21) and PRF + collagen plug (+ 0.13 ± 0.74) in comparison to the control (− 1.45 ± 0.51) (p ≤ 0.001) [17]. The second study was by Kollati et al., have shown significant difference between the test group (97.29 ± 2.67%) and the control group (88.5 ± 10.69%) (p ≤ 0.000) [18]. The last study, Dhamija et al. mentioned a gain in bone density in the test group (57.13%) and compared to the control group (52.70%) (p ≤ 0.05) [16].
On the other hand, the rest of the studies were not significant, Kumar et al. [20] mentioned the amount of bone filled for one of the test groups that combined PRF and POP (79.16 ± 0.13%) was better than control (74.3 ± 0.13%) however, other test group with only PRF showed lesser improvement (73.76 ± 0.14%) (p > 0.05) [20].
In contrast to the previous findings, Yewale et al. reported lesser values in BD in the test group (− 1783.10 ± 772.09) compared to the control (− 1393.10 ± 449.8) (p = 0.17) [14].
Secondary outcomes
Pain assessment:
Yewale et al. reported that both groups experienced mild to moderate levels of pain with no significant difference (p > 0.05) [14]. De Angelis et al., Kumar et al. and Zhang et al. reported significant difference in favour to the PRF group, De Angeles mentioned that the control group which was from a Xenograft had greater pain scores when compared to the PRF groups during the first five days (p < 0.05) [23], Kumar et al. reported that 18.1% of control group complained of pain during the first 24 h while PRF groups did not complain of any pain [20], Zhang et al. Reported that control group felt pain during the first three days and some of the participants continues suffering from pain up to seven days compared to PRF group which felt mild pain during the first day and completely disappeared by third day [25].
Healing and maturation of soft tissue:
Azangookhiavi et al. reported accelerated healing and maturation of soft tissue coverage in the PRF group after two weeks but nothing regarding the control [15]. Ahmed et al. mentioned superior healing after seven days in the PRF group (94.1%) followed by the PRF + Collapug group (88.2%) and the control (86.7%) [17]. Finally, De Angelis et al. also reported better healing outcome in the PRF and PRF + xenograft groups during the first three weeks compared to the control (p < 0.05) [23].
Bone to implant contact
Dhamija et al. reported the percentage of bone to implant contact was better for the control at three months (84.20 ± 15.51) and six months (79.80 ± 18.67), as well for the test group at three (81.47 ± 18.77) and six months (78.13 ± 17.99) but there was no significant difference between the groups after both three months (p = 0.334) and six months (p = 0.143) [16].
Crestal bone level and Socket depth
Kollati et al. reported that after six months, test group showed a lesser amount of crestal bone loss (0.1 mm) compared to control (0.5 mm) with no significant difference reported (p = 0.027) [18].
Marginal gingiva junction (MGJ)
Mendoza-Azpur et al. reported regarding the marginal gingival junction, that for the control group (β-TCP), coronal migration at different time points was significantly less at the mesial, buccal, and distal sites of the study site when compared to the PRF group after four months (p < 0.01) [24].
Histological evaluation:
Mendoza-Azpur et al. 2019 reported more newly formed osteocytes in the test group (123.25 (5.12) mm2 compared to 84.02 (26.53) mm2). Also, mentioned more mineralized tissue gain in the test group (77.33 (9.80) % compared to 26.14 (7.49) %) [24]. Clark et al. 2018 and Zhang et al. 2018 also reported more vital bone in the PRF groups [19, 25].
Bone microarchitecture and intrinsic quality:
Hauser et al. [21] reported that after the Micro-CT analysis the samples that received PRF showed better results and they also reported in terms of trabecular bone The results of the PRF group (+ 1.30%) were not significantly different from the control group (+ 3.68%) [21].
