Abstract
A basidiomycetous fungus Flavodon flavus (Klotzsch) Ryvarden (strain 312), isolated from decaying sea grass from a coral lagoon off the west coast of India, mineralized nearly 24% of 14C-labeled synthetic lignin to 14CO2 in 24 days. When grown in low-nitrogen medium (2.4 mM N) this fungus produced three major classes of extracellular lignin-modifying enzymes (LMEs): manganese-dependent peroxidase (MNP), lignin peroxidase (LIP), and laccase. Low MNP and laccase activities were seen in high-nitrogen medium (24 mM N), but no LIP activity was seen. In media containing lignocellulosic substrates such as pine, poplar, or sugarcane bagasse as the sole source of carbon and nitrogen, relatively high MNP and moderate levels of laccases were seen, but LIP production either was not seen or was minimal. LME production was also seen in media prepared with artificial seawater. Fast protein liquid chromatography and isoelectric focusing resolved LMEs into four isozymes each of MNP and LIP, while laccase isozymes were resolved into two groups, one group containing seven isozymes (pIs 4 to 6) and the other group containing at least three isozymes (pIs < 3). The molecular masses of the different isozymes were 43 to 99 kDa for MNP, 40 and 41.5 kDa for LIP, and 43 and 99 kDa for laccase. F. flavus showed effective degradation of various dye pollutants in media prepared with or without artificial seawater. This is the first report on the production of all three major classes of LMEs by F. flavus and points to the bioremediation potential of this organism in terrestrial as well as marine environments.
Research on lignin-degrading enzymes has greatly intensified in recent years because of their potential applications in a variety of biotechnological applications. These applications include biotransformation of lignocellulosic biomass to feeds, fuels, and chemicals; biopulping; biobleaching of paper pulps; decolorizing and detoxifying kraft bleach plant effluents; and degradation of highly toxic environmental chemicals such as dioxins, polychlorinated biphenyls, various dye pollutants, and polyaromatic hydrocarbons (1, 44). Basidiomycetous fungi involved in white rot decay of wood are known to play a major role in the mineralization of the lignin polymer to carbon dioxide and water in the terrestrial environment, while bacteria are believed to be relatively unimportant in this process (8, 21). Three major classes of extracellular enzymes designated manganese-dependent peroxidases (MNPs), lignin peroxidases (LIPs), and laccases are believed to be important in the fungal degradation of lignin (8, 10, 18, 21). LIPs and MNPs are heme proteins, while laccases are copper-containing proteins. Some wood-degrading fungi contain all three classes of lignin-modifying enzymes (LMEs), while the others contain only one or two of these enzymes (8, 18).
The sea grasses and mangrove plants are the major contributors of lignocellulose in the highly productive coastal marine environments (30, 38, 42). Microbial degradation of the lignin component is the rate-limiting step affecting the availability of lignocellulose-derived dissolved organic carbon in the food chain (4, 5, 39, 40). Fungi associated with decaying sea grass and mangrove plants contribute about 3% of the biomass per g (dry weight) of detritus (49) and contribute nearly 75 to 100% of nitrogen in decaying salt marsh grass ecosystems (26, 27). The association and importance of terrestrial species of fungi with plant detritus in the coastal marine environment have also been documented (12, 15, 40, 41). However, there is little published information on the lignin-degrading ability of obligate or facultative marine fungi. Only a few isolated reports indicate low-level degradation of [14C]lignin-labeled lignocellulose by marine fungi (5, 39, 52). Moreover, the presence of MNPs, LIPs, and laccases in these fungi has not been investigated except for two recent surveys (37, 40). In this study, we describe the production and characteristics of LMEs, mineralization of synthetic, side chain-labeled [14C]lignin, and degradation of various heterocyclic and azo dyes by a marine isolate of Flavodon flavus isolated from decaying sea grass leaves in a tropical coral lagoon.
MATERIALS AND METHODS
Organism and culture conditions.
The basidiomycetous fungus Flavodon flavus (Klotzsch) Ryvarden strain 312 was isolated from decaying brown leaves of the sea grass Thalassia hemprichii (Ehrenberg) Ascherson from the intertidal lagoon on Kavaratti island of the Lakshadweep archipelago (10°30′ to 11.0°N latitude, 72.0° to 73.0°E longitude) off the west coast of India in the Arabian Sea. Within 2 h after collection, discs (0.5-cm diameter) cut from the sea grass leaves were surface sterilized in 0.5% sodium hypochlorite for 3 min (42), washed thrice with 50% (vol/vol) filter-sterilized artificial seawater (see below) as previously described (40), and placed on plates of Poly-R 478 (Sigma Chemical Co., St. Louis, Mo.) (Poly-R). Poly-R medium contained the following (per 100 ml): the usual components of low-nitrogen (LN) medium (13), 1.5 g of Instant Ocean (IO) salts (24) (Instant Ocean of Aquarium System, Mentor, Ohio), 2 g of Bacto Agar (Difco), 0.02% Poly-R, 10,000 U of sodium benzylpenicillin, and 0.05 g of streptomycin sulfate. One of the Poly-R-decolorizing isolates designated strain 312 was identified as F. flavus (Klotzsch) Ryvarden by using the key given by Ryvarden and Johansen (48). F. flavus 312 and Phanerochaete chrysosporium (strain BKM-F 1767; ATCC 24725), a well-known lignin-degrading white rot fungus (21) which was used as a positive control in this study, were maintained on slants of malt extract agar (MEA) as previously described (57).
To test for LME activities, the fungus was cultured in the LN medium (13) except where mentioned otherwise. High-nitrogen medium (HN medium) was identical to LN medium except that the nitrogen concentration was increased to 24 mM. Sterile media were dispensed in 9-ml amounts into 125-ml sterile rubber-stoppered Erlenmeyer flasks. Since strain 312 was isolated from a coastal marine environment, the effect of salinity on production of LMEs was tested by growing the strain in LN medium prepared with 50% artificial seawater (LN+IO) (i.e., 1.5 g of IO in 100 ml of medium, which gives 50% of the salinity found in natural seawater). To prepare media with lignocellulosic substrates as the sole source of carbon, energy, and nitrogen, sawmill-cut (60/100-mesh screen) Eastern pine wood (Pinus strobus) representing gymnosperms or soft woods, poplar wood (Populus × euramericana eugenei) representing angiosperms or hardwoods, and sugarcane bagasse (16-mesh screen) were added at 1% (wt/vol) to distilled water (15) or to 50% (vol/vol) artificial seawater. Sugarcane bagasse was washed under running tap water for 12 h to minimize contamination by residual soluble sugars, washed with 3 volumes of distilled water, and dried at room temperature. To test for the production of LMEs under alkaline conditions (simulating the marine environment), the LN medium was modified as follows: trace mineral solution was deleted, glycerol was substituted for glucose at the 3% level, yeast extract (0.1%, wt/vol) and sodium acetate buffer with 0.2 M bicine (Sigma Chemicals) were added, and the pH was adjusted to 7.8 with 0.2 M NaOH.
