Abstract
Background:
Autologous vessels graft (Inner diameter < 6 mm) harvesting always challenged during bypass grafting surgery and its complication shows poor outcome. Tissue engineered vascular graft allow to generate biological graft without any immunogenic complication. The approach presented in this study is to induce graft remodeling through heparin coating in luminal surface of small diameter (Inner diameter < 1 mm) decellularized arterial graft.
Methods:
Decellularization of graft was done using SDS, combination of 0.5% sodium dodecyl sulfate and 0.5% sodium deoxycholate and only sodium deoxycholate. Decellularization was confirmed on basis of histology, and DAPI. Characterization of extracellular matrix was analyzed using histology and scanning electron microscopy. Surface modification of decellularized vascular graft was done with heparin coating. Heparin immobilization was evaluated by toluidine blue stain. Heparin-coated graft was transplanted end to end anastomosis in femoral artery in rat.
Results:
Combination of 0.5% sodium dodecyl sulfate and 0.5% Sodium deoxycholate showed complete removal of xenogeneic cells. The heparin coating on luminal surface showed anti-thrombogenicity and endothelialization. Mechanical testing revealed no significant differences in strain characteristics and modulus between native tissues, decellularized scaffolds and transplanted scaffold. Collectively, this study proposed a heparin-immobilized ECM coating to surface modification offering functionalize biomaterials for developing small-diameter vascular grafts.
Conclusion:
We conclude that xenogeneic decellularized arterial scaffold with heparin surface modification can be fabricated and successfully transplanted small diameter (inner diameter < 1 mm) decellularized arterial graft.
Keywords: Xenogeneic, Vascular graft, Decellularization, Small diameter vessels, Heparin surface modification
Introduction
Every year a large number of cardiac surgeries are performed [1] among them coronary artery bypass graft surgeries are more needed to save life affected by coronary artery disease [2]. Patients at high risk who have coronary artery disease (CAD) with peripheral artery disease (PAD) of successive cardiovascular events and mortality [3]. In developed countries mortality rate caused by CAD have declined [4] but it is a major cause of death in developing countries [5]. PAD is also progressive disorder causing stenosis or occlusion in upper and lower extremities and it is estimated that more than 200 million people have been affected by PAD worldwide [6]. PAD increases risk for cardiovascular morbidity and mortality and is associated with reduced functional capacity [7, 8]. Atherosclerosis formation is a highly predominant and underlying pathophysiological cause for coronary artery disease (CAD), carotid artery disease, cerebrovascular (CVD) disease, peripheral artery disease (PAD) [9, 10]. Atherosclerosis causes ischemic risk, reducing it is the main goal of all therapies focused on patients with acute coronary syndrome and peripheral vascular ischemic disease. Although the incidence of CAD and PAD continues to upsurge, the existing options for therapy are quite limited. In recent times, the improvements in tissue engineering and regenerative medicine provide us with unique confidence aimed at treating CAD and PAD [11–14]. In coronary artery bypass surgery (CABG) autologous grafts e.g. internal thoracic artery, radial artery, saphenous vein is used with high patency rate [15]. Thrombus formation, intimal hyperplasia and atherosclerosis are the failure reasons of autologous and allogeneic grafts [16–18]. Synthetic vascular grafts have been used successfully in large arteries (> 6 mm internal diameter) replacement treatment but it has been shown low patency rate in replacing the smaller diameter (< 6 mm internal diameter) vessels [19]. Several synthetic vascular grafts such as polyurethane (PU) [20], expanded poly (tetrafluoroethylene) (ePTFE) [21], poly(ethylene terephthalate) (PET) [22], and polycaprolactone (PCL) [23] have low patency rate when used as small diameter (< 6 mm) bypass grafts [24]. Therefore, innovative sources of blood vessels are vastly necessitated for small diameter vascular grafts surgeries in patients short of accessible grafts.
Decellularized small diameter vascular grafts generated from xenogeneic origin will provide a better answer to the dearth of commercially available vascular grafts. These decellularized vascular scaffolds can be used as grafts for vascular repair directly or patients’ specific cells will be seeded on vascular scaffolds and then implants for vascular repair in patients. Bai et al. showed that a decellularized carotid artery has 100% patency rate with reendothelialization after arteriovenous graft transplant in rat model [25]. Thrombus formation is the main cause of small diameter vascular grafts failure. Zhou et al. found that the decellularized vascular scaffold after heparin coating possessed excellent antithrombogenicity [26]. Another study by Bai et al. showed that after coating of heparin on the luminal side of human Saphenous Vein patches decrease neointimal thickness used for venoplasty and arterioplasty in rat model [27]. Decellularized biomaterials showed good biochemical activity and biological compatibility. During the process of decellularization and handling of natural materials the normal 3D structure is changed, or some biological components are lost [28]. Surface modification of decellularized scaffold materials play an important role in improving its biocompatibility and functionality [29]. Several researchers proposed that surface modification of natural or synthetic materials for vascular replacements enhance the endothelialization of small diameter vascular grafts [29–31].
Our aim is to present work which focuses on the surface modification with heparin immobilization of a decellularization small diameter artery for reendothelialization.
Materials and methods
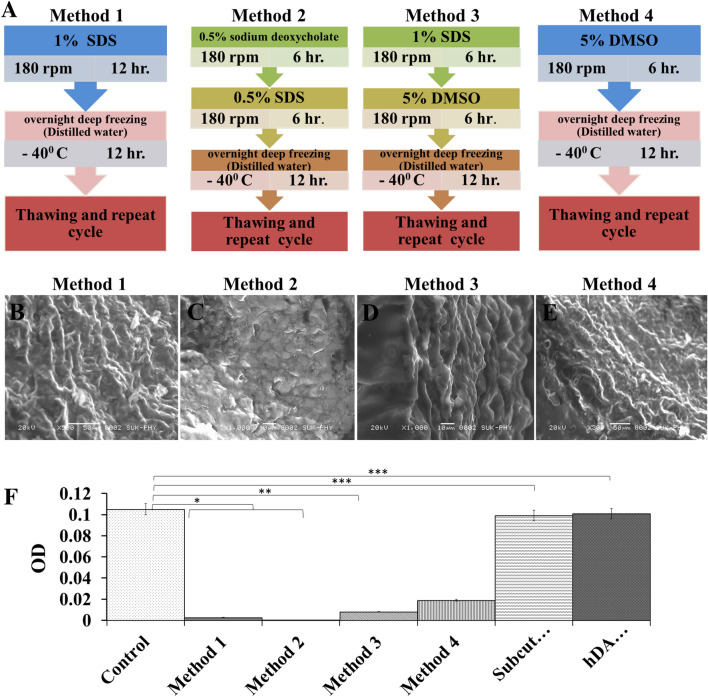
Tissue Harvesting: 10–12 blood vessels (Arteries) of goat where collected from slaughterhouse and carried in antibiotic, antimycotic (penicillin, streptomycin, and amphotericin B) solution 1 × liquid (Himedia Laboratories Pvt. Ltd. Mumbai, India) solution in lab. Each segment was rinsed three times with Dulbecco’s phosphate buffered saline (PBS) containing penicillin, streptomycin, and amphotericin B and stored in the freezer at 20 °C for further use. Four methods were followed to achieve decellularization as follows: Decellularization: Vessels were decellularized using in-house developed protocols. Briefly, the treatment consisted around 4 to 5 cycles of four methods as follows: The whole process was done under agitation on shaker (Fig. 1A).
Fig. 1.
A Four different methods used with different chemicals concentration for decellularization of native goat artery. B–E FE-SEM images of decellularized artery (DA) scaffolds obtained after completion of four different decellularization processes. F DNA content in control (Native artery), different scaffolds obtained by different decellularization methods, subcutaneously transplanted DA for 14 days and after transplantation hDA studies. Methods 2 showed complete removal of DNA from scaffold. In vivo experiment showed equal amount of cellular DNA in DA and hDA grafts after transplantation compared to native vessel DNA
Decellularization process
Method 1: This method was performed using SDS (sodium dodecly sulphate, Loba chemicals, Mumbai, India). Sample was treated with 1% SDS for 12 h on shaker (REMI RX-12R-DX) at 180 rpm. After 12 h the sample was transferred into D/W and stored in a deep freezer overnight. It was thawed the next morning at room temperature and the cycle was repeated again. Such many cycles were repeated to achieve complete decellularization of the sample.
Method 2: This method was performed using sodium deoxycholate (SDC)(Loba chemicals) and SDS (sodium dodecly sulphate, Loba chemicals). Sample was treated with 0.5% sodium deoxycholate for 6 h followed by 0.5% SDS for 6 h on shaker (REMI RX-12R-DX) at 180 rpm. After 12 h the sample was transferred into D/W and stored in a deep freezer overnight. It was thawed the next morning at room temperature and the cycle was repeated again. Such many cycles were repeated to achieve complete decellularization of the sample.
Method 3: This method was performed using DMSO (Molychem) and SDS (sodium dodecly sulphate, Loba chemicals). Sample was treated with 1% DMSO for 6 h followed by 1% SDS for 6 h on shaker (REMI RX-12R-DX) at 180 rpm. After 12 h the sample was transferred into D/W and stored in a deep freezer overnight. It was thawed the next morning at room temperature and the cycle was repeated again. Such many cycles were repeated to achieve complete decellularization of the sample.
Method 4: This method was performed using DMSO. Sample was treated with 2% DMS0 by keeping it overnight at -40 °c for 12 h. It was thawed the next morning at room temperature followed by 6 h wash in D/W on shaker (REMI RX-12R-DX) at 180 rpm. Such many cycles were repeated to achieve complete decellularization of the sample.
Decellularized samples from all 4 methods were washed with distilled water five times for 4 h each and then incubated with PBS for 48 h for further use.