Meta-analysis results
Reduction in width (RW)
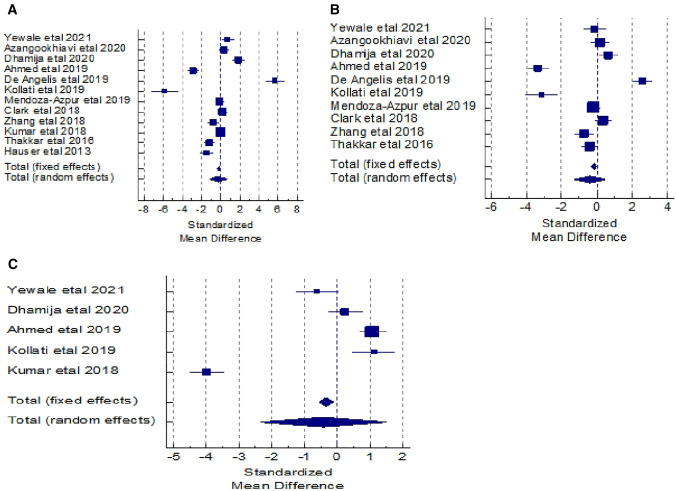
The meta-analysis for the outcome variable “Reduction in width” was carried out to assess the statistical significance, by combining the difference in its mean values which were extracted from the 12 studies and compared between the test and control groups. The total sample size for this analysis was 472. The results show no statistically significant difference in the values of pooled standardized mean difference (SMD) by random effects model. But the pooled SMD is statistically significant by fixed effects model (SMD = − 0.184, t = − 2.509, p = 0.012). The Cochran’s Q value is statistically significant (Q = 418.082, DF = 11, p < 0.0001) and I2 value (97.37%) is higher, which implies heterogeneity among the 12 studies which is statistically significant. Hence, the pooled SMD by random effect was used to infer no significant difference in the mean values of reduction in width between the two groups (SMD = − 0.253, t = − 0.543, p = 0.587). The overall effect (0.253) is low effect. The publication bias was assessed using Egger’s test and Begg’s test. The corresponding p-values of these two tests indicates non-statistical significance for the absence of publication bias. (Table 2).
Table 2.
Meta-Analysis for the outcome variable: Reduction in width
| Test group | Control group | SMD | 95% CI | Weight (%) | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Mean (SD) | N2 | Mean (SD) | t-value | p-value | Fixed | Random | |||
| 20 | 1.68 (1.28) | 20 | 0.59 (1.59) | 0.742 | 0.09 to 1.39 | 5.21 | 8.35 | Yewale et al. [14] | ||
| 32 | 1.1 (2.0) | 32 | 0.50 (1.4) | 0.343 | − 0.15 to 0.84 | 8.66 | 8.49 | Azangookhiavi et al. [14] | ||
| 30 | 3.27 (0.2) | 30 | 2.67 (0.4) | 1.873 | 1.26 to 2.49 | 5.69 | 8.38 | Dhamija et al. [16] | ||
| 54 | 0.47 (0.36) | 54 | 1.71 (1.49) | − 2.864 | − 3.40 to − 2.32 | 7.20 | 8.45 | Ahmed et al. [17] | ||
| 45 | 2.8 (0.31) | 45 | 1.12 (0.28) | 5.639 | 4.71 to 6.57 | 2.43 | 7.99 | De Angelis et al. [23] | ||
| 23 | 1.47 (0.21) | 23 | 2.75(0.22) | − 5.850 | − 7.21 to − 4.48 | 1.18 | 7.35 | Kollati et al. [18] | ||
| 51 | 1.0 (0.14) | 51 | 1.02 (0.21) | − 0.111 | − 0.50 to 0.28 | 13.86 | 8.57 | Mendoza-Azpur et al. [24] | ||
| 40 | 1.8 (1.8) | 40 | 1.50 (1.20) | 0.194 | − 0.25 to 0.64 | 10.88 | 8.53 | Clark et al. [19] | ||
| 28 | 1.05 (0.78) | 28 | 2.08 (1.67) | − 0.779 | − 1.39 to -0.23 | 7.16 | 8.45 | Zhang et al. [25] | ||
| 90 | 3 (0.8) | 90 | 3.0 (0.83) | 0.000 | − 0.29 to 0.29 | 24.33 | 8.62 | Kumar et al. [20] | ||
| 8.41 | 8.48 | |||||||||
| 36 | 0.75 (0.51) | 36 | 1.36 (0.53) | − 1.160 | − 1.664 to − 0.66 | Thakkar et al. [22] | ||||
| 4.99 | 8.34 | |||||||||
| 23 | 0.06 (0.28) | 23 | 0.43 (0.21) | − 1.469 | − 2.13 to − 0.81 | Hauser et al. [21] | ||||
| 472 | 472 | − 0.184 | − 0.33 to − 0.04 | − 2.509 | 0.012 | 100.00 | 100.00 | Total (fixed effects) | ||
| 472 | 472 | − 0.253 | − 1.17 to 0.66 | − 0.543 | 0.587 | 100.00 | 100.00 | Total (random effects) | ||
The corresponding forest plot for reduction in width shows the effect size of each study and combined effect size by fixed and random effects models. Also, the funnel plot shows the pattern of studies in the funnel. (Fig. 3A).