For inoculum preparation, mycelial mats grown in malt extract (ME) broth (25 ml in 250 ml of foam-plugged Erlenmeyer flasks) for 10 days at room temperature were washed twice with 150 ml of sterile water and blended in 20 ml of LN medium with a Sorvall blender (with four 30-s pulses). One milliliter of this blended mycelium (equivalent to 3 mg [dry weight] of mycelium) was added to each flask and flushed with 100% O2 at the time of inoculation and every other day thereafter as described previously (7). Culture flasks were incubated at room temperature (25 to 27°C) without agitation or on a rotary shaker (200 rpm). Cultures were routinely monitored for purity by phase-contrast microscopy as described previously (57).
Since strain 312 was isolated from the marine environment, its growth (measured as the weight of mycelium [dry weight]) was determined in ME broth and in LN medium prepared with distilled water as well as 50% artificial seawater.
Mineralization of 14C-labeled synthetic lignin.
Synthetic, side chain-labeled [14C]lignin was a gift from Kenneth Hammel, U.S. Forest Products Laboratory, Madison, Wis. A total of 30,000 dpm was added to 3-day-old cultures grown in LN medium. The flasks were flushed immediately after inoculation and every third day thereafter with carbon dioxide-free air for 20 min, and the 14CO2 produced in the flasks was trapped in ethanolamine-containing scintillation fluid by the method of Boominathan et al. (7). After the CO2 was collected, the cultures were reoxygenated (57). A culture of P. chrysosporium BKM-F 1767 was used as a positive control and was incubated at 37°C, whereas strain 312 was incubated at room temperature.
Enzyme assays.
On selected days of incubation, extracellular culture fluid from three flasks was collected by filtration with Whatman GF/C filters (42.5-mm diameter) and assayed for LME activities. Laccase activity was determined by the method of Niku-Paavola et al. (28). The laccase reaction mixture contained 14 mM 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS) in 0.1 M glycine-HCl buffer at pH 3.0 in a final volume of 1.0 ml. The reaction was monitored by measuring the change in absorbance at 405 nm (14). The enzyme units were expressed as nanokatals per liter. One katal is defined as one mole of product formed per second. MNP activity was determined by the method of Paszczynski et al. (33) by monitoring the oxidation of Mn2+ to Mn3+, while the LIP activity was determined by measuring the rate of H2O2-dependent oxidation of veratryl alcohol to veratraldehyde (13, 53), and the activities were expressed as units per liter (shown as U/L in the figures). One unit of enzyme is defined as 1 μmol of veratryl alcohol oxidized per min and 1 μmol of Mn(II) oxidized per min for LIP and MNP, respectively.
FPLC.
The extracellular fluids from cultures grown in LN medium were concentrated 50-fold by using a 10-kDa cutoff membrane (PM-10; Amicon Div., W. R. Grace & Co., Danvers, Mass.). The concentrate was passed through 0.2-μm-pore-size filters, and the filtrate was fractionated by fast protein liquid chromatography (FPLC) using a Mono-Q HR 5/5 column (Pharmacia) and a gradient of 10 mM to 1 M sodium acetate buffer at pH 6.0. Elution of heme proteins (i.e., LIP and MNP) was monitored by using a 405-nm filter as described by Dass and Reddy (13). Peak fractions were assayed for MNP and LIP activities as described above.
Laccase in the extracellular culture fluid was similarly fractionated by FPLC as described above except that an elution gradient of 0 to 1 M NaCl in 20 mM Tris buffer (pH 8.0) was used and a 280-nm filter was used to monitor protein elution. Fractions were assayed for laccase activity as described above.
IEF.
Concentrated extracellular fluids from culture grown in LN medium were separated by isoelectric focusing (IEF) on premade, nondenaturing, 5% polyacrylamide gels (Novex, San Diego, Calif.), and pI markers (range, 3.0 to 7.0) provided by the manufacturer were used as standards. A constant voltage of 100 V was applied to the gels for the first hour, 200 V was used for another hour, and 500 V was used for the last 30 min. The gels were sliced with a surgical blade into sections: one for visualization of protein and the others for activity staining for LIP, MNP, and laccase. For detection of MNP activity, the gels were stained in a solution containing 2 mM diaminobenzidine in sodium acetate buffer at pH 4.5, 2 mM MnSO4, and 0.5 mM H2O2 (55). Activity staining for LIP was done with 2 mM o-dianisidine in sodium tartrate buffer at pH 3.0 and 1 mM H2O2 (55). The gels were stained for laccase activity by using ABTS in glycine-HCl buffer at pH 3.0 (29). The pI markers were detected on the IEF gel by first fixing the gels in a solution containing 0.7 M trichloroacetic acid and 0.14 M sulfosalicylic acid, followed by protein staining with Coomassie blue (as per the manufacturer’s instructions).
Pooled FPLC peak fractions showing MNP, LIP, and laccase activities were subjected to IEF fractionation as described above, and the results obtained were compared to those obtained with the concentrated extracellular fluid.
SDS-PAGE.
To estimate the molecular masses of MNP, LIP, and laccase, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the concentrated extracellular fluid and pooled FPLC fractions showing enzyme activities was done by the method of Laemmli (23) as modified by Srinivasan et al. (50). The gels were stained for protein with 0.2% Coomassie brilliant blue R-250 (56). A GIBCO-BRL marker protein mixture (BenchMark) with a molecular mass range from 23 to 241 kDa was used as the standard.
For activity staining of various enzymes, concentrated extracellular culture fluid was subjected to nondenaturing SDS-PAGE as described by Kelley and Reddy (20). Activity staining for detecting MNP, LIP, and laccase was performed at room temperature as described above.
Analytical techniques.
For determination of the weight of mycelium (dry weight), the contents of three flasks were vacuum filtered through tared Whatman GF/C filter papers, rinsed with 100 ml of deionized water, and dried to a constant weight, and the net mycelial weight (dry weight) was calculated as described earlier (25).