Histological characterizations
At retrieval and after decellularization specimens of the vessels were isolated and processed in paraformaldehyde 4% in PBS, dehydrated through alcohol grades, enlightened in xylene, embedded in paraffin wax, and 4–5 μm slices were taken on the glass slides using a microtome (YORCO sales Pvt. Ltd, India). Sections were stained for nuclear organization and cytoplasm using HE technique according to the standard procedure [32]. Extracellular matrix was characterized for presence of collagen fibers by massion’s trichrome stain [33]. The sections were treated with a 0.1% alcian blue acetic acid solution of pH 2.5 for 20 min, rinsed in water, dehydrated with ethanol and then cleared and mounted [34]. Analysis of glycosaminoglycans (GAG) was determined using AB pH 2.5 (Sigma, A5268) stained. Images were taken in brightfield at, 20 × using a Nikon Ts Eclipse 108 microscope fitted with a nikon camera.
6-Diamidino-2-phenylindole (DAPI) staining
DAPI is a nuclear stain used to counterstain slides at every cycle and to confirm cell removal from scaffolds by decellularization. After serial grading of xylene and alcohol sections were treated with DAPI (Invitrogen, CA, USA) for 1 min and mounted with fluorescent medium (Dako, Denmark). Stained sections were examined under fluorescent microscopy (Nikon Ti-S, Eclips, Japan).
DNA quantification
At native condition, in decellularization by all four methods, in subcutaneous implant scaffold and in transplanted graft the total amount of DNA was quantified using UV Spectrophotometer at 260 nm. All Samples were collected at concentration of 1 mg/ml and freeze at − 20 °C. After 15 min all samples were crushed with mortar pastel and D/W was added to it. This DNA solution was poured in 2 ml microcentrifuge tubes which were used to obtain DNA content. From the above prepared DNA solution 40 µl of solution was taken using a Hamilton syringe in 4 ml cuvette to which 3.96 ml of D/W was added. At most care was taken to ensure that solution is air bubble free. Solution was left to stand for 10 min to ensure the complete diffusion of DNA throughout the solution. This represents a 1:100 dilution of the standards and DNA samples. The spectrophotometer was set at 260 nm and readings were noted down [35].
Scanning electron microscopy (SEM) of scaffolds
Dehydration of tissue samples was done by keeping small sections of tissue overnight in an oven at 37 °C. A Scanning Electron Microscope (SEM) was done at the Department of Physics, Shivaji University, Kolhapur, MS, India. Since resolution was required at the high magnifications, the specimen sputtered with gold to improve image quality. SEM analysis was done using JEOL JSM-6360 Scanning Electron Microscope model. Several sets of images were taken of each sample at the scales of 10, 20, 50, 100 and 200 µm. Image-J open-source imaging software was used to analyze the images taken with the SEM.
Mechanical properties
Native and decellularized vessels were cut into cylindrical-shaped specimens of 3 cm length for mechanical analysis. Native tissue samples were tested within 4 h of cold ischemia after retrieval. Mechanical testing was performed according to a previously developed protocol by the Civil engineering department of KIT engineering college, Kolhapur. Ultimate Stress and elasticity were calculated using Tensometer and burst pressure was calculated using concrete permeability machine.
Suture retention strength
Suture retention strength test was performed by using methods similar to Schaner et al. [36]. Six samples from three groups, control (native artery) and decellularized scaffold obtained by decellularization protocol 2 (DA = decellularized artery graft) (Method 2) and heparin-treated scaffold (hDA = heparin-coated decellularized Artery graft) were used for this test. Prolene sutures (8–0) were threaded through one side of the vessel in four quadrants 1 mm from the vessel end and other end was clamped in the stage clamp of tensometer (Instron 5565) from the force transducer at a constant displacement rate of 0.1 mm/s. Displacement was continued until the suture pulled out from the vessel edge. The maximum force was recorded on the suture as the suture retention strength (MPa).
Loading of heparin on decellularized vascular scaffold
Before implantation, decellularized vascular scaffolds were treated for heparin immobilization. Heparin loading in a decellularized vascular scaffold was done by a similar method used by Kong et al. [37]. Decellularized vascular scaffold (DA) (Method 2) incubate at 37 °C for 4 h. in 50 mL MES buffer (0.05 mol/L, pH 5.60) containing 100 mg EDC (1–(3-Dimethylaminopropyl)-3-ethylcarbodiimide) (Sigma), 100 mg heparin (Sigma) and 60 mg NHS (N-hydroxysuccinimide) (Sigma). After rinsing in disodium hydrogen phosphate solution (0.1 mol/L) for 2 h, sodium chloride solution (4 mol/L) for 24 h 3 4 times, and 24 h in distilled water for 3 3 times, the heparinized vascular scaffold was obtained. All heparinized grafts were sterilized by ethylene oxide sterilization atmosphere (concentration of 321 g/m3) (RUGIKON Semi-Automatic KANC-125, Mumbai, India) [38]. Sterile grafts were stored in 0.9% NaCl + 1% penicillin/streptomycin at 4 °C for future use. Toluidine blue (0.05 mg/mL) was used for heparin content staining on heparin treated graft slides.
Anticoagulant assay
The anticoagulant activity before and after heparin-coated (DA and hDA) decellularized vascular scaffolds were determine by thrombin time (TT), prothrombin time (PTT), activated partial thromboplastin time (APTT) assay [39]. Healthy human blood and an automated machine were used for this test.
Hemolysis assay
The hemolysis assay was performed to study the hemolytic activity of hDA with human erythrocytes. 10 ml of blood was taken from healthy human volunteer. Blood sample was added in a centrifuge tube containing ethylenediaminetetraacetic acid (EDTA) and centrifuged at 3000 rpm for 15 min. RBC pellet were isolated and washed with phosphate-buffered saline (PBS). 200 μl of Prediluted RBC with PBS was added with hDA. 200 μl of diluted fresh whole blood incubated with 10 ml of distilled water (D/W) and 10 ml of 0.9% NaCl (Normal Saline) aqueous solution were used positive and as negative controls respectively. All the samples were incubated at 37 °C for 2 h, followed by centrifugation at 3000 rpm for 15 min and the supernatant was used for spectroscopic analysis at 540 nm. The percentage of hemolysis was calculated from the formula: OD540 (sample) − OD540 (0% lysis)/OD540 (100% lysis) − OD540 (0% lysis) × 100%.
Platelet adhesion test
Risk of thrombosis on modified surface with heparin coating was evaluated by a platelet adhesion assay. The DA (decellularized artery) and hDA samples were used in the platelet.
adhesion test. 10 ml human whole blood was collected by venous puncture from healthy donor with informed consent. For obtaining platelet-rich plasma (PRP), blood was added into the centrifuge tube and centrifuged at 2000 rpm for 10 min. Before performing platelet adhesion test, the samples were washed with PBS. Samples were added into the 24-well plate then 200 μL of PRP were added to each sample and incubated at 37 °C for 2 h. After incubation, the samples were rinsed in PBS three times and fixed with 2.5 wt% glutaraldehyde at 4 °C overnight. For SEM analysis samples were dehydrated with different concentrations of alcohol for 15 min each and sufficiently dried in a freeze dryer.
Biocompatibility study of Decellularized goat artery (DA and hDA) scaffold in vivo
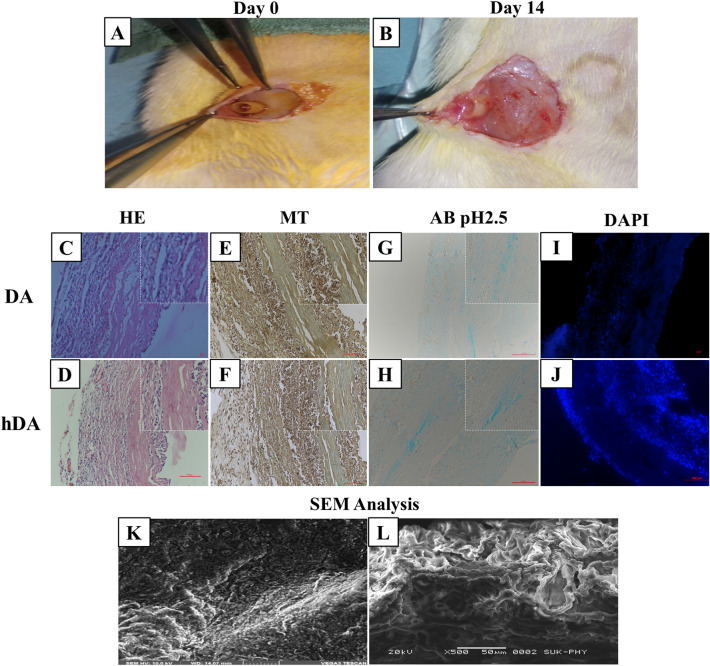
In vivo experiments were performed according to guidelines of the Institutional Ethical Committee (IAEC) (Approval No: Item No.04 - Sub-item 7), D Y Patil Education Society, (Deemed University), Kolhapur, India. Wistar rats obtained from Central Animal House, D Y Patil Education Society, Kolhapur, India. The animals were acclimated for a week before the surgical procedure. The animals were divided into two groups, DA and hDA. Rat (male/female) weighing 200–250 gm were anesthetized by injection Inj. Thiosol (Thiopentone injection, NEON 173,251 Neon Laboratories Ltd, Mumbai, India), intraperitoneal route (45 mg/kg/body wt.) [40]. DA and hDA scaffolds (n = 3) implanted subcutaneously (Fig. 2A). An incision was closed with 3–0 prolene surgical sutures. After 14 days (Fig. 2B), implanted scaffolds were excised and fixed in 10% formalin buffer for further histological analysis.
Fig. 2.
In vivo biocompatibility test DA and hDA grafts. A Subcutaneously implant of graft, B grafts after 14 days of subcutaneously implants. Histological studies and SEM analysis of subcutaneous transplanted DA and hDA after 14 days. C, D HE images of DA and had. E, F MT images. G, H AB images. I, J DAPI staining images and K, L SEM images of DA and hDA grafts placed subcutaneously in rat model
Study design and animals
The animals were acclimated for a week before the surgical procedure. A total of 16 rats were randomly divided into 2 groups (8 rats in each group). The first group acted as a control group, in the second group 4 hDA (Method 2) grafts were transplanted in the right femoral artery and 4 hDA (Method 2) grafts were transplanted in the left femoral artery. In the control group, the same surgical procedure was performed: an excised artery segment was implanted at its position. Clopidogrel (1.5 mg/kg/day) and Aspirin (5.5 mg/kg/day) as anticoagulants were administered in the drinking water beginning three days before surgery.