Fig. 3.
Forest plots for A reduction in width, B reduction in height, C change in bone density
Reduction in height (RH)
For the outcome variable “Reduction in height” the meta-analysis was done to assess the statistical significance, by combining the difference in its mean values which were extracted from the 10 studies and compared between the test and control groups. The total sample size for this analysis was 359. The results show no statistically significant difference in the values of pooled standardized mean difference (SMD) by random effects model. But the pooled SMD is statistically significant by fixed effects model (SMD = − 0.177, t = − 2.145, p = 0.032). The Cochran’s Q value is statistically significant (Q = 277.13, DF = 9, p < 0.0001) and I2 value (96.75%) is higher, which implies heterogeneity among the 10 studies which is statistically significant. Hence, the pooled SMD by random effect was used to infer no significant difference in the mean values of reduction in height between the two groups (SMD = − 0.397, t = − 0.861, p = 0.390). The overall effect (0.397) is low effect. The publication bias was assessed using Egger’s test and Begg’s test. The corresponding p-values of these two tests (p = 0.303 & p = 0.421) indicates non-statistical significance for the absence of publication bias. (Table 3).
Table 3.
Meta Analysis for the Outcome variable: Reduction in height
| Test group | Control group | SMD | 95% CI | Weight (%) | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Mean (SD) | N2 | Mean (SD) | t-value | p-value | Fixed | Random | |||
| 20 | 1.48 (1.53) | 20 | 1.67 (1.61) | − 0.119 | − 0.75 to 0.51 | 7.04 | 9.92 | Yewale et al. [14] | ||
| 32 | 0.1 (0.7) | 32 | 0.0( 0.2) | 0.192 | − 0.30 to 0.69 | 11.06 | 10.09 | Azangookhiavi et al. [14] | ||
| 30 | − 1.6 (0.5) | 30 | − 1.87 (0.30) | 0.646 | 0.12 to 1.17 | 9.91 | 10.05 | Dhamija et al. [16] | ||
| 54 | 0.17 (0.44) | 54 | 2.12 (0.69) | − 3.346 | − 3.93 to 2.76 | 7.67 | 9.96 | Ahmed et al. [17] | ||
| 45 | 1.89 (0.55) | 45 | 0.61 (0.42) | 2.593 | 2.03 to 3.16 | 8.36 | 9.99 | De Angelis et al. [23] | ||
| 23 | 1.0 (0.34) | 23 | 2.22 (0.42) | − 3.138 | − 4.02 to 2.26 | 3.55 | 9.50 | Kollati et al. [18] | ||
| 51 | 0.50 (0.24) | 51 | 0.56 (0.33) | − 0.206 | − 0.59 to 0.18 | 17.45 | 10.20 | Mendoza-Azpur et al. [24] | ||
| 40 | 1.80 (2.10) | 40 | 1.0 (2.30) | 0.360 | − 0.085 to 0.80 | 13.60 | 10.14 | Clark et al. [19] | ||
| 28 | 1.60 (1.46) | 28 | 2.80 (1.81) | − 0.720 | − 1.26 to − 0.17 | 9.15 | 10.03 | Zhang et al. [25] | ||
| 36 | 1.08 (0.66) | 36 | 1.36 (0.71 | − 0.404 | − 0.87 to 0.06 | 12.21 | 10.12 | Thakkar et al. [22] | ||
| 359 | 359 | − 0.177 | − 0.34 to − 0.015 | − 2.145 | 0.032 | 100.00 | 100.00 | Total (fixed effects) | ||
| 359 | 359 | − 0.397 | − 1.30 to 0.51 | − 0.861 | 0.390 | 100.00 | 100.00 | Total (random effects) | ||
The corresponding forest plot for reduction in height shows the effect size of each study and combined effect size by fixed and random effects models. Also, the funnel plot shows the pattern of studies in the funnel. (Fig. 3B).