The levels of protein in the extracellular culture fluid were estimated by the method of Bradford (9) using the Bio-Rad protein dye reagent and bovine serum albumin as the standard (Bio-Rad Laboratories, Richmond, Calif.) following a protocol described by the manufacturer.
Decolorization of synthetic dyes.
The dyes Poly-R, Poly B-411 (Poly-B), azure B, brilliant green, Congo red, and Remazol brilliant blue R used were of analytical grade. Stock solutions were prepared in distilled water (0.5 g/100 ml) and were filter sterilized. The decolorization ability of strain 312 was tested in ME broth as well as in LN medium prepared with and without 50% artificial seawater. Individual dye solutions were added to 4-day-old cultures of 312 to a final concentration of 0.02%. The flasks were flushed with oxygen immediately after inoculation and after every sampling. Autoclaved 4-day-old cultures, to which dyes have been added served as heat-killed controls. The degradation of dyes was monitored by measuring changes in absorbance at the respective absorbance maxima for the different dyes (17, 19). Decolorization of Poly-B and Poly-R was monitored by determining the ratios of absorbance at 593 nm versus 483 nm for Poly-B and at 513 nm versus 362 nm for Poly-R. Aliquots (0.5 ml) of culture fluid were aseptically removed from the flasks at 2-day intervals and diluted appropriately with distilled water, and the absorption was measured with a Varian Cary 1 Bio UV-visible light spectrophotometer (Varian, Mulgrave, Australia). The results were calculated as differences in percent decolorization between heat-killed cultures and experimental cultures. At the end of the experiments, degradation of various dyes was also monitored by comparing the visible spectrum (400 to 800 nm) of a given dye in heat-killed controls versus that in experimental cultures.
RESULTS
Description of the organism.
Strain 312 was identified as F. flavus (48), a tropical polypore that is believed to be closely related to the temperate polypore genus Irpex, which in turn is related to the genera Trametes, Ganoderma, and Fomes. It is found on dead and deciduous woods in terrestrial environments and on decaying lignocellulosic compounds in brackish areas. Strain 312 produced hyaline to off-white aerial mycelia and did not sporulate on plates of MEA or cornmeal agar media but produced fertile basidiomata on alphacel agar. On plates of Poly-R medium, strain 312 actively decolorized Poly-R in about 7 days. It grew in ME medium as well as in ME medium prepared with 50% artificial seawater and yielded 52 and 66 mg (dry wt) of mycelium per 10 ml of medium, respectively. The corresponding growth yields in LN medium and in LN medium with 50% artificial seawater were 23 and 44 mg, respectively, indicating the salt tolerance of this organism.
Mineralization of 14C-labeled synthetic lignin.
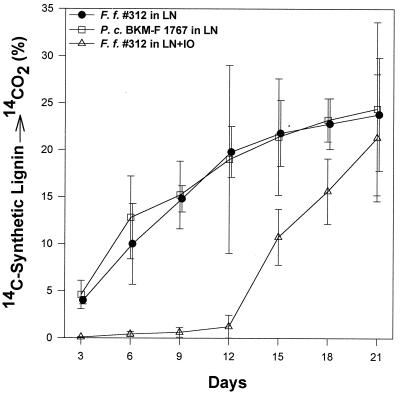
Strain 312 mineralized 23.8% of synthetic, side chain-labeled [14C]lignin after 24 days of incubation (Fig. 1). This was comparable to lignin mineralization (24%) by the widely studied white rot fungus P. chrysosporium which was used as a positive control. Lignin mineralization by strain 312 in the presence of 50% artificial seawater showed an initial lag period of about 12 days, but the final mineralization on day 24 was 22% (Fig. 1). These results indicate the ligninolytic ability of this organism under marine conditions.
FIG. 1.
Lignin degradation (14C-labeled synthetic lignin → 14CO2) by F. flavus (F. f.) 312 grown in LN medium (13) and in LN+IO medium. P. chrysosporium (P. c.), included as a positive control, was grown in LN medium. The cumulative percentage of 14CO2 released during incubation was plotted.
Production of LMEs.
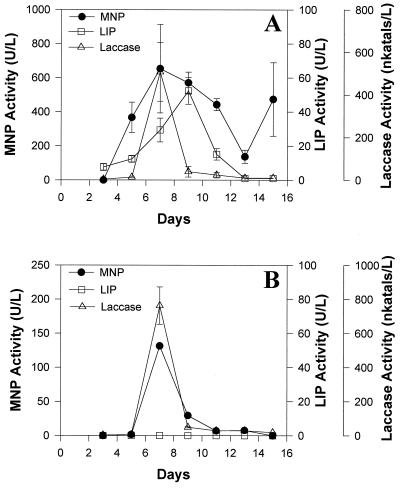
Strain 312 produced both extracellular MNP and laccase in LN medium in stationary as well as shaken cultures, and peak activities were seen around day 7 (Fig. 2). LIP activity, on the other hand, was seen only in stationary cultures and reached a peak around day 9. Growth in LN medium, measured as mycelial dry weight, usually reached a peak on day 5 (data not shown).
FIG. 2.
Production of MNP, LIP, and laccase by strain 312 in LN medium incubated under stationary (A) or shaken conditions (B).
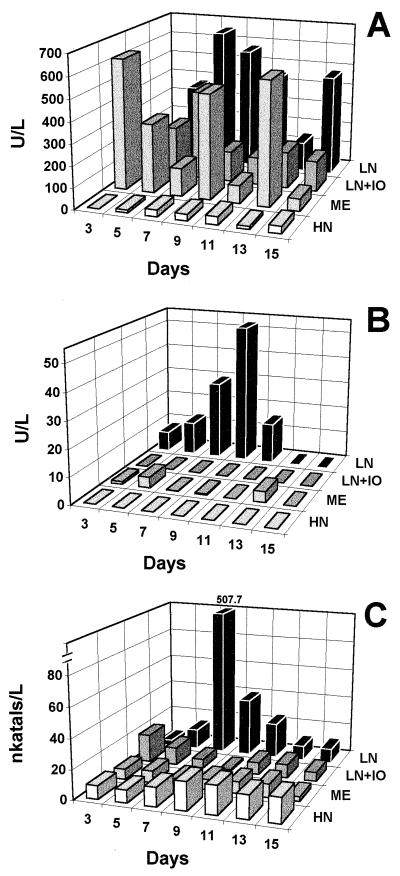
Comparison of enzyme production in LN, LN+IO, HN, and ME media revealed that MNP production was reduced by about 50% in LN medium prepared with artificial seawater. Maximal levels of MNP were produced in LN medium and generally peaked between days 7 and 9. MNP production in ME medium was comparable to that seen in LN medium but peaked on day 3, decreased through day 7, and consistently showed secondary peaks on days 9 and 13 (Fig. 3A). LIP production was rather minimal in all these media except in LN medium (Fig. 3B). Maximum laccase activity was seen in LN medium, but the activities in the other three media were substantially lower (Fig. 3C).