Transplantation surgery
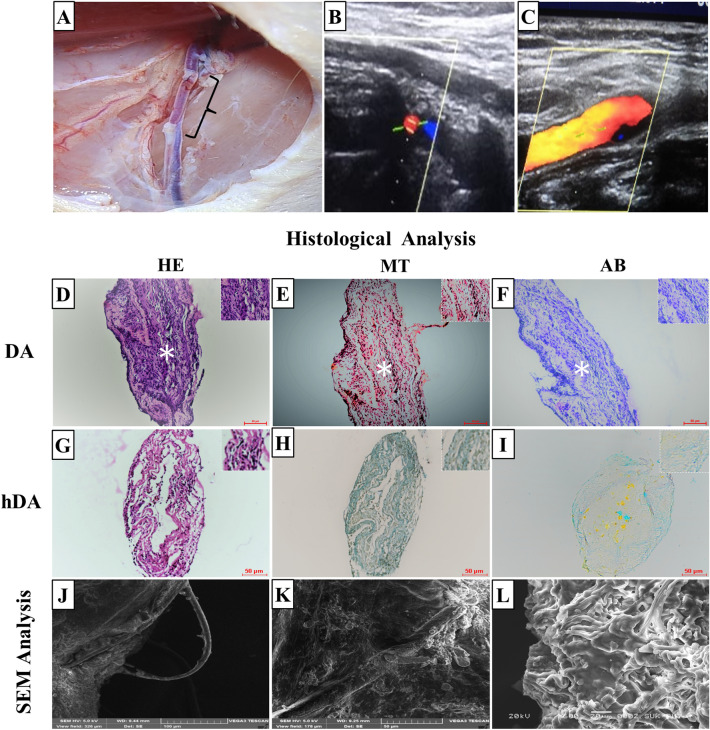
Rats were anesthetized with Inj. Thiosol (45 mg/kg/body wt.) by intraperitoneal route. Inj. Heparin (Caprin, Samarth Lifesciences Pvt Ltd, Himachal Pradesh, India) (100 U/kg) was administered intraperitoneally before surgery and half dose (50 U/kg) everyone hour, was administered. At the inguinal site, oblique incision was taken, and skin and fat tissue were cut to expose femoral vessels. The femoral artery was dissected and separated from the femoral vein. At the proximal and distal region micro clamp was applied in the femoral artery and between the clamps, 7–8 mm arterial segment was excised. The hDA (Method 2) graft in the length of 10 mm was used to substitute for the excised artery segment in the femoral artery. End-to-end anastomosis was done with 8–0 monofilament sutures (Fig. 3). After confirmation of no leakage from the anastomosis site, micro-clamps at the distal and proximal regions were removed. The fat tissue was sutured by 7–0 prolene and the skin was closed in an interrupted manner. During the surgical procedure, an animal’s body temperature was maintained at 37 °C by using a heating pad. After surgery animal was returned to the cage and allowed to freely access food and water.
Fig. 3.
A hDA graft transplant in right femoral artery of rat (B, C) color doppler images after one month of transplantation. D–I Histological analysis of DA and hDA transplanted grafts. Hematoxylin Eosin stain (D, G), Masson’s trichrome stain (E, H), and Alcian blue stain (F, I) images of transplanted DA and hDA graft in rat femoral artery. D–F Graft completely occluded with thrombus observed in lumen of DA graft. Thrombus indicated with white artistic. J–L SEM analysis showed cellular lining on surface of transplanted hDA graft
Blood investigation after transplantation surgery
Blood samples were collected from retro-orbital sinus and collected in two vials (1 ml in each vial), with and without anticoagulant day before transplant and 30 days later. Hematological parameters including hemoglobin (Hb) (g/dL), packed cell volume (PCV; %), total red cell count (RBC), total white cell count (WBC; (lymphocytes, monocytes, neutrophils, eosinophils, and basophiles) were calculated morphologically by blood smears), absolute erythrocyte indices, differential WBC count. Separated serum was utilized for evaluation of serum biochemistry parameters such as liver enzymes and creatinine and urea were evaluated on Erba Chem-5 Plus (Erba Mannheim, Germany) semi-autoanalyzer using commercial reagent kits. CBC was performed using Abacus (Nihon Kohedon, Japan) hematology analyzer.
Postoperative evaluation
For evaluation of patency of transplanted graft, arterial color doppler (Philips, Japan) was done on the 45th day in the control and experimental group. Total 7 animals were euthanized after 3 months with anesthesia overdose. After skin and fat tissue reopened, arterial graft explanted with native artery from proximal and distal end for histochemical and electron microscopy analysis.
Explanted specimens cut into segments and were fixed in 10% neutral buffered formalin for histology and immunofluorescence analysis and in 4% glutaraldehyde for electron microscopy analysis.
Immunofluorescence analysis
For the immunofluorescence analysis, 3 µm thickness sections of Paraffin-embedded tissue were used. The sections were processed by xylene, alcohol grading for rehydration. Tris citrate buffers were used for heat induced epitope retrieval techniques. Goat and rabbit serum were used for blocking. Then the samples were incubated with primary antibodies and fluorescence secondary antibodies [41]. The primary antibodies for endothelial cell biomarkers were von Willebrand factor (vWF; Cat. #PA5-16,634, 1/100 dilution, Thermo Fisher Scientific), vascular endothelial growth factor (VEGF-A; Cat. #bs-1665R, 1/400 dilution, Bioss Antibodies. The primary antibodies for vascular smooth muscle biomarkers were Alpha-smooth muscle actin (Cat. #MA1-06,110, 1/1000 dilution, Invitrogen), β-actin (C4; Cat. #sc-47778, 1:500 dilution, Santa Cruz biotechnology). The primary antibodies for cell adhesion were VCAM-1 (E-10; Cat. #sc13160, 1/200 dilution, Santa Cruz Biotechnology), VAP-1 (A-8; Cat.#sc-166713, 1/100 dilution, Santa Cruz Biotechnology). The primary antibodies for cell migration and antigen presentation Anti–CD3 Antibody ((PS1) Cat. #sc-59013, 1:100 dilution, Santa Cruz Biotechnology), Anti-CD4 Antibody ((W3/25); Cat. #sc-20079, 1:500 dilution, Santa Cruz Biotechnology). The primary antibodies incubated at 4 °C overnight. The secondary antibodies Goat anti-mouse (Alexa Fluor Cat. #A-11001, 1:500 dilution, Invitrogen) & Donkey anti-rabbit (Alexa Fluor Cat. #A-21206, 1:500 dilution, Invitrogen) incubated at room temperature for 1 h. The fluorescence reagent DAPI (4′6-diamidino-2 phenylindole, dihydrochloride) (Cat. #D1306, 1/2000 dilution, Life Technology) was for cellular nuclei visualization. The various biomarkers were visualized using Nikon Ti inverted microscope (Nikon Eclipse Ti, Japan) with Nikon Nis Elements software, ver. 5.2 (Nikon, Japan).
Statistical analysis
Data are reported as the mean ± SD. Comparisons between the two groups were performed by Student's t-test. Differences between three or more groups were assessed using one-way ANOVA. Significance level p = 0.05 was set for all the tests. In the figures, statistical significance is denoted as * for p-value ≤ 0.05, ** for p-value ≤ 0.001, *** for p-value ≤ 0.0001.
Results
Decellularization methods
On the basis of histological analysis, DAPI staining, DNA quantification and mechanical properties of all artery scaffolds, we found that scaffold obtained from decellularization by method 2 possess all the qualities needed for transplantation in rat model.
Histological characterization of scaffold
HE stain
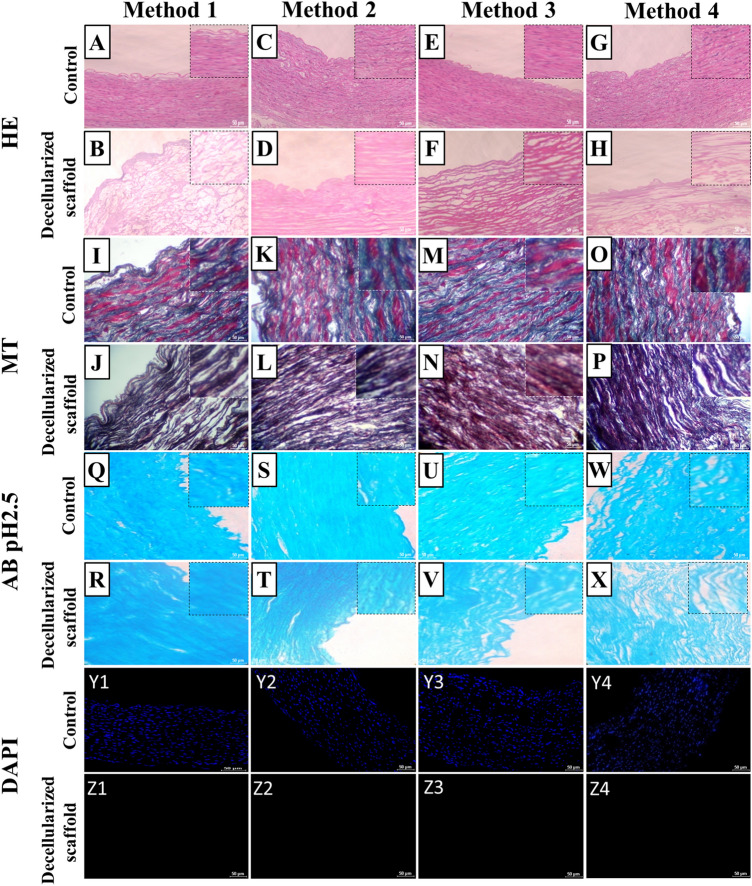
Process of decellularization was cross checked at each cycle with H & E staining to keep track of cell removal. It was observed that complete decellularization was achieved by the 4th cycle in all methods (Fig. 4). In DA (Method 2) scaffold showing complete removal of cells with intact microarchitecture (Fig. 4C, D). H&E staining demonstrated the decellularized scaffolds free of nuclei in all methods. In method 1, 3 and 4 HE is showing complete removal of cells, but microstructure of scaffold was disturbed (Fig. 4A–H).