Change in bone density (BD)
For this outcome variable, only 5 studies have been included in the meta-analysis. The total sample size in each of the test and control group was 217. The difference in mean values from these 5 studies were extracted to get the combined SMD to compare between the test and control groups. The results show no statistically significant difference in the values of pooled standardized mean difference (SMD) by random effects model. But the pooled SMD is statistically significant by fixed effects model (SMD = − 0.341, t = − 2.947, p = 0.032). The Cochran’s Q value is statistically significant (Q = 276.18, DF = 4, p < 0.0001) and I2 value (98.55%) is higher, which implies heterogeneity among the 5 studies which is statistically significant. Hence, the pooled SMD by random effect was used to infer no significant difference in the mean values of change in bone density between the two groups (SMD = -0.431, t = -0.441, p = 0.659). The overall effect (0.431) is low effect. The publication bias was assessed using Egger’s test and Begg’s test. The corresponding p-values of these two tests (p = 0.765 & p = 0.327) indicates non-statistical significance for the absence of publication bias. (Table 4).
Table 4.
Meta Analysis for the outcome variable: change in bone density
| Test group | Control group | SMD | 95% CI | Weight (%) | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Mean (SD) | N2 | Mean (SD) | t-value | p-value | Fixed | Random | |||
| 20 | − 1783.1(772.1) | 20 | − 1393.1 (449.8) | − 0.605 | − 1.25 to 0.03 | 13.34 | 19.89 | Yewale et al. [14] | ||
| 30 | 57.13( 20.41) | 30 | 52.7 (15.53) | 0.241 | − 0.27 to 0.75 | 20.52 | 20.04 | Dhamija etal. [16] | ||
| 54 | − 0.44 (1.21) | 54 | − 1.45 (0.51) | 1.080 | 0.67 to 1.48 | 32.03 | 20.14 | Ahmed et al. [17] | ||
| 23 | 97.29 (2.67) | 23 | 88.5 (10.69) | 1.109 | 0.48 to 1.74 | 13.79 | 19.90 | Kollati et al. [18] | ||
| 90 | 73.76 (0.14) | 90 | 74.3 (0.13) | − 3.980 | − 4.49 to 3.47 | 20.33 | 20.03 | Kumar et al. [20] | ||
| 217 | 217 | − 0.341 | − 0.57 to 0.11 | − 2.947 | 0.003 | 100.00 | 100.00 | Total (fixed effects) | ||
| 217 | 217 | − 0.431 | − 2.35 to 1.49 | − 0.441 | 0.659 | 100.00 | 100.00 | Total (random effects) | ||
The corresponding forest plot for change in bone density shows the effect size of each study and combined effect size by fixed and random effects models. Also, the funnel plot shows the pattern of studies in the funnel. (Fig. 3C).
Discussion
In this systematic review and Meta analysis, all clinical studies comparing PRF as a socket preservation material with other commonly used grafting materials until July 2021 were selected. The aim was to evaluate the effects of PRF when used in socket preservation procedures on the amount bone width, hight and density after augmentation.
Regarding the PRF effect on the bone width, most studies included in the review concluded positive effects wither it was statistically significant or not, they mentioned lesser loss of bone width when compared to no grafting at all, but when compared to other commonly utilized grafting materials some studies reported the results were either almost similar or leaning toward the other grafting materials. However, almost all the included studies showed superior results when the PRF were mixed with grafting materials. Similar findings were concluded regarding bone height after grafting. In regard to its effect on bone density, PRF alone showed better outcomes only when compared with sockets that were not grafted with anything. However, when mixed with a bone graft most included studies showed better results when compared to grafts alone.
Twelve studies were heterogenous regarding width reduction, ten for height reduction, and five for bone density and showed statistical significance. The meta-analysis conducted in the review, revealed that for socket preservation procedures, the use of PRF alone or in combination with other graft materials, was correlated with a significant difference in lesser loss of bone height and width, as well as improved bone density when compared to empty sockets and grafts alone.
In a systematic review made by Pan et al., they reported after analysing their included studies, that PRF had positive results on bone density in one study and non-significant results in the other, which is almost like the present review’s findings. Authors have also reported its effect on the width and height bone reduction, they mentioned in terms of width, PRF alone showed better results in most included studies and when mixed with bone grafts it showed better results than grafts alone which agrees with our findings. However, when it comes to the PRF effect on height, their outcomes mostly show non-significant results which differs from the findings presented in our review [28].