FIG. 3.
Production of MNP (A), LIP (B), and laccases (C) by strain 312 in different media. LN (2.4 mM N), HN (24 mM N), ME, and LN+IO media were used. The values shown are means of three replicates, and the standard deviation was less than 1%. In panel C, note the break in the y axis; the number shown above the tall bar is the actual laccase activity.
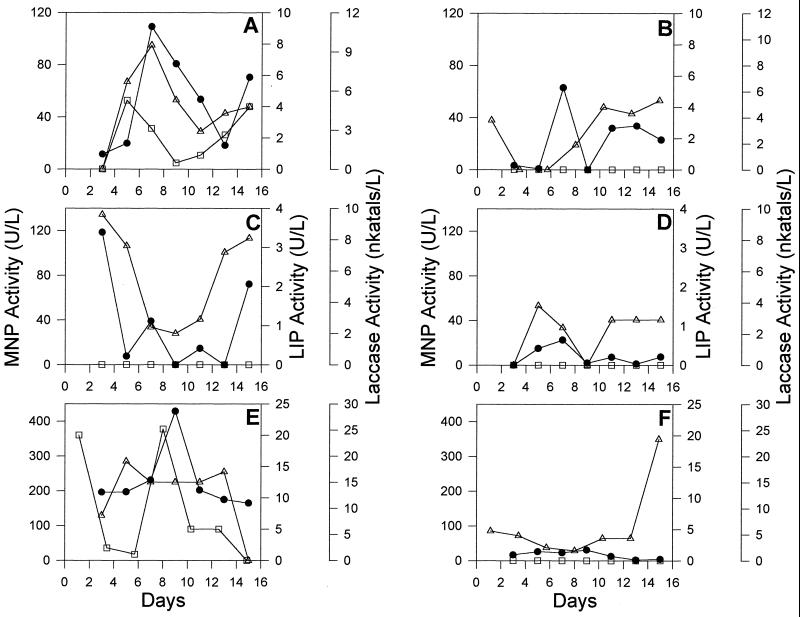
Wood and other lignocellulosic compounds are the natural substrates for ligninolytic fungi. Therefore, LME production was compared in cultures grown with pine, poplar, or sugarcane bagasse as the sole source of carbon, energy, and nitrogen. Our results show that sugarcane bagasse medium supported maximal levels of LMEs compared to the other two media (Fig. 4). While MNP and laccase production was seen in all three media, low levels of LIP production was seen in cultures grown with pine and sugarcane bagasse but not in cultures grown with poplar. Identical media prepared with 50% artificial seawater gave comparable growth (visual observation), but LME activities were substantially lower (Fig. 4).
FIG. 4.
Production of MNP, LIP, and laccase by strain 312 in cultures grown with pine (A and B), poplar (C and D), or sugarcane bagasse (E and F) in the presence (B, D, and F) or absence (A, C, and E) of 50% artificial seawater. Symbols: ●, MNP; □, LIP; ▵, laccase.
Isoenzymes and molecular masses of LMEs.
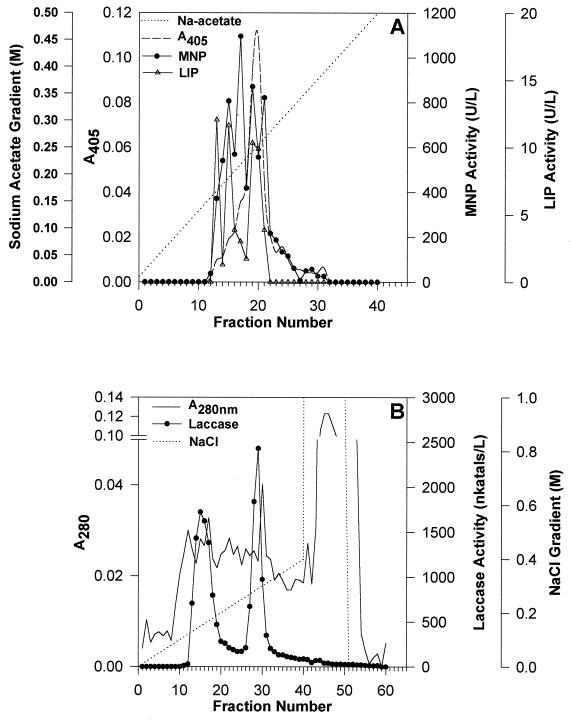
FPLC fractionation of the extracellular fluid on a Mono-Q column using a 10 mM to 1 M sodium acetate gradient followed by MNP and LIP assays for each fraction showed four protein peaks with MNP activity and three with LIP activity of which the third peak had a pronounced shoulder (Fig. 5A). FPLC fractionation of the same extracellular culture fluid on a Mono-Q column using a 0 to 1 M NaCl gradient, followed by activity staining for laccase, showed two major laccase peaks (Fig. 5B).
FIG. 5.
FPLC of the concentrated extracellular culture fluid of F. flavus 312. Extracellular culture fluid was collected from cultures of the organism grown in LN medium for 9 days and concentrated 50-fold as described in the text. (A) MNP and LIP profiles; (B) laccase profile. Gradients of sodium acetate (10 mM to 1 M at pH 6.0) and of sodium chloride (0 to 1 M in 20 mM Tris buffer at pH 8.0) were used.
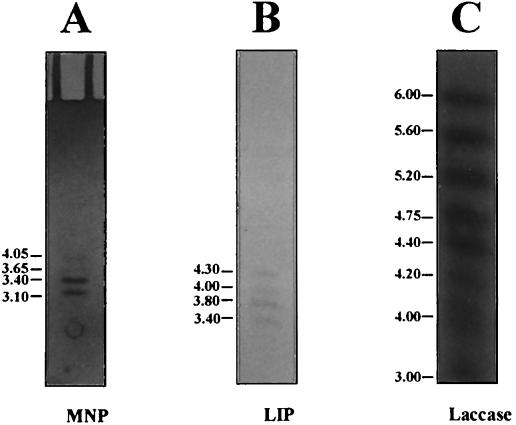
IEF of concentrated and dialyzed extracellular fluid of cultures grown in LN medium showed four isozymes of MNP with pIs of 4.0, 3.65, 3.4, and 3.1 (Fig. 6A) and four isozymes of LIP with pIs of 4.3, 4.0, 3.8, and 3.4 (Fig. 6B). Ten laccase isozymes were seen; one group of seven had pIs of 4.0, 4.2, 4.4, 4.75, 5.2, 5.6, and 6.0, and a second group containing at least three isozymes had pIs of <3.0 (Fig. 6C). Aliquots of the pooled FPLC peak fractions were also fractionated by IEF and stained for MNP, LIP, and laccase activities. The isozymes observed were similar to those seen with concentrated and dialyzed extracellular culture fluid (data not shown).