Fig. 4.
Histochemical study of control (native goat artery) and decellularized scaffolds obtained by different methods. (A to H) HE image of native tissue and complete cell free DA scaffolds. In method 2 decellularized artery scaffold (D) showed intact intimal and medial structure compared to other three methods. (I to P) Masson’s trichrome image of native artery and DA scaffold. Q to X Alcian blue stain images of native artery and DA scaffold. (Y1 toY4, Z1 to Z8) DAPI images of native vessel and DA scaffolds obtained by different decellularization methods. In all DA scaffolds blue stain absent it indicates that complete removal of cellular componenet. Scale bar, 100 μm
MT stain
Masson’s Trichrome stain was used to see the presence of collagen in blood vessels and compared native vessels. MT staining indicated that collagen fibers of the ECM retained in scaffold resulted from all methods. Results were promising and an appreciable amount of collagen was seen being present in treated vessels when compared to native. Method 1, Method 2 and Method 3 (Fig. 4I–N) showed good results compared to method 4 (Fig. 4O, P).
AB pH 2.5 stain
The Alcian Blue stain showed glycosaminoglycans (GAGs) contents were preserved in the scaffolds prepared by all 4 methods (Fig. 4Q–X). Out of all four methods used Method 2 and Method 3 showed optimum results.
DAPI stain
DAPI staining revealed that complete decellularization was achieved with removal of all cell nucleuses by 4th cycle in all methods (Fig. 4Y1–4, Z1–4).
Scanning electron microscopy (SEM) of scaffolds
The reported images refer to the luminal surface of the vessels; no evidence of major damage can be observed after the decellularization process. Scanning electron microscopy of Method 1 showed (Fig. 1B) intact elastic and muscle tissue in their tunica media and porous decellularized membrane. Whereas SEM of Method 2 showed (Fig. 1C) preserved ECM including elastic fibers or elastin. In Method 3 ultrastructure of the ECM appeared to be well-preserved following decellularization with thick bundles of fibrous collagen; at higher magnification nano-fibrous collagen with minimal damage to the matrix during decellularized protocol was observed (Fig. 1D). Method 4 showed (Fig. 1E) collagen network architecture and parallel arranged collagen fibers.
DNA quantification
The spectrophotometric analysis revealed that the decellularization process removed the majority of the DNA content in the treated tissue compared to native one in Fig. 1F. Compared to native vessels Method 1 showed that there was no DNA content in the scaffold. Method 2 and Method 3 also showed fairly less DNA content whereas the highest amount of DNA content was observed in Method 4 compared to all methods. ANOVA was calculated from readings obtained and it showed untreated vessels were statistically significant to all three Methods used except for Method 4. After subcutaneous implant and transplanted graft DNA content showed no statistical difference compared to native vessel DNA content it indicates that cellular component was present in both scaffolds after subcutaneous implant and transplanted graft.
Mechanical properties
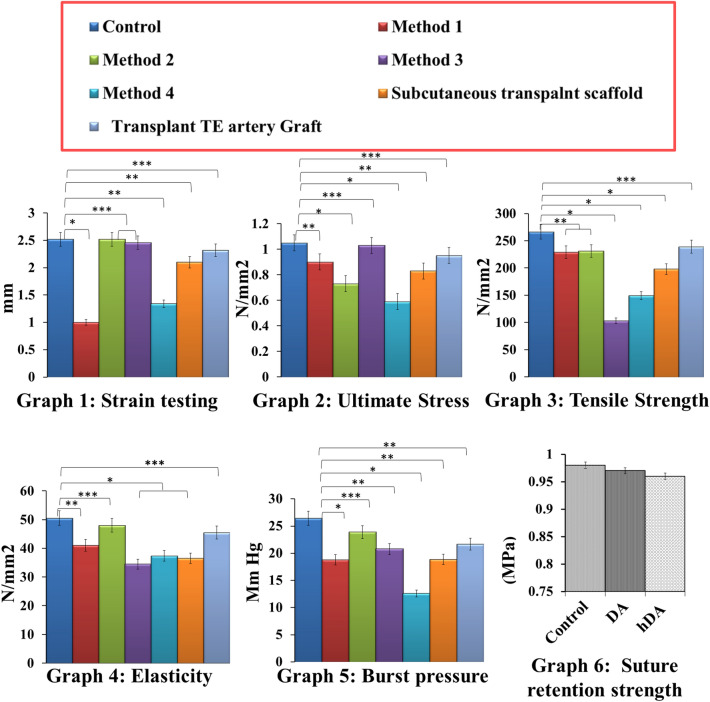
Values obtained from mechanical testing are shown in Fig. 5. The mechanical testing analysis resulted in no statistically significant differences for Young’s modulus, ultimate circumferential stress, elasticity and burst pressure on the other hand, comparison between native and decellularized vessels showed there was not much significant loss in ultimate strain and elasticity of Method 1 and Method 3 samples but Method 2 and Method 4 showed significant loss of ultimate strain and elasticity (Graph 1 to 5). The decellularized vessels hold promises of in vivo performance closely resembling the physiological ones. Considering the overall outcome, the decellularization process leads to limited modifications of the treated tissues compared to native ones.
Fig. 5.
Mechanical testing of control (Native goat artrey), different decellularized artery scaffold obtained from different decellularization methods, subcutaneously transplanted scaffold (DA) and transplantation Studies (hDA). Mechanical testing of all samples compared with native HSV. Graph1: Strain testing in DHSV was significantly lower, after implantation in CAM, subcutaneous transplantation, and graft transplantation there was no significant difference. Graph2: Stress test was showing in native and after graft transplantation, there was no significant difference. Graph3: Tensile strength in native HSV and biocompatibility in CAM, subcutaneous implant, and transplanted graft was a significant difference. Graph4: Elasticity testing showing there was no significant difference in biocompatibility in CAM, subcutaneous implant, and transplanted graft. Graph5: Burst pressure statistically no difference in native HSV and transplanted graft. Graph6: Suture retention strength (MPa) measured of control (Native goat artery), decellularized scaffold and heparinized graft. The suture retention strength of all three groups shows no significant difference and no harmful effect by loading of heparin in decellularized scaffold
Suture retention strength
The average strength recorded was 0.98 ± 0.10, 0.90 ± 0.12, and 0.89 ± 0.12 MPa, respectively in control (Native artery), decellularized scaffold DA and hDA. The result showing that there was no significant difference in the suture retention strength among all three groups indicating that there were no harmful effects of decellularization and heparinization on mechanical property of the grafts (Fig. 5).
Validation of heparin treatment
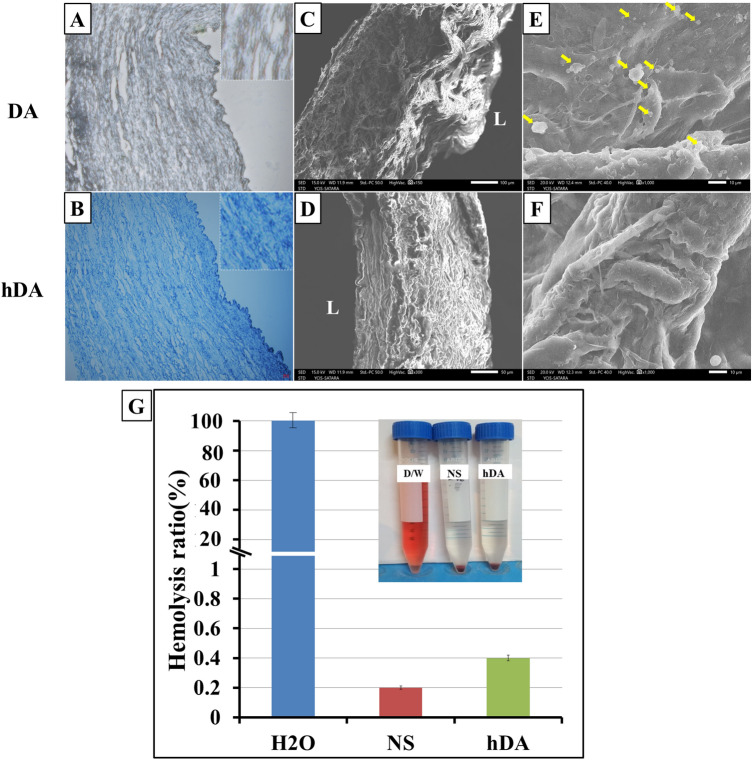
Toluidine blue staining revealed that grafts with heparin treatment showed positive staining in vessel walls (Fig. 6A) and without heparin-coated decellularized scaffold DA did not show toluidine blue stain (Fig. 6B). These results confirmed that heparin was well linked through the entire thickness of decellularized grafts.
Fig. 6.
A, B Toluidine blue stain for immobilized heparin after coating in decellularized scaffold. B In hDA blue stain indicates immobilization of heparin in scaffold. Scanning electron microscopy of DA and hDA vascular scaffold. C, D Cross-sectional scan of DA and hDA (E) inner surface scan of the DA (F) inner surface scan of heparin coating decellularized artery graft after platelet adhesion test. The arrowheads indicate adhered platelets on luminal surface of DA. (L-Luminal area) (G) Hemolysis assay for hDA where distilled water (DW) and 0.9% NaCl (Normal saline NS) were used as positive and negative controls, respectively
Anticoagulation assay
Data summarizes in Table 1 obtained in these tests. Data showed that all clotting times for hDA were significantly larger than DA. It was noted that all clotting times for hDA exceeded the instrument limit set (PT, 50 s; APTT, 150 s; and TT, 200 s). It indicates that heparin coating on decellularized artery enhance the anticoagulant activities.
Table 1.