Furthermore, Bastami and Khojasteh briefly addressed the effects of PRF on socket preservations surgeries, and they mentioned PRF alone did not pose any significant improvement. However, significant improvement was shown when PRF was combined with bone grafts in terms of preserving the level of bone [29]. Another review by Moraschini and Barboza addressed the beneficial effects of PRF in socket preservation surgeries, they mentioned regarding hard tissue dimensional changes, PRF showed significant result in half of the studies included and non-significant results in the other half, both halves compared it to natural healing [30]. Another review by Najeeb et al. discussed the positive effects of PRF in treating intra-bony defects, they concluded that PRF can augment the effect of other bone substitutes in treating bony defects which aligns with the current review findings [31].
The results of this review might have been affected by many factors. One of these factors is the PRF preparation protocol as it was not uniform in all included studies, in addition, some authors added their own slight modifications. Another factor was variation in follow up periods among studies which might affected the results based on healing period reached. Other factors to consider is the utilization of different surgical techniques and the post-surgical care. Therefore, further standardized randomized clinical trials are recommended in order to confirm present findings. In conclusion, the meta-analysis of the studies included in this study, proved the beneficial effect of PRF in socket preservation surgeries alone or in combination with other graft materials.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Deanship of scientific research in King Saud University for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR).
Declarations
Conflict of interest
The authors report no conflict of interest.
Ethical statement
The review was registered on PROSPERO under Registration Number: CRD42021261939 on 27th of July 2021.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Annunziata M, Guida L, Nastri L, Piccirillo A, Sommese L, Napoli C. The role of autologous platelet concentrates in alveolar socket preservation: a systematic review. Transfus Med Hemother. 2018;45:195–203. [DOI] [PMC free article] [PubMed]
- 2.Zhang Y, Ruan Z, Shen M, Tan L, Huang W, Wang L, et al. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp Ther Med. 2018;5:2277–2286. doi: 10.3892/etm.2018.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grecu AF, Reclaru L, Ardelean LC, Nica O, Ciucă EM, Ciurea ME. Platelet rich fibrin and its emerging therapeutic benefits for musculoskeletal. injury treatment. Medicina (Kaunas). 2019;55:141. [DOI] [PMC free article] [PubMed]
- 4.Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;9:48–55. doi: 10.1080/09537104.2017.1293807. [DOI] [PubMed] [Google Scholar]
- 5.Magalhães VS, Ribeiro RA, Leite do Amaral JMB, Pimentel AC, Paulim LA. The use platelet rich fibrin in dental implants: a literature review. Trends in Transplant. 2018;11:2–3.
- 6.Neiva RF, Gil LF, Tovar N, Janal MN, Marao HF, Bonfante EA, et al. The synergistic effect of leukocyte platelet-rich fibrin and micrometer/nanometer surface texturing on bone healing around immediately placed implants: an experimental study in dogs. Biomed Res Int. 2016;2016:9507342. doi: 10.1155/2016/9507342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao JH, Tsai CH, Chang YC. Clinical application of platelet rich fibrin as the sole grafting material in maxillary sinus augmentation. J Formosan Med Assoc. 2015;114:779–780. doi: 10.1016/j.jfma.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Meshram VS, Lambade PN, Meshram PV, Kadu A, Tiwari MS. The autologous platelet rich fibrin: a novel approach in osseous regeneration after cystic enucleation: a pilot study. Indian J Dent Res. 2015;26:560–4. [DOI] [PubMed]
- 9.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed]
- 10.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. (Zagreb) 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration. 2011. https://www.cochrane-handbook.org
- 13.McGuinness LA. Robvis: An R Package and Web Application for Visualising Risk-of-Bias Assessments. 2019. https://github.com/mcguinlu/robvis [DOI] [PubMed]