FIG. 6.
MNP, LIP, and laccase isozymes separated by IEF. The cultures of F. flavus were grown in LN medium for 9 days, and the extracellular fluid was used for IEF. The gels were stained with 3,3′-diaminobenzidine in the presence of MnSO4 and H2O2 for MNP (A) (2), o-dianisidine and H2O2 for LIP (B) (30), and ABTS without H2O2 for laccase (C) (34). The numbers on the left are the pI values.
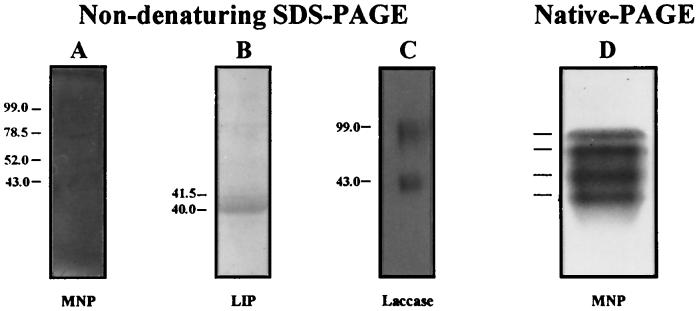
Nondenaturing SDS-PAGE of concentrated and dialyzed extracellular culture fluid containing LMEs, followed by activity staining, gave four MNP bands, two bands of LIP, and two broad bands of laccase. The molecular masses of the bands were as follows: 43, 52, 78.5, and 99 kDa for the four MNP bands (Fig. 7A), 40 and 41.5 kDa for the two LIP bands (Fig. 7B), and 43 and 99 kDa for the two laccase bands (Fig. 7C). The faint bands seen in the gel stained for LIP activity (Fig. 7B) were artifacts.
FIG. 7.
Nondenaturing SDS-PAGE of concentrated extracellular culture fluid (ECF) of F. flavus followed by activity staining. F. flavus was grown in LN medium for 9 days, and the ECF was concentrated 50-fold as described in the text. Activity staining for MNP (A), LIP (B), and laccase (C) was done as described in the legend for Fig. 6. Numbers on the left indicate molecular masses in kilodaltons. (D) Native PAGE of concentrated ECF stained for MNP activity with 3,3′-diaminobenzidine. The four lines to the left of the gel show the positions of MNP activity bands.
Decolorization of synthetic dyes.
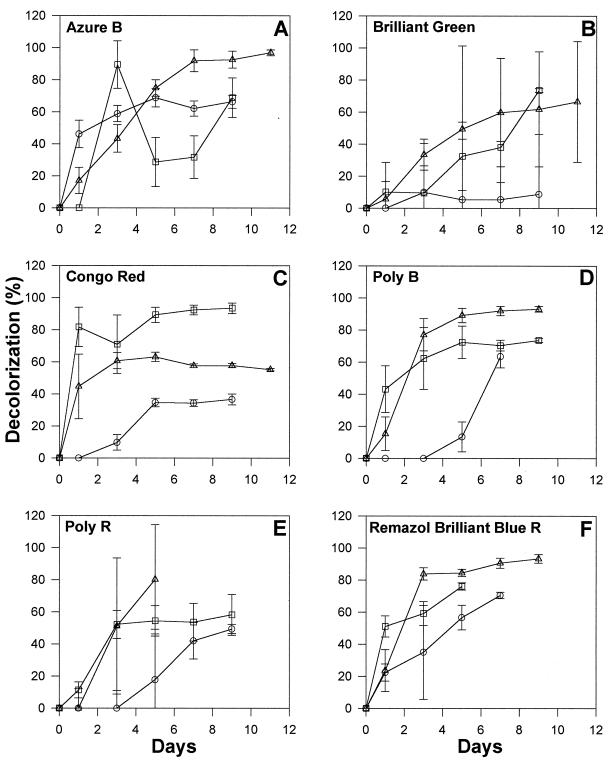
Ligninolytic white rot basidiomycetes have previously been shown to degrade a variety of xenobiotics and have been extensively investigated for bioremediation of soils and various industrial effluents containing dyes and other aromatic pollutants (44). In light of this, we tested strain 312 for its ability to degrade various synthetic dyes when grown in LN medium with and without 50% artificial seawater as well as in nutrient-rich media such as ME medium. Maximal decolorization of five of the six dyes was seen in LN+IO medium (Fig. 8). Efficient decolorization was also seen in LN medium, but decolorization tended to be much less efficient in ME medium. Changes in the visible spectra of the degraded dyes in experimental cultures versus heat-killed controls were positively correlated to decolorization and/or degradation in the case of Congo red, Poly-B, Poly-R, and Remazol brilliant blue R. The spectral patterns of the other two dyes were not affected (data not shown), but the visible spectrum of brilliant green changed in cultures grown in LN+IO medium. Decolorization was not due to pH changes, since the pH of the culture media remained essentially unchanged throughout the experiment.
FIG. 8.
Decolorization of selected synthetic dyes by strain 312 in LN, LN+IO, and ME media. Percent decolorization values are the net differences between experimental cultures and parallel heat-killed controls. Symbols: ○, ME; □, LN; ▵, LN+IO.