Coagulation times for PDS and HEP-PDS
| Surface type | PT (s) | APTT (s) | TT (s) |
|---|---|---|---|
| DA | 12.6 ± 1.4* | 31.7 ± 0.2* | 21.3 ± 0.1* |
| hDA | > 50 | > 150 | > 200 |
DA: decellularized artery scaffold obtained by method 2; hDA: heparin-coated decellularized artery scaffold PT: prothrombin time; APTT: activated partial thromboplastin time; TT: thrombin time
*p < 0.05
Hemolysis assay
It is reported that blood clotting will happen when red cells are exposed to water. When RBC contact with allogenic or xenogeneic material then the condition of the red will be aggravated. For evaluation of blood compatibility of biomaterials, hemolysis test is an important index. With high hemolysis rate indicates the bad blood biocompatibility. According to ISO 10993-4, materials with hemolysis values < 5% are considered as safe. The results of the hemolytic studies of had with human RBC for 2 h of incubation are shown in Fig. 6G. Our study finds that the hemolysis rate for hDA is 0.4. The hemolytic ratios smaller than 5.0%, which means no hemolytic activities.
Platelet adhesion test
Thrombus formation in vascular graft is another important issue in implantation of vascular grafts. The implantable vascular grafts need appropriate amendment otherwise they cause thrombosis. A platelet adhesion test was performed to evaluation the effect of surface modification with heparin coating for the risk of thrombosis. If the platelets attach on the surface, the material or graft will be considered thrombogenic. As shown in Fig. 6C–F SEM analysis of the morphologies of the platelets adhered on the DA and hDA surface to study the thrombogenicity of the modified surfaces. Many platelets were found on the DA surfaces (Fig. 6C, E). Platelets not adhered to had surface, suggesting improved hemocompatibility. From Fig. 6D, F hDA had more anti-thrombogenic behavior than the DA due to surface modification with heparin coating. These observations indicate that the DA surface was thrombogenic. These platelet adhesion results suggest that hDA successfully inhibiting the attachment of platelets.
In vivo implantation surgery
We have observed that after implantation of scaffold in subcutaneous space and graft transplant in rat femoral artery did not show any postoperative infection at surgical site and all animals survived after transplant surgery. Weight gain or weight loss did not occur after surgery.
Assessment of the body and organs weight of animals
Body weight measured on day 1, 7, 30, 60, 90 demonstrated that all experimental rats lose weight in the first week. (Table 2). From the second week, all experimental rats gain their normal weight compared with the control group. There was no significant result found for the body weight of the experimental groups after a 3 month study period. There was no significant difference observed in liver, kidney, lung, heart weight of graft transplanted rats group compared to the control (without graft transplant) rats (Table 3).
Table 2.
Comparison of the body weight of rats during the three month study period
| Day Weight of the rats (mg) | Control group (Without graft transplant) | Graft transplanted group |
|---|---|---|
| 1 | 228.85 ± 14.92 | 234.34 ± 12.32 |
| 7 | 230.50 ± 14.94 | 213.97 ± 10.02 |
| 30 | 229.47 ± 15.43 | 232.97 ± 12.29 |
| 60 | 232.33 ± 15.08 | 232.98 ± 09.64 |
| 90 | 232.15 ± 13.98 | 233.43 ± 13.56 |
Data was analyzed by one-way measures ANOVA. Here, N = 7 for both groups
Significant value was considered as *p < 0.01
Table 3.
Weight comparison of the organs of the experimental rats at the end of the experiment period
| Weight of the organs (mg/kg body weight) | Control group | Graft transplanted group (Without graft transplant) |
|---|---|---|
| Kidney | 1.29 ± 0.06 | 1.30 ± 0.09 |
| Liver | 7.12 ± 0.19 | 7.13 ± 0.30 |
| Lungs | 0.94 ± 0.15 | 0.94 ± 0.02 |
| Heart | 0.71 ± 0.02 | 0.70 ± 0.01 |
Data are expressed as Mean (± SD). Sample t-test was used for the analysis. N = 7 for both groups
Here, *p < 0.05, compared to the healthy control are significant
Hematology and biochemical analysis
In the control (without graft transplant) and graft transplanted group there was no significant difference in CBC and differential leukocytes counts after 30 days (Table 4). Evaluation of serum biochemistry parameters, including serum AST, ALT, ALKP, urea, creatinine demonstrated no significant differences in both control and graft transplanted animals (Table 5). The hematology and biochemistry data indicate the existence of the decellularized graft had no harmful effect after transplantation in the host animal. The values in a normal range for controls and treated animals were of the Charles River Laboratories reference [42].
Table 4.
Complete blood count measured after 30 days of graft transplantation in rats. [Reference values from: Baseline Hematology and Clinical Chemistry Values for Charles River Wistar Rats (1998)]
| CBC | Reference range | Control group (without graft transpalnt) |
Graft transplanted group |
|---|---|---|---|
| (Mean ± SD) | |||
| Hb (g/dl) | 14.1–17.1 | 15.34 ± 0.11 | 15.88 ± 0.24 |
| WBC (cells/μl) | 4.77–12.08 × 103 | 7.35 × 103 ± 0.67 | 6.11 × 103 ± 2.65 |
| RBC (cells/μl) | 6.86–8.75 × 106 | 7.22 × 106 ± 0.78 | 8.01 × 106 ± 1.42 |
| Plt (cells/μl) | 500–1300 × 103 | 924.00 × 103 ± 123.22 | 911.11 × 103 ± 122.56 |
| Neu (cells/μl) | 0.68–3.87 × 103 | 0.47 × 103 ± 0.32 | 0.46 × 103 ± 0.14 |
| Lymph (cells/μl) | 3.20–11.84. × 103 | 6.32 × 103 ± 0.12 | 5.38 × 103 ± 0.25 |
| Mono (cells/μl) | 0–0.72 × 103 | 0.05 × 103 ± 0.02 | 0.06 × 103 ± 0.01 |
| Eos (cells/μl) | 0–0.36 × 103 | 0.03 × 103 ± 0.04 | 0.04 × 103 ± 0.02 |
| Baso (cells/μl) | 0–0.24 × 103 | 0.02 × 103 ± 0.03 | 0.02 × 103 ± 0.005 |
All values are expressed as mean ± SD (N = 7). Abbreviations: Hb, hemoglobin; WBC, white blood cell; RBC, red blood cell; Plt, platelets; Neu, neutrophils; Lymph, lymphocytes; Mon, monocytes; Eos, eosinophils; Baso, basophils
* Statistically significant difference (p < 0.05)
Table 5.
Biochemistry analysis after 30 days of graft transplantation in rats. (Reference values from: Baseline Hematology and Clinical Chemistry Values for Charles River Wistar Rats (1998))
| Reference range | Control group (Without graft transplant) |
Scaffold implanted group | |
|---|---|---|---|
| (Mean ± SD) | |||
| Urea (mg/dl) | 21.43–45 | 29.23 ± 2.78 | 31.22 ± 1.15 |
| Cr (mg/dl) | 0.4–1 | 00.62 ± 0.01 | 00.61 ± 0.07 |
| AST (IU/L) | 39–84 | 62.39 ± 16.24 | 64.38 ± 28.63 |
| ALT (IU/L) | 35–80 | 55.14 ± 12.37 | 52.16 ± 21.26 |
| ALKP (IU/L) | 16–50 | 42.18 ± 14.18 | 41.32 ± 34.16 |
All values expressed as means ± SD (n = 7) Abbreviations: Cr, creatinine; AST, Aspartate transaminase; ALT, Alanine aminotransferase; ALKP alkaline phosphatase
*Statistically significant difference (p < 0.05.)
Arterial Color Doppler
Arterial Color Doppler (Philips, Japan) was done after 45 days of graft transplantation to check patency of the graft (Fig. 3B, C). The blood flow rate in the control (without graft transplant) and graft transplanted femoral artery of rats were recorded. In control group (n = 7) blood flow rate was 0.61 ± 0.3 m/second. Blood flow rate in graft transplant group (n = 7) at proximal end was 0.57 ± 0.01 m/s and at distal end 0.51 ± 0.01. Result of arterial color doppler indicates that the transplanted graft was patent till 45 days.
Histological studies after subcutaneous implantation
HE: DA and hDA showed (Fig. 2C, D) that cells were engrafted in the intimal and adventitial layer. In hDA graft thin cellular lining was observed on luminal surface of heparin-coated artery scaffold (Fig. 2C, D).
MT: Collagen was observed in both DA and hDA grafts. There was no destruction to collagen structure observed after heparin coating on decellularized artery scaffold (Fig. 2E, F).
AB: Expression of GAGs was observed after subcutaneous implantation of DA and hDA (Fig. 2G, H).
DAPI: After subcutaneous implantation blue stained nucleus were seen in the luminal and outer site of both DA and hDA scaffold (Fig. 2I, J). It indicates that cells were attached on scaffold.
DNA quantification: There was no statistical difference between DNA content in subcutaneously implanted DA graft compared to native vessel. It indicates that cellular component was present in DA after implant subcutaneously in rat model (Fig. 1F).
SEM analysis: In subcutaneous transplant scaffold cellular lining were observed on luminal and outer surface of both DA and hDA scaffold (Fig. 2K, L).
Mechanical properties in in vivo studies
There was no significant statistical difference in subcutaneous implanted scaffold and transplanted graft compared to native vessels. No significant loss in ultimate strain, tensile strength and elasticity of subcutaneously implanted scaffold and transplanted graft (Graph1, 2 and 4). Young’s modulus (elasticity) and burst pressure showed one-way ANOVA p < 0.001 for all groups.
Histological studies after transplantation
HE: Transplantation of DA graft showed early thrombus fornation. Figure 3A showed that total occlusion of DA graft after transplantation in rat model. After 3 months of transplantation in a rat femoral artery, the graft scaffold recruited cells in all three layers of a scaffold resulting in preservation of the microvascular structure (Fig. 3G).
MT: After transplantation of hDA in rat femoral artery, collagen fibers were more accumulated in hDA shown in Fig. 3H. In DA graft after transplantation, collagen stain observed in Fig. 3E.