- 14.Yewale M, Bhat S, Kamath A, Tamrakar A, Patil V, Algal AS. Advanced platelet-rich fibrin plus and osseous bone graft for socket preservation and ridge augmentation: a randomized control clinical trial. J Oral Biol Craniofac Res. 2021;11:225–233. doi: 10.1016/j.jobcr.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azangookhiavi H, Ghodsi S, Jalil F, Dadpour Y. Comparison of the efficacy of platelet-rich fibrin and bone allograft for alveolar ridge preservation after tooth extraction: a clinical trial. Front Dent. 2020;17:1–6. doi: 10.18502/fid.v17i1.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhamija R, Shetty V, Vineeth K, Nagaraju R, Rao RS. Socket preservation with demineralized freeze-dried bone allograft and platelet-rich fibrin for implant site development: A randomized controlled trial. J Indian Prosthodont Soc. 2020;20:304–311. doi: 10.4103/jips.jips_2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed N, Gopalakrishna V, Shetty A, Nagraj V, Imran M, Kumar P. Efficacy of PRF vs PRF + biodegradable collagen plug in post-extraction preservation of socket. J Contemp Dent Pract. 2019;20:1323–1328. doi: 10.5005/jp-journals-10024-2673. [DOI] [PubMed] [Google Scholar]
- 18.Kollati P, Koneru S, Dwarakanath CD, Gottumukkala SNVS. Effectiveness of naturally derived bovine hydroxyapatite (Cerabone™) combined with platelet-rich fibrin matrix in socket preservation: A randomized controlled clinical trial. J Indian Soc Periodontol. 2019;23:145–151. doi: 10.4103/jisp.jisp_400_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark D, Rajendran Y, Paydar S, Ho S, Cox D, Ryder M, et al. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: a randomized controlled clinical trial. J Periodontol. 2018;89:379–387. doi: 10.1002/JPER.17-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girish Kumar N, Chaudhary R, Kumar I, Arora SS, Kumar N, Singh H. To assess the efficacy of socket plug technique using platelet rich fibrin with or without the use of bone substitute in alveolar ridge preservation: a prospective randomised controlled study. Oral Maxillofac Surg. 2018;22:135–142. doi: 10.1007/s10006-018-0680-3. [DOI] [PubMed] [Google Scholar]
- 21.Hauser F, Gaydarov N, Badoud I, Vazquez L, Bernard JP, Ammann P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent. 2013;22:295–303. doi: 10.1097/ID.0b013e3182906eb3. [DOI] [PubMed] [Google Scholar]
- 22.Thakkar DJ, Deshpande NC, Dave DH, Narayankar SD. A comparative evaluation of extraction socket preservation with demineralized freeze-dried bone allograft alone and along with platelet-rich fibrin: a clinical and radiographic study. Contemp Clin Dent. 2016;7:371–376. doi: 10.4103/0976-237X.188567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Angelis P, De Angelis S, Passarelli PC, Liguori MG, Manicone PF, D'Addona A. Hard and soft tissue evaluation of different socket preservation procedures using leukocyte and platelet-rich fibrin: a retrospective clinical and volumetric analysis. J Oral Maxillofac Surg. 2019;77:1807–1815. doi: 10.1016/j.joms.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza-Azpur G, Olaechea A, Padial-Molina M, Gutiérrez-Garrido L, Ovalle F, Mesa F, et al. Composite alloplastic biomaterial vs. autologous platelet-rich fibrin in ridge preservation. J Clin Med. 2019;8:223. doi: 10.3390/jcm8020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Ruan Z, Shen M, Tan L, Huang W, Wang L, et al. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp Ther Med. 2018;15:2277–2286. doi: 10.3892/etm.2018.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–121. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 28.Pan J, Xu Q, Hou J, Wu Y, Liu Y, Li R, et al. Effect of platelet-rich fibrin on alveolar ridge preservation: a systematic review. J Am Dent Assoc. 2019;150:766–778. doi: 10.1016/j.adaj.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Bastami F, Khojasteh A. Use of leukocyte- and platelet-rich fibrin for bone regeneration: a systematic review. RRR. 2016;1:47–68. [Google Scholar]
- 30.Moraschini V, Barboza ES. Effect of autologous platelet concentrates for alveolar socket preservation: a systematic review. Int J Oral Maxillofac Surg. 2015;44:632–641. doi: 10.1016/j.ijom.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Najeeb S, Khurshid Z, Agwan MAS, Ansari SA, Zafar MS, Matinlinna JP. Regenerative potential of platelet rich fibrin (PRF) for curing intrabony periodontal defects: a systematic review of clinical studies. Tissue Eng Regen Med. 2017;14:735–42. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.