DISCUSSION
Sea grasses, besides mangrove plants, are the major contributors of lignocellulose in coastal marine ecosystems (16), and fungi are believed to be important in lignocellulose degradation in these ecosystems (27, 40, 41). Sutherland et al. (52) reported 4 to 5% mineralization of lignin in 30 days by several species of marine fungi. An ascomycete Phaeospheria spartinicola was also shown to mineralize 3.3% of the [14C]lignin-labeled lignocellulose and contribute 2.7% to 14C-labeled dissolved organic carbon (5). Qualitative screening of lignocellulose-degrading enzymes such as cellulase, xylanase, laccase, and tyrosinase in selected marine fungi was reported by Rohrmann and Molitoris (46). Raghukumar et al. (40) presented quantitative data on laccase, cellulase, and xylanase activities in several marine and facultative marine fungi isolated from 4mangrove and sea grass leaves and from sediments of the mangrove ecosystem. We report here the presence of the three major classes of LMEs (MNP, LIP, and laccase) and mineralization of 14C-labeled synthetic lignin by a marine isolate of F. flavus (strain 312). The fact that this fungus was isolated with LN+IO medium, showed growth and production of LMEs in media with and without 50% artificial seawater, and degraded 14C-labeled lignin in the presence of 50% artificial seawater (simulating marine conditions) suggest its importance as a degrader of lignocellulosic substrates under marine conditions.
The ability of F. flavus to mineralize 14C-labeled synthetic lignin to 14CO2 in LN medium was comparable to that of P. chrysosporium BKM-F 1767, an extensively studied terrestrial white rot basidiomycete, which has previously been shown to mineralize up to 33% of 14C-labeled synthetic lignin in 25 to 30 days (7, 11, 34). Other white rot fungi such as Phlebia brevispora, Dichomitus squalens, and Ceriporiopsis subvermispora have been shown to mineralize 24, 40, and 28% of synthetic lignin, respectively (34, 36, 47). Thus, the lignin-degrading ability of our marine isolate is comparable to that of selected widely studied terrestrial white rot fungi.
Relatively few of the known white rot basidiomycetes have been reported to produce all the three major classes of LMEs: MNPs, LIPs, and laccases (18). These basidiomycetes include well-known organisms such as Trametes versicolor (1), P. chrysosporium (51, 53), and Phlebia radiata (29). Our marine isolate, F. flavus 312, also belongs to this category of white rot fungi in that it produces all the three LMEs. However, the physiology of LME production by F. flavus differs in a number of respects from the other white rot fungi. For example, MNP production was completely suppressed in P. chrysosporium cultures under nutrient-rich conditions such as in ME medium (57), but strain 312 produced relatively high levels of MNP in this medium. Moreover, strain 312 produced MNP in HN medium (although at low levels), whereas MNP production by P. chrysosporium and several other white rot fungi is completely suppressed in this medium (21, 43). F. flavus is similar to T. versicolor, perhaps the second best-studied white rot fungus, in producing MNP, LIP, and laccase in LN medium with glucose, whereas P. chrysosporium and a few others produce only LIP and MNP; other white rot fungi produce some combinations of LIP, MNP, and laccase, but not all three (18). Our results further show that F. flavus, similar to a number of previously studied white rot fungi (54), requires Mn2+ in the growth medium for the production of MNP and increasing levels of Mn2+ in the medium are positively correlated with increasing levels of MNP production (data not shown). Roch et al. (45) previously showed that some strains of P. chrysosporium produce high levels of LIP but poor growth in glycerol medium. In a similar fashion, strain 312 showed poor growth but substantial production of MNP, LIP, and laccase in LN medium with glycerol as the carbon source either at pH 4.5 or 7.8.
Strain 312 produces minimal levels of laccase in HN and ME media as well as in wood-grown cultures but produces relatively high levels (ca. 650 to 700 nkat/liter) in LN medium with glucose or glycerol as the carbon source. In this respect, it is different from Phanerochaete flavido-alba which produced much higher levels of laccase in HN medium than in LN medium (35). The white rot fungus P. chrysosporium was shown to produce laccase in HN medium with cellulose as the carbon source but not with glucose (50), whereas Ganoderma lucidum (14) and the soil basidiomycete Irpex lacteus (3) produce high levels of laccase in HN medium with glucose.
F. flavus 312 grew in media with pine, poplar, or sugarcane bagasse as the sole source of energy, carbon, and nitrogen. LME production was, however, variable in these media. Sugarcane bagasse supported maximal levels of LME production compared to the other two media. It is of interest that MNP, which has been shown to be important in the delignification of kraft pulp (32) and in the decolorization of kraft bleach plant effluents (25), was produced at high levels in cultures of strain 312 grown with sugarcane bagasse, which is used for making paper pulp in a number of developing countries. These results further suggest that sugarcane bagasse can be used for bulk production of MNP by F. flavus (strain 312) for possible applications in pulp and paper industry. MNP production in cultures of strain 312 grown with lignocellulosic substrates is consistent with prior reports on MNP production by P. chrysosporium (51), G. lucidum (14), T. versicolor (6), and other organisms. Moreover, Archibald et al. (2) demonstrated MNP production in aspen and spruce wood pulp by T. versicolor and indicated the importance of this enzyme in delignification of kraft pulp. Low but negligible levels of LIP were produced by strain 312 cultures grown with sugarcane bagasse or pine, whereas previous studies showed high levels of LIP production in wood-grown cultures of P. chrysosporium (51) and T. versicolor (6). G. lucidum on the other hand, did not produce LIP in cultures grown with poplar, pine, or spruce (14). F. flavus differs from G. lucidum in that the former produced low levels of laccase in the wood media tested, whereas high titers of laccase are produced by the latter in pine- and poplar-grown cultures (14).
Most of the wood-rot fungi studied to date have been reported to produce multiple molecular forms of MNPs and LIPs, and the number and types of isoforms produced were shown to vary based on the culture conditions employed (8, 43). For example, P. chrysosporium, the more extensively studied organism in this respect, has been shown to produce seven readily identifiable isoforms of LIP and four isoforms of MNP in LN medium (13, 53). Consistent with this, our current results showed that F. flavus cultures grown in LN medium produce four isoforms of MNP and two isoforms of LIP as evidenced by SDS-PAGE, native PAGE, and FPLC. However, IEF revealed four isoforms each of MNP and LIP. The laccase of strain 312 was resolved into 2 isoforms by FPLC and SDS-PAGE, whereas IEF revealed about 10 isozymes. In comparison, the white rot fungus G. lucidum (14) produces 5 to 7 laccase isoforms, and the soil basidiomycete I. lacteus (3) produces 10 to 14 laccase isoforms in HN medium. It is not clearly known why these fungi produce such a broad array of closely related isozymes and whether each isoform has a specific role in lignin degradation; however, it has been suggested that these isozymes contribute to the versatility of the lignin-degrading fungi in degrading a wide variety of lignocellulose substrates and xenobiotics (6, 13, 21).