AB: After transplantation of hDA in rat femoral artery, GAG was preserved well compared to native vessel shown in Fig. 3I. GAG was observed in DA graft after transplantation in Fig. 3F.
DNA quantification: Data showed that DNA content in hDA graft after transplant in rat femoral artery was equal amount as compared to native vessel DNA. There was no statistical difference between transplanted hDA and native vessel DNA (Fig. 1F).
SEM analysis: In grafted hDA scaffold, SEM images revealed newly formed capillary on graft and also cell lining on luminal and outer site of tubular scaffold (Fig. 3J–L).
Immunohistochemistry
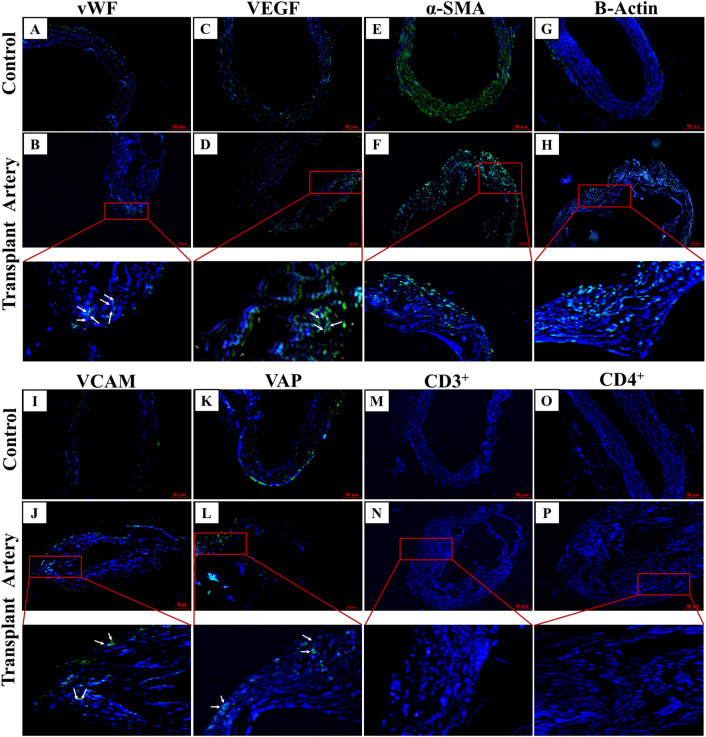
The immunohistochemical results of the native and explanted grafts showed in Fig. 7. Endothelial cells expressing vWF were observed in the lumen of the native artery and transplanted xenografts (Fig. 7A, B). VEGF positive cells were observed in both native arteries and explanted xenograft but VEGF was weak in the xenograft (Fig. 7C, D). Smooth muscle cells expressing α-SMA were observed in the tunica media of the native artery and transplanted xenograft (Fig. 7E, F). In xenograft α-SMA positive cells were abundantly observed (Fig. 7F). β-actin positive cells were observed in explanted xenograft in a mid-layer and adventitial region (Fig. 7G, H). Expression of VCAM was observed in adventitial layer in native arteries and explanted xenograft (Fig. 7I, J). VAP positive cells were also detected in the sub-endothelial region of the intima, as well as the adventitia, of the explanted xenografts (Fig. 7K, L). Specific localized CD3+ cells were observed in the adventitia of the explanted xenografts, but it was absent in native vessels (Fig. 7M, N). CD4+ cells were absent in both native vessel and transplanted graft (Fig. 7O, P).
Fig. 7.
(A–P) Immunohistochemistry analysis of transplanted hDA graft done by vWF, VEGF, α-SMA, VCAM, B-Actin, VAP and immunological respose were analysed by CD3 + , CD4 + markers. B Edothelial cells were stained with von Willebrand factor (vWF, green) and F Smooth muscle cells are stained with α-Smooth muscle actin (α-SMA, green). Scale bars: 50 µm
Discussion
The aim of present study was to generate a small diameter xenogenic arterial graft with luminal surface modification to improve hemocompatibility of vascular grafts for clinical applications. Our work was focused on generating small diameter decellularized arterial xenografts (< 1 mm) with retaining the ability of arterial compatibility, biomechanical integrity and less immunogenic under in vivo conditions. Xenogeneic decellularized tissue-engineered small diameter vascular grafts have excessive possibility of an ideal substitute for limited autologous vein grafts. SDS is an anionic surfactant and strong detergent, widely used for tissue or organs decellularization with the different concentration. In recent studies it was reported that SDS in lower concentration more effective for decellularization of porcine carotid artery [43]. Sodium Deoxycholate is an ionic detergent that is especially useful for disrupting and dissociating protein interactions. In another study, combination of SDS and SDC were used for decellularization of bovine pericardium in lower concentration [44]. Dimethyl sulfoxide (DMSO) is a colorless solvent widely used as a cryopreservative in cryopreservation of cells, tissues and organs. Another property of DMSO is used as a penetration enhancer in layers of skin without causing much damage [45]. Study was performed by Selcan Guler and group, they found that use of DMSO does not only decline exposure time of ionic SDS, but also preserves ECM structure and important constituents of ECM [35, 46]. Different decellularization methods using SDS, Sodium deoxycholate (SDC) and DMSO were employed on goat arteries. Among the all 4 methods, we obtain an ideal xenograft scaffold from method 2 with an intact 3D natural geometrical alignment of ECM following complete and effective decellularization of the goat artery using anionic detergent SDS and SDC in lower concentration. Preservation of ECM is a key factor for recruitment and proliferation of native cells in scaffold [47]. Histological analysis showed that combination of 0.5% SDS and 0.5% SDC preserved histoarchitecture intact compared to other methods used. HE showed that the preservation on the luminal layer in method 2 and mechanical integrity of artery collagen and elastin was intact in method 2 confirmed by MT staining. Alcian blue stain used for staining of GAG which is another important factor for preventing the calcification formation in blood vessels which was well preserved by method 2 [48]. Thus, GAG and Collagen are key components of scaffolding materials that can imitate the natural matrix niche and play important roles in cellular behavior, migration, proliferation and cell differentiation [49]. Quantification DNA content was performed for the determination of removal of cells from the scaffold. Complete removal of DNA from biological scaffolds is very important or it causes inflammatory response in patients [50]. Result showed that in method 2, there was complete removal of the DNA component achieved.
Tong et al. showed that transplanted porcine decellularized vascular graft after surface modification patency remained for 6 months in diabetic ischemic lower limb in humans [51]. This result potentially concretes the way for resolving a problem of extensive clinical need, i.e., the necessity for small-diameter vascular grafts. Use of synthetic vascular conduit for repair of damaged blood vessels has been reported since the 1950 [52]. Advancement in new techniques in developing small diameter vascular grafts from biological and synthetic materials have been successful but there is a lack of achievement with total functional graft has remained unclear as a result of thrombus formation, calcification, intimal hyperplasia, decreased compliance, decreased elasticity and aneurysmal failure [16, 53–55]. Although numerous advantages, graft patency is a major problem in small diameter vascular grafts developed from biological or synthetic materials [56]. To enhance the function of material, surface modification is essential because degradation of surface directly affects the functionality of material and surface functionality is beneficial in tissue engineering and regenerative medicine [31]. Skovrind et al. conclude in their data survey, scaffold surface modification with recellularization with endothelial cells inside the lumen of the TEVG for several days is an essential and preconditioning for patency [57]. In vascular grafts surface modification of biomaterials and endothelialization in luminal surface are common tactics for improving hemocompatibility and to achieve long-term patency of artificial vascular grafts [58]. To maintain long-term vascular patency and reduce thrombosis, suitable endothelial cell seeding methods should be designated in diverse atmospheres for vascular endothelialization [59]. In much research it is stated that heparin immobilization on a decellularized scaffold can prevent thrombus formation [60]. Along with clotting time platelet response to the foreign material are the parameters of blood compatibility. It has been reported that heparin coating surfaces reduce platelet adhesion. After heparinization of biomaterials it prevents adsorption of nonspecific protein and improves cell attachment, proliferation and differentiation. Such modified surfaces have proved anticoagulant actions, help in angiogenesis and shown good hemocompatibility and cytocompatibility effects in vitro and in vivo [61].
Small-diameter artificial vascular grafts less than 6 mm inner diameter shows poor performance leading to risk of thrombosis [62]. Yu et al. showed in their in vitro experiment, heparin coating in PTFE vascular graft promote human umbilical vein endothelial cell (HUVEC) proliferation but inhibit human umbilical artery smooth muscle cell (HUASMC) growth and obtain the satisfactory hemocompatibility [63]. Wenhui et al. showed in their experiments that electrospun nano-PCL coating around decellularized rat aorta (lumen diameters from 1.5 to 2.0 mm) and lumen was coated with heparin to modify the acellular vascular intima, patent for 6 weeks after interposition transplant in abdominal aorta in rats [64]. In another study heparin were incorporated into a synthetic vascular graft showed rapidly endothelialized within 30 days with good biocompatibility and maintained patency after transplant in rabbit carotid artery [65]. Mostly transplant study of heparin-coated synthetic or biosynthetic vascular graft’s inner diameter were more than 6 mm. In our study, we were succeed to develop very small size xenogeneic heparin-coated vascular grafts (diameter < 1 mm).
In animal experiments it was revealed that heparin coated graft implanted subcutaneously, and transplanted graft showed DNA content in abundance. SEM analysis was performed for scaffold morphology and cellular attachment on heparinized graft after transplantation in animals. In explanted grafts, newly formed blood capillaries were revealed by SEM (Fig. 3). In our study we found that no significant differences in mechanical properties in terms of strain, tensile strength, elasticity, burst pressure and suture holding strength of decellularized arteries compared with native arteries. In vivo experiments revealed that heparinized decellularized grafts had 92% patency rate at 3 months after transplantation. Color doppler was performed after 45 days of graft transplantation and showed that graft was patent till 45 days of transplant. Hsia K showed that surface modification with sphingosine-1-phosphate possibly will prevent thrombus formation and promote reendothelialization to improve the patency rate of decellularized vascular grafts in vivo [66].
Cellular infiltration after transplant in hDA was confirmed by immunohistochemistry analysis. Anti-thrombogenic and anti-inflammatory molecules such as nitric oxide, prostacyclin, thrombomodulin secreted by endothelial cells in the luminal layer of vessel [67]. In previous study it reported that host endothelial and progenitor cell repopulate on acellular graft turns into functional multilayered living blood vessel after transplant in human [68]. vWF positive cells were present intimal layer of transplanted hDA it indicates that neointimal layer was formed after transplantation (Fig. 7B). Yamanaka et al. showed that surface modified with synthesized peptides of decellularized small vascular bioscaffold material promotes neointima formation remained patent for three weeks [69]. Vascular tone maintained by smooth muscles cells and its expression in graft indicates maturation of blood vessels and which is regulated by endothelial-derived and circulating agonists and antagonists factors [70, 71]. Expression of α-Smooth muscle actin in transplanted hDA (Fig. 7F) denotes the presence of vascular MSCs which may be beneficial for maintaining graft compliance, and long-term patency [72]. Vascular endothelial growth factor (VEGF) is a signaling molecule involved in vasculogenesis and angiogenesis which were present in outer layer of transplanted graft [73]. β-actin positive cells are also vastly expressed in the transplanted graft (Fig. 7J) which shows the regulation of the vascular nature of the graft. VCAM used as a surface marker for endothelial cells and direct leukocytes movement towards the inflamed tissue [74]. Expressions of CD3 and CD4 were very low in the transplanted graft which suggests that the graft was well accepted and there was no any immune response after transplantation. Immunohistochemistry study of transplanted graft showed that endothelial cells were attached on the luminal side of the transplanted grafts. The heparinization of graft indicates its anticoagulant effect on the early endothelialization which efficiently improved the patency rate of blood vessels. Formation of the vascular endothelium revealed by immunohistochemistry and scanning electron microscopy.
We conclude that xenogeneic decellularized scaffolds using combination of detergents and modified in luminal area with immobilized heparin improve re-endothelialization. Surface modified heparinized small diameter vascular grafts resist early thrombus formation. Transplanted graft was repopulated by host cells resulted in functional multilayered living blood vessel. This xenogeneic small diameter vascular graft is a promising approach for the treatment of patients undergoing coronary artery bypass graft surgery (CABG) and/or peripheral arterial diseases and in vascular complications.
Acknowledgements
Authors are grateful to the Department of Botany, Shivaji University, Kolhapur for extending SEM facility. Dr. Meghnad G. Joshi acknowledges the research funding support from the Department of Science and Technology (DST), Govt. of India (SB/SO/HS/0198/2013) and D.Y. Patil Education Society Deemed University (DYPES/DU/R&D/3104). M. J. conceived the study and designed the experiments. K.T., T.M., N.B., L.C. performed the experiments. M.J. reviewed, M.J., J.K. analysed and interpreted the data. M. J. and K. T. wrote the manuscript. All authors contributed to the analysis of the data and discussed the manuscript.
Declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical statement
The animal studies were performed after receiving approval Institutional Animal Ethical Committee (IAEC). D Y Patil Education Society,(Deemed Universty) Kolhapur, India. (Approval No: Item No.04 - Sub-item 7).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg M, Gunn J, Rautava P, Sipilä J, Kytö V. Outcome of acute myocardial infarction versus stable coronary artery disease patients treated with coronary bypass surgery. Ann Med. 2021;53:70–7. doi: 10.1080/07853890.2020.1818118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kshersagar J, Kshirsagar R, Desai S, Bohara R. Decellularized amnion scaffold with activated PRP: a new paradigm dressing material for burn wound healing Decellularized amnion scaffold with activated PRP: a new paradigm dressing material for burn wound healing. Cell Tissue Bank. 2018;19:423–436. doi: 10.1007/s10561-018-9688-z. [DOI] [PubMed] [Google Scholar]
- 4.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendis S, Puska P, Norrving B, World Health Organization. Global atlas on cardiovascular disease prevention and control. World Health Organization; 2011.
- 6.Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis. 2018;1:379–81. doi: 10.1016/j.atherosclerosis.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association 9. Cardiovascular disease and risk management: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 8.Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS, Blomster J. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;5:32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res. 2015;116:1527–1539. doi: 10.1161/CIRCRESAHA.116.303566. [DOI] [PubMed] [Google Scholar]
- 10.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, Hsia K, Tsai CH, Ma H, Lu JH, Tsay RY. Decellularized porcine coronary artery with adipose stem cells for vascular tissue engineering. Biomed Mater. 2019;14:045014. doi: 10.1088/1748-605X/ab2329. [DOI] [PubMed] [Google Scholar]
- 12.Hielscher D, Kaebisch C, Braun BJV, Gray K, Tobiasch E. Stem cell sources and graft material for vascular tissue engineering. Stem Cell Rev Rep. 2018;14:642–667. doi: 10.1007/s12015-018-9825-x. [DOI] [PubMed] [Google Scholar]
- 13.Carrabba M, Madeddu P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front Bioeng Biotechnol. 2018;6:1–12. doi: 10.3389/fbioe.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muangsanit P, Shipley RJ, Phillips JB. Vascularization strategies for peripheral nerve tissue engineering. Anat Rec. 2018;301:1657–1667. doi: 10.1002/ar.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann FEM, Lamm P, Wellmann P, Milz S, Hagl C, Juchem G. Autologous endothelialized vein allografts in coronary artery bypass surgery—long term results. Biomaterials. 2019;212:87–97. doi: 10.1016/j.biomaterials.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Mahara A, Sakuma T, Mihashi N, Moritan T, Yamaoka T. Accelerated endothelialization and suppressed thrombus formation of acellular vascular grafts by modifying with neointima-inducing peptide: a time-dependent analysis of graft patency in rat-abdominal transplantation model. Colloids Surf B. 2019;18:806–13. doi: 10.1016/j.colsurfb.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Ilanlou S, Khakbiz M, Amoabediny G, Mohammadi J. Preclinical studies of acellular extracellular matrices as small-caliber vascular grafts. Tissue Cell. 2019;60:25–32. doi: 10.1016/j.tice.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y, Ali F. Atherosclerotic cardiovascular disease: a review of initiators and protective factors. Inflammopharmacology. 2016;24:1–10. doi: 10.1007/s10787-015-0255-y. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Chen X, Liang X, Zhang G, Xu J, He L, et al. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci USA. 2011;108:9466–9471. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jun H, Taite LJ, West JL. Nitric oxide-producing polyurethanes. Biomacromolecules. 2005;6:838–44. doi: 10.1021/bm049419y. [DOI] [PubMed] [Google Scholar]
- 21.Zhu AP, Ming Z, Jian S. Blood compatibility of chitosan/heparin complex surface modified ePTFE vascular graft. Appl Surf Sci. 2005;241:485–492. doi: 10.1016/j.apsusc.2004.07.055. [DOI] [Google Scholar]
- 22.Li J, Lin F, Li L, Li J, Liu S. Surface engineering of poly(ethylene terephthalate) for durable hemocompatibility via a surface interpenetrating network technique. Macromol Chem Phys. 2012;213:2120–2129. doi: 10.1002/macp.201200251. [DOI] [Google Scholar]
- 23.Williamson MR, Black R, Kielty C. PCL-PU composite vascular scaffold production for vascular tissue engineering: attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials. 2006;27:3608–3616. doi: 10.1016/j.biomaterials.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Shin YM, Lee YB, Kim SJ, Kang JK, Park JC, Jang W, et al. Mussel-inspired immobilization of vascular endothelial growth factor (VEGF) for enhanced endothelialization of vascular grafts. Biomacromolecules. 2012;13:2020–8. doi: 10.1021/bm300194b. [DOI] [PubMed] [Google Scholar]
- 25.Bai H, Dardik A, Xing Y. Decellularized carotid artery functions as an arteriovenous graft. J Surg Res. 2019;234:33–9. doi: 10.1016/j.jss.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Liu Z, Liu C, Jiang X, Wei Z, Qiao W, et al. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J Biomed Mater Res Part B. 2012;100:111–20. doi: 10.1002/jbm.b.31928. [DOI] [PubMed] [Google Scholar]
- 27.Bai H, Wang Z, Li M, Liu Y, Wang W, Sun P, Wei S, Wang Z, Li JA, Dardik A. Hyaluronic acid–heparin conjugated decellularized human great saphenous vein patches decrease neointimal thickness. J Biomed Mater Res Part B. 2020;108:2417–25. doi: 10.1002/jbm.b.34574. [DOI] [PubMed] [Google Scholar]
- 28.Ji Y, Zhou J, Sun T, Tang K, Xiong Z, Ren Z, et al. Diverse preparation methods for small intestinal submucosa (SIS): decellularization, components, and structure. J Biomed Mater Res Part A. 2019;107:689–697. doi: 10.1002/jbm.a.36582. [DOI] [PubMed] [Google Scholar]
- 29.Zhao P, Li X, Fang Q, Wang F, Ao Q, Wang X, et al. Surface modification of small intestine submucosa in tissue engineering. Regen Biomater. 2020;7:339–348. doi: 10.1093/rb/rbaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng G, Yao D, Niu Y, Liu H, Fan Y. Surface modification of multiple bioactive peptides to improve endothelialization of vascular grafts. Macromol Biosci. 2019;19:e1800368. doi: 10.1002/mabi.201800368. [DOI] [PubMed] [Google Scholar]
- 31.Chen JP, Su CH. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011;7:234–43. doi: 10.1016/j.actbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:pdb.prot4986. [DOI] [PubMed]
- 33.Garvey W. Modified elastic tissue-Masson trichrome stain. Stain Technol. 1984;59(4):213–216. doi: 10.3109/10520298409113858. [DOI] [PubMed] [Google Scholar]
- 34.Yamada K. An acriflavine alcian blue technique for dual staining of cartilage and mast cells in paraffin sections. Yamada Laboratory of Histology, Department of Anatomy School of Medicine, Nagoya Received for Publication November. Acta Histochem Cytochem. 1970;3:1–6.
- 35.Tardalkar K, Desai S, Adnaik A, Bohara R. Novel approach toward the generation of tissue engineered heart valve by using combination of antioxidant and detergent: a potential therapy in cardiovascular tissue engineering. Tissue Eng Regen Med. 2017;14:755–762. doi: 10.1007/s13770-017-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, DiMuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–53. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Kong X, Kong C, Wen S, Shi J. The use of heparin, bFGF, and VEGF 145 grafted acellular vascular scaffold in small diameter vascular graft. J Biomed Mater Res Part B. 2019;107:672–679. doi: 10.1002/jbm.b.34160. [DOI] [PubMed] [Google Scholar]
- 38.Komohara Y, Nakagawa T, Ohnishi K, Kosaki Y, Saito Y, Horlad H, et al. Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin-embedded tissue samples. J Clin Exp Hematop. 2017;57:31–6. doi: 10.3960/jslrt.17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YQ, Ma Y, Xia YY, Shen WD, Mao JP, Zha XM, Shirai K, Kiguchi K. Synthesis of silk fibroin-insulin bioconjugates and their characterization and activities in vivo. J Biomed Mater Res Part B. 2006;79:275–83. doi: 10.1002/jbm.b.30539. [DOI] [PubMed] [Google Scholar]
- 40.Narayan J, Kumar P, Gupta A, Tiwari S. To compare the blood pressure and heart rate during course of various types of anesthesia in wistar rat: a novel experiences. Asian J Med Sci. 2018;9:37–39. doi: 10.3126/ajms.v9i6.20625. [DOI] [Google Scholar]
- 41.Hira VVV, de Jong AL, Ferro K, Khurshed M, Molenaar RJ, Van Noorden CJF. Comparison of different methodologies and cryostat versus paraffin sections for chromogenic immunohistochemistry. Acta Histochem. 2019;121:125–34. doi: 10.1016/j.acthis.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Charles R. Baseline hematology and clinical chemistry values for Charles River Wistar rats (CRL (W) BR) as a function of sex and age. Charles River Technical Bulletin. 1982;1:1–4.
- 43.Cheng J, Li J, Cai Z, Xing Y, Wang C, Guo L, et al. Decellularization of porcine carotid arteries using low-concentration sodium dodecyl sulfate. Int J Artif Organs. 2020;45:497–508. doi: 10.1177/0391398820975420. [DOI] [PubMed] [Google Scholar]
- 44.Li N, Li Y, Gong D, Xia C, Liu X, Xu Z. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact Cardiovasc Thorac Surg. 2018;26:768–776. doi: 10.1093/icvts/ivx416. [DOI] [PubMed] [Google Scholar]
- 45.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–137. doi: 10.1016/j.addr.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 46.Guler S, Aydin HM, Lü LX, Yang Y. Improvement of decellularization efficiency of porcine aorta using dimethyl sulfoxide as a penetration enhancer. Artif Organs. 2018;42:219–230. doi: 10.1111/aor.12978. [DOI] [PubMed] [Google Scholar]
- 47.Schneider KH, Rohringer S, Kapeller B, Grasl C, Kiss H, Heber S, et al. Riboflavin-mediated photooxidation to improve the characteristics of decellularized human arterial small diameter vascular grafts. Acta Biomater. 2020;116:246–58. doi: 10.1016/j.actbio.2020.08.037. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Li B, Jing H, Wu Y, Kong D, Leng X, et al. Swim bladder as a novel biomaterial for cardiovascular materials with anti-calcification properties. Adv Healthc Mater. 2020;9:1–13. doi: 10.11648/j.am.20200901.11. [DOI] [PubMed] [Google Scholar]
- 49.Li YY, Choy TH, Ho FC, Chan PB. Scaffold composition affects cytoskeleton organization, cell – matrix interaction and the cellular fate of human mesenchymal stem cells upon chondrogenic differentiation Biomaterials Scaffold composition affects cytoskeleton organization, cell e matrix. Biomaterials. 2018;52:208–20. doi: 10.1016/j.biomaterials.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 50.Li Q, Chang Z, Oliveira G, Xiong M, Smith LM, Frey BL, et al. Biomaterials Protein turnover during in vitro tissue engineering. Biomaterials. 2016;81:104–13. doi: 10.1016/j.biomaterials.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong Z, Xu Z, Tong Y, Qi L, Guo L. Effectiveness of distal arterial bypass with porcine decellularized vascular graft for treating diabetic lower limb ischemia. Int J Artif Org J. 2021;44:580–586. doi: 10.1177/0391398820980021. [DOI] [PubMed] [Google Scholar]
- 52.Voorhees AB, Jaretzki A, Blakemore AH. The use of tubes constructed from vinyon “N” cloth in bridging arterial defects. Ann Surg. 1952;135:332–6. doi: 10.1097/00000658-195203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang B, Suen R, Wang JJ, Zhang ZJ, Wertheim JA, Ameer GA. Vascular scaffolds with enhanced antioxidant activity inhibit graft calcification. Biomaterials. 2017;144:166–75. doi: 10.1016/j.biomaterials.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong Y, Yao Y, Yim EKF. Current understanding of intimal hyperplasia and effect of compliance in synthetic small diameter vascular grafts. Biomater Sci. 2020;8:4383–4395. doi: 10.1039/D0BM00226G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopera Higuita M, Griffiths LG. Small diameter xenogeneic extracellular matrix scaffolds for vascular applications. Tissue Eng Part B. 2020;26:26–45. doi: 10.1089/ten.teb.2019.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stowell CET, Wang Y. Quickening: translational design of resorbable synthetic vascular grafts. Biomaterials. 2018;173:71–86. doi: 10.1016/j.biomaterials.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skovrind I, Harvald EB, Juul Belling H, Jørgensen CD, Lindholt JS, Andersen DC. Concise review: patency of small-diameter tissue-engineered vascular grafts: a meta-analysis of preclinical trials. Stem Cells Transl Med. 2019;8:671–680. doi: 10.1002/sctm.18-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren X, Feng Y, Guo J, Wang H, Li Q, Yang J, et al. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem Soc Rev. 2015;44:5680–5742. doi: 10.1039/C4CS00483C. [DOI] [PubMed] [Google Scholar]
- 59.Cai Q, Liao W, Xue F, Wang X, Zhou W, Li Y, et al. Selection of different endothelialization modes and different seed cells for tissue-engineered vascular graft. Bioact Mater. 2021;6:2557–68. doi: 10.1016/j.bioactmat.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M, Bao L, Qiu X, Yang X, Liu S, Su Y, et al. Immobilization of heparin on decellularized kidney scaffold to construct microenvironment for antithrombosis and inducing reendothelialization. Sci China Life Sci. 2018;61:1168–1177. doi: 10.1007/s11427-018-9387-4. [DOI] [PubMed] [Google Scholar]
- 61.Aslani S, Kabiri M, HosseinZadeh S, Hanaee-Ahvaz H, Taherzadeh ES, Soleimani M. The applications of heparin in vascular tissue engineering. Microvasc Res. 2020;131:104027. doi: 10.1016/j.mvr.2020.104027. [DOI] [PubMed] [Google Scholar]
- 62.Boni R, Ali A, Shavandi A, Clarkson AN. Current and novel polymeric biomaterials for neural tissue engineering. J Biomed Sci. 2018;25:1–21. doi: 10.1186/s12929-018-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu C, Yang H, Wang L, Thomson JA, Turng LS, Guan G. Surface modification of polytetrafluoroethylene (PTFE) with a heparin-immobilized extracellular matrix (ECM) coating for small-diameter vascular grafts applications. Mater Sci Eng C. 2021;128:112301. doi: 10.1016/j.msec.2021.112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gong W, Lei D, Li S, Huang P, Qi Q, Sun Y, et al. Hybrid small-diameter vascular grafts: anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials. 2015;76:359–70. doi: 10.1016/j.biomaterials.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 65.Zhu T, Gu H, Zhang H, Wang H, Xia H, Mo X, et al. Covalent grafting of PEG and heparin improves biological performance of electrospun vascular grafts for carotid artery replacement. Acta Biomater. 2021;119:211–224. doi: 10.1016/j.actbio.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 66.Hsia K, Lin CH, Lee HY, Chen WM, Yao CL, Chen CC, et al. Sphingosine-1-phosphate in endothelial cell recellularization improves patency and endothelialization of decellularized vascular grafts in vivo. Int J Mol Sci. 2019;20:1641. doi: 10.3390/ijms20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu X, Han L, Kassab GS. In vivo self-assembly of small diameter pulmonary visceral pleura artery graft. Acta Biomater. 2019;83:265–76. doi: 10.1016/j.actbio.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Kirkton RD, Santiago-Maysonet M, Lawson JH, Tente WE, Dahl SL, Niklason LE, Prichard HL. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019 doi: 10.1126/scitranslmed.aau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamanaka H, Yamaoka T, Mahara A, Morimoto N, Suzuki S. Tissue-engineered submillimeter-diameter vascular grafts for free flap survival in rat model. Biomaterials. 2018;179:156–63. doi: 10.1016/j.biomaterials.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 70.Zanetta L, Marcus SG, Vasile J, Dobryansky M, Cohen H, Eng K, et al. Expression of von Willebrand factor, an endothelial cell marker, is up- regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int J Cancer. 2000;85:281–288. doi: 10.1002/(SICI)1097-0215(20000115)85:2<281::AID-IJC21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 71.Sun KH, Chang Y, Reed NI, Sheppard D. α-smooth muscle actin is an inconsistent marker of fibroblasts responsible for force-dependent TGFβ activation or collagen production across multiple models of organ fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;310:L824–L836. doi: 10.1152/ajplung.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, et al. α-Smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- 73.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer. 2010;102:8–18. doi: 10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kung CT, Su CM, Chang HW, Cheng HH, Hsiao SY, Tsai TC, et al. Serum adhesion molecules as outcome predictors in adult severe sepsis patients requiring mechanical ventilation in the emergency department. Clin Biochem. 2014;47:38–43. doi: 10.1016/j.clinbiochem.2014.06.020. [DOI] [PubMed] [Google Scholar]