It has been widely reported that lignin-degrading enzymes of white rot fungi are relatively nonspecific and are capable of oxidizing a variety of aromatic and halo-aromatic environmental pollutants such as polyaromatic hydrocarbons, azo dyes, heterocyclic and polymeric dyes, polychlorinated biphenyls, chlorolignols, and other xenobiotics (reviewed in reference 44). Various heterocyclic and azo dyes, widely used by the textile and dyestuff industry are resistant to conventional wastewater treatments and thus persist in the environment (19, 22, 31). Such pollutants are of concern both in freshwater and marine ecosystems. F. flavus, strain 312, showed efficient degradation of the dyes Poly-B, Poly-R, Congo red, Remazol brilliant blue R, and azure B, but decolorization of brilliant green was relatively less efficient. Better degradation of all the dyes was seen in LN+IO medium, suggesting the potential of this marine isolate of F. flavus for bioremediation of aromatic pollutants under marine conditions.
ACKNOWLEDGMENT
This research was supported in part by grant DE-FG02-85ER 13369 from the U.S. Department of Energy.
Footnotes
NIO contribution number 2632.
REFERENCES
- 1.Archibald F, Paice M G, Jurasek L. Decolorization of kraft bleachery effluent chromophores by Coriolus (Trametes) versicolor. Enzyme Microb Technol. 1990;12:846–853. [Google Scholar]
- 2.Archibald F S, Bourbonnais R, Jurasek L, Paice M G, Reid I D. Kraft pulp bleaching and delignification by Trametes versicolor. J Biotechnol. 1997;53:215–236. [Google Scholar]
- 3.Balajee S, D’Souza T M, Thorn R G, Reddy C A. Program and Abstracts of the 8th International Symposium on Microbial Ecology. 1998. Lignin-modifying enzymes of a soil basidiomycete Irpex lacteus; p. 299. [Google Scholar]
- 4.Benner R, Newell S Y, Maccubbin A E, Hodson R E. Relative contributions of bacteria and fungi to rates of degradation of lignocellulosic detritus in salt-marsh sediments. Appl Environ Microbiol. 1984;48:36–40. doi: 10.1128/aem.48.1.36-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergbauer M, Newell S Y. Contribution of lignocellulose degradation and DOC formation from a salt marsh macrophyte by the ascomycete Phaeosphaeria spartinicola. FEMS Microbiol Ecol. 1992;86:341–348. [Google Scholar]
- 6.Black A K. Structure and molecular regulation of the lignin peroxidase genes from the white-rot fungus Trametes versicolor. Ph.D. dissertation. East Lansing: Michigan State University; 1993. [Google Scholar]
- 7.Boominathan K, Dass S B, Randall T A, Reddy C A. Nitrogen-deregulated mutants of Phanerochaete chrysosporium, a lignin-degrading basidiomycete. Arch Microbiol. 1990;153:521–527. doi: 10.1007/BF00245259. [DOI] [PubMed] [Google Scholar]
- 8.Boominathan K, Reddy C A. Fungal degradation of lignin: biotechnological applications. In: Arora D K, Elander R P, Mukerji K G, editors. Handbook of applied mycology. 4. Fungal biotechnology. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 763–822. [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Buswell J A, Odier E. Lignin biodegradation. Crit Rev Biotechnol. 1987;6:1–60. [Google Scholar]
- 11.Chau M G S, Choi S, Kirk T K. Mycelium binding and depolymerization of synthetic 14C-labeled lignin during decomposition by Phanerochaete chrysosporium. Holzforschung. 1983;37:55–61. [Google Scholar]
- 12.Cundell A M, Brown M S, Stanford R, Mitchell R. Microbial degradation of Rhizophora mangle leaves immersed in the sea. Estuar Coast Mar Sci. 1979;9:281–286. [Google Scholar]
- 13.Dass S B, Reddy C A. Characterization of extracellular peroxidases produced by acetate-buffered cultures of Phanerochaete chrysosporium. FEMS Microbiol Lett. 1990;69:221–224. doi: 10.1111/j.1574-6968.1990.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza T M, Merritt C S, Reddy C A. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Lignin-modifying enzymes from the white-rot basidiomycete Ganoderma lucidum, abstr. Q-267; p. 432. [Google Scholar]
- 15.Fell J W, Newell S Y. Role of fungi in carbon flow and nitrogen immobilisation in coastal marine plant litter systems. In: Wicklow D T, Carroll G C, editors. The fungal community. New York, N.Y: Marcel Dekker Inc.; 1981. pp. 665–678. [Google Scholar]
- 16.Godshalk G L, Wetzel R G. Decomposition of aquatic angiosperms. II. Particulate components. Aquat Bot. 1978;5:301–327. [Google Scholar]
- 17.Gold M H, Glenn J K, Alic M. Use of polymeric dyes in lignin biodegradation assays. Methods Enzymol. 1988;161:74–78. [Google Scholar]
- 18.Hatakka A. Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev. 1994;13:125–135. [Google Scholar]
- 19.Heinfling A, Bergbauer M, Szewzyk U. Biodegradation of azo and phthalocyanine dyes by Trametes versicolor and Bjerkandera adusta. Appl Microbiol Biotechnol. 1997;48:261–266. [Google Scholar]
- 20.Kelley R L, Reddy C A. Identification of glucose oxidase activity as the primary source of hydrogen peroxide production in ligninolytic cultures of Phanerochaete chrysosporium. Arch Microbiol. 1986;144:248–253. [Google Scholar]
- 21.Kirk T K, Farrell R L. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 22.Knapp J S, Newby P S, Reece L P. Decolorization of dyes by wood-rotting basidiomycete fungi. Enzyme Microb Technol. 1995;17:664–668. [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.McLachlan J. Growth media-marine. In: Stein J R, editor. Handbook of phycological methods. Cambridge, United Kingdom: Cambridge University Press; 1972. pp. 25–52. [Google Scholar]
- 25.Michel F C, Jr, Dass S B, Grulke E A, Reddy C A. Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of kraft bleach plant effluent. Appl Environ Microbiol. 1991;57:2368–2375. doi: 10.1128/aem.57.8.2368-2375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell S Y. Decomposition of shoots of a salt-marsh grass. Methodology and dynamics of microbial assemblages. Adv Microb Ecol. 1993;13:301–326. [Google Scholar]
- 27.Newell S Y. Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. J Exp Mar Biol Ecol. 1996;200:187–206. [Google Scholar]
- 28.Niku-Paavola M-L, Karhunen E, Salola P, Raunio V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem J. 1988;254:877–884. doi: 10.1042/bj2540877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niku-Paavola M-L, Karhunen E, Kantelinen A, Viikari L, Lundell T, Hatakka A. The effect of culture conditions on the production of lignin modifying enzymes by the white-rot fungus Phlebia radiata. J Biotechnol. 1990;13:211–221. [Google Scholar]
- 30.Odum W E, Kirk P W, Zieman J C. Non-protein nitrogen compounds associated with particles of vascular plant detritus. Oikos. 1979;32:363–367. [Google Scholar]
- 31.Ollikka P, Alhonmaki K, Leppanen V M, Glumoff T, Raijola T, Souminen I. Decolorization of azo, triphenyl methane, heterocyclic and polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Appl Environ Microbiol. 1993;59:4010–4016. doi: 10.1128/aem.59.12.4010-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paice M G, Reid I D, Bourbonnais R, Archibald F S, Jurasek L. Manganese peroxidase, produced by Trametes versicolor during pulp bleaching, demethylates and delignifies kraft pulp. Appl Environ Microbiol. 1993;59:260–265. doi: 10.1128/aem.59.1.260-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paszczynski A, Crawford R L, Huynh V-B. Manganese peroxidase of Phanerochaete chrysosporium: purification. Methods Enzymol. 1988;161:264–270. [Google Scholar]
- 34.Perez J, Jeffries T W. Mineralization of 14C-ring-labeled synthetic lignin correlates with the production of lignin peroxidase, not of manganese peroxidase or laccase. Appl Environ Microbiol. 1990;56:1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez J, Martinez J, de la Rubia T. Purification and partial characterization of a laccase from the white-rot fungus Phanerochaete flavido-alba. Appl Environ Microbiol. 1996;62:4263–4267. doi: 10.1128/aem.62.11.4263-4267.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perie F H, Gold M H. Manganese regulation of manganese-peroxidase expression and lignin degradation by the white-rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991;57:2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pointing S B, Vrijmoed L L P, Jones E B G. A qualitative assessment of lignocellulose degrading activity in marine fungi. Bot Mar. 1998;41:293–298. [Google Scholar]
- 38.Pomeroy L R. Detritus and its role as a food source. In: Barnes R S K, Mann K H, editors. Fundamentals of aquatic ecosystems. Oxford, United Kingdom: Blackwell Scientific Publications; 1980. pp. 84–102. [Google Scholar]
- 39.Raghukumar C, Chandramohan D, Michel F C, Jr, Reddy C A. Degradation of lignin and decolorization of paper mill bleach plant effluent (BPE) by marine fungi. Biotechnol Lett. 1996;18:105–108. [Google Scholar]
- 40.Raghukumar C, Raghukumar S, Chinnaraj A, Chandramohan D, D’Souza T M, Reddy C A. Laccase and other lignocellulose modifying enzymes of marine fungi isolated from the coast of India. Bot Mar. 1994;37:515–523. [Google Scholar]
- 41.Raghukumar S, Sharma S, Raghukumar C, Sathe-Pathak V, Chandramohan D. Thraustochytrid and fungal component of marine detritus. IV. Laboratory studies on decomposition of leaves of the mangrove Rhizophora apiculata Blume. J Exp Mar Biol Ecol. 1994;183:113–131. [Google Scholar]
- 42.Raghukumar S, Sathe-Pathak V, Sharma S, Raghukumar C. Thraustochytrid and fungal component of marine detritus. III. Field studies on decomposition of leaves of the mangrove Rhizophora apiculata. Aquat Microb Ecol. 1995;9:117–125. [Google Scholar]
- 43.Reddy C A. An overview of the recent advances on the physiology and molecular biology of lignin peroxidases of Phanerochaete chrysosporium. J Biotechnol. 1993;30:91–107. doi: 10.1016/0168-1656(93)90030-q. [DOI] [PubMed] [Google Scholar]
- 44.Reddy C A. The potential for white-rot fungi in the treatment of pollutants. Curr Opin Biotechnol. 1995;6:320–328. [Google Scholar]
- 45.Roch P, Buswell J A, Cain R B, Odier E. Lignin peroxidase production by strains of Phanerochaete chrysosporium grown on glycerol. Appl Microbiol Biotechnol. 1989;31:587–591. [Google Scholar]
- 46.Rohrmann S, Molitoris P. Screening of wood-degrading enzymes in marine fungi. Can J Bot. 1992;70:2116–2123. [Google Scholar]
- 47.Ruttiman C, Schwember E, Salas L, Cullen D, Vicuna R. Ligninolytic enzymes of the white-rot basidiomycetes Phlebia brevispora and Ceriporiopsis subvermispora. Biotechnol Appl Biochem. 1992;16:64–75. [Google Scholar]
- 48.Ryvarden L, Johansen I. A preliminary polypore flora of East Africa. Oslo, Norway: Fungiflora; 1980. [Google Scholar]
- 49.Sathe V, Raghukumar S. Fungi and their biomass in detritus of the seagrass Thalassia hemprichii (Ehrenberg) Ascherson. Bot Mar. 1991;34:271–277. [Google Scholar]
- 50.Srinivasan C, D’Souza T M, Boominathan K, Reddy C A. Demonstration of laccase in the white-rot basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1995;61:4274–4277. doi: 10.1128/aem.61.12.4274-4277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan, C., K. Boominathan, and C. A. Reddy. Unpublished data.
- 52.Sutherland J B, Crawford D L, Speedie M K. Decomposition of 14C-labeled maple and spruce lignin by marine fungi. Mycologia. 1982;74:511–513. [Google Scholar]
- 53.Tien M, Kirk T K. Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 1988;161:238–249. [Google Scholar]
- 54.Van der Woude M W, Boominathan K, Reddy C A. Nitrogen regulation of lignin peroxidase and manganese-dependent peroxidase production is independent of carbon and manganese regulation in Phanerochaete chrysosporium. Arch Microbiol. 1993;160:1–4. doi: 10.1007/BF00258138. [DOI] [PubMed] [Google Scholar]
- 55.Vares T, Lundell T K, Hatakka A I. Novel heme-containing enzyme possibly involved in lignin degradation by the white-rot fungus Junghuhnia separabilima. FEMS Microbiol Lett. 1992;99:53–58. [Google Scholar]
- 56.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 57.Yadav J, Reddy C A. Mineralization of 2,4-dichlorophenoxyacetic acid (2,4-D) and mixtures of 2,4-D and 2,4,5-trichlorophenoxyacetic acid by Phanerochaete chrysosporium. Appl Environ Microbiol. 1993;59:2904–2908. doi: 10.1128/aem.59.9.2904-2908